Abstract
Curcuma longa (turmeric) has traditionally been used in Ayurvedic, Unani and herbal drugs to cure numerous ailments. Due to the high demand, the quantitative standardization of herbal products is challenging to maintain their quality. We aim to develop a rapid, sensitive and validated high-performance thin-layer chromatography (HPTLC) method for the simultaneous determination and quantification of curcumin I, curcumin II and curcumin III in C. longa and herbal formulation. The three standards were separated using centrifugal preparative thin-layer chromatography (CPTLC) silica gel and identified by different spectroscopic methods. The developed HPTLC method was validated by following ICH guidelines (linearity; limit of detection, LOD; limit of quantitation; accuracy; precision; and robustness). The calibration curves of both the compounds were linear (50–500 ng/spot), with a correlation coefficient (r2) of >999. The developed HPTLC method was effectively applied to the concurrent detection and quantification of curcumins I–III in fresh, dry rhizomes and the herbal formulation of C. longa extracts was obtained by hot and cold extraction methods.
1. Introduction
The genus Curcuma is a rhizomatous annual or perennial herb in the Zingiberaceae family comprising about 120 species [1]. Most of the species of Curcuma are naturally present in tropical evergreen areas [2,3]. Many members of the genus are well known in traditional medicine. C. amada rhizomes are used as an appetizer, carminative, digestive, stomachic, demulcent, vulnerary, aphrodisiac, laxative, diuretic, expectorant, anti-inflammatory and antipyretic [4]. The rhizomes of C. angustifolia are used as a demulcent and antipyretic, are effective against gravel Stomatitis and aid in blood coagulation [5]. In combination with astringents and aromatics, the rhizomes of C. aromatic are used for bruises, sprains, hiccough, bronchitis, cough, leukoderma and skin eruptions [6]. The rhizome of C. zedoaria are used as an appetizer and tonic, and are particularly prescribed to ladies after childbirth. In Ayurveda, it is an ingredient of “Braticityādi kwatha”, used in high fever [7].
Ayurvedic systems have widely used Curcuma longa (turmeric) for centuries in the treatment of many inflammatory conditions and diseases such as biliary disorders, anorexia, cough, diabetic wounds, hepatic disorders, rheumatism and sinusitis [8]. Turmeric has been used as a remedy for all kinds of poisonous conditions, ulcers and wounds [9]. It gives a good complexion to the skin and is applied to face as a depilatory and facial tonic. The drug is also useful in cold, cough, bronchitis, conjunctivitis and liver affections [10,11]. Several pharmacological studies were conducted to support the use of C. longa. Water- and fat-soluble extracts of turmeric and its main active component, curcumin, exhibited strong antioxidant activity, comparable to vitamins C and E. Curcumin was found to be eight times more powerful than vitamin E in preventing lipid peroxidation [12]. Turmeric’s hepatoprotective effects have been proven in a number of animal studies. Curcumin has choleretic activity that increases bile output and solubility, which may be helpful in treating gallstones [13]. An open, phase II trial was performed on 25 patients with endoscopically diagnosed gastric ulcer treated with 600 mg of powdered turmeric five times daily. After four weeks, the ulcers had completely healed in 48 percent of patients and the percentage reached 76 after 12 weeks of treatment. No significant adverse reactions or blood abnormalities were noted [14].
The active constituents of C. longa are the curcumins [15]. Turmeric contains up to 5% curcumin and up to 5% of essential oils. Other constituents include sugars, proteins and resins [16]. The main oil components were identified as ar-turmerone (31.7%), α-turmerone (12.9%), β-turmerone (12.0%) and (Z) β-ocimene (5.5%), α-bisabolene (13.9%), trans-ocimene (9.8%), myrcene (7.6%), 1,8-cineole (6.9%), thujene (6.7%) and thymol (6.4%) [17].
Curcumin has anti-inflammatory properties [18] mediated via downregulation of the nuclear factor (NF)-κB [19] and cyclooxygenase 2 (Cox-2) [20]. Animal studies have indicated that oral curcumin possess antinociceptive [21] and indicated the involvement of ATP-sensitive potassium channels [22]. Pilot human studies of curcumin have demonstrated promise for improving the symptoms of rheumatoid arthritis and inflammatory bowel disease [23,24]. Curcumin also demonstrated antiviral, anti-inflammatory, antibacterial, antifungal, antidiabetic, antifertility and cardiovascular protective and immunostimulant activity [11]. Curcumin, one of the most studied chemopreventive agents, allows suppression, retardation or inversion of carcinogenesis. Curcumin is also described as an antitumoral, anti-oxidant and anti-inflammatory agent capable of inducing apoptosis in numerous cellular systems [25]. Both in vitro studies utilizing human cell lines and in vivo studies have demonstrated curcumin’s ability to inhibit carcinogenesis at three stages: tumor promotion, angiogenesis and tumor growth [26]. Curcumin exhibits anticoagulant activity by inhibiting collagen and adrenaline-induced platelet aggregation in vitro as well as in vivo in rat thoracic aorta [27].
The aim of the current study is to develop and validate the high-performance thin-layer chromatography (HPTLC) method for the quantification of curcumins I–III in different extracts of C. longa and formulation. There are several advantages of using HPTLC for the analysis, such as the ability to analyze crude samples containing multi-components. Several samples can be separated parallel to each other on the same plate, resulting in a high output and a rapid low-cost analysis. The choice of solvents for the HPTLC development is wide, as the mobile phases evaporated before the spot detection. Spray reagents can be used to detect separated spots [28]. The HPTLC method uses much less amounts of the mobile phase, minimizing exposure to organic solvents and reducing environment pollution [29,30].
