Separation of Microalgae by a Dynamic Bed of Magnetite-Containing Gel in the Application of a Magnetic Field
Abstract
1. Introduction
2. Materials and Methods
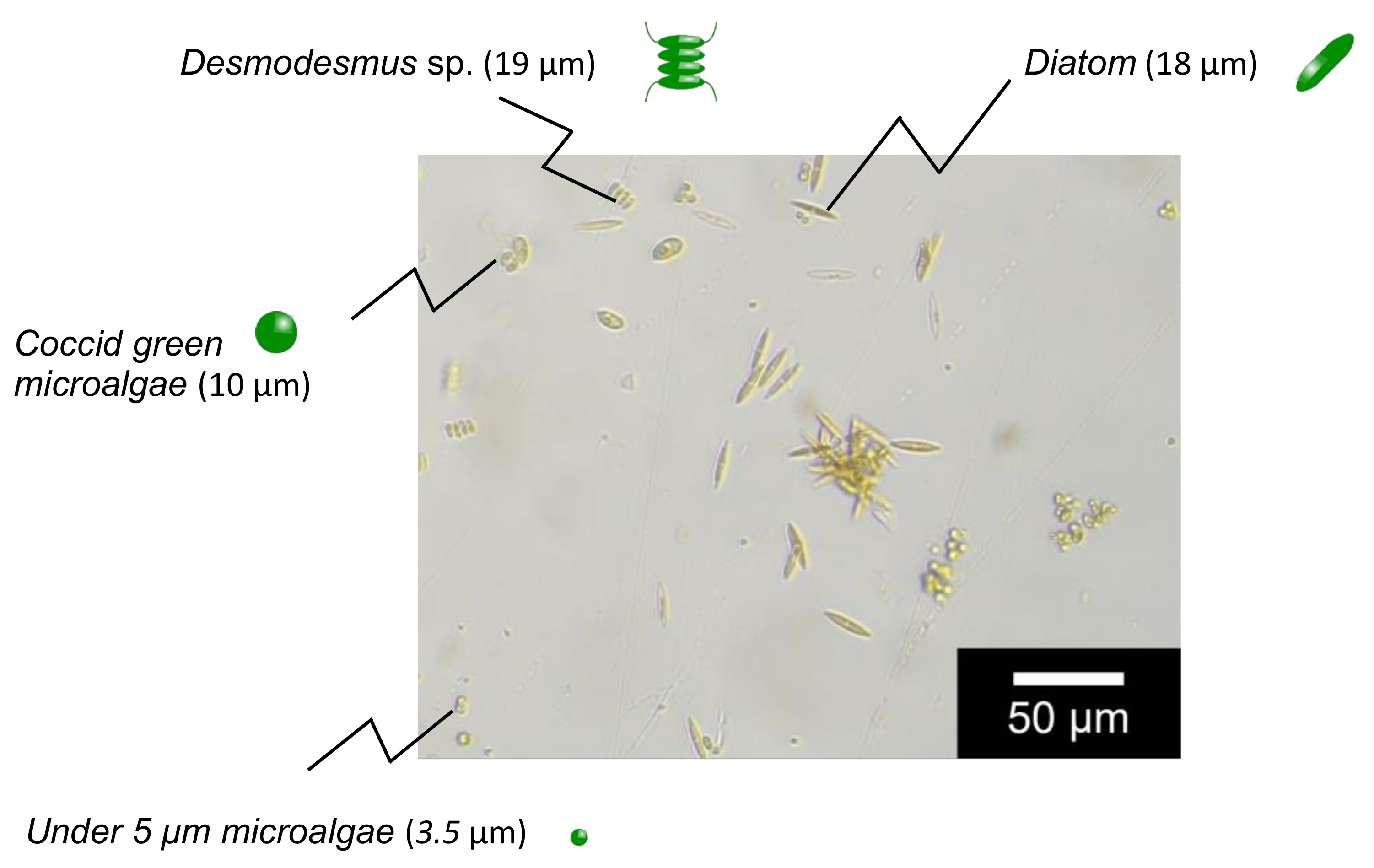
2.1. Materials
2.2. Preparation of Magnetite-Containing Gel
2.3. Adsorption of Microalgae to Mag Gel
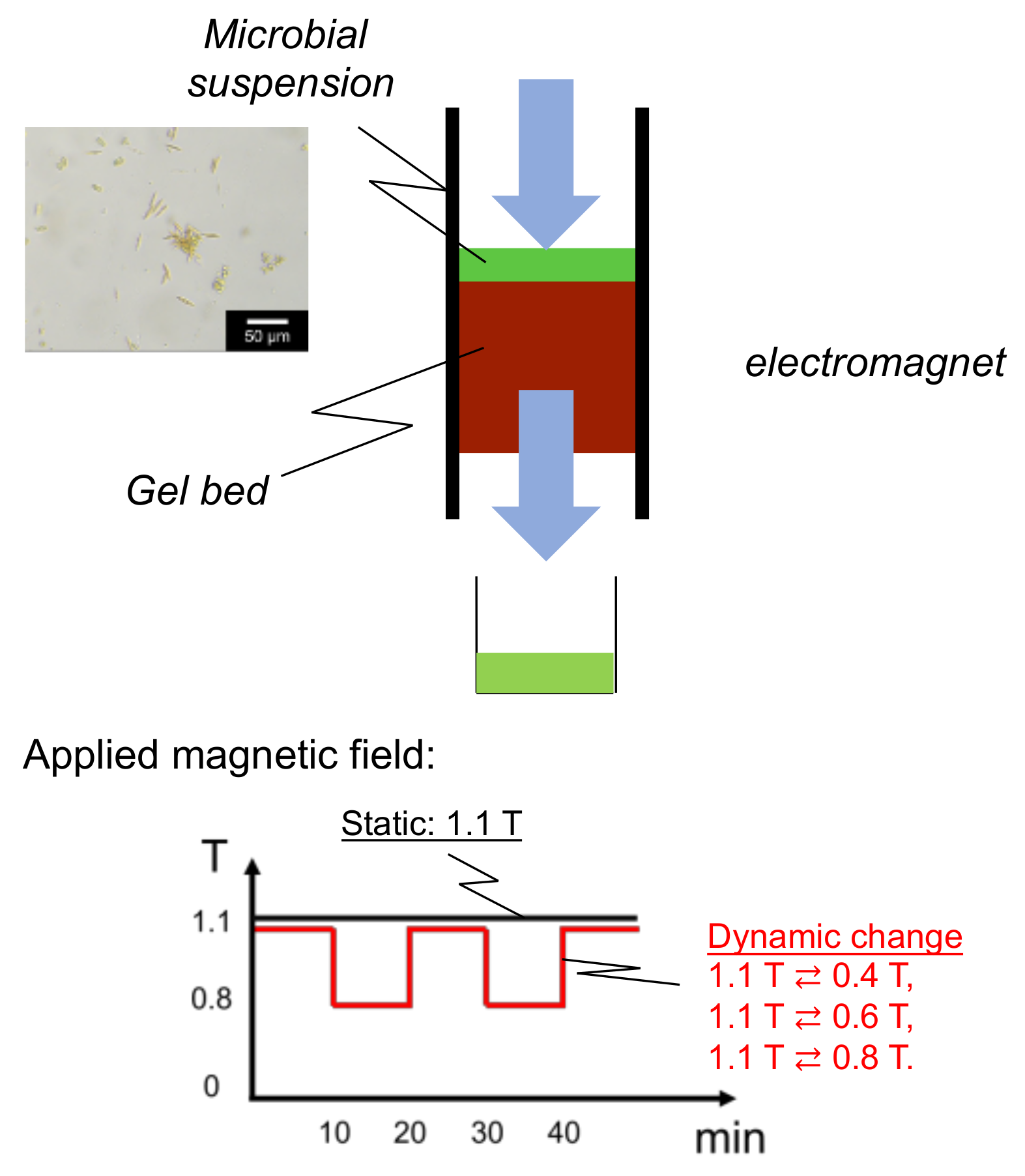
2.4. Mag Gel Packed in Column by Application of Magnetic Field for Separation of Microalgae
3. Results and Discussion
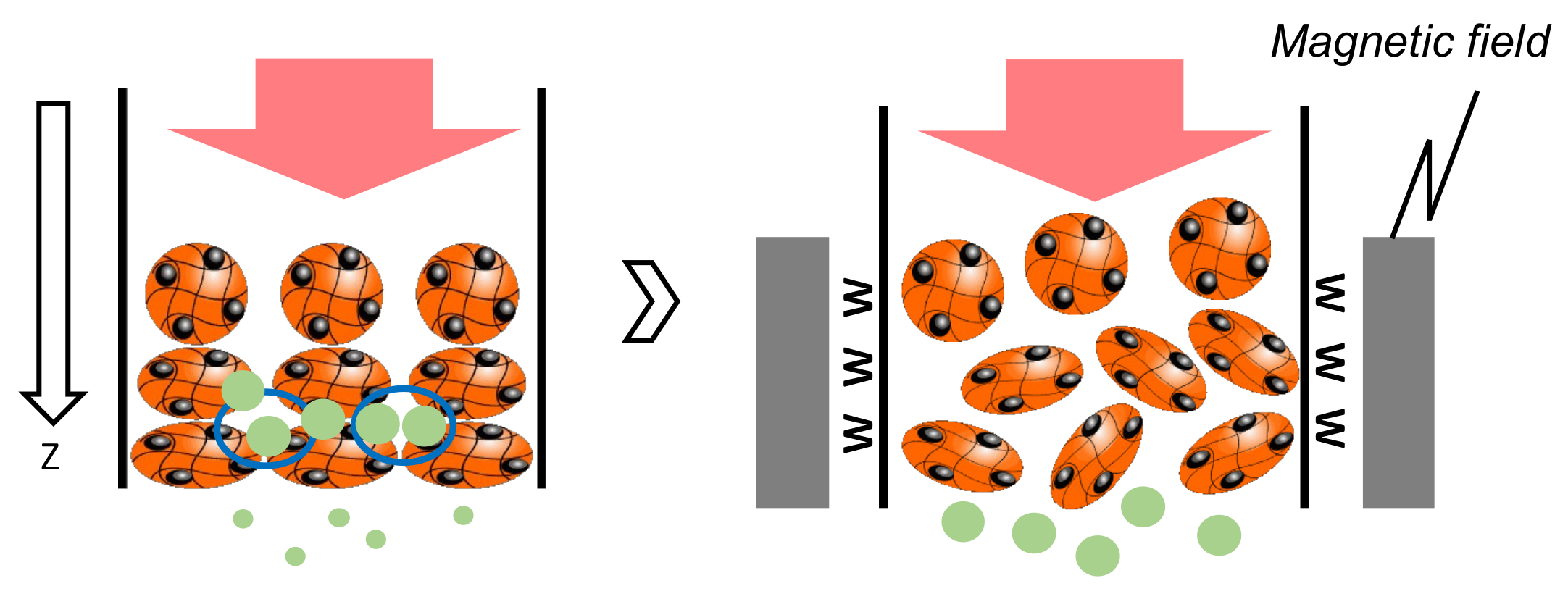
3.1. Leakage of Mag Gel from Column in Application of Magnetic Field
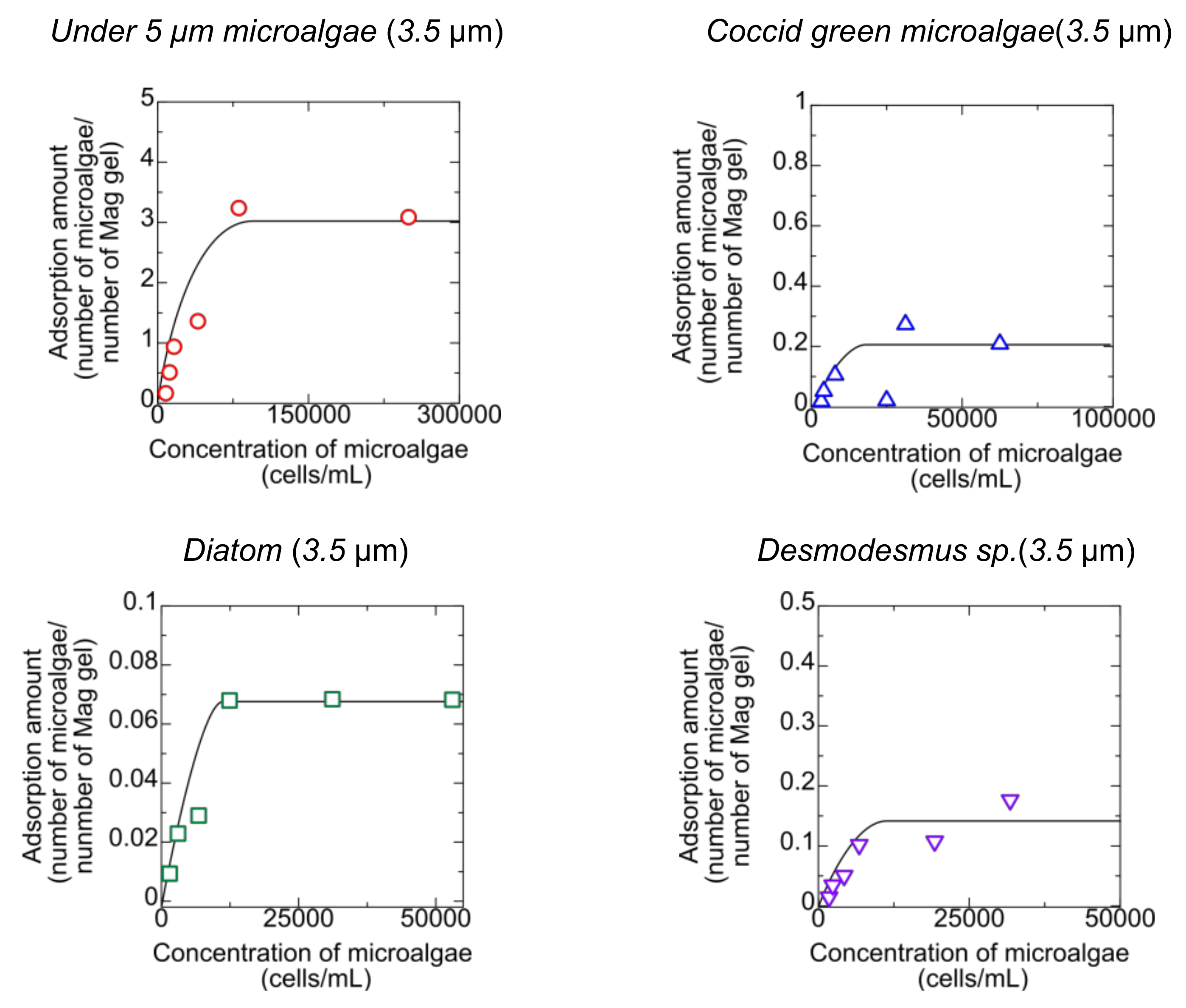
3.2. Adsorption of Microalgae to Mag Gel
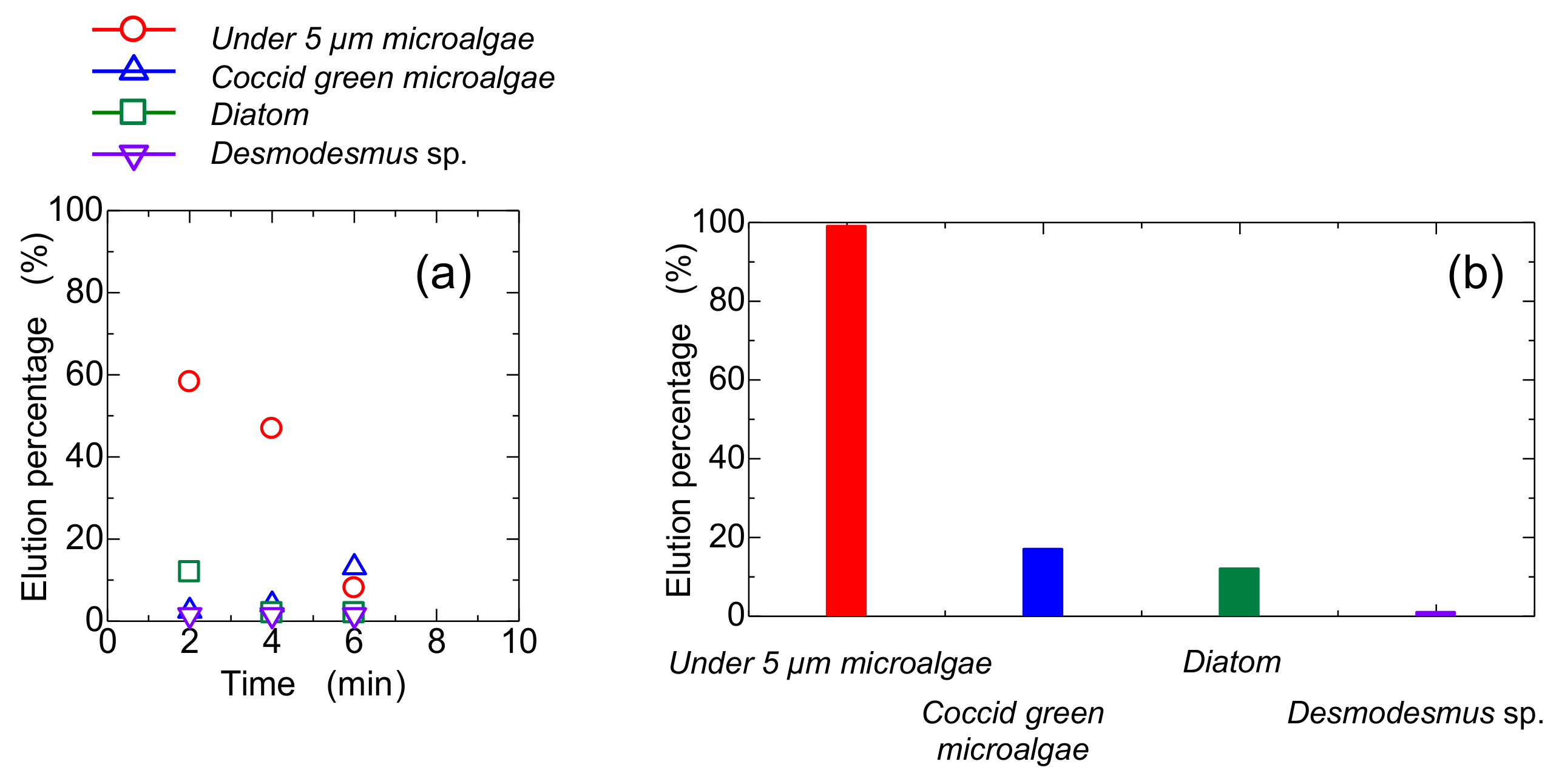
3.3. Separation of Microalgae in Static Mag Gel in Application of Magnetic Field
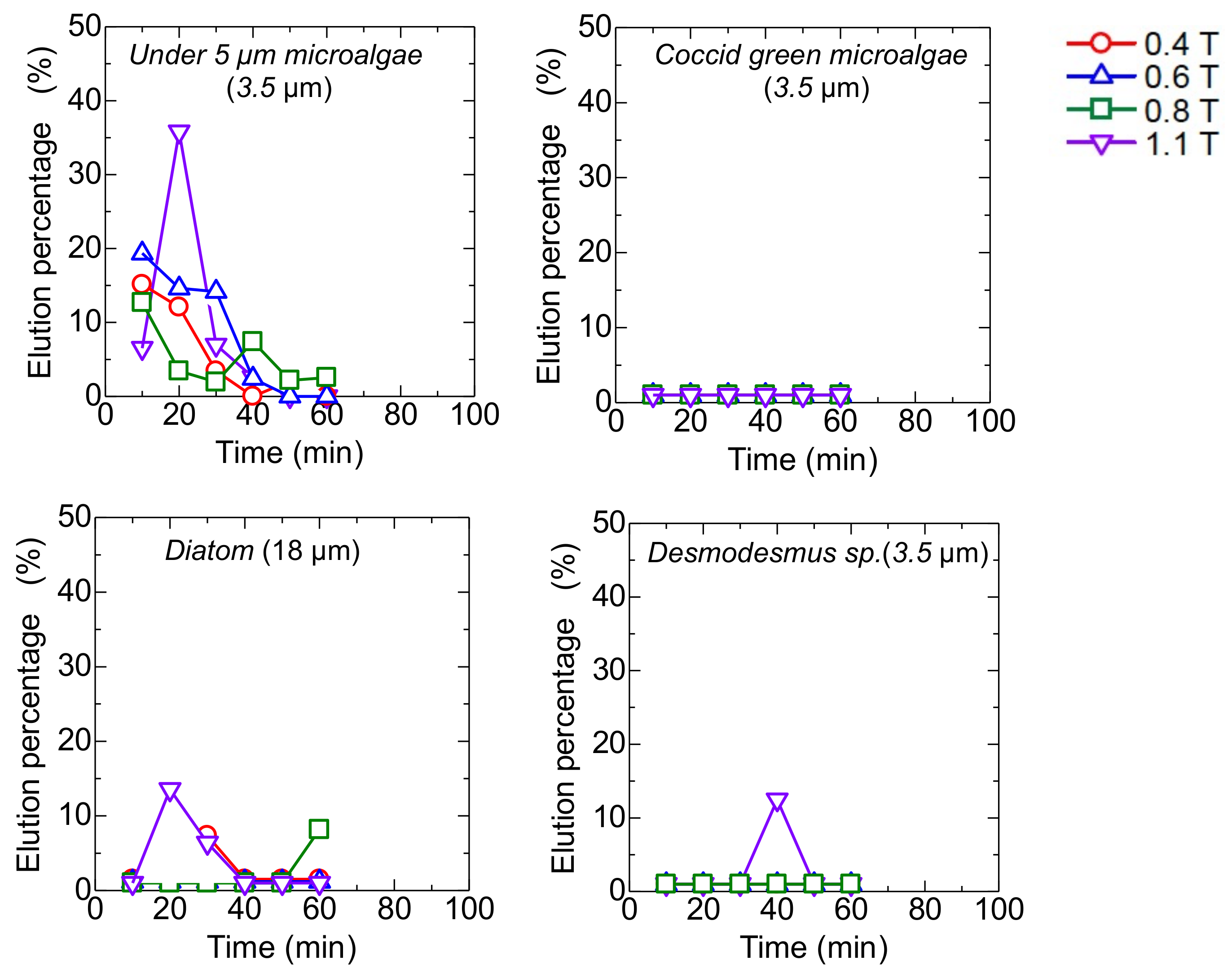
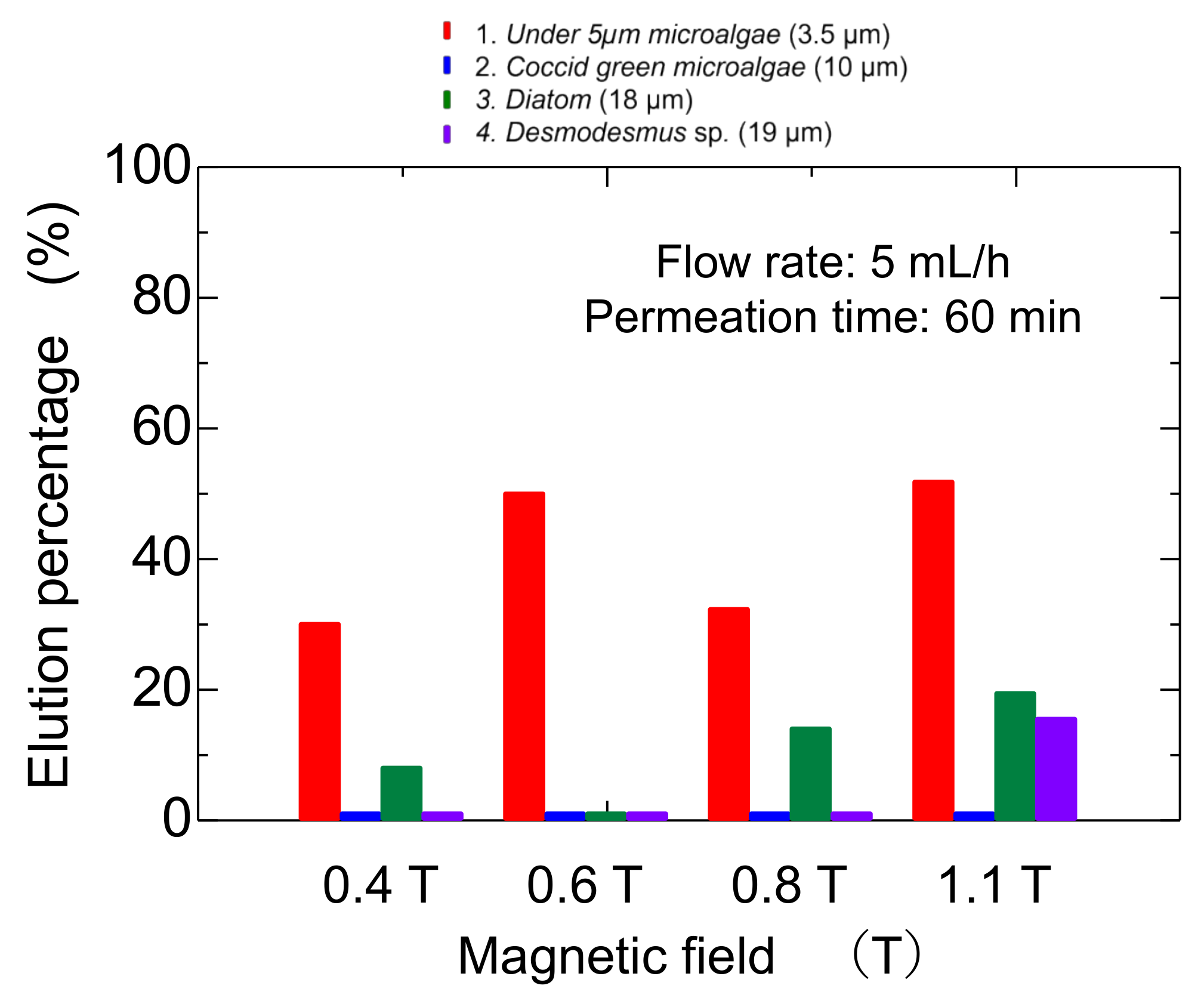
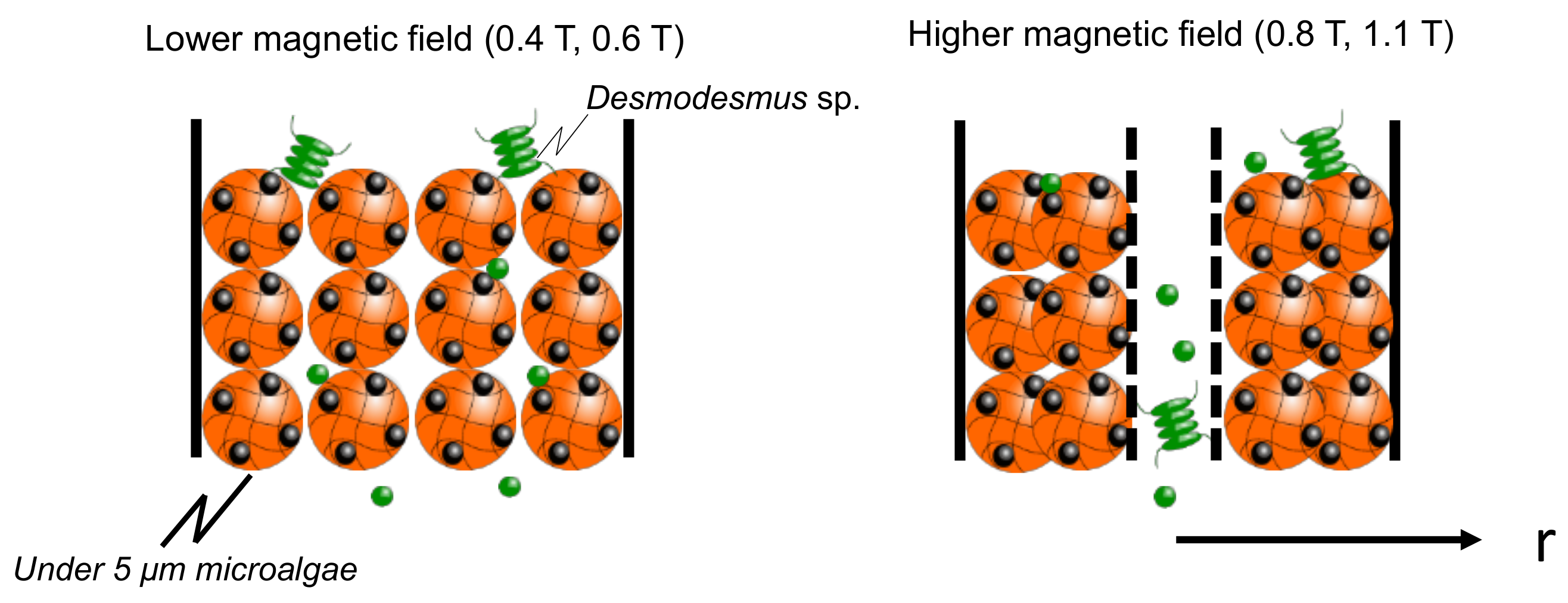
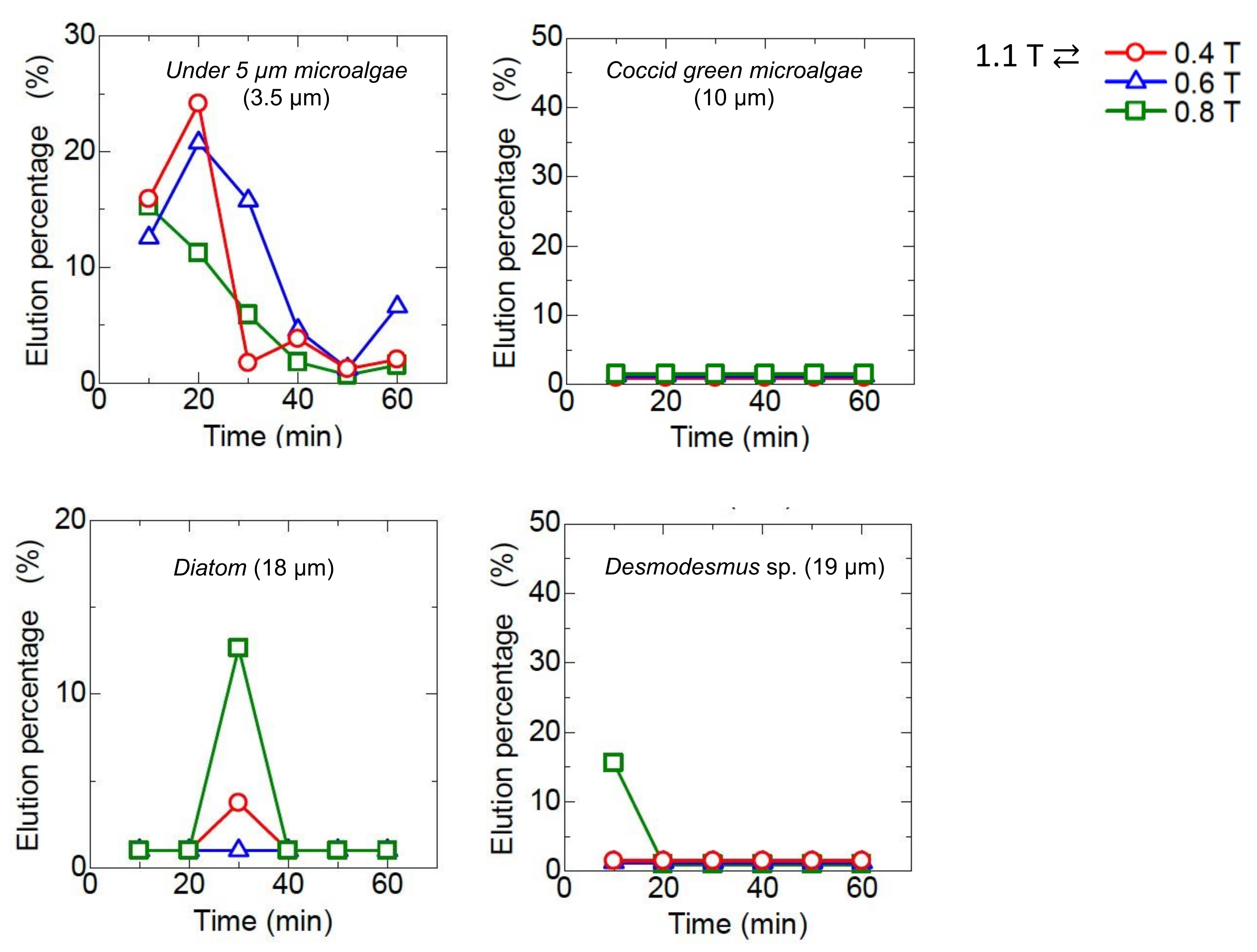
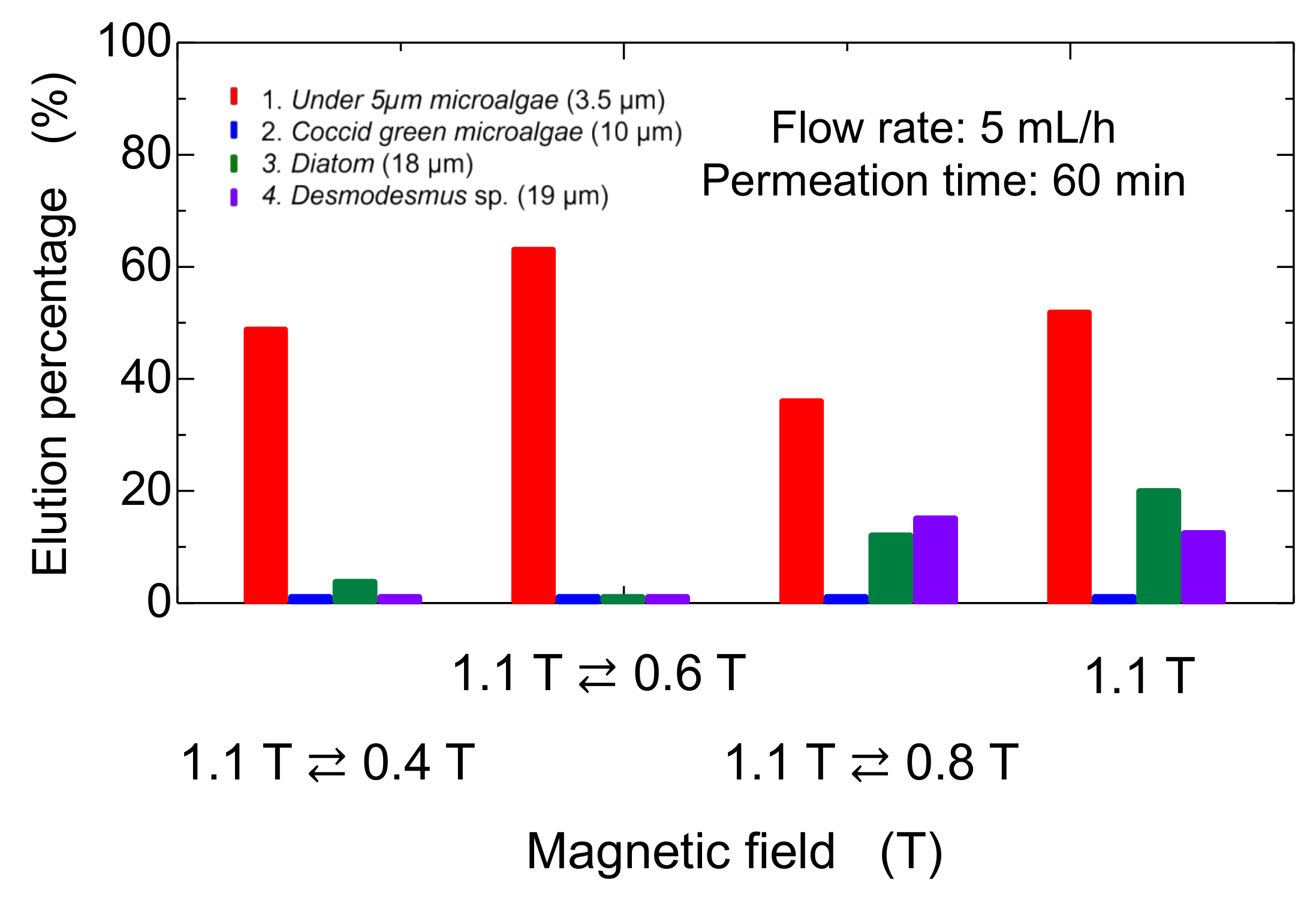
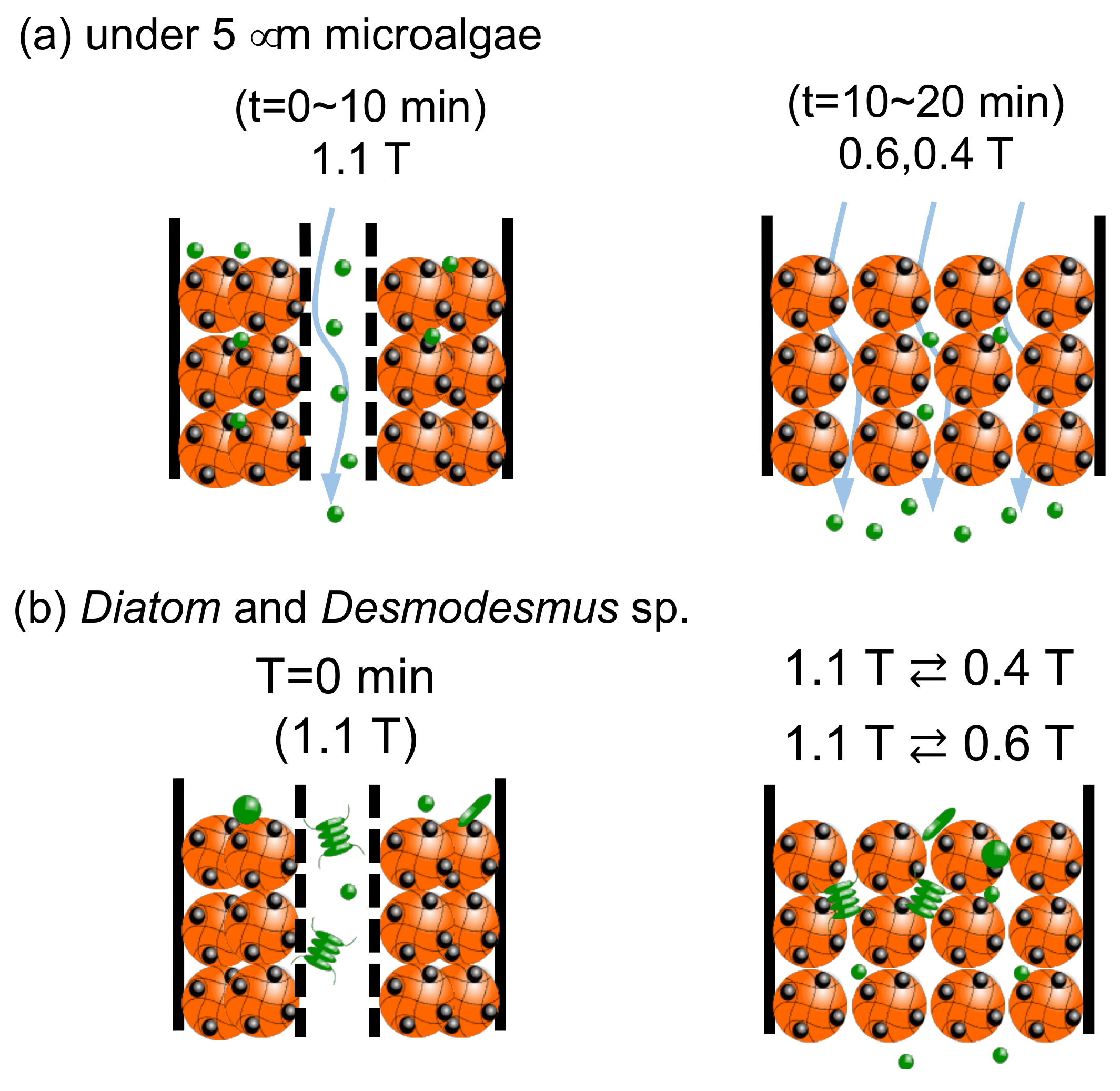
