Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling Site
2.2. Standards and Reagents
2.3. Extraction Protocols
2.4. Analytical Determinations
2.4.1. Determination of Total Phenolic (TP) and Total Flavonoid (TF) Contents and Antioxidant Activity (AA) by UV-Vis Spectrophotometric Determinations
2.4.2. Phenolic Compounds Profile by UHPLC-DAD-ESI/MS
2.4.3. Sugars Profile by HPLC-ELSD
2.4.4. Elemental Content Determination
2.5. Data Analysis
3. Results
3.1. Evaluation of Total Polyphenolics (TP), Total Flavonoids (TF) and Antioxidant Activity (AA) of Chestnut Cultivars
3.2. Target UHPLC-MS/MS Analysis of Phenolic Compounds Biomarkers in the Investigated Chestnut Cultivars
3.3. Untarget UHPLC-MS/MS Analysis of Biomarkers and Their Metabolite Profiles in Fruits of Different Chestnut Cultivars
Vitamins
3.4. Sugars Profile of Different Chestnut Cultivars Harvested in Two Years
3.5. Macro and Micronutrients Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Míguez-Soto, B.; Fernández-Cruz, J.; Fernández-López, J. Mediterranean and Northern Iberian gene pools of wild Castanea sativa Mill. are two differentiated ecotypes originated under natural divergent selection. PLoS ONE 2019, 14, e0211315. [Google Scholar] [CrossRef] [PubMed]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. Agrobiodiversity Conservation: Genotype Influence on Chemical and Sensorial Traits of Cultivars Grown on the Same Clonal Rootstock. Foods 2020, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Choupina, A.B. Nutritional and health potential of European chestnut. Rev. Ciências Agrárias 2019, 42, 801–807. [Google Scholar] [CrossRef]
- Akbulut, M.; Bozhuyuk, M.R.; Ercisli, S.; Skender, A.; Sorkheh, K. Chemical Composition of Seed Propagated Chestnut Genotypes from Northeastern Turkey. Not. Bot. Horti Agrobot. 2017, 45, 425–430. [Google Scholar] [CrossRef]
- Otles, S.; Selek, I. Phenolic compounds and antioxidant activities of chestnut (Castanea sativa Mill.) fruits. Qual. Assur. Saf. Crop. Foods 2012, 4, 199–205. [Google Scholar] [CrossRef]
- Poljak, I.; Vahčić, N.; Vidaković, A.; Tumpa, K.; Žarković, I.; Idžojtić, M. Traditional Sweet Chestnut and Hybrid Varieties: Chemical Composition, Morphometric and Qualitative Nut Characteristics. Agronomy 2021, 11, 516. [Google Scholar] [CrossRef]
- Gonçalves, B.; Borges, O.; Costa, H.S.; Bennett, R.; Santos, M.; Silva, A.P. Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: Proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010, 122, 154–160. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef]