2. Materials and Methods
2.1. Chemicals and Plant Materials
A standard curcumin mixture was procured from Sigma-Aldrich, St. Louis, MO, USA. All other reagents utilized for the extraction and method development were of analytical grade. The rhizomes of Curcuma longa and its herbal formulation containing 500 mg of curcuma extract per capsule were obtained randomly from the hypermarket in Al-Kharj, Saudi Arabia. Chromatographic and analytical grade reagent (AR) were used for the extraction and method development. Centrifugal preparative TLC (CPTLC) was preformed using a chromatotron (Harrison Research Inc. model 7924, Harrison Research, Palo Alto, CA, USA): a 4 mm silica gel P254 disc. Pre-coated, glass-baked TLC plates obtained from E. Merck (silica gel-60F254; thickness: 0.2 mm; area: 20 × 10 cm) were used for quantification.
2.2. Purification of Individual Curcumins
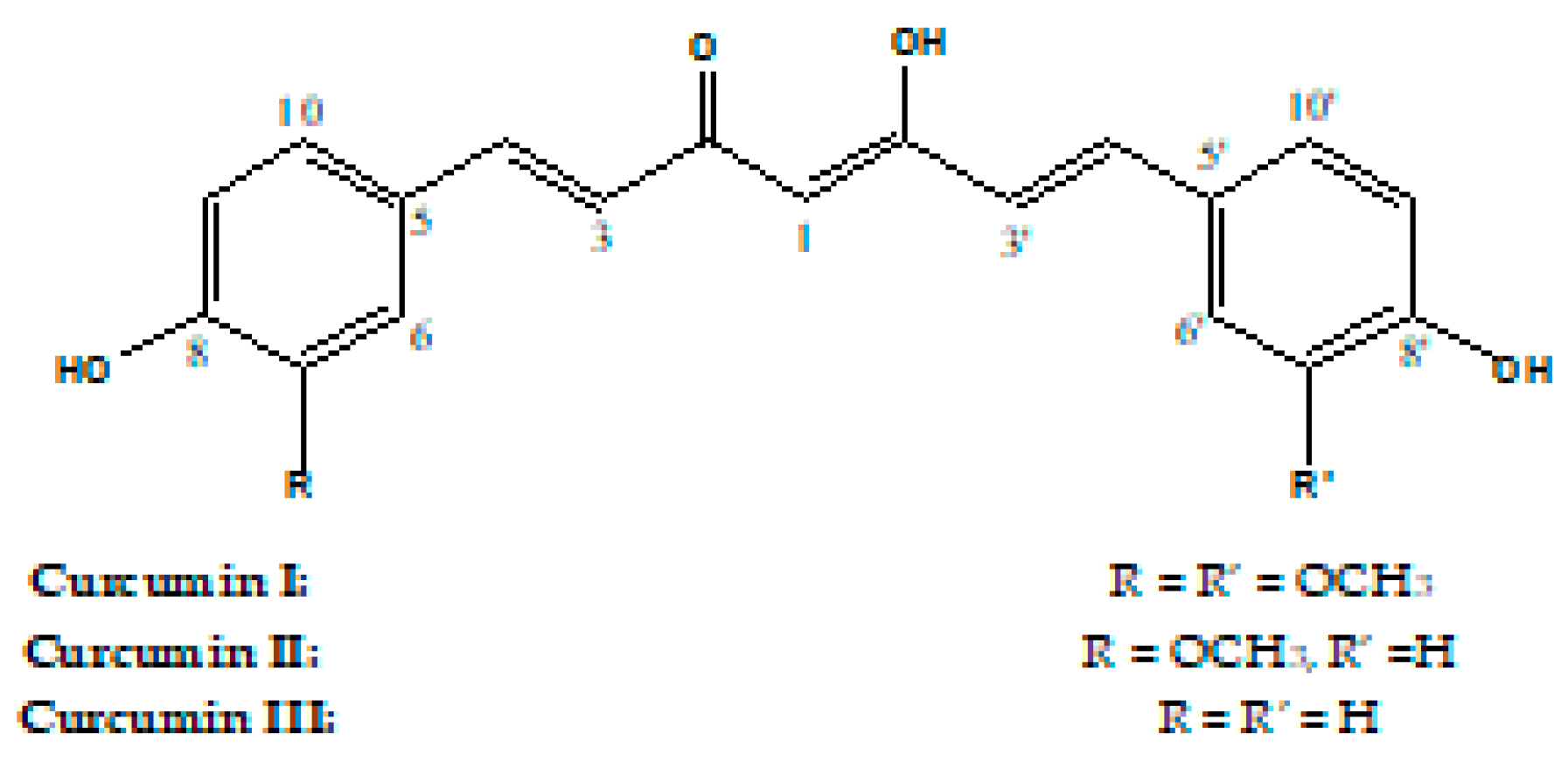
The standardly composed mixture of the three major curcumins was purified to obtain the individual compounds for the quantification. From the standard mixture, 100 mg were separated using CPTLC on 4 mm silica gel GF254 disks (solvents: 0.5% MeOH in CHCl3). Three zones were collected from the chromatotron and corresponded to curcumins I–III (Figure 1).
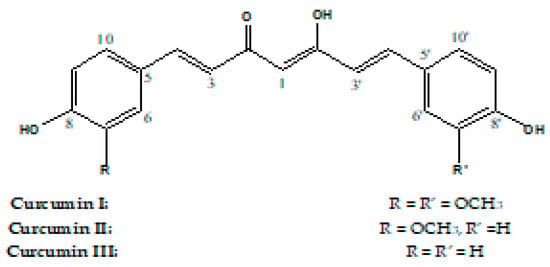
Figure 1.
Structures of Curcumins I–III.
