Abstract
The aim of the project was to investigate Peat-derived Fulvic acid for its propensity to form co-crystals with quercetin and curcumin and characterize it by using different analytical techniques. The formation of co-crystals generally enhances water solubility and the overall bioavailability of molecules. Co-crystals were synthesized using a 1:1 stoichiometric ratio of fulvic acid with quercetin and curcumin, respectively, using solvent crystallization techniques taking tetrahydrofuran and water in a 1:1 v/v ratio. The co-crystals were characterized by spectroscopic methods, FTIR and Differential scanning calorimetry. Further confirmation was made by morphological studies using SEM. A structural analysis was also carried out, using 13C solid-state NMR analysis. The studies confirmed the formation of semi crystalline forms. Furthermore, the saturation solubility displayed the enhancement in solubility of up to 10, 5-folds for Quercetin and Curcumin, respectively. The in vitro dissolution results showed that T50% was achieved within 30 min for both the drugs. The literature supports that the nutraceutical co-crystals offer advantages, particularly in the improvement of biopharmaceutical properties and addressing the challenges of the lab and manufacturing scale process. Both the semi crystalline powders exhibited enhanced solubility and a better dissolution profile.
1. Introduction
Co-crystallization is a technique where more than two molecular and or ionic compounds are directed to be held together to attain a single homogeneous crystalline phase by utilizing intermolecular interactions. Typically, either by halogen (XB) bonding, hydrogen bonding (HBs), Van der Waal’s force, π–π stacking, and other non-covalent interactions [1]. HBs form several supramolecular synthons, i.e., O–H•••O, C–H•••O, N–H•••N, O–H•••N. Amongst them, the O•••H•••N interactions are the most practicable supramolecular synthons for the formation of co-crystals [2]. It requires at least one co-former (CCF) for co-crystal formation, which may be excipient, polymer, organic acids, or other active pharmaceutical ingredient [3]. Usually, the CCF selection is based on its inheritance with the supramolecular synthon hierarchy or its intermolecular interaction ability [4]. The CCF plays a crucial role in altering the raw drug’s physicochemical and mechanical properties in the co-crystal structure. For instance, high solubility co-formers impart enhanced solubility to the co-crystal, while high melting co-formers offer a high melting co-crystal [5]. Thus, several new CCFs have been investigated and found to be fruitful [6,7,8].
A General Prerequisite for Co-Former Includes
- It should be nontoxic;
- Generally, a neutral co-former is of paramount importance as it is unaffected by the “pH” in the microenvironment;
- It should be compatible with other excipients used in the formulation;
- It must tend to form intermolecular hydrogen bonding or strong π-π stacking or halogen bonds with APIs [8].
Fulvic acid (FA) meets all the essential requirements, signifying its usage as a co-former. However, it has not been accepted as a commercial pharmaceutical excipient and is not included in the GRAS category [9,10]. We have already carried out its safety and compatibility evaluation with other excipients [11]. FA is marketed globally as a dietary supplement/nutraceutical/novel food. Japan’s regulatory agency (Ministry of Health, Labour and Welfare) accepted FA as foodstuff in 2004 [12]. This naturally occurring geo-polymer is categorized as a humic substance abundantly found in Peat and other organic deposits [13] and has vital functional groups such as COOH, OH, and NH2 are essential to forming a bond with phytochemicals/APIs. Typically, FA has a unique set of physicochemical properties such as low molecular weight, soluble in water at any pH, and contains substantial acidic functional groups, out of which the majority are carboxylic and phenolic acids [14].
On the other hand, Schnitzer has summarized FA as a group of humic substances assembled by aromatic oxy-carboxylic acids linked to each other via hydrogen bonding [15]. To date, FA derived from shilajit has been mainly employed in supramolecular chemistry in the formation of inclusion complexes (such as cyclodextrins) with water insoluble drugs in the form of a host–guest type of interaction [16,17]. Being soluble in water at all pHs (0–14), it is unaffected by the gastric environment. Nevertheless, few macromolecules such as crown ethers and calixarenes [18,19] (commonly employed in inclusion complexes) were employed successfully in crystallization techniques. So, referring to the prior art, this is the first attempt of the crystallization of phytochemicals using FA to the best of our knowledge. We have also reported a supra-molecular assembly of bacogenins from Bacopa monnieri -Fulvic acid-soy lecithin under the same umbrella project [20].