- Rodrigues, P.; Ferreira, T.; Nascimento-Gonçalves, E.; Seixas, F.; da Costa, R.M.G.; Martins, T.; Neuparth, M.J.; Pires, M.J.; Lanzarin, G.; Félix, L.; et al. Dietary Supplementation with Chestnut (Castanea sativa) Reduces Abdominal Adiposity in FVB/n Mice: A Preliminary Study. Biomedicines 2020, 8, 75. [Google Scholar] [CrossRef]
- Mert, C.; Ertürk, Ü. Chemical Compositions and Sugar Profiles of Consumed Chestnut Cultivars in the Marmara Region, Turkey. Not. Bot. Horti Agrobot. 2017, 45, 203–207. [Google Scholar] [CrossRef]
- Da, L.R.; Silva, B.M. Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016. [Google Scholar]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2017, 32, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Brochard, M.; Correia, P.; Barroca, M.J.; Guiné, R.P.F. Development of a New Pasta Product by the Incorporation of Chestnut Flour and Bee Pollen. Appl. Sci. 2021, 11, 6617. [Google Scholar] [CrossRef]
- Pinto, D.; Rodrigues, F.; Braga, N.; Santos, J.; Pimentel, F.B.; Palmeira-De-Oliveira, A.; Oliveira, M.B.P.P. The Castanea sativa bur as a new potential ingredient for nutraceutical and cosmetic outcomes: Preliminary studies. Food Funct. 2017, 8, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the Phenolic Profile of Castanea sativa Mill. By-Products and Their Antioxidant and Antimicrobial Activity against Multiresistant Bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- You, T.T.; Zhou, S.K.; Wen, J.L.; Ma, C.; Xu, F. Chemical Composition, Properties, and Antimicrobial Activity of the Water-Soluble Pigments from Castanea mollissima Shells. J. Agric. Food Chem. 2014, 62, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Gullón, B.; Dávila, I.; Eibes, G.; Labidi, J.; Gullón, P. Optimization of alkaline pretreatment for the co-production of biopolymer lignin and bioethanol from chestnut shells following a biorefinery approach. Ind. Crops Prod. 2018, 124, 582–592. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: A review on phytochemical composition, bioactivity and waste management approaches for industrial valorization. Food Res. Int. 2021, 144, 110364. [Google Scholar] [CrossRef]
- Corona, P.; Frangipane, M.T.; Moscetti, R.; Feudo, G.L.; Castellotti, T.; Massantini, R. Chestnut Cultivar Identification through the Data Fusion of Sensory Quality and FT-NIR Spectral Data. Foods 2021, 10, 2575. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Casal, S.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Nutritional, Fatty Acid and Triacylglycerol Profiles of Castanea sativa Mill. Cultivars: A Compositional and Chemometric Approach. J. Agric. Food Chem. 2009, 57, 2836–2842. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Casal, S.; Ferreira, I.C.F.R.; Peres, A.M.; Pereira, J.A.; Oliveira, M.B.P.P. Chemical characterization of chestnut cultivars from three consecutive years: Chemometrics and contribution for authentication. Food Chem. Toxicol. 2012, 50, 2311–2317. [Google Scholar] [CrossRef]