2.3. Charaterization of Curcumins
NMR data (Figures S1 and S2) were measured using a Bruker UltraShield Plus 500 MHz spectrometer (Bruker, Fällanden, Switzerland) at the NMR Unite, College of Pharmacy, Prince Sattam Bin Abdulaziz, and was operated at 500 MHz for protons and 125 MHz for carbon atoms, respectively. Chemical shift values were reported in δ (ppm) relative to the residual solvent peaks. HRESIMS (Figures S3–S5) were determined by direct injection using a Thermo Scientific UPLC RS Ultimate 3000-Q Exactive hybrid quadrupole-Orbitrap mass spectrometer (Thermo Scientific, Waltham, MA, United States) combined with the high-performance quadrupole precursor selection with high resolution and accurate-mass (HR/AM) Orbitrap™ detection.
2.4. Extraction Procedure
Fresh and dried curcuma purchased from the local market and a formulation containing 500 mg of curcuma extract per capsule were extracted with MeOH by maceration (×3) at room temperature, using the soxhlet apparatus for 6 h to obtain the corresponding six extracts, two for each sample. All extracts were filtered and dried using the rotary vacuum evaporator to obtain the corresponding dry extracts.
2.5. HPTLC Conditions
HPTLC (Camag, Muttenz, Switzerland) was performed on glass-backed TLC silica-gel plates (20 cm × 10 cm). Purified curcumin I, curcumin II and curcumin III and sample solutions were separately spotted with nitrogen flow on a plate with a 6-mm-wide band at 8-mm from the bottom by using the automatic Camag-TLC (Linomat V, Camag, Muttenz, Switzerland) applicator. The (linear ascending) development of TLC plates was performed in a twin glass Camag chamber (automatic ADC2, Camag). The chamber was filled with 20 mL of mobile phase for a fixed time of 15 min.
2.6. Method Validation
Different validation parameters for the simultaneous determination of curcumin I, curcumin II and curcumin III were determined as per the ICH-Q2 (R1) guidelines [31].
2.6.1. Accuracy
Accuracy was determined by the standard addition method. The preanalyzed samples of curcumin I, curcumin II and curcumin III were spiked with the extra 0, 50, 100 and 150% of the standard curcumin I, curcumin II and curcumin III, and the solutions were reanalyzed in six replicates by the proposed method. The accuracy of the HPTLC technique for the simultaneous determination of curcumin I, curcumin II and curcumin III was estimated as % recovery.
2.6.2. Precision
Precision of the proposed method was determined at two levels i.e., repeatability and intermediate precision. Repeatability was determined as the intraday precision, whereas the intermediate precision was determined by carrying out an inter-day variation for the determination of curcumin I, curcumin II and curcumin III. The precision of the HPTLC technique for the simultaneous estimation of curcumin I, curcumin II and curcumin III was expressed as the percent relative standard deviation (% RSD).
2.6.3. Robustness
The robustness of the proposed HPTLC method was determined to evaluate the influence of small deliberate changes in the chromatographic conditions during the determination of curcumin I, curcumin II and curcumin III. The robustness of the HPTLC technique for the simultaneous determination of curcumin I, curcumin II and curcumin III was evaluated by introducing small deliberate changes in the composition of mobile phase components, total run length, saturation time and detection wavelength.
2.6.4. Limit of Detection and Quantification (Sensitivity)
The sensitivity of the HPTLC technique for the simultaneous determination of curcumin I, curcumin II and curcumin III was determined in terms of detection (LOD) and quantification (LOQ) limits. The limit of detection (LOD) and limit of quantification (LOQ) were determined by the standard deviation (SD) method. They were determined from the slope of the calibration (S) curve and SD of the blank sample using the following equations:
LOD = 3.3 × SD/S
LOQ = 10 × SD/S
2.6.5. Specificity
The specificity of the proposed TLC densitometric method was confirmed by the Rf and spectra of the spot with that of the standards of curcumin I, curcumin II and curcumin III.
2.7. Quantification of Curcumins I–III in C. longa and Herbal Formulation
Curcumin I, curcumin II and curcumin III peaks in C. longa and herbal formulation were identified by comparing their spots at that of the standard curcumin I, curcumin II and curcumin III. The amount of curcumin I, curcumin II and curcumin III present in the C. longa and herbal formulation was quantified from the regression equation obtained from the calibration plot of HPTLC.
3. Results
3.1. Method Validation
3.1.1. Linearity
Calibration curves for curcumins I–III are presented in Figures S6–S8. The results for the least square regression analysis of the calibration curves (CCs) of curcumins I–III are included in Table 1.

Table 1.
Linear regression data for the calibration curve of curcumins I–III (n = 6).
3.1.2. Accuracy
The accuracy of the HPTLC technique for the simultaneous determination of curcumins I–III was estimated as % recovery, and the results are included in Table 2.

Table 2.
Accuracy of the proposed method of curcumins I–III (n = 6).
3.1.3. Precision
The precision of the HPTLC technique for the simultaneous estimation of curcumin I, curcumin II and curcumin III was estimated in terms of instrumental and intra/inter-assay precision and expressed as the percent relative standard deviation (% RSD). The results of intra/inter-assay precisions for the simultaneous determination of curcumins I–III using the HPTLC technique are included in Table 3.

Table 3.
Precision of the proposed method of curcumins I–III.
3.1.4. Robustness
The robustness of the HPTLC technique for the simultaneous determination of curcumins I–III was evaluated by introducing small deliberate changes in the composition of mobile phase components, total run length, saturation time, and detection wavelength. The results of the robustness analysis after changing the mobile phase components are included in Table 4.

Table 4.
Robustness of the proposed HPTLC method of curcumins I–III.
3.1.5. Sensitivity
The sensitivity of the HPTLC technique for the simultaneous determination of curcumin I, curcumin II and curcumin III was determined in terms of detection (LOD) and quantification (LOQ) limits.
