Profiling the Volatile and Non-Volatile Compounds along with the Antioxidant Properties of Malted Barley
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
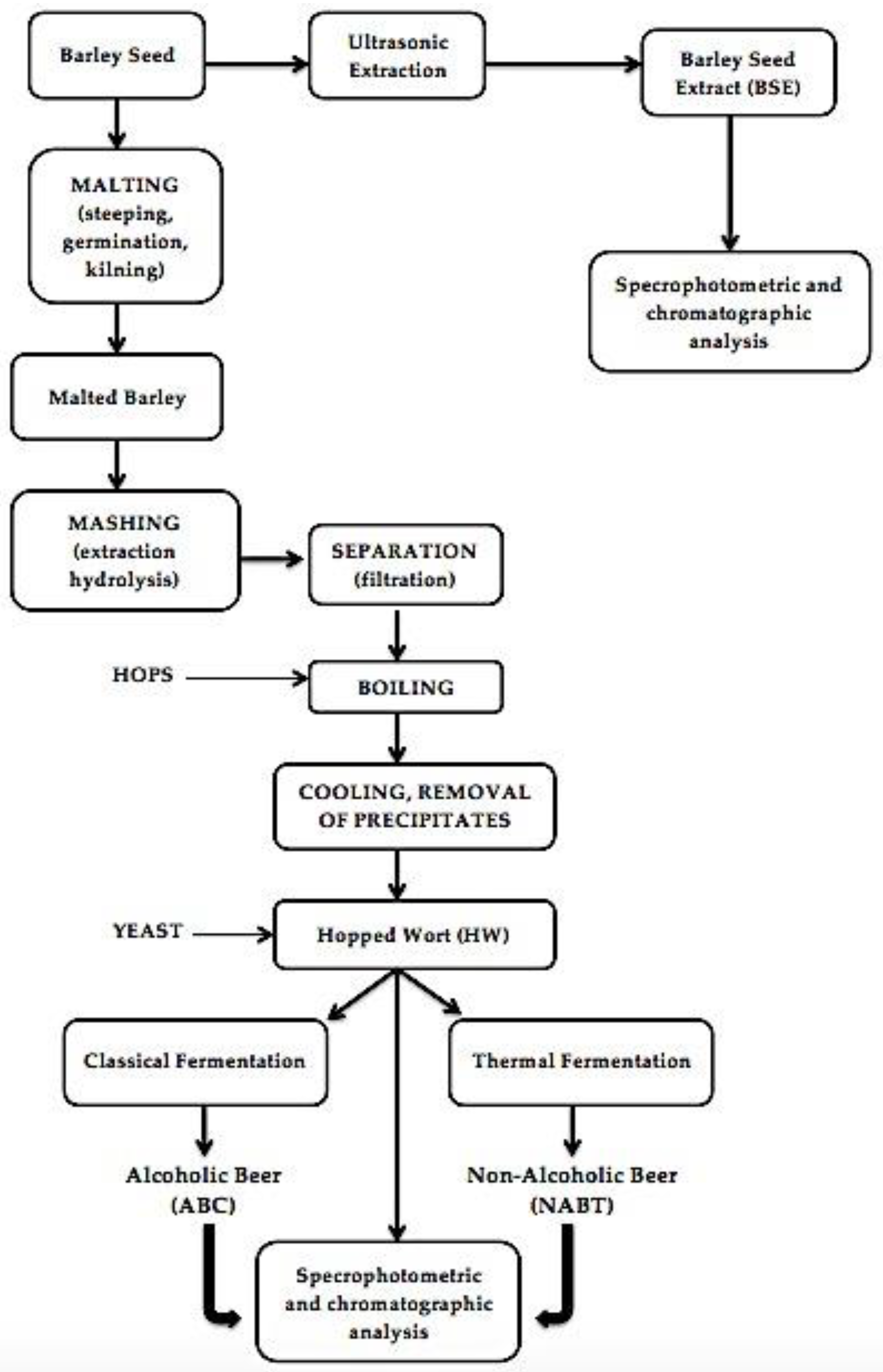
2.2. Extraction Method
2.3. Analysis by GC–MS and HPLC–PDA/ESI–MS
2.3.1. Standard Compounds (Reagents)
2.3.2. SPME Extraction Conditions
2.3.3. GC–MS Analyses
2.3.4. HPLC–PDA/ESI–MS Analysis
2.4. Spectroscopic Analysis
2.4.1. Spectroscopic Analysis of Polyphenols
2.4.2. Determination of Total Phenolic Content (TPC)
2.4.3. Determination of Flavonoid Content (TFC)
2.4.4. Determination of Free Radical Scavenging Potential by DPPH (2,2-Diphenyl-1-Picrylhydrazyl)
3. Results and Discussion
3.1. GC–MS Analyses
3.2. Phenolic Profile by HPLC–DAD–ESI/MS
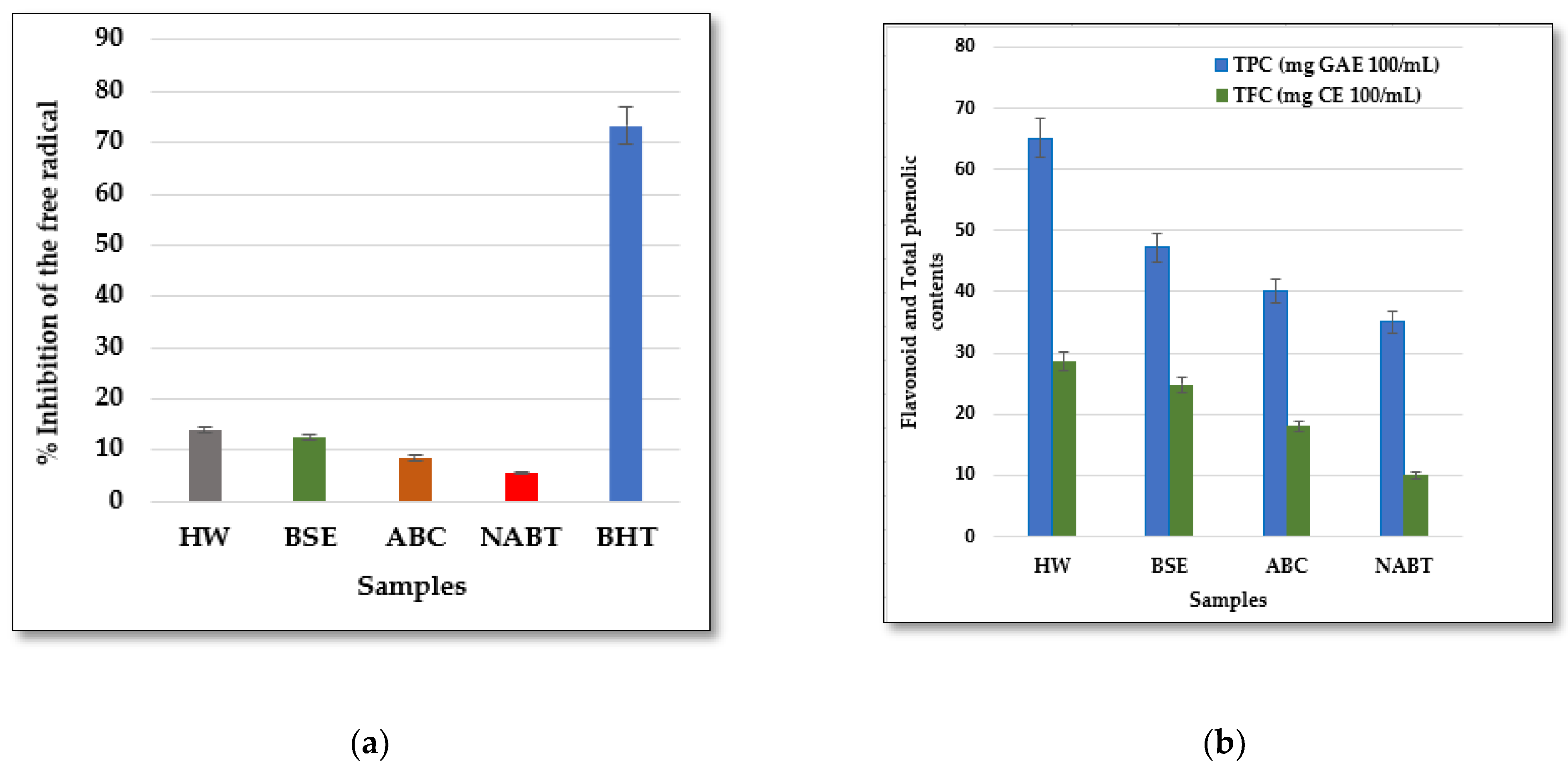
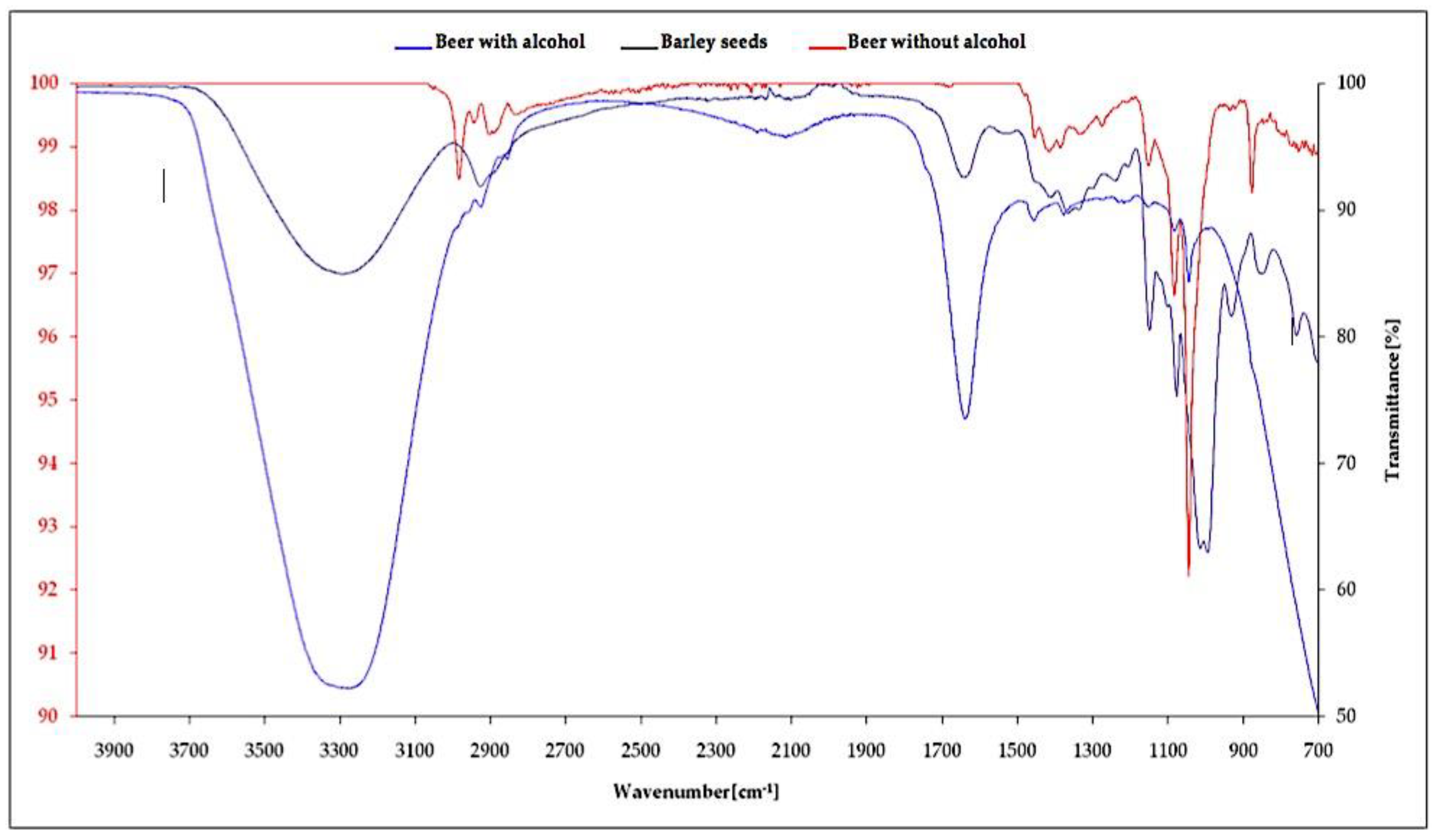
3.3. Interpretation of Spectroscopic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Shao, S.; Chen, M.; Hou, M.; Yu, X.; Xiong, F. Morphology and physicochemical properties of starch from waxy and non-waxy barley. Starch-Stearke 2020, 72, 5–6. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, R. Bioactive Factors and Processing Technology for Cereal Foods; Springer: Singapore, 2019. [Google Scholar]
- Suriano, S.; Iannucci, A.; Codianni, P.; Fares, C.; Russo, M.; Pecchion, N.; Savino, M. Phenolic acids profile, nutritional and phytochemical compounds, antioxidant properties incolored barley grown in southern Italy. Food Res. Int. 2018, 113, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.; Wang, X.; Zhang, C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, K.; Huang, S.; Wang, H.; Mu, X.; He, C.; Ji, X.; Zhang, J.; Huang, F. Antioxydant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel. Food Chem. 2008, 106, 1264–1270. [Google Scholar] [CrossRef]