3.4. Dynamic Elution of Microalgae in Convex Change of Applied Magnetic Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Brennan, L.; Owende, P. Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557. [Google Scholar] [CrossRef]
- Yoshida, M.; Tanabe, Y.; Yonezawa, N.; Watanabe, M.M. Energy innovation potential of oleaginous microalgae. Biofuels 2012, 3, 761. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci. Technol. 2021, 116, 176. [Google Scholar] [CrossRef]
- Demura, M.; Yoshida, M.; Yokoyama, A.; Ito, J.; Kobayashi, H.; Kayano, S.; Tamagawa, Y.; Watanobe, M.; Date, N.; Osaka, M.; et al. Biomass productivity of native algal communities in Minamisoma city, Fukushima Prefecture, Japan. Algal Res. 2018, 29, 22. [Google Scholar] [CrossRef]
- Aoi, Y.; Kaneko, Y.; Tsuneda, S. pH-gradient ion-exchange microbial cell chromatography as a simple method for microbial separation. J. Biosci. Bioeng. 2017, 123, 431. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Galaev, I.Y.; Mattiasson, B. Affinity cryogel monoliths for screening for optimal separation conditions and chromatographic separation of cells. J. Chromatogr. A 2006, 1123, 145. [Google Scholar] [CrossRef]
- Schneiderheinze, J.M.; Armstrong, D.W.; Schulte, G.; Westenberg, D.J. High efficient separation of microbial aggregates using capillary electrophoresis. FEMS Microbiol. Lett. 2000, 189, 39. [Google Scholar] [CrossRef][Green Version]
- Desai, M.J.; Armstrong, D.W. Separation, identification, and characterization of microorganisms by capillary electrophoresis. Microbiol. Mol. Biol. Rev. 2003, 67, 38. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, W.; Zhou, X.; Han, S.-F.; Tu, R.; Feng, X.; Jensen, P.D.; Wang, Q. Efficient harvesting of Chlorella pyrenoidosa and Scenedesmus obliquus cultivated in urban sewage by magnetic flocculation using nano-Fe3O4 coated with polyethyleneimide. Biores. Technol. 2019, 290, 121771. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, P.; Liu, S.; Zhao, H.; Cui, Y.; Qin, S. Purification of 6×His-tagged phycobiliprotein using zinc-decorated silica-coated magnetic nanoparticles. J. Chromatogr. B 2011, 879, 993. [Google Scholar] [CrossRef]
- Franzreb, M.; Siemann-Herzberg, M.; Hobley, T.J.; Thomas, O.R.T. Protein purification using magnetic adsorbent particles. Appl. Microbiol. Biotechnol. 2006, 70, 505. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Panda, N.; Pramanik, P. Boronic acid functionalized superparamagnetic iron oxide nanoparticle as a novel tool for adsorption of sugar. Mater. Sci. Eng. 2009, 29, 2254. [Google Scholar] [CrossRef]
- Raghavarao, K.S.M.S.; Dueser, M.; Todd, P. Multistage magnetic and electrophoretic extraction of cells, particles and macromolecules. Adv. Biochem. Eng./Biotechnol. 2000, 68, 139. [Google Scholar]
- Yang, W.; Jazaiz, M.E.; Levenspiel, O.; Fitzgerald, T.J. A magnetic control valve for flowing solids: Exploratory studies. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 717. [Google Scholar] [CrossRef]
- Jaraiz, M.E.; Levenspiel, O.; Fitzgerald, T.J. The uses of magnetic fields in the processing of solids. Chem. Eng. Sci. 1983, 38, 107. [Google Scholar] [CrossRef]
- Hristov, J. Magnetic field assisted fluidization-unified approach Part 1. Fundamentals and relevant hydrodynamics of gas-fluidized beds (batch solids mode). Rev. Chem. Eng. 2002, 18, 295. [Google Scholar] [CrossRef]
- Hristov, J.; Fachikov, L. An overview of separation by magnetically stabilized beds: State-of-the-art and potential applications. China Particuol. 2007, 5, 11. [Google Scholar] [CrossRef]
- Tanaka, H.; Uno, Y.; Morisada, S.; Ohto, K.; Kawakita, H. Filtration and recovery of starch granules using assembled magnetite filter. Chem. Eng. Proc. Proc Intensif. 2016, 110, 128. [Google Scholar] [CrossRef]
- Miyoshi, M.; Takayanagi, K.; Morisada, S.; Ohto, K.; Kawakita, H.; Morita, S. Size separation of silica particles using a magnetite-containing gel-packed column. Processes 2019, 7, 201. [Google Scholar] [CrossRef]