3.1.6. Specificity
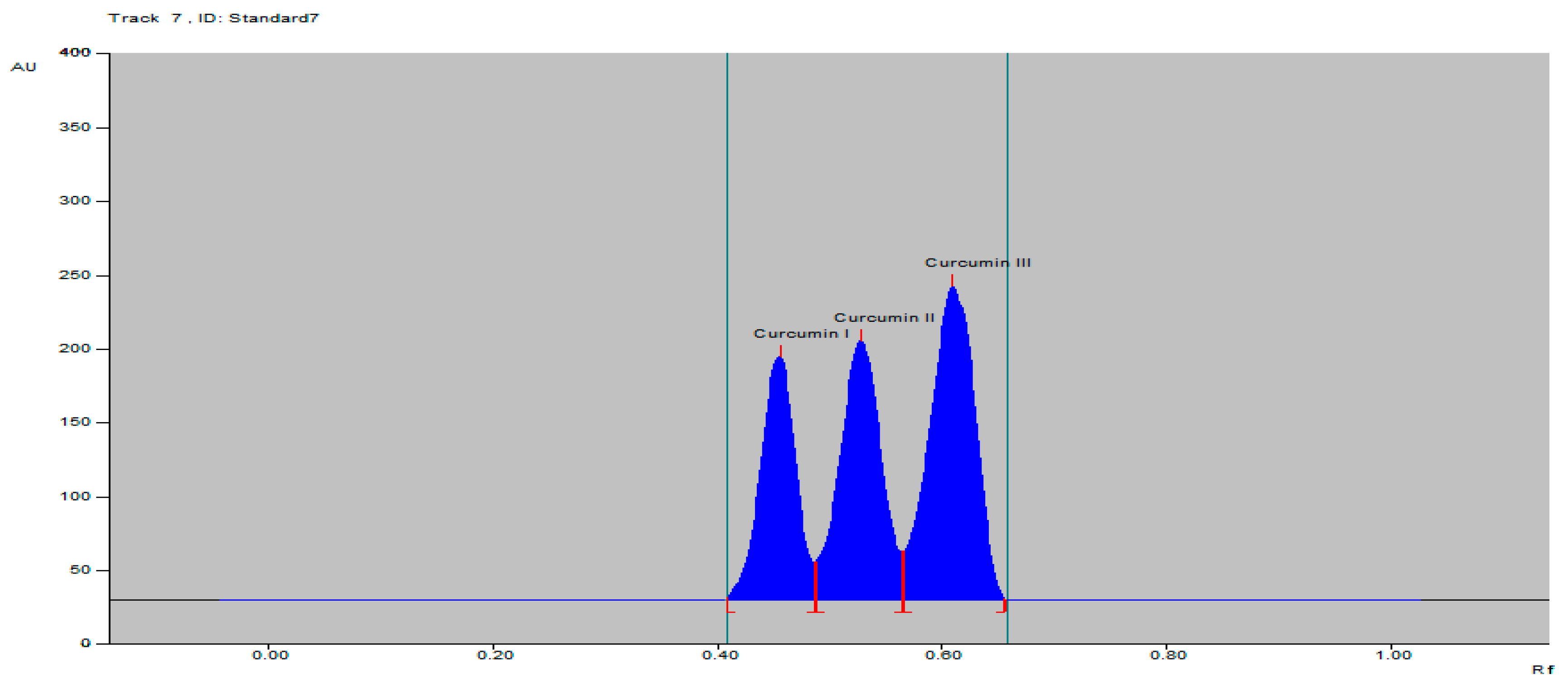
The specificity of the proposed TLC densitometric method was confirmed by the Rf at 0.45 ± 0.02, 0.52 ± 0.02 and 0.61 ± 0.04 and the spectra of the spot with that of the standards curcumins I–III (Figure 2). The proposed method was found to be specific by comparing the Rf of C. longa rhizomes, herbal formulation and standard, as well as the overlaid spectra at peak start, peak apex and peak end position of the spot, showing λmax 423 nm for curcumin I, curcumin II and curcumin III (Figure S9).
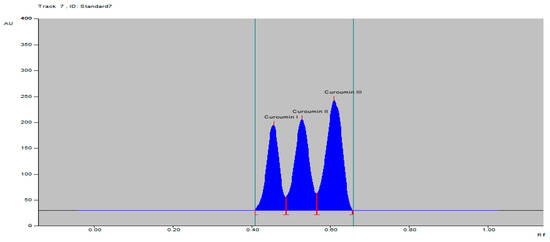
Figure 2.
HPTLC densitogram of standard curcumins I–III.