- Castilla, P.; Echarri, R.; Dávalos, A.; Cerrato, F.; Ortega, H.; Luis Teruel, J.; Fernández Lucas, M.; Gómez-Coronado, D.; Ortuño, J.; Lasunción, M.A. Concentrated red grape juice exerts antioxidant. hypolipidemic. and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutrit. 2006, 84, 252. [Google Scholar] [CrossRef]
- Greenspan, P.; Bauer, J.D.; Pollock, S.H.; David Gangemi, J.; Mayer, E.P.; Ghaffar, A.; Hargrove, J.L.; Hartle, D.K. Antiinflammatory Properties of the Muscadine Grape (Vitis rotundifolia). J. Agric. Food Chem. 2005, 53, 8481. [Google Scholar] [CrossRef]
- Akiyama, H.; Sakushima, J.; Taniuchi, S.; Kanda, T.; Yanagida, A.; Kojima, T.; Teshima, R.; Kobayashi, Y.; Goda, Y.; Toyoda, M. Antiallergic effect of apple polyphenols on the allergic model mouse. Biol. Pharm. Bull. 2000, 23, 1370. [Google Scholar] [CrossRef][Green Version]
- Ghedira, K. Les flavonoïdes: Structure. propriétés biologiques. rôle prophylactique et emplois en thérapeutique. Phytothérapie 2005, 3, 162–169. [Google Scholar] [CrossRef]
- Morré, D.M.; James Morre, D. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2006, 238, 202. [Google Scholar] [CrossRef]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food. Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D.; Ba, G.N.; Mathe, G. Polyphenols: Do they play a role in the prevention of human pathologies. Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. Biomed Pap. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- King, P.J.; Robinson, W.E., Jr. Resistance to the anti-human immunodeficiency virus type 1 compound L-chicoric acid results from a single mutation at amino acid 140 of integrase. Virol. J. 1998, 72, 8420–8424. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.W. Current perspectives on the role of enzymes in brewing. J. Cereal Sci. 2009, 50, 353–357. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal., L.; Mokoena., M.P.; Pillay., B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Guido, L.F.; Rodrigues., P.G.; Rodrigues., J.A.; Gonçalves., C.R.; Barros., A.A. The impact of the physiological condition of the pitching yeast on beer flavor stability: An industrial approach. Food Chem. 2004, 87, 187–193. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira., J.A.; Branyik., T.; Vicente., A.A. Yeast: The soul of beer’s aroma a review of flavor Active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing and Malting; VLB Berlin: Berlin, Germany, 1996. [Google Scholar]
- Piddocke, M.; Olsson., L. Beer Brewing. In Applications of Metabolic Engineering; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Saerens, S.M.G.; Duong., C.T.; Nevoigt., E. Genetic improvement of brewer’s yeast: Current state. perspectives. and limits. Appl. Microbiol. Biotechnol. 2010, 86, 1195–1212. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Kanellaki., M.; Bekatorou., A. Low temperature brewing using cells immobilized on brewer’s spent grains. Food Chem. 2007, 104, 480–488. [Google Scholar] [CrossRef]
- Witrick, K.; Pitts., E.R.; O’Keefe., S.F. Analysis of Lambic Beer Volatiles during Aging Using Gas Chromatography–Mass Spectrometry (GCMS) and Gas Chromatography–Olfactometry (GCO). Beverages 2020, 6, 31. [Google Scholar] [CrossRef]