- Peña-Méndez, E.M.; Hernández-Suárez, M.; Díaz-Romero, C.; Rodríguez-Rodríguez, E. Characterization of various chestnut cultivars by means of chemometrics approach. Food Chem. 2008, 107, 537–544. [Google Scholar] [CrossRef]
- De Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Pinto, T.; Vilela, A.; Marques, T.; Borges, A.; Caço, J.; Ferreira-Cardoso, J.; Raimundo, F.; Gomes-Laranjo, J. Irrigation positively affects the chestnut’s quality: The chemical composition, fruit size and sensory attributes. Sci. Hortic. 2018, 238, 177–186. [Google Scholar] [CrossRef]
- Furones-Pérez, P.; Fernández-López, J. Morphological and phenological description of 38 sweet chestnut cultivars (Castanea sativa Miller) in a contemporary collection. Spanish J. Agric. Res. 2009, 7, 829–843. [Google Scholar] [CrossRef]
- De Vasconcelos, M.D.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Cardoso, J.V.F. Primary and secondary metabolite composition of kernels from three cultivars of Portuguese chestnut (Castanea sativa Mill.) at different stages of industrial transformation. J. Agric. Food Chem. 2007, 55, 3508–3516. [Google Scholar] [CrossRef]
- Ertürk, Ü.; Mert, C.; Soylu, A. Chemical composition of fruits of some important chestnut cultivars. Braz. Arch. Biol. Technol. 2006, 49, 183–188. [Google Scholar] [CrossRef]
- Delgado, T.; Ramalhosa, E.; Pereira, J.A.; Casal, S. Organic acid profile of chestnut (Castanea sativa Mill.) as affected by hot air convective drying. Int. J. Food Prop. 2018, 21, 557–565. [Google Scholar] [CrossRef]
- Suárez, M.H.; Galdón, B.R.; Mesa, D.R.; Romero, C.D.; Rodríguez, E.R. Sugars, Organic Acids and Total Phenols in Varieties of Chestnut Fruits from Tenerife (Spain). Food Nutr. Sci. 2012, 3, 705–715. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Pereira, J.A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Sugars Profiles of Different Chestnut (Castanea sativa Mill.) and Almond (Prunus dulcis) Cultivars by HPLC-RI. Plant Foods Hum. Nutr. 2010, 65, 38–43. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Sustainable Recovery of Preservative and Bioactive Compounds from Food Industry Bioresidues. Antioxidants 2021, 10, 1827. [Google Scholar] [CrossRef]