3.2. Quantification of Curcumins I–III in C. longa and Herbal Formulation
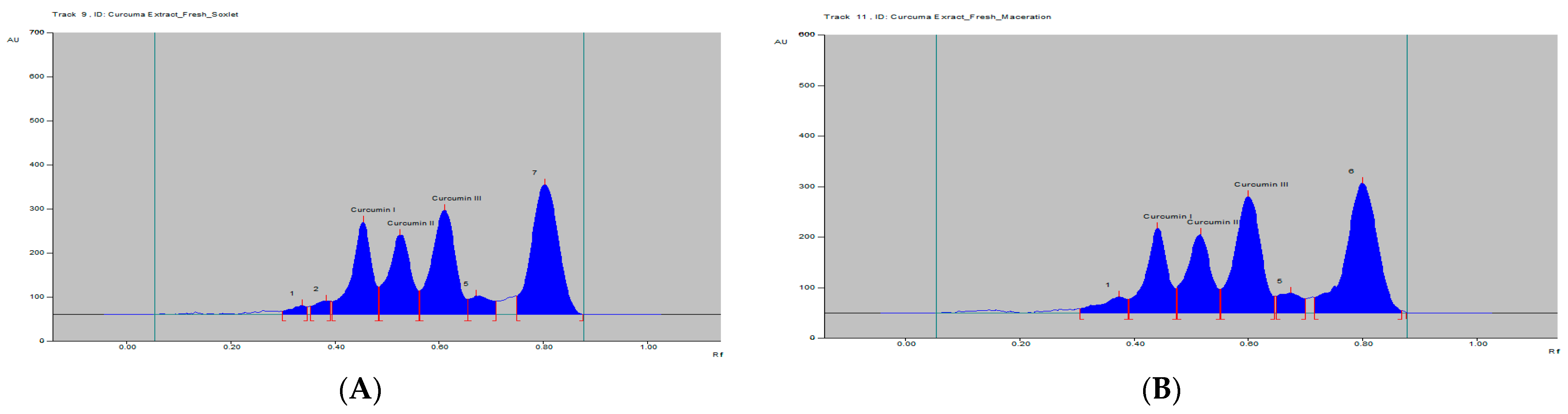
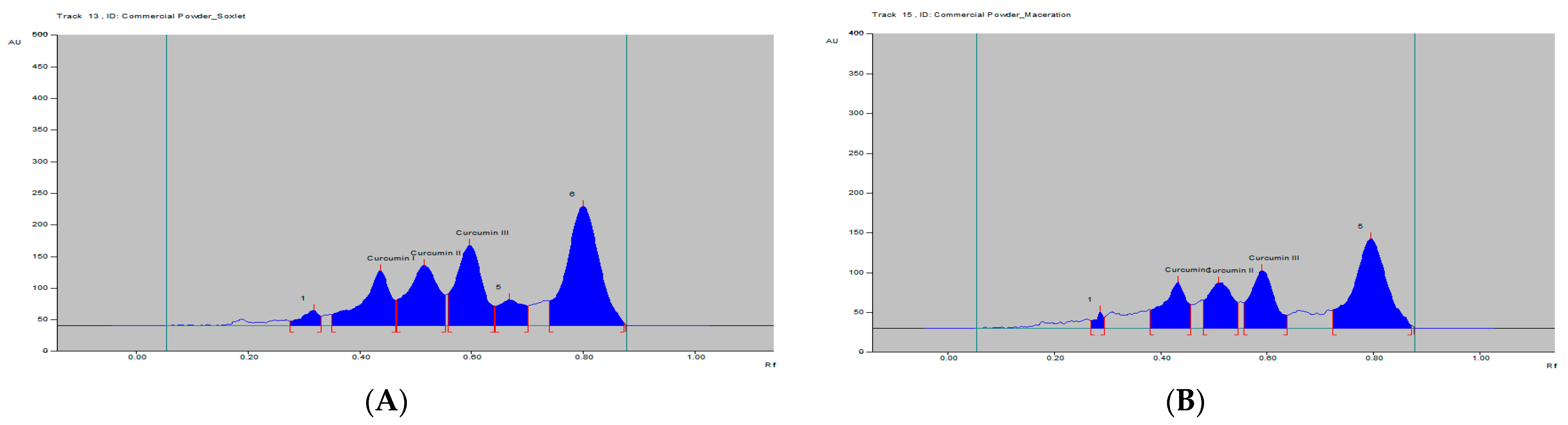
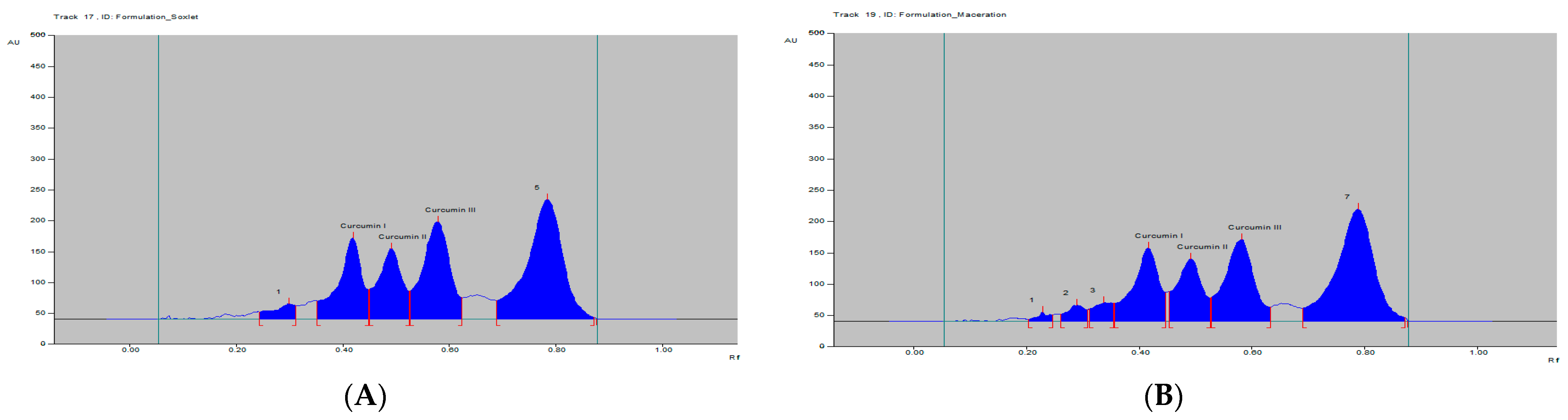
The curcumins I–III peaks in the C. longa and herbal formulation were identified by comparing their spots at Rf = 0.45, 0.52 and 0.61 with that of purified curcumins I–III (Figure 2) as well as the overlay UV spectra of the spots with the same Rf value (Figure S9). The amount of curcumins I–III present in the C. longa (Figure 3, Figure 4 and Figure 5) and herbal formulation was quantified from the regression equation obtained from the calibration plot of HPTLC. The amounts of curcumins I–III in C. longa and herbal formulation are presented in Table 5.