- Washino, T.; Demura, M.; Morisada, S.; Ohto, K.; Kawakita, H. Separation of microalgae using a compacted magnetite-containing gel bed. Bioproc. Biosystems Eng. 2022, 45, 321–331. [Google Scholar] [CrossRef]
- Morisada, S.; Namazuda, K.; Kanda, H.; Hirokawa, Y.; Nakano, Y. Temperature-swing adsorption of proteins in water using N-isopropylacrylamide copolymer gel particles. Adv. Powder Technol. 2010, 21, 28. [Google Scholar] [CrossRef]
- Baudelet, P.-H.; Ricochon, G.; Lnder, M.; Muniglia, L. A new insight into cell walls of Chlorophyta. Algal Res. 2017, 25, 333. [Google Scholar] [CrossRef]
- Safi, C.; Frances, C.; Ursu, A.V.; Laroche, C.; Pouzet, C.; Vaca-Garcia, C.; Pontalier, P.-Y. Understanding the effect of cell disruption methods on the diffusion of Chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res. 2015, 8, 61. [Google Scholar] [CrossRef]
- Winson, M.K.; Davey, H.M. Flow cytometry analysis of microorganisms. Methods 2000, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Hyka, P.; Lickova, S.; Přibyl, P.; Melzoch, K.; Kovar, K. Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol. Adv. 2013, 31, 2. [Google Scholar] [CrossRef] [PubMed]