- Chira, D.; Bolea, V.; Botu, M.; Giorgi, N.; Juveloiu, E. Sweet chestnut (Castanea sativa Mill.) forest in Romania: Distribution, current state, management and research activities. Revista Silvicultură Cinegetică 2013, 33, 15–20. [Google Scholar]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Botu, M. Physical and compositional characteristics of chestnut fruits. Rom. J. Hortic. 2020, 1, 51–58. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geană, E.I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Geană, E.-I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh.) Cultivars. Foods 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Geană, E.-I.; Ciucure, C.T.; Costinel, D.; Ionete, R.E. Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control 2020, 109, 106919. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chiţescu, C.L.; Sandru, C.; Geană, E.-I.; Lupoae, M.; Dobre, M.; Borda, D.; Gird, C.E.; Boscencu, R. Comparative study of the bioactive properties and elemental composition of red clover (Trifolium pratense) and alfalfa (Medicago sativa) sprouts during germination. Appl. Sci. 2020, 10, 7249. [Google Scholar] [CrossRef]
- Daramola, B. Preliminary investigation on antioxidant interactions between bioactive components of Solanum anguivi and Capsicum annuum. J. Food Sci. Technol. 2018, 55, 3827–3832. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Zavalloni, C.; Andresen, J.A.; Flore, J.A. Phenological Models of Flower Bud Stages and Fruit Growth of `Montmorency’ Sour Cherry Based on Growing Degree-day Accumulation. J. Am. Soc. Hortic. Sci. 2006, 131, 601–607. [Google Scholar] [CrossRef]
- Dinis, L.T.; Peixoto, F.; Pinto, T.; Costa, R.; Bennett, R.N.; Gomes-Laranjo, J. Study of morphological and phenological diversity in chestnut trees (‘Judia’ variety) as a function of temperature sum. Environ. Exp. Bot. 2011, 70, 110–120. [Google Scholar] [CrossRef]
- Santos, J.A.; Costa, R.; Fraga, H. Climate change impacts on thermal growing conditions of main fruit species in Portugal. Clim. Change 2017, 140, 273–286. [Google Scholar] [CrossRef]
- Soifoini, T.; Donno, D.; Jeannoda, V.; Rakoto, D.D.; Msahazi, A.; Farhat, S.M.M.; Oulam, M.Z.; Beccaro, G.L. Phytochemical composition, antibacterial activity, and antioxidant properties of the artocarpus altilis fruits to promote their consumption in the comoros islands as potential health-promoting food or a source of bioactive molecules for the food industry. Foods 2021, 10, 2136. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Camilla Bergonzi, M.; Heard, C.M.; Lupaescu, A.-V.; Iavorschi, M.; Covasa, M. The Use of Bioactive Compounds in Hyperglycemia and Amyloid Fibrils-Induced Toxicity in Type 2 Diabetes and Alzheimer’s Disease. Pharmaceutics 2022, 14, 235. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef]
- Hohrenk, L.L.; Itzel, F.; Baetz, N.; Tuerk, J.; Vosough, M.; Schmidt, T.C. Comparison of Software Tools for Liquid Chromatography-High-Resolution Mass Spectrometry Data Processing in Nontarget Screening of Environmental Samples. Anal. Chem. 2020, 92, 1898–1907. [Google Scholar] [CrossRef]