Recently, nutraceutical co-crystals have begun to offer a wide range of benefits, particularly improving the biopharmaceutical properties and overcoming the shortcomings of the formulation and manufacturing process, as shown by several scientific studies [21,22,23,24]. We selected Curcumin (CUR) and Quercetin (Q) for the given study. Both the drugs, despite their different pharmacological merits, such as antioxidant, anti-inflammatory, and anticancer, also share a common stumbling block, i.e., inadequate solubility which results into reduced bioavailability [25,26]. Several techniques have been implemented to enhance their bioavailability, in which the crystalline forms are considered strikingly more stable and affordable than other nano-formulations [27].
Furthermore, both these phytochemicals offer several hydrogen-bond donor and acceptor sites owing to their flexibility in forming co-crystals. We employed a trial-and-error approach to evaluate FA’s propensity to form co-crystals with phytochemicals in this context. Nevertheless, most drug formulations on the shelf are solids due to their possible benefits such as easy handling, storage, enhanced physical, chemical stability, and cost-effectiveness [28]. A novel solid form can be the subject of patentability and therefore is of commercial interest.
2. Materials and Methods
Both Quercetin (Q) and Curcumin (CUR) (>95% purity) were purchased from Kuber Impex Ltd. (Indore, India). Peat-derived FA was received from NZ Fulvic Ltd. (Mount Maunganui, New Zealand) as a gift sample and the sample was used after lyophilizing the aqueous solution. The extraction process of FA from Peat was mostly reliant on the acid-base method of extraction, which was not divulged to the authors. At the same time, all the other solvents used were of AR grade.
2.1. Elemental Analysis of FA Using TEM EDX
Elemental analysis of FA was carried out using Energy-dispersive X-ray spectroscopy (EDS, EDX, or XEDS) technique. The FA sample was prepared in 1 mg/mL aqueous solution, sonicated for 15 min. Then, 1–2 drops of the solution were placed on a TEM copper mesh grid and dried for at least 1 h. The details of the operating conditions are Instrument (TalosL120C), High Voltage (120 keV), counting rate 30 kcps and Energy range 0–80 keV.
2.2. Methodology Used for the Preparation of Co-Crystals
Co-crystals were synthesized by solution methods viz. the solution crystallization technique, where the targeted phytochemical and FA (CCF) were simultaneously co-dissolved in THF: water (1:1 v/v). The solution was concentrated by boiling it until it was half its original volume, and then cooled to room temperature to crystallize in an open beaker. It is likely to take several days for the solvent to evaporate [29].
2.3. Solubility and In Vitro Dissolution Studies
Solubility studies were performed for Cur and Q, along with its co-crystals. Briefly, an excess amount (~50 mg) of the pure drugs and co-crystal were placed in 10 mL of water. They were kept in an orbital shaker at 37 ± 1 °C for 24 h at 150 rpm and subsequently filtered and analyzed using ultraviolet UV spectroscopy at 430 nm and 360 nm for Curcumin and Quercetin, respectively.
In dissolution studies, 50 mg of pure Cur and Q and Curcumin-Fulvic acid (CFA) and Quercetin-Fulvic acid (QFA) were put in HPMC capsules (Size no 5). In brief, the study was performed in a Type-I dissolution apparatus, (Model: TDT-06T, Electro lab India Pvt. Ltd., India); the dissolution medium comprised of 900 mL 40% ethanol-water at 37 ± 0.5 °C. One capsule was placed in each basket. The dissolution was performed for 4 h at 100 rpm. At the intervals of 1 h, samples of 5 mL were collected, and the dissolution vessels were reloaded with an exact amount of fresh medium in order to maintain the sink condition. The collected samples were filtered using a membrane filter (0.45 μm), diluted properly with methanol, and analyzed using the UV method at 430 nm and 360 nm for Curcumin and Quercetin, respectively [25,26].