- Otálora, P.; Guzmán, J.L.; Acién, F.G.; Berenguel, M.; Reul, A. Microalgae classification based on machine learning techniques. Algal Res. 2021, 55, 102256. [Google Scholar] [CrossRef]

| Chemical | Mass [g] | Mole [mmol] | |
|---|---|---|---|
| Water phase | N,N-dimethylacrylamide | 9.4 | 94 |
| N,N’-Methylene bisacrylamide | 0.161 | 1 | |
| Ethylenediamine tetraacetic acid | 0.148 | 0.53 | |
| Ammonium persulfate | 0.335 | 1.4 | |
| Distilled water | 25.2 | 138 | |
| Magnetite | 2.06 | 8.63 | |
| Organic phase | Span 80 | 9.28 | 21.5 |
| Tween 80 | 3.44 | 2.59 | |
| hexane | 100 | 116 |
| Mean Size [μm] | 190 |
|---|---|
| size of manunetite used [nm] | 280 |
| number of magnetite in Mag gel * | 3.3 × 106 |
| Volume occupation percentage in Mag gel [%] * | 1.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washino, T.; Demura, M.; Morisada, S.; Ohto, K.; Kawakita, H. Separation of Microalgae by a Dynamic Bed of Magnetite-Containing Gel in the Application of a Magnetic Field. Separations 2022, 9, 120. https://doi.org/10.3390/separations9050120
Washino T, Demura M, Morisada S, Ohto K, Kawakita H. Separation of Microalgae by a Dynamic Bed of Magnetite-Containing Gel in the Application of a Magnetic Field. Separations. 2022; 9(5):120. https://doi.org/10.3390/separations9050120
Chicago/Turabian StyleWashino, Takehiro, Mikihide Demura, Shintaro Morisada, Keisuke Ohto, and Hidetaka Kawakita. 2022. "Separation of Microalgae by a Dynamic Bed of Magnetite-Containing Gel in the Application of a Magnetic Field" Separations 9, no. 5: 120. https://doi.org/10.3390/separations9050120
APA StyleWashino, T., Demura, M., Morisada, S., Ohto, K., & Kawakita, H. (2022). Separation of Microalgae by a Dynamic Bed of Magnetite-Containing Gel in the Application of a Magnetic Field. Separations, 9(5), 120. https://doi.org/10.3390/separations9050120