- Ruiz-Cruz, S.; Chaparro-Hernández, S.; Hernández-Ruiz, K.L.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Gassos Ortega, L.E.; De, J.; Ornelas-Paz, J.; Lopez Mata, M.A. Flavonoids: Important Biocompounds in Food. In Flavonoids From Biosynthesis to Human Health; Intechopen: London, UK, 2017. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Jucá, M.M.; Filho, F.M.S.C.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.d.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2018, 34, 692–705. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A.; Feliciano, A.S.; Santos-Buelga, C. Variation of the Phytochemical Constituents and Antioxidant Activities of Zingiber officinale var. rubrum Theilade Associated with Different Drying Methods and Polyphenol Oxidase Activity. Molecules 2016, 21, 780. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Diez, E.; Hofmann, M.A.; Bravo, B.; Malgazhdarova, G.; Katkhanova, O.A.; Yutskovskaya, Y. Azelaic Acid in the Treatment of Acne in Adult Females: Case Reports. Skin Pharmacol. Physiol. 2014, 27, 18–25. [Google Scholar] [CrossRef]
- Zadernowski, R.; Czaplicki, S.; Naczk, M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana). Food Chem. 2009, 112, 685–689. [Google Scholar] [CrossRef]
- Méndez-Cuesta, C.A.; Laura, A.; Campos, E.; Sánchez, D.S.; González, C.P.; Gutiérrez, S.P. Cytotoxic and Antitumoral Activities of Compounds Isolated from Cucurbitaceae Plants. In Pharmacognosy Medicinal Plants; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef]
- Gonçalves, M.J.; Cruz, M.T.; Tavares, A.C.; Cavaleiro, C.; Lopes, M.C.; Canhoto, J.; Salgueiro, L. Composition and biological activity of the essential oil from Thapsia minor, a new source of geranyl acetate. Ind. Crop. Prod. 2012, 35, 166–171. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Zhang, C.; Tanabe, K.; Tani, H.; Nakajima, H.; Mori, M.; Sakuno, E. Biologically Active Gibberellins and Abscisic Acid in Fruit of Two Late-maturing Japanese Pear Cultivars with Contrasting Fruit Size. J. Am. Soc. Hortic. Sci. 2007, 132, 452–458. [Google Scholar] [CrossRef]
- Toner, P.; Nelson, D.; Rao, J.R.; Ennis, M.; Moore, J.E.; Schock, B. Antimicrobial properties of phytohormone (gibberellins) against phytopathogens and clinical pathogens. Access Microbiol. 2021, 3, 278. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, P.; Guo, M.; Li, M.; Wang, L.; Adeel, M.; Shakoor, N.; Rui, Y. Effects of age on mineral elements, amino acids and fatty acids in Chinese chestnut fruits. Eur. Food Res. Technol. 2021, 247, 2079–2086. [Google Scholar] [CrossRef]