- Montanari, L.; Marconi, O.; Mayer, H.; Fantozzi, P. Production of Alcohol-Free Beer. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 61–75. [Google Scholar]
- EL Mansouri, F.; Lovillo, M.P.; El Farissi, H.; Oufdou, H.; Brigui, J. Extraction analysis of polyphenols and antioxidant properties of morrocan barley seed extracts (Hordeum vulgare L.). Mater. Today Proc. 2021, 43, 1896–1902. [Google Scholar] [CrossRef]
- Mansouri, F.E.; Farissi., H.E.; Cacciola., F.; Bouhcain., B.; Silva., J.C.G.E.d.; Lovillo., M.P.; Brigui., J. Optimal Design Approach Applied to Headspace GC for the Monitoring of Diacetyl Concentration. Spectrophotometric Assessment of Phenolic Compounds and Antioxidant Potential in Different Fermentation Processes of Barley. Appl. Sci. 2022, 12, 37. [Google Scholar] [CrossRef]
- Trovato, E.; Arigò, A.; Vento, F.; Micalizzi, G.; Dugo, P.; Mondello, L. Influence of Citrus Flavor Addition in Brewing Process: Characterization of the Volatile and Non-Volatile Profile to Prevent Frauds and Adulterations. Separations 2021, 8, 18. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Benkeblia, N. Free radical scavenging capacity and antioxidant properties of some selected onions (Aliium cepa L.) and garlic (Aliium sativum L.) extracts. Braz. Arch. Biol. Technol. 2005, 48, 1–8. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A Comprehensive Characterisation of Beer Polyphenols by High Resolution Mass Spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef]