2.4. Characterization of Solid-State Co-Crystals
2.4.1. Fourier Transformed Infrared Spectroscopy (FTIR)
Pure compounds and their co-crystals were analyzed by employing an FTIR spectrophotometer (ALPHA Bruker) by using the KBr pellet method. The spectrum was recorded using ALPHA Bruker, in the region between 4000 and 400 cm−1. Respective spectra were analyzed and observed for any noticeable shift(s) in the representative peak(s), compared with pure forms.
2.4.2. Differential Scanning Calorimetry (DSC)
Thermograms of Pure compounds and their co-crystals were obtained from TG/DTA/DSC (Hitachi STA7300). Precisely weighed test compounds (5 mg) were exposed to one heating cycle from 30 °C to 300 °C at a heating rate of 10 °C/min. The data obtained were analyzed with the software Originlab® Origin.
2.4.3. X-ray Diffraction (XRD)
Powder X-ray diffraction of the Cur, Q and FA along with its physical mixture and co-crystal achieved using Rigaku X-ray diffractometer (D/MAX-B) with Ni-filtered Cu-K (α) radiation, a current of 30 mA and a voltage of 40 kV. The samples were examined over the 2° range of 10–90°.
2.4.4. Solid-State NMR Analysis (SNMR)
The structure of pure drugs and the synthesized co-crystals were analyzed experimentally by solid-state 13C NMR analysis. The Bruker AV III 500 MHz NMR Instrument at SAIF, Chennai, India was outsourced to carry out the analysis.
2.5. In Vitro Anticancer Activity
- Morphological studies
U87MG and U118 Cells were treated for 12 h post-seeding with 150 and 300 mg/mL concentration of each formulation, namely CFA and QFA, for morphological study. Cells were observed for 24, 48, 72 h after treatment of the test formulations. An Axiovert 200 M phase-contrast microscope, at the magnification of 10×, was used to take the images. Axiovision Rel.4.2 software was used to acquire the images.
- b.
- MTT cytotoxic assay
U87MG, U118 were chosen and mounted on 96-well plates at 5.0 × 104 cells/well in a medium of 100 mL. At 37 °C the cells were incubated with 5% CO2 for one night and allowed to stick on the wells. Subsequently, after washing the cultured cells twice with PBS, CFA and QFA were dissolved in the media added in triplicate to achieve the final concentrations of 5 to 300 g/mL (5, 50, 100, 150, 200, 250, 300 g/mL). After another 24 h of incubation, 15 µL of 0.5 mg/mL MTT solution was dispensed to each well. The cells were further incubated for an additional 3 h at 37 °C before being assessed on an absorbance plate reader at OD 570 nm for each well. Blank DMSO was prepared at the same time for the correction of sample color absorbance, as well as a sample’s intrinsic ability to diminish MTT in the absence of cells. The optical density of the formazan generated by the untreated control cells was taken to indicate 100% vitality [30].
3. Results
3.1. Elemental Analysis of FA Using TEM EDX
The results of elemental analysis have been given in Table 1.

Table 1.
Results of elemental analysis of pure FA using EDX technique.
3.2. Saturation Solubility and In Vitro Dissolution Studies
From the literature reports, the aqueous solubility of Cur and Q was found to be very low, i.e., 0.0087 mg/mL and 0.00215 mg/mL, respectively [24,25]. In our observations, CFA and QFA co-crystals showed 30 µg/mL and 108 µg/mL, respectively while pure components (Cur and Q) could not be quantified using UV, in complete agreement with previous reports.