- Akram, M.; Daniyal, M.; Ali, A.; Zainab, R.; Muhammad, S.; Shah, A.; Munir, N.; Tahir, I.M. Role of Phenylalanine and Its Metabolites in Health and Neurological Disorders. In Synucleins—Biochemisty and Role in Diseases; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- James, S.P.; Bondugji, D. Gamma-Aminobutyric Acid (GABA) and the Endocannabinoids: Understanding the Risks and Opportunities. In Natural Drugs from Plants; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.Y.; Omara, E.A.; Sleem, A.A. Citric Acid Effects on Brain and Liver Oxidative Stress in Lipopolysaccharide-Treated Mice. J. Med. Food 2014, 17, 588. [Google Scholar] [CrossRef]
- Qiang, F. Effect of Malate-oligosaccharide Solution on Antioxidant Capacity of Endurance Athletes. Open Biomed. Eng. J. 2015, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Ferrero, M. Strategies for the Synthesis of 19-nor-Vitamin D Analogs. Pharmacology 2020, 13, 159. [Google Scholar] [CrossRef]
- Devaki, S.J.; Raveendran, R.L. Vitamin C: Sources, Functions, Sensing and Analysis. In Vitamin C; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Zhang, Y.; Qian, D.; Tang, Y.; Zhu, Z.; Wang, H.; McPhee, D.J. Contents Changes of Triterpenic Acids, Nucleosides, Nucleobases, and Saccharides in Jujube (Ziziphus jujuba) Fruit During the Drying and Steaming Process. Molecules 2015, 20, 22329–22340. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Vitamin E intestinal absorption: Regulation of membrane transport across the enterocyte. IUBMB Life 2019, 71, 416–423. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, F.; Lan, J.; Sun, F.; Li, J.; Li, M.; Song, K.; Wu, X. Ultra-small micelles based on polyoxyl 15 hydroxystearate for ocular delivery of myricetin: Optimization, in vitro, and in vivo evaluation. Drug Deliv. 2019, 26, 158–167. [Google Scholar] [CrossRef]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, J. Fatty Acids and Their Analogues as Anticancer Agents. Fatty Acids 2017, 21, 72–86. [Google Scholar] [CrossRef]
- Chehade, L.A.; Angelini, L.G.; Tavarini, S. Genotype and Seasonal Variation Affect Yield and Oil Quality of Safflower (Carthamus tinctorius L.) under Mediterranean Conditions. Agronomy 2022, 12, 122. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Lê, K.A.; Samra, R.A.; Roger, O.; Green, H.; Macé, K. The scientific basis for healthful carbohydrate profile. Crit. Rev. Food Sci. Nutr. 2017, 59, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Míguelez, J.d.l.M.; Bernárdez, M.M.; Queijeiro, J.M.G. Composition of varieties of chestnuts from Galicia (Spain). Food Chem. 2004, 84, 401–404. [Google Scholar] [CrossRef]