- Cortese, M.; Gigliobianco, M.R.; Peregrina, D.V.; Sagratini, G.; Censi, R.; Di Martino, P. Quantification of Phenolic Compounds in Different Types of Crafts Beers. Worts. Starting and Spent Ingredients by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1612, 460622. [Google Scholar] [CrossRef]
- Meilgaard, M. Stale flavor carbonyls in brewing. Brew. Dig. 1972, 47, 48–57. [Google Scholar]
- Zufall, C.; Wackerbauer, K. Verfahrenstechnische parameter bei der entalkoholisierung von bier mittels fallstromverdampfung und ihr einfluß auf die bierqualität. Mon. f. Brauwiss 2000, 53, 124–179. [Google Scholar]
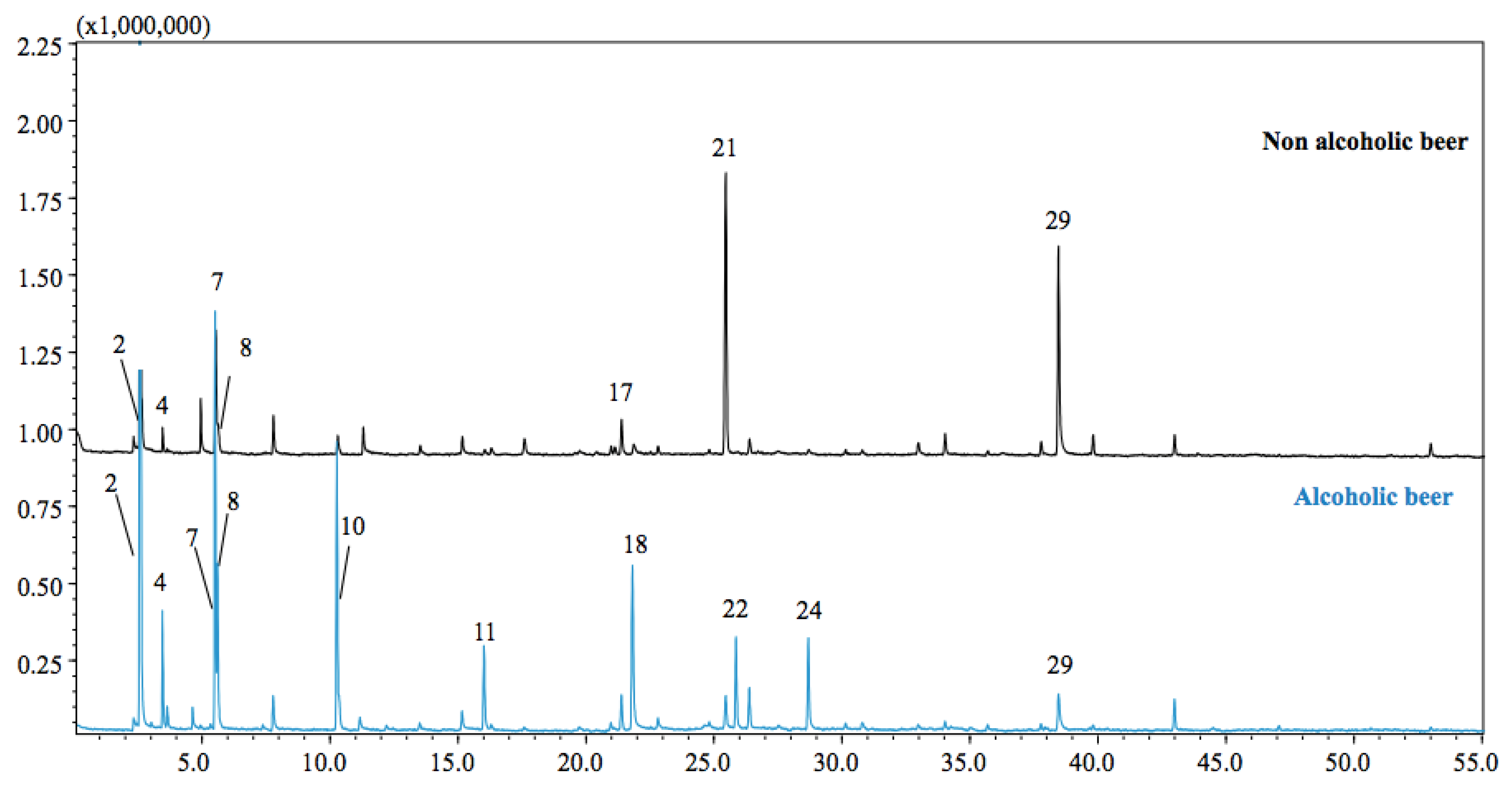

| Non-Alcoholic Beer | Alcoholic Beer | |||||
|---|---|---|---|---|---|---|
| Peak | Compound | LRIexp | LRIlib | Similarity | Area% | Area% |
| 1 | Acetaldehyde | 611 | nd | 89 | 0.58 | 0.08 |
| 2 | Ethanol | 616 | nd | 98 | 2.65 | 6.14 |
| 3 | Propyl alcohol | 633 | 638 | 91 | nd | 0.19 |
| 4 | Acetic acid ethyl ester | 649 | 606 | 97 | 1.57 | 4.29 |
| 5 | Isobutyl alcohol | 656 | 621 | 89 | 0.21 | 0.95 |
| 6 | Ethyl propanoate | 705 | 708 | 89 | nd | 0.08 |
| 7 | Isopentyl alcohol | 727 | 729 | 92 | 11.48 | 18.19 |
| 8 | sec-Butylcarbinol | 730 | 733 | 93 | 2.97 | 8.52 |
| 9 | Ethyl butyrate | 803 | 807 | 90 | nd | 0.18 |
| 10 | Isoamyl acetate | 871 | 871 | 96 | 1.19 | 13.81 |
| 11 | Ethyl hexanoate | 995 | 1003 | 93 | 0.43 | 4.77 |
| 12 | Octanal | 1001 | 1006 | 91 | 0.71 | 0.4 |
| 13 | 2-Ethyl hexanol | 1026 | 1030 | 88 | 2.03 | 0.24 |
| 14 | n-Octanol | 1069 | 1076 | 89 | 0.54 | 0.27 |
| 15 | p-Tolualdehyde | 1082 | 1086 | 91 | 0.21 | nd |
| 16 | Linalool | 1097 | 1100 | 94 | 0.69 | nd |
| 17 | Nonanal | 1102 | 1107 | 96 | 3.83 | 2.06 |