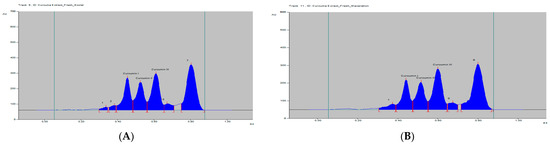
Figure 3.
HPTLC densitogram of fresh C. longa extract by (A) soxhlet and (B) maceration.
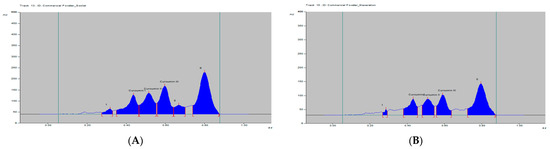
Figure 4.
HPTLC densitogram of dry C. longa extract by (A) soxhlet and (B) maceration.
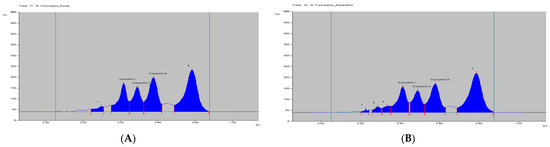
Figure 5.
HPTLC densitogram of C. longa formulation extract by (A) soxhlet and (B) maceration.

Table 5.
Contents (% w/w) of curcumins I–III in different samples of C. longa.
4. Discussion
4.1. Characterization of Curcumins
The three purified curcumins I–III shared the same carbon chain composed of C-1, C-2, C-2′, C-3, C-3′, C-4 and C-4′ with two trans-oriented methine carbons assigned for C-3, 3′ and C-4, 4′, which have trans orientation based on the J value of 16.0 Hz between H-3, 3′ and H-4, 4′ (Supplementary Materials). Curcumin I 1H, 13C NMR spectrum, showed ABX spin system in a 1, 3, 4 trisubstituted aromatic ring (Figures S1A and S2A). Two overlapped OCH3 appeared at δH 3.98 and δC 55.84 ppm. High resolution electrospray ionization mass spectroscopy (HRESIMS) (Figure S3) showed an M+−1 at m/z 367.1188, M++1 at 369.1328 and M++Na 391.1147. The data were identical with those reported for curcumin I [32,33,34]. 1H NMR of curcumin II (Figure S1B) showed one system ABX system assigned for 6, 9 and 10 at δH 7.19 (s), 6.80 (bd, J = 7.5 Hz) and 7.08 (d, J = 7.0 Hz) in a tri-substituted benzene ring and an AX system assigned for a para-substituted benzene ring at δH 6.80 (bd, J = 7.5 Hz) and 7.46 (d, J = 7.5 Hz). Signals for one OCH3 at δH 3.83 and δC 56.04 ppm were also observed. HRESIMS (Figure S4) showed an M+−1 at m/z 337.1084, M++1 at 339.1226 and M++Na at 361.1043. The data were identical with those reported for curcumin II [32,33,34]. The 1H NMR of curcumin III (Figure S1C) showed only two aromatic proton signals at δH 6.80 (d, J = 8.2) and 7.47 (d, J = 8.2) each integrated for four protons diagnostic for two identical para-substituted benzene rings. HRESIMS showed (Figure S5) an M+−1 at m/z 307.0977, M++1 at 309.1120 and M++Na at 331.0938. The data were identical with those reported for curcumin III [32,33,34].
4.2. HPTLC Analysis
Standard curcumins are available as a mixture of the three main curcumins. For the purpose of quantification, the mixture was purified on chromatoron using normal silica gel and the resulted compounds were used as a standard after their identification spectroscopically. The calibration curve for curcumins I–III was observed as linear in the range of 50–500 ng band-1. The results demonstrated a good linear relationship between the concentration and spot area of curcumins I–III. The determination coefficient (R2) value for curcumins I–III was obtained as 0.998, 0.999 and 0.997, respectively, which were highly significant (p < 0.05) (Table 1). The R2 values represents the strength of the correlations between the two variables. All these observations and results suggested that the sustainable HPTLC technique was linear and acceptable for the simultaneous estimation of curcumin I, curcumin II and curcumin III.
The preanalyzed sample of curcumins I–III were spiked with the extra 0, 50, 100 and 150% of the standard curcumins I–III, and the solutions were reanalyzed in six replicates by the proposed method. The % of recoveries of curcumins I–III were estimated as 97.22–98.67, 97.83–98.22 and 99.32–100.84%, respectively, using the HPTLC technique (Table 2). The estimated % recoveries within the limit that exhibited the HPTLC technique was accurate for the simultaneous determination of curcumins I–III.
The precision of the proposed method was determined at two levels, i.e., repeatability and intermediate precision. The repeatability was determined as an intra-day precision, whereas the intermediate precision was determined by carrying out inter-day variation for the determination of curcumins I–III. Intra-day and inter-day precisions (n = 6) for curcumin I, curcumin II and curcumin III were found to be 0.78–1.56% and 1.08–1.72%, 0.49–1.06 and 0.74–1.21 and 0.36–1.04 and 0.44–1.43, respectively (Table 3), which demonstrated the good precision of the proposed method.
The robustness of the HPTLC technique for the simultaneous determination of curcumins I–III was evaluated by introducing small deliberate changes in the composition of mobile phase components. The % RSD for curcumins I–III after this change were determined as 1.01–1.33, 0.36–0.75 and 0.45–0.55%, respectively. In addition, the Rf values for curcumins I-III were recorded as 0.43–0.46, 0.49–0.52 and 0.61–0.63, respectively (Table 4). The small differences in the Rf values and lower % RSD demonstrated the robustness of the HPTLC technique for the simultaneous determination of curcumins I–III.