In dissolution studies, both CFA and QFA showed a better dissolution profile than its free Cur and Q, as shown in Figure 1 and Figure 2 showing the free Cur and Q (that exhibited only 30–40% of drug release in 250 min), while CFA and QFA showed more than 90% of drug release in 3 h of dissolution in ethanol-water media. These results are in agreement with reported results, wherein only 34% release of curcumin occurred after 5 h of dissolution period [25].
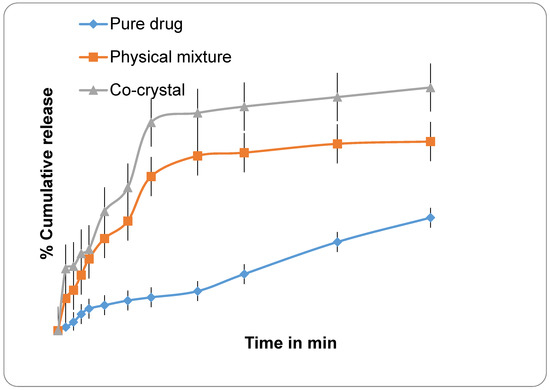
Figure 1.
Graphs showing % cumulative release profile of pure drug Quercetin; Physical mixture; and Co-crystal.
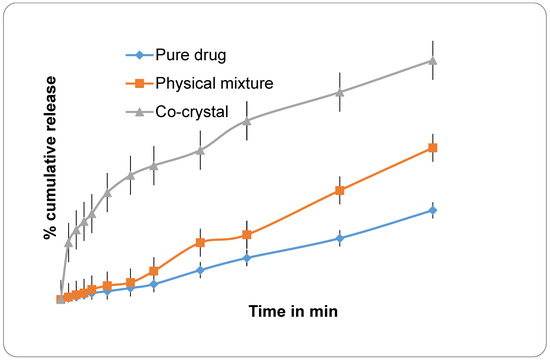
Figure 2.
Graphs showing % cumulative drug profile of where (Pure) CUR-Curcumin; (Mixture)-Physical mixture; (Co-crystal) CFA- Curcumin co-crystal.
FA is highly water-soluble, as evidenced in reports, and has a surfactant-like property, tending to form an inclusion complex with BCS II and IV drugs. That is why a marked improvement in the dissolution profiles of CFA and QFA were observed. Further from the cumulative interpretation of FTIR, DSC, XRD and SNMR analysis, it can be concluded that there is a new solid state. Any slight change in the drugs’ crystallinity contributes to a change in solubility profile [31,32].
3.3. Solid State Characterization of Co-Crystals
3.3.1. Fourier Transformed Infrared Spectroscopy (FTIR)
In general, FTIR data reflect a molecule’s vibrational form as a result of changes in hydrogen bonding and molecular conformations, as well as deviations in the sample’s physical state. In the given FTIR data (Table 2), FA, CUR and Q exhibited their distinguishing peaks in their consequent regions such as carbonyl stretching, -CH stretching, -OH stretching and C-O-C stretching vibration. Its co-crystals showed a slight deviation in the O-H, C=O, and C-O stretching frequencies, suggesting forming a new phase.

Table 2.
FT-IR Stretching Vibration Modes Wave number (cm−1) of pure compound along with its cocrystals.
3.3.2. Differential Scanning Calorimetry (DSC)
For the thermal behaviors of FA, CUR and Q, its crystals were characterized by DSC, as shown in Figure 3. Both CUR and Q exhibited single sharp endothermic peaks at 183.39 °C and 139.06 °C, respectively; FA exhibited a broad endothermic peak at 226 °C. On the other hand, both co-crystals showed a slight shift in the melting point compared to pure components, indicating a new phase formation. It is reported that the DSC thermograms display a single endothermic peak, either at lower or in between and or greater than the melting points of the individual components, indicating the formation of a new solid phase.