| 18 | Phenethyl alcohol | 1111 | 1113 | 96 | 1.39 | 10.6 |
| 19 | n-Octanoic acid | 1168 | 1192 | 90 | nd | 0.44 |
| 20 | (Z)-3-Nonen-1-ol | 1171 | 1173 | 89 | 0.31 | 0.21 |
| 21 | Naphthene | 1184 | 1188 | 92 | 32.88 | 2.27 |
| 22 | Ethyl octanoate | 1194 | 1202 | 96 | 0.17 | 4.83 |
| 23 | n-Decanal | 1203 | 1208 | 96 | 1.62 | 2.24 |
| 24 | 2-Phenethyl acetate | 1252 | 1257 | 88 | 0.46 | 5.74 |
| 25 | Bornyl acetate | 1283 | 1285 | 93 | 0.41 | 0.31 |
| 26 | Ethyl decanoate | 1391 | 1399 | 91 | nd | 0.34 |
| 27 | n-Dodecanal | 1407 | 1410 | 92 | 0.48 | 0.37 |
| 28 | Verdyl acetate | 1421 | 1424 | 88 | 0.51 | nd |
| 29 | Dodecanol | 1472 | 1480 | 98 | 24.35 | 2.9 |
| TOTAL IDENTIFIED | 91.69 | 90.44 |
| Peak N | tR (min) | MS | MS Fragments | Compound | Alcoholic Beer | Non-Alcoholic Beer | References |
|---|---|---|---|---|---|---|---|
| 1 | 16.15 | 451- | - | 3-Hydroxyphloretin 2′-O-glucoside | x | - | [33] |
| 2 | 25.42 | 357+ | 193- | Ferulic acid-O-hexoside | x | x | [34,35] |
| 3 | 26.24 | 387+ | 223- | Sinapic acid-O-hexoside | x | x | [34,35] |
| 4 | 28.22 | 291+ | 245- | Catechin | x | x | [34,35] |
| 5 | 28.38 | 291+ | 245- | Epicatechin | x | x | [34,35] |
| 6 | 35.81 | 329- | 314- | 3,7-Dimethylquercetin | x | x | [34,35] |
| 7 | 36.43 | 365- | 349- | Unknown | x | x | [34,35] |
| 8 | 44.17 | 347- | 235- | Cohumulone I | x | x | [34,35] |
| 9 | 44.81 | 347- | 278- | Cohumulone II | x | x | [34,35] |
| 10 | 46.24 | 361- | 292- | n-Humulone | x | x | [34,35] |
| 11 | 46.73 | 361- | 265- | Iso-a-ad/n-humulone | x | x | [34,35] |
| 12 | 47.13 | 361- | 265- | Iso-a-ad/n-humulone | x | x | [34,35] |