- Borges, O.; Gonçalves, B.; de Carvalho, J.L.S.; Correia, P.; Silva, A.P. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 2008, 106, 976–984. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B.; Chwil, S.; Chwil, M. Macronutrient composition of nickel-treated wheat under different sulfur concentrations in the nutrient solution. Environ. Sci. Pollut. Res. 2016, 23, 5902–5914. [Google Scholar] [CrossRef]
- Mensah, E.; Kyei-Baffour, N.; Ofori, E.; Obeng, G. Influence of Human Activities and Land Use on Heavy Metal Concentrations in Irrigated Vegetables in Ghana and Their Health Implications. In Appropriate Technologies for Environmental Protection in the Developing World; Sel. Pap. from ERTEP 2007; Springer: Dordrecht, The Netherlands, 2009; pp. 9–14. [Google Scholar] [CrossRef]
- Udensi, U.K.; Tchounwou, P.B. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111. [Google Scholar] [CrossRef]
- Sunyecz, J.A. The use of calcium and vitamin D in the management of osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164. [Google Scholar]
- Da Silva, E.C.; De Albuquerque, M.B.; Dias, A.; Neto, A.; Dias, C.; Junior, S. Drought and Its Consequences to Plants—From Individual to Ecosystem. In Responses of Organisms to Water Stress; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Influence of Climate Change on Chestnut Trees: A Review. Plants 2021, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, M.d.C.B.M.; Nunes, F.; Viguera, C.G.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Industrial processing effects on chestnut fruits (Castanea sativa Mill.) 3. Minerals, free sugars, carotenoids and antioxidant vitamins. Int. J. Food Sci. Technol. 2010, 45, 496–505. [Google Scholar] [CrossRef]


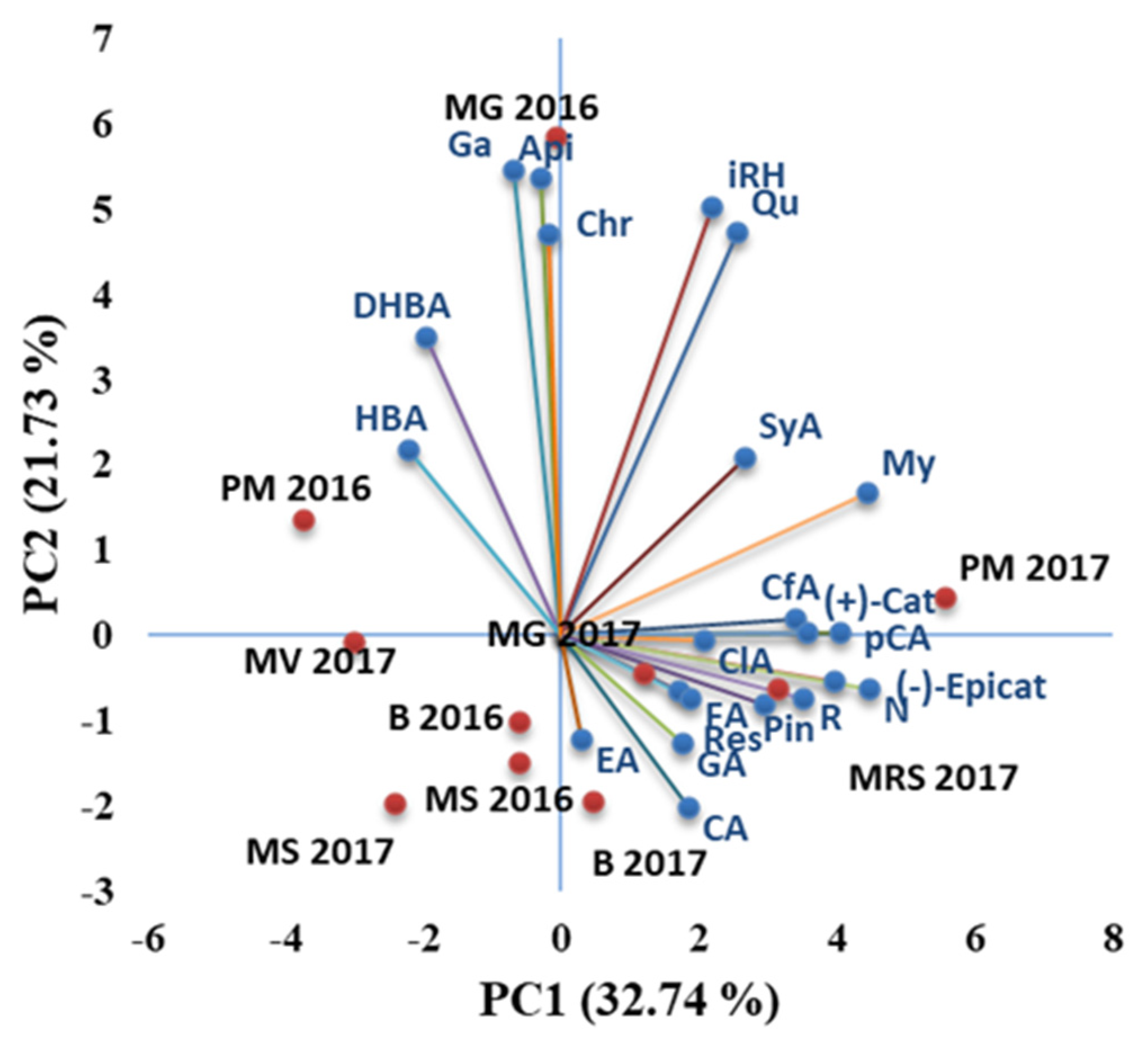

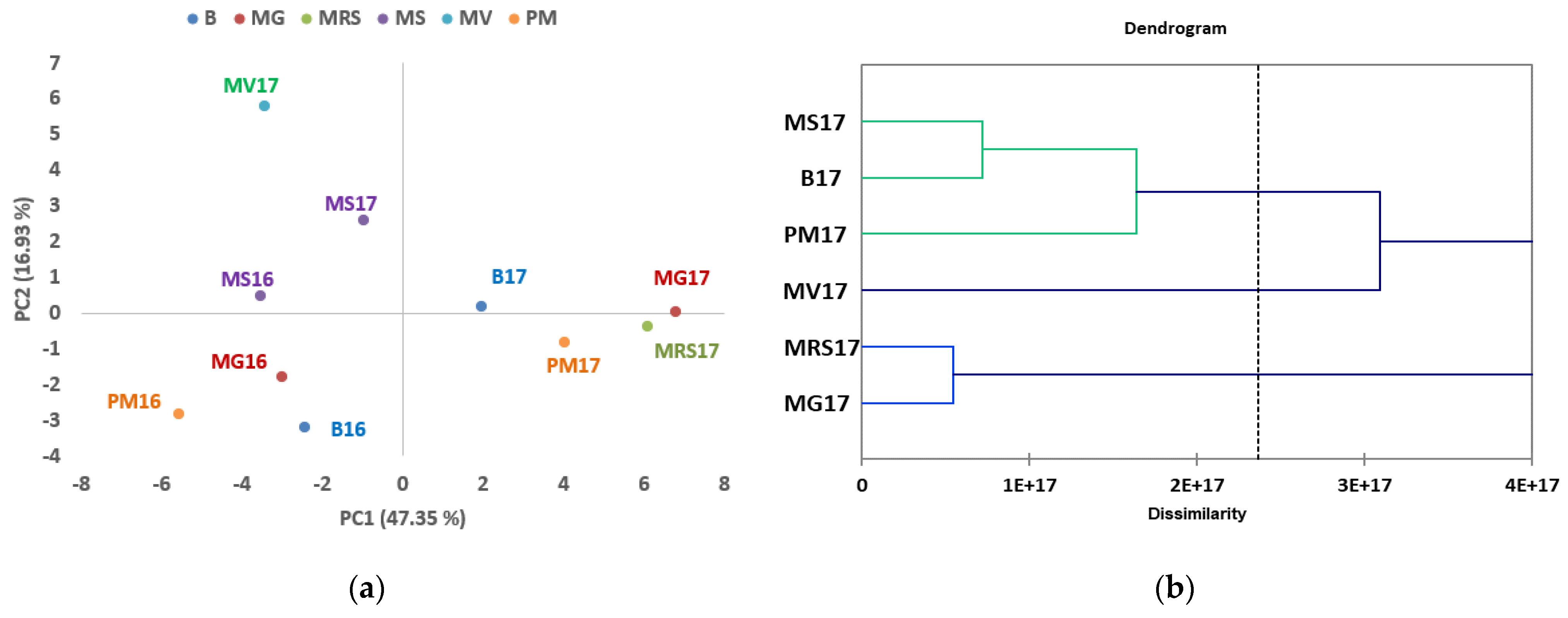
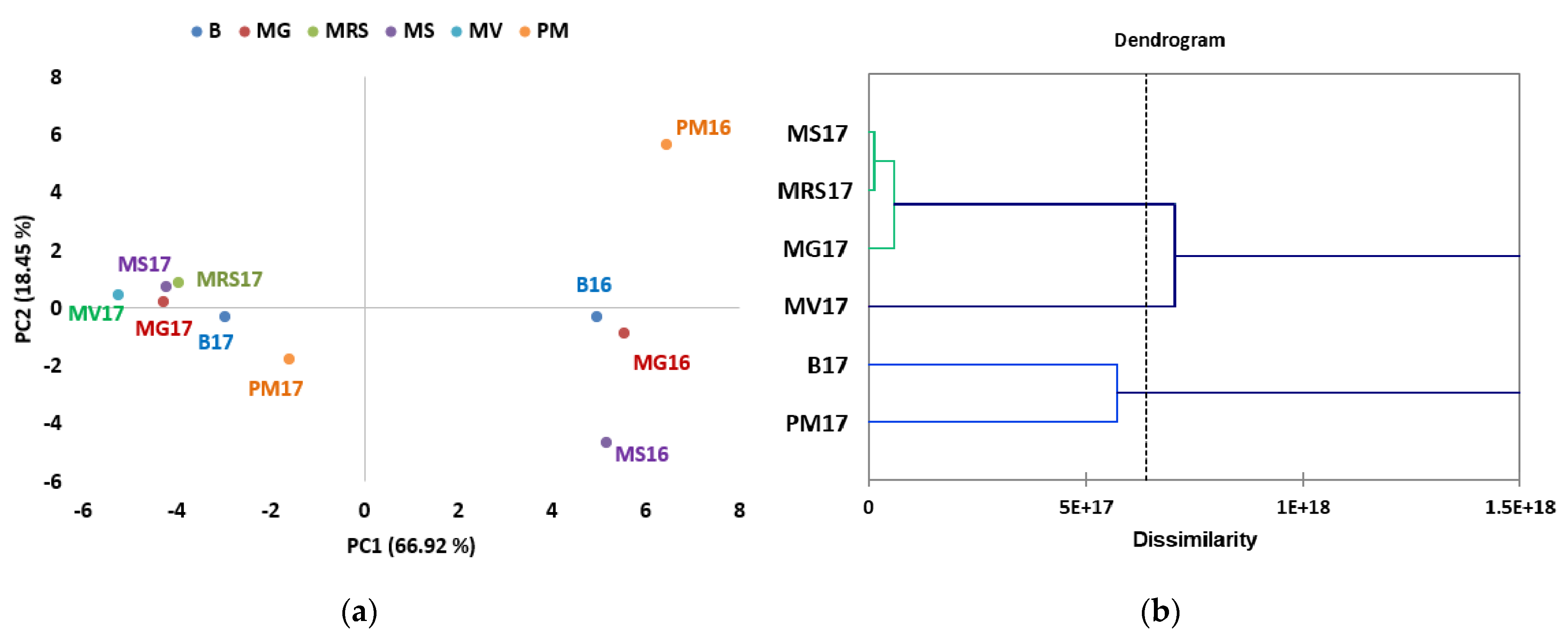


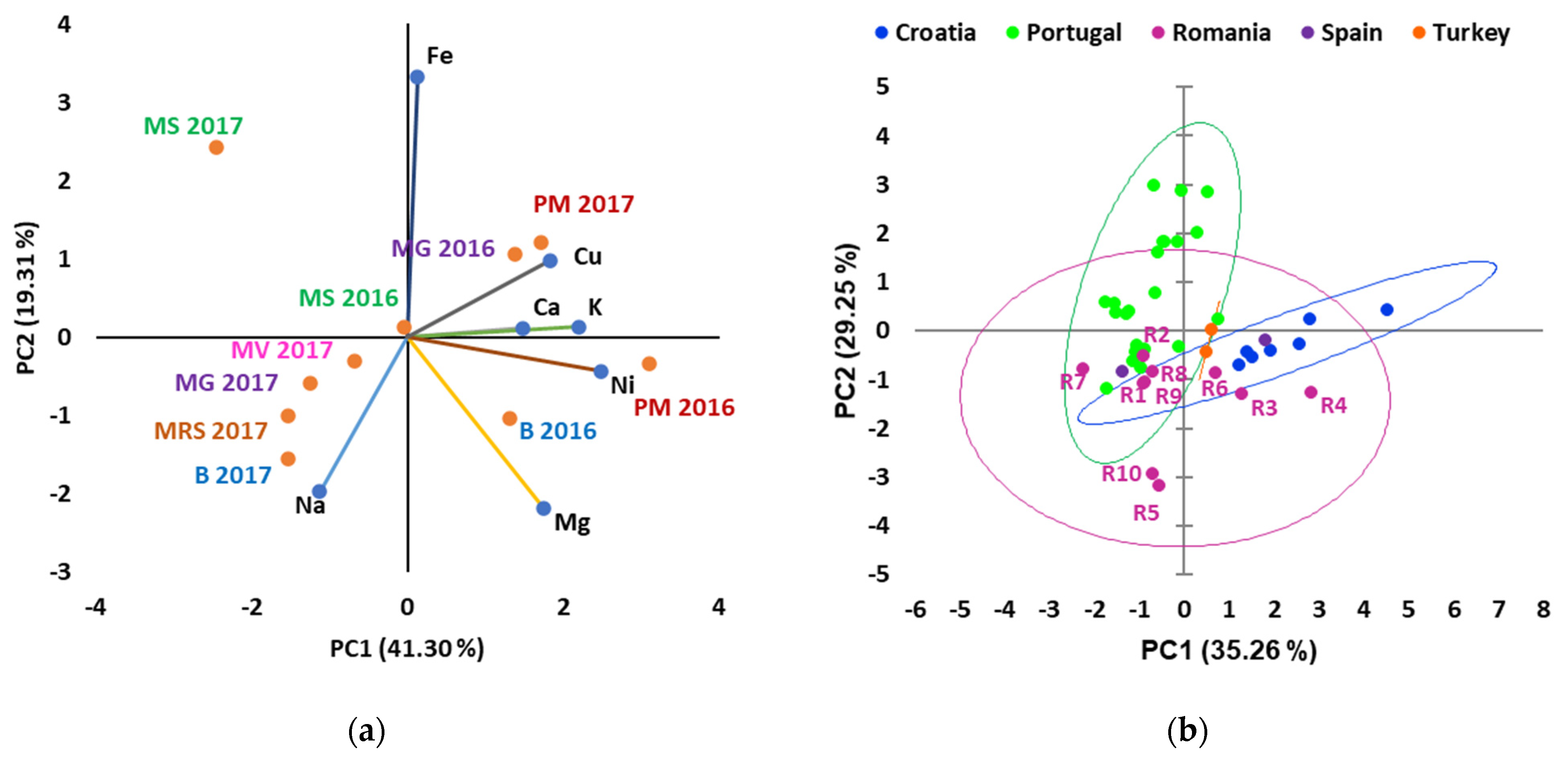
| Year | Temperature at 2 m Height | Rainfall (mm) | Relative Humidity (%) at a Height of 2 m | Number of Days with Rainfall | ||
|---|---|---|---|---|---|---|
| Mean Temperature Tavr, (°C) | Minimum Temperature Tmin, (°C) | Maximum Temperature Tmax, (°C) | ||||
| 2015 | 12.6 | −16.1 | 37.3 | 706 | 70 | 126 |
| 2016 | 11.9 | −14.0 | 35.2 | 746 | 73 | 135 |
| 2017 | 12.0 | −19.1 | 38.6 | 802 | 72 | 136 |
| Phenolic Compounds | ‘Marigoule’ (MG) | ‘Précoce Migoule’ (PM) | ‘Bournette’ (B) | ‘Marsol’ (MS) | ‘Maraval’ (MV) | ‘Marissard’ (MRS) |
|---|---|---|---|---|---|---|
| Gallic acid | 1.035 ± 0.466 | 0.659 ± 0.156 | 1.670 ± 0.574 | 0.508 ± 0.118 | 0.158 ± 0.005 | 1.282 ± 0.006 |
| 3,4-Dihydroxybenzoic acid | 0.068 ± 0.088 | 0.140 ± 0.187 | NF | NF | NF | 0.009 ± 0.001 |
| 4-Hydroxybenzoic acid | 0.052 ± 0.051 | 0.200 ± 0.262 | 0.016 ± 0.018 | 0.026 ± 0.025 | 0.003 ± 0.001 | 0.008 ± 0.001 |