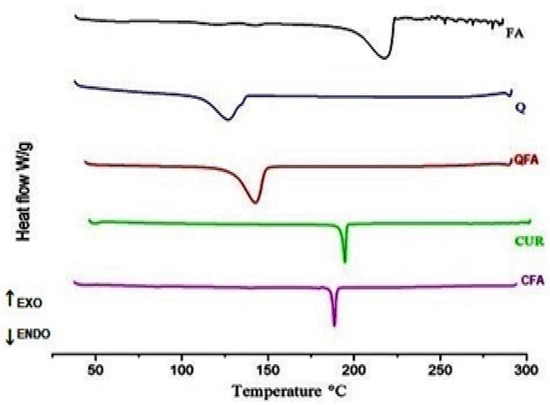
Figure 3.
DSC thermograms of pure compounds along with its co-crystals; where FA-Fulvic acid; Q-Quercetin; QFA-indicates Quercetin-Fulvic acid co-crystal; while CUR-Curcumin, CFA indicates Curcumin-Fulvic acid co-crystal.
When compared to pure forms, their co-crystals showed a very slight shift in endotherm peaks, i.e., a shift in peak to the higher temperature is observed in the case of QFA, while the shift of peak to the lower temperature is noticed for CFA, indicating the formation of co-crystals.
3.3.3. Powder X-ray Diffraction (XRD)
In powered XRD, pure Q and Cur displayed a crystalline nature with characteristic 2θ values, while FA exposed their amorphous nature and did not even display any peaks. However, their co-crystals exhibited slight amorphous nature, indicating the formation of semi crystalline forms. A considerable shift in 2θ values crystalline forms to pure components indicates changes in both the drugs’ physical nature, as shown in Figure 4. Interestingly, the change in crystallinity was also noticed from earlier studies carried out on a different co-former. Furthermore, any small change in physical forms (crystalline) also gives an indication of alteration in the drugs’ physicochemical properties, leading to solubility enhancement.
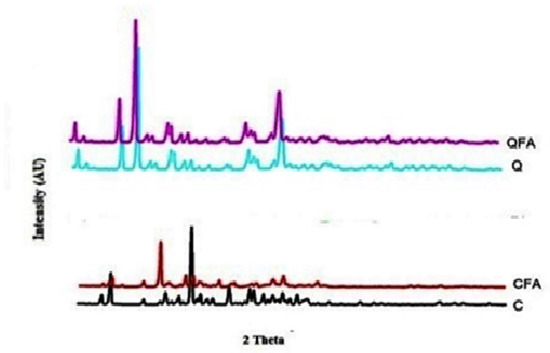
Figure 4.
XRD overlay image of pure compounds along with its crystals. Q-Quercetin; QFA- Quercetin-Fulvic acid co-crystal while C-curcumin, CFA-Curcumin Fulvic acid co-crystal results show a shift in 2 theta values for both CFA and QFA indicates the formation of a new solid-state.
3.3.4. Solid State SNMR Analysis
In SNMR and its peaks (Carbon environment), the sample results’ physical nature correlates with powdered XRD data. Nevertheless, all the characteristic peaks related to pure compounds were confirmed by supporting the previously published data [25,26]. In our observations, co-crystals exhibited shifts in 13C NMR, either the up-field or downfield and even the disappearance of some peaks due to non-bonding interactions, as shown in Figure 5. Moreover, similar studies carried out using the polymers also showed semi crystalline forms.
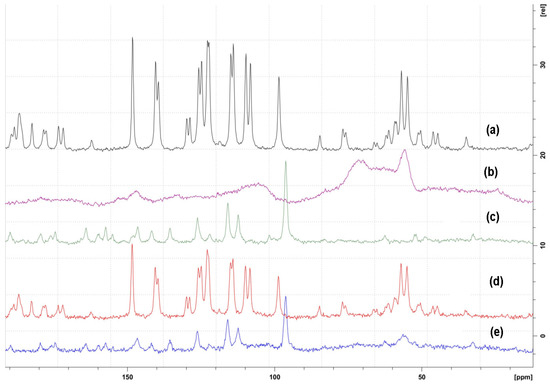
Figure 5.