| 13 | 47.93 | 361- | 265- | Iso-a-ad/n-humulone | x | x | [34,35] |
| Detected Compounds | Original Sample Using Classical Fermentation | Dealcoholized Sample Using Thermal Process |
|---|---|---|
| Original gravity (wt.%) | 11.60 | 3.16 |
| Ethanol (% ABV) | 4.98 | 0.48 |
| Color (EBC) | 8.0 | 9.5 |
| pH | 4.72 | 4.71 |
| Turbidity (IBC) | 0.5 | 2.5 |
| 1-Propanol (mg/L) | 6.1 | 0.8 |
| 2-Methylpropanol (mg/L) | 10.99 | nd |
| 2-Methyl-1-butanol (mg/L) | 15.2 | nd |
| 3-Methyl-1-butanol (mg/L) | 49.8 | nd |
| 2-Phenylethanol (mg/L) | 15.6 | 22.4 |
| Furfuryl alcohol (mg/L) | 0.07 | nd |
| Ethyl acetate (mg/L) | 16.8 | nd |
| Isoamyl acetate (mg/L) | 1.8 | nd |
| 2-Phenyl ethyl acetate (mg/L) | 0.4 | 0.03 |
| Total of Higher Aliphatic Alcohols (mg/L) | 100.4 | 23.2 |
| Total of Esters Acetic Acids (mg/L) | 18.91 | 0.04 |
| Diacetyl (mg/L) | 0.16 | 0.07 |
| DMS (μg/L) | 25 | nd |
| Type of Analysis | Samples | Main | Std. Error | 95% Confidence Interval | Test ANOVA | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Std. Deviation | Significance. | ||||
| Total Phenol Content (mg GAE 100 mL−1) | HW | 65.120 | 0.001 | 65.117 | 65.123 | 0.003 | 0.001 S |
| BSE | 47.220 | 0.001 | 47.218 | 47.222 | 0.002 | ||
| ABC | 40.150 | 0.001 | 40.148 | 40.152 | 0.002 | ||
| NABT | 35.100 | 0.001 | 35.097 | 35.103 | 0.003 | ||
| Flavonoid Content (mg CE 100 mL−1) | HW | 28.650 | 0.002 | 28.643 | 28.656 | 0.006 | 0.001 S |
| BSE | 24.850 | 0.000 | 24.85 | 24.85 | 0.000 | ||
| ABC | 18.050 | 0.006 | 18.043 | 18.056 | 0.006 | ||
| NABT | 10.012 | 0.001 | 10.010 | 10.014 | 0.001 | ||
| DPPH assay (% Inhibition of the free radical) | HW | 14.00 | 0.057 | 13.841 | 14.158 | 0.057 | 0.001 S |