The LOD and LOQ values for curcumin I were determined as 4.36 and 12.79 ng band-1, respectively. The LOD and LOQ values for curcumin II, were determined as 4.59 and 12.67 ng band−1, respectively. However, the LOD and LOQ values for curcumin III, were determined as 4.66 and 12.84 ng band-1, respectively. The recorded values of LOD and LOQ suggested that the HPTLC technique was highly sensitive for the simultaneous estimation and quantification of curcumins I–III.
The specificity was proven by comparing the Rf at 0.45 ± 0.02, 0.52 ± 0.02 and 0.61 ± 0.04 and spectra of the spots in the C. longa extract and formulation with that of the purified curcumins I–III. The proposed method was found to be specific by comparing the Rf of rhizomes of C. longa and herbal formulation and standard as well as the overlaid spectra at peak start, peak apex and peak end position of the spot showing λmax 423 nm for curcumins I–III (Figure S9). The specificity or peak purity for the proposed analytical method was suggested by the similar UV-absorption spectra, Rf values and detection wavelength of curcumins I–III.
4.3. Quantification of Curcumins I–III in C. longa and Herbal Formulation
Extraction methods selection is mainly based on the nature of the components to be obtained. Solvent extraction is the most widely used method. Solvent extraction includes maceration, percolation and reflux extraction. It usually requires a large volume of the organic solvents and long extraction time. Some modern extraction methods, such as the super critical fluid extraction, pressurized liquid extraction and microwave-assisted extraction, have been used in extraction. These methods lower organic solvent consumption, shorten extraction time and are more selective. However, they require more sophisticated and expensive instrumentation [35]. Maceration is a very simple extraction method with the disadvantage of long extraction time and low extraction efficiency. It could be used for the extraction of thermolabile components. On the other hand, the soxhlet extraction method integrates the advantages of the reflux extraction and percolation, which utilizes the principle of reflux and siphoning to continuously extract the plant materials with fresh solvent. The soxhlet extraction is an automatic continuous extraction method with high extraction efficiency that requires less time and solvent consumption than maceration or percolation. However, soxhlet extraction is not suitable for plant materials with thermolabile or volatile components [35]. In the current study, for comparison, both the maceration and soxhlet extraction were applied for the extraction of curcumins from C. longa fresh rhizomes, dry powdered rhizomes and the formulation, using methanol as an extraction solvent. The amounts of the three main curcumins were estimated by applying the proposed and validated HPTLC method. The results of the analysis are presented in Figure 3, Figure 4 and Figure 5 and Table 5. The content of curcumin I–III in fresh rhizome and dry powdered by maceration and soxlet extraction was determined as 0.44, 0.28, 0.65 and 0.53, 0.37, 0.76 and 0.23, 0.15, 0.33 and 0.34, 0.25, 0.47% w/w, respectively, using the proposed analytical method. The content of curcumin I–III in formulation by maceration and soxhlet extraction was determined as 0.26, 0.19, 0.41 and 0.28, 0.22, 0.46% w/w, respectively, using the proposed analytical method.
As expected in all cases, the soxhlet extraction was more efficient than the maceration and gives better yield of the extracted compounds. Moreover, the soxhlet saves much time and solvent consumption. The results also indicated that the fresh plant materials were superior to the dry powdered plants in term of the percentage w/w of the three curcumins. This fact may be explained by the loss of some contents during the drying and processing of the plant material by oxidation or other factors. The amount of curcumin III was the highest in all estimations followed by curcumin I, while curcumin II was present in a lower concentration.
5. Conclusions
In conclusion, curcumins I–III were purified and characterized by various spectroscopic methods from the standard curcumin mixture available on the market. An HPTLC method was developed, validated and applied to estimate the amount of curcumins in the fresh, dry plant materials and formulation containing C. longa. Two extraction methods were applied to obtain the target curcumins from the raw plant materials. The soxhlet extraction gives better yields of the three curcumins than maceration, indicating the thermal stability of the compounds under used conditions. The fresh plant materials contained more amounts of curcumins than the dry plant materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9040094/s1. Figure S1. 1HNMR of the aromatic protons of curcumins I–III (A–C). Figure S2. 13CNMR of the aromatic carbons of curcumins I–III (A–C). Figure S3. Mass spectra of curcumin I (A: Negative Mode B: Positive Mode). Figure S4. Mass spectra of curcumin II (A: Negative Mode B: Positive Mode). Figure S5. Mass spectra of Curcumin III (A: Negative Mode B: Positive Mode). Figure S6. Calibration curve of curcumin I. Figure S7. Calibration curve of curcumin II. Figure S8. Calibration curve of curcumin III. Figure S9: Overlay UV absorption spectra of curcumins I–III and corresponding spots in C. longa extracts and formulations.