Solid-state 13C NMR spectra of (a) CUR-Curcumin; (b) FA-Fulvic acid; (c) Q- Quercetin; (d) CFA- Curcumin-fulvic acid co-crystal; (e) QFA Quercetin -fulvic acid co-crystal.
3.4. In Vitro Anti-Cancer Activity
- Morphological analysis
The morphological analysis revealed both CFA and QFA against two cell lines, U 87MG and U118, showed cytotoxicity. Among both CFA, potent cytotoxic activity at both the concentrations tested (150 and 300 μg/mL) was observed, while QFA demonstrated cytotoxicity at 350 μg/mL against both cell lines, as shown in Figure 6a,b.
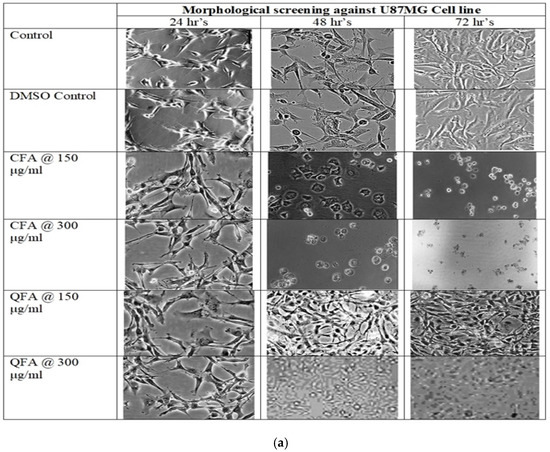
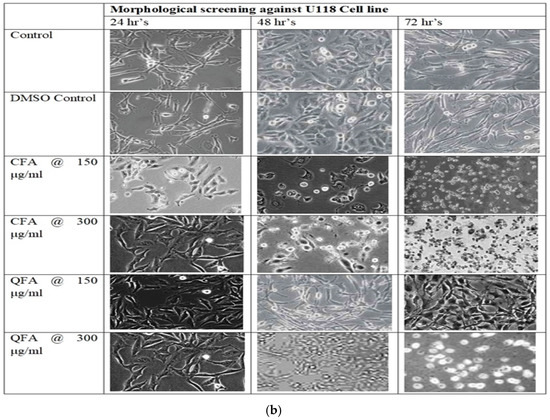
Figure 6.
(a): Cell morphology of Curcumin Fulvic acid co-crystal (CFA) and Quercetin Fulvic acid QFA at two different concentrations 150 and 300 μg/mL for 24 h, 48 h and 72 h, respectively where severally modified cell morphology is noticed for CFA at 150 μg/mL within 24 h but for QFA modified cell morphology was noticed only at 350 μg/mL after 48 h. (b): Cell morphology of Curcumin Fulvic acid co-crystal (CFA) and Quercetin Fulvic acid QFA at two different concentrations 150 and 300 μg/mL for 24 h, 48 h and 72 h, respectively where severally modified cell morphology is noticed for CFA at 150 μg/mL within 24 h but for QFA modified cell morphology was noticed only at 350 μg/mL after 48 h.
- b.
- MTT Assay against U87MG and U118
Cytotoxic effects shown by CFA are in in-complete agreement with the effects observed in the morphological screening. The IC50 values were calculated based on the nonlinear regression method. The IC50 values of CFA at three-time points, i.e., at 24 h, 48 h, and 72 h against U87MG and U118 are shown below in Table 3. The FA did not show a cytotoxic effect against any of the two cell lines tested at any time point.

Table 3.
Results showing cytotoxicity effects of Curcumin-Fulvic acid cocrystal (CFA) and Quercetin-Fulvic acid cocrystal (QFA) against U87 MG, U118 cell lines at three different time intervals where both CFA and QFA showed significant cytotoxicity.