| BSE | 12.40 | 0.240 | 11.731 | 13.068 | 0.240 | ||
| ABC | 8.50 | 0.018 | 8.448 | 8.551 | 0.041 | ||
| NABT | 5.50 | 0.011 | 5.468 | 5.531 | 0.025 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansouri, F.E.; Farissi, H.E.; Cacciola, F.; Esteves da Silva, J.C.G.; Lovillo, M.P.; Majdoub, Y.O.E.; Trovato, E.; Mondello, L.; Khaddor, M.; Brigui, J. Profiling the Volatile and Non-Volatile Compounds along with the Antioxidant Properties of Malted Barley. Separations 2022, 9, 119. https://doi.org/10.3390/separations9050119
Mansouri FE, Farissi HE, Cacciola F, Esteves da Silva JCG, Lovillo MP, Majdoub YOE, Trovato E, Mondello L, Khaddor M, Brigui J. Profiling the Volatile and Non-Volatile Compounds along with the Antioxidant Properties of Malted Barley. Separations. 2022; 9(5):119. https://doi.org/10.3390/separations9050119
Chicago/Turabian StyleMansouri, Fouad El, Hammadi El Farissi, Francesco Cacciola, Joaquim C. G. Esteves da Silva, Miguel Palma Lovillo, Yassine Oulad El Majdoub, Emanuela Trovato, Luigi Mondello, Mohamed Khaddor, and Jamal Brigui. 2022. "Profiling the Volatile and Non-Volatile Compounds along with the Antioxidant Properties of Malted Barley" Separations 9, no. 5: 119. https://doi.org/10.3390/separations9050119
APA StyleMansouri, F. E., Farissi, H. E., Cacciola, F., Esteves da Silva, J. C. G., Lovillo, M. P., Majdoub, Y. O. E., Trovato, E., Mondello, L., Khaddor, M., & Brigui, J. (2022). Profiling the Volatile and Non-Volatile Compounds along with the Antioxidant Properties of Malted Barley. Separations, 9(5), 119. https://doi.org/10.3390/separations9050119