Author Contributions
Conceptualization, M.S.A.-K. and P.A.; methodology, P.A., A.A.S., K.A.A. and M.H.A.; software, A.I.F. and M.H.A.; validation, A.I.F. and M.H.A.; formal analysis, M.S.A.-K., A.I.F. and M.H.A.; investigation, P.A., A.A.S. and K.A.A.; resources, M.S.A.-K.; data curation, P.A., A.A.S. and K.A.A.; writing—original draft preparation, P.A., A.A.S. and K.A.A.; writing—review and editing, M.S.A.-K., M.H.A. and A.I.F.; visualization, M.S.A.-K.; supervision, M.S.A.-K.; project administration, M.S.A.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K. A preliminary checklist of the Zingiberaceae of Thailand. Thai For. Bull. 1996, 24, 35–49. [Google Scholar]
- Xia, Q.; Zhao, K.J.; Huang, Z.G.; Zhang, P.; Dong, T.T.; Li, S.P.; Tsim, K.W. Molecular genetic and chemical assessment of Rhizoma Curcumae in China. J. Agric. Food Chem. 2005, 53, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Shaw, B.P.; Mukherjee, A. A new glycosidic flavonoid from Jwarhar mahakashay (antipyretic) Ayurvedic preparation. Int. J. Ayurveda Res. 2010, 1, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Nigam, S.S. Antifungal activity of the essential oil of Curcuma angustifolia. Indian J. Pharmacol. 1977, 39, 143–145. [Google Scholar]
- Warrier, P.K.; Nambiar, V.P.; Ramankutty, C. Indian Medicinal Plants; Orient Longman Ltd.: Madras, India, 1994; pp. 1–5. [Google Scholar]
- Thakur, R.S.; Puri, H.S.; Husain, A. Major Medicinal Plants of India; CIMAP: Lucknow, India, 1989; pp. 50–52. [Google Scholar]
- Hanif, R.; Qiao, L.; Shiff, S.J. Curcumin., a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J. Laborat. Clin. Med. 1997, 130, 576–584. [Google Scholar] [CrossRef]
- Kolammal, M. Pharmacognosy of Ayurvedic Drugs; Ayurveda College: Trivandrum, India, 1979. [Google Scholar]
- Kurup, P.N.; Ramdas, V.N.; Joshi, P. Handbook of Medicinal Plants; New Delhi, Oxford and IBH Publishing Co pvt Ltd.: Delhi, India, 1979. [Google Scholar]
- Nadkarni, A.K. Indian Materia Medica; Popular Prakashay: Bombay, India, 1954; Volume 408, p. 1476. [Google Scholar]
- Toda, S.; Miyase, T.; Arich, H. Natural antioxidants. Antioxidative compounds isolated from rhizome of Curcuma longa. Laborat Chem. Pharmacol. Bullet. 1985, 33, 1725–1728. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Jeon, C.H.; Ko, G. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J. Pharm. Pharmacol. 2000, 52, 437–440. [Google Scholar] [CrossRef]
- Ammon, H.P.T.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Goh, C.L.; Ng, S.K. Allergic contact dermatitis to Curcuma longa (turmeric). Cont. Dermat. 1987, 17, 186. [Google Scholar]
- Chempakam, B.; Parthasarathy, V.A.; Zachariah, T.J. Chemistry of Spices; Chempakam, B., Parthasarathy, V.A., Eds.; Turmeric; CABI Publishing: Oxford, UK, 2008. [Google Scholar]
- Asher, G.N.; Spelman, K. Clinical utility of curcumin extract. Altern. Ther. Health Med. 2013, 19, 20–22. [Google Scholar]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.; Abdi, S. Cyclooxygenase-2 and antagonists in pain management. Curr. Opin. Anaesthesiol. 2001, 14, 527–532. [Google Scholar] [CrossRef]
- Tajik, H.; Tamaddonfard, E.; Hamzeh-Gooshchi, N. The effect of curcumin (active substance of turmeric) on the acetic acidinduced visceral nociception in rats. Pak. J. Biol. Sci. 2008, 11, 312–314. [Google Scholar] [CrossRef] [Green Version]
- De Paz-Campos, M.A.; Chavez-Pina, A.E.; Ortiz, M.I.; Castaneda-Hernandez, G. Evidence for the participation of ATP sensitive potassium channels in the antinociceptive effect of curcumin. Korean J. Pain. 2012, 25, 221–227. [Google Scholar] [CrossRef]
- Chandran, B.; Goel, A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Digest Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef] [Green Version]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Srivastava, R.; Puri, V.; Srimal, R.C.; Dhawan, B.N. Effect of curcumin on platelet aggregation and vascular prostacyclin synthesis. Arzneimittelforschung 1986, 36, 715–717. [Google Scholar]
- Limtrakul, P.; Lipigorngoson, S.; Namwong, O. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997, 116, 197203. [Google Scholar] [CrossRef]
- Wagner, H. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Shewiyo, D.H.; Kaaleb, E.; Rishab, P.G.; Dejaegherc, B.; Verbekec, J.S.; Heydenc, Y.V. HPTLC methods to assay active ingredients in pharmaceutical formulations: A review of the method development and validation steps. J. Pharm. Biomed. Anal. 2012, 66, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Shuijun, L.; Gangyi, L.; Jingying, J.; Xiaochuan, L.; Chen, Y. Liquid chromatography-negative ion electrospray tandem mass spectrometry method for the quantification of ezetimibe in human. Plasma J. Pharm. Biomed. Anal. 2006, 40, 987–992. [Google Scholar]
- ICH. Q2 (R2): Validation of Analytical Procedures–Text and Methodology. In Proceedings of the International Conference on Harmonization (ICH), Geneva, Switzerland, 31 March 2022. [Google Scholar]
- Chearwae, W.; Wu, C.P.; Chu, H.Y.; Lee, T.R.; Ambudkar, S.V.; Limtrakul, P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother. Pharmacol. 2006, 57, 376–388. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdul, Q.M.; Imtiaz, S.M.; Muddassar, M.; Hameed, A.; Nadeem, A.M.; Asiri, A.M. Curcumin: Synthesis optimization and in silico interaction with cyclin dependent kinase. Acta Pharm. 2017, 67, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, M.M.; Shyamala, B.N.; Manjunatha, J.R.; Sulochanamma, G.; Srinivas, P. Simple HPLC method for resolution of curcuminoids with antioxidant potential. J. Food Sci. 2009, 74, C312–C318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).