4. Conclusions
Although curcumin and quercetin have several pharmacological activities, their application in the pharmaceutical field is restricted, owing to their poor water solubility and therefore meager oral bioavailability. To improve the solubility and dissolution rate of curcumin and quercetin, formulating co-crystals with the highly soluble co-former, Fulvic acid, has been attempted in this work. Several attempts were made to attain a single homogenous crystal form. However, no decent quality single crystals were obtained due to the highly amorphous nature of Fulvic acid. Instead, semi crystalline forms were developed through the solvent evaporation technique. These semi crystalline forms were characterized by FTIR, DSC, PXRD, and solid-state 13C NMR spectroscopy indicated the formation of a new solid-state that was dissimilar from the starting material. Nevertheless, semi crystalline forms of both the moieties (curcumin and quercetin) showed better solubility and dissolution rate than raw drugs. Our investigation in this regard is still continuing and if any conclusive result is obtained, it will be available in the public domain.
Author Contributions
Conceptualization, K.G., K.P.K. and M.A.M.; methodology, K.G. and K.P.K.; software, K.S.N.; validation, Y.R.P. and K.S.N.; formal analysis, M.A.M., M.T. and Y.R.P.; investigation, K.G. and K.P.K.; resources, Y.R.P., K.S.N. and M.A.M.; data curation, M.J.A.; writing—original draft preparation, K.G.; writing—review and editing, M.A.M., M.T. and M.J.A.; visualization, K.S.N.; supervision, M.A.M., Y.R.P. and K.S.N.; project administration, M.A.M.; funding acquisition, M.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arafa, M.F.; El-Gizawy, S.A.; Osman, M.A.; El Maghraby, G.M. Xylitol as a potential cocrystal co-former for enhancing dissolution rate of felodipine: Preparation and evaluation of sublingual tablets. Pharm. Dev. Technol. 2018, 23, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.L.; Zhou, J.R.; Cai, F.Y.; Lu, J.; Cao, R. Two-component pharmaceutical cocrystals regulated by supramolecular synthons comprising primary N··· H··· O interactions. Cryst. Growth Des 2018, 19, 3–16. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical cocrystals: New solid phase modification approaches for the formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavuru, P. Hierarchy of Supramolecular Synthons in the of Design Multi-Component Crystals; University of South Florida: Tampa, FL, USA, 2012. [Google Scholar]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.N.; Hu, S.; Ng, W.W.; Xu, X.; Lai, K.L.; Lee, W.Y.; Chow, A.H.; Sun, C.C.; Chow, S.F. Cocrystallization of curcumin with benzenediols and benzenetriols via rapid solvent removal. Cryst. Growth Des 2018, 18, 5534–5546. [Google Scholar] [CrossRef]
- Nugroho, D.; Sugih, A.K. Determination of Process Parameters for Curcumin–Dextrose Cocrystallization; IOP Publishing: Bristol, UK, 2018; Volume 299, pp. 1–6. [Google Scholar]
- Lu, J.; Rohani, S. Preparation and characterization of theophylline− nicotinamide cocrystal. Org. Process Res. Dev. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- Stahl, P.H. Handbook of Pharmaceutical Salts Properties, Selection, and Use; Burdock, G.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 4. [Google Scholar]
- Wagner, B.M.; Smith, R.L.; Munro, I.C.; Newberne, P.M. 15. GRAS substances. Food Technol. 1990, 44, 78. [Google Scholar]
- Gnananath, K.; Nataraj, K.S.; Rao, B.G.; Kumar, K.P.; Mahnashi, M.H.; Anwer, M.K.; Umar, A.; Iqbal, Z.; Mirza, M.A. Exploration of Fulvic acid as a functional excipient in line with regulatory requirement. Environ. Res. 2020, 187, 109642. [Google Scholar] [CrossRef]
- Motojima, H.; Yamada, P.; Yamada, P.; Ozaki, M.; Han, J.; Shigemori, H.; Isoda, H. Properties of fulvic acid extracted from excess sludge and its inhibiting effect on βhexosaminidase release. Biosci. Biotechnol. Biochem. 2009, 73, 2210–2216. [Google Scholar] [CrossRef]
- Winkler, J.; Ghosh, S. Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J. Diabetes Res. 2018, 2018, 5391014. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.; Xu, L.; Zhang, Y.; Liu, W.; Wang, M.; Zhao, Y.; Yuan, X.; Li, Y. Extraction of fulvic acid from lignite and characterization of its functional groups. ACS Omega 2020, 5, 27953–27961. [Google Scholar] [CrossRef] [PubMed]
- Ogner, G.; Schnitzer, M. Chemistry of fulvic acid, a soil humic fraction, and its relationto lignin. Can. J. Chem. 1971, 49, 1053–1063. [Google Scholar] [CrossRef]
- Mirza, A.M.; Iqbal, Z.; Agarwal, S.P. Effect of fulvic acid on oral delivery of Carbamazepine. Sci. Adv. Mater. 2011, 3, 223–232. [Google Scholar] [CrossRef]
- Mirza, A.M.; Answer, K.M.; Agarwal, P.S.; Mahmood, D.; Ahmad, N.; Iqbal, Z. Comparative evaluation of humic substances in oral drug delivery. Results Pharma Sci. 2011, 1, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Liu, X.; Luo, Q.; Duan, X.; Pei, C. Cocrystals of ammonium perchlorate with a series of crown ethers: Preparation, structures, and properties. CrystEngComm 2016, 18, 8487–8496. [Google Scholar] [CrossRef]
- Coleman, A.W.; Lazar, A.N.; Suwinska, K.; Danylyuk, O. Cocrystals of Calixarenes and Biologically Active Molecules. U.S. Patent US20090233941A1, 18 April 2007. [Google Scholar]
- Gnananath, K.; Sri Nataraj, K.; Rao, B.G.; Kumar, K.P.; Chandrasekhar, K.; Jain, P.; Mirza, M. Ternary System of Bacogenins with Fulvic Acid and Hydrogenated Soy Lecithin: Preparation, Characterization and, In-Vivo Studies. Curr. Top. Med. Chem. 2022. [Google Scholar] [CrossRef]
- Khandavilli, U.R.; Skořepová, E.; Sinha, A.S.; Bhogala, B.R.; Maguire, N.M.; Maguire, A.R.; Lawrence, S.E. Cocrystals and a salt of the bioactive flavonoid: Naringenin. Cryst. Growth Des. 2018, 18, 4571–4577. [Google Scholar] [CrossRef]
- Selivanova, I.A.; Terekhov, R.P. Crystal engineering as a scientific basis for modification of physicochemical properties of bioflavonoids. Russ. Chem. Bull. 2019, 68, 2155–2162. [Google Scholar] [CrossRef]
- Smith, A.J.; Kavuru, P.; Arora, K.K.; Kesani, S.; Tan, J.; Zaworotko, M.J.; Shytle, R.D. Crystal engineering of green tea epigallocatechin-3-gallate (EGCG) cocrystals and pharmacokinetic modulation in rats. Mol. Pharm. 2013, 10, 2948–2961. [Google Scholar] [CrossRef]
- Chadha, K.; Karan, M.; Bhalla, Y.; Chadha, R.; Khullar, S.; Mandal, S.; Vasisht, K. Cocrystals of hesperetin: Structural, pharmacokinetic, and pharmacodynamic evaluation. Cryst. Growth Des. 2017, 17, 2386–2405. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.R.; Nangia, A. Fast dissolving curcumin cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, S.; Zhang, R.; Jia, X.; Yang, T.; Zhang, X. Drug-Drug Cocrystals:Opportunities and Challenges. Asian J. Pharm. Sci. 2021, 16, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’Donnell, E.; Park, A. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Vidya, K.; Lakshmi, P.K. Cytotoxic effect of transdermal invasomalanastrozole gel on MCF-7 breast cancer cell line. J. Appl. Pharm. Sci. 2019, 9, 50–58. [Google Scholar]
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in solubility of poor water-solubledrugs by solid dispersion. Int. J. Pharm. Investig. 2012, 2, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).