| Chlorogenic acid | 0.001 ± 0.0004 | 0.001 ± 0.001 | NF | 0.002 ± 0.001 | NF | 0.001 ± 0.0009 |
| Syringic acid | 0.007 ± 0.008 | 0.024 ± 0.012 | 0.008 ± 0.010 | 0.002 ± 0.003 | NF | 0.010 ± 0.001 |
| Caffeic acid | 0.010 ± 0.000 | 0.007 ± 0.010 | 0.011 ± 0.001 | 0.006 ± 0.008 | 0.009 ± 0.001 | 0.011 ± 0.001 |
| p-Coumaric acid | 0.017 ± 0.002 | 0.030 ± 0.020 | 0.023 ± 0.005 | 0.015 ± 0.009 | 0.009 ± 0.002 | 0.023 ± 0.002 |
| t-Ferulic acid | 0.018 ± 0.006 | 0.015 ± 0.006 | 0.022 ± 0.020 | 0.024 ± 0.017 | 0.009 ± 0.001 | 0.023 ± 0.001 |
| Ellagic acid | 0.757 ± 0.600 | 0.349 ± 0.143 | 6.211 ± 6.807 | 0.217 ± 0.242 | 0.056 ± 0.006 | 2.902 ± 0.037 |
| Cinnamic acid | 0.012 ± 0.000 | 0.019 ± 0.001 | 0.018 ± 0.011 | 0.023 ± 0.003 | 0.002 ± 0.001 | 0.029 ± 0.002 |
| (+)-Catechin | 0.332 ± 0.304 | 0.258 ± 0.294 | 0.090 ± 0.082 | 0.036 ± 0.051 | 0.174 ± 0.007 | 0.363 ± 0.013 |
| (−)-Epicatechin | 0.005 ± 0.004 | 0.007 ± 0.008 | 0.002 ± 0.002 | 0.003 ± 0.003 | 0.001 ± 0.001 | 0.010 ± 0.001 |
| Quercetin | 0.006 ± 0.004 | 0.005 ± 0.002 | 0.001 ± 0.0002 | 0.001 ± 0.001 | NF | 0.004 ± 0.001 |
| Myricetin | 0.044 ± 0.001 | 0.044 ± 0.010 | 0.042 ± 0.002 | 0.038 ± 0.002 | 0.031 ± 0.008 | 0.045 ± 0.002 |
| Naringin | 0.006 ± 0.004 | 0.009 ± 0.012 | 0.006 ± 0.002 | 0.003 ± 0.001 | NF | 0.009 ± 0.001 |
| Rutin | 0.001 ± 0.0004 | 0.004 ± 0.001 | 0.004 ± 0.0002 | 0.001 ± 0.0003 | NF | 0.010 ± 0.001 |
| Isorhamnetin | 0.002 ± 0.002 | 0.001 ± 0.0007 | 0.001 ± 0.0001 | NF | NF | 0.001 ± 0.0010 |
| Apigenin | NF | NF | NF | NF | NF | NF |
| Hesperidin | NF | NF | NF | NF | NF | NF |
| Kaempferol | NF | NF | NF | NF | NF | NF |
| Pinocembrin | 0.002 ± 0.0002 | <LOQ | 0.002 ± 0.0002 | 0.002 ± 0.0002 | <LOQ | <LOQ |
| Chrysin | 0.001 ± 0.001 | NF | NF | NF | NF | NF |
| Galangin | 0.003 ± 0.003 | <LOQ | NF | NF | <LOQ | NF |
| t-Resveratrol | 0.007 ± 0.010 | 0.002 ± 0.0002 | 0.001 ± 0.0003 | 0.001 ± 0.0008 | NF | 0.050 ± 0.056 |
| Compound Name | Formula | R.T. (min) | Exact Mass | Accurate Mass [M − H] | Experimental Adduct Ion (m/z) | MS2 Fragments (m/z) |
|---|---|---|---|---|---|---|
| Phytochemical Compounds | ||||||
| Flavonoids | ||||||
| (+)-gallocatechin | C15 H14 O7 | 5.72 | 306.0740 | 305.0667 | 305.0668 | 109.0295, 124.016, 125.0249, 137.0248, 139.0400 |
| (+)-taxifolin/(−)-taxifolin | C15H12O7 | 7.44/8.37 | 304.0583 | 303.0510 | 303.0510 | 273.0412, 125.0241, 259.0611, 178.9977, 151.0028 |
| afzelechin/(−)-epiafzelechin | C15H14O5 | 11.40/8.67 | 274.0841 | 273.0769 | 273.0770 | 229.0867; 205.0864; 187.0758; 166.02628; 137.02335; 97.02821 |
| (−)-dihydrokaempferol/(+)-(aromadendrin) | C15H12O6 | 8.09/9.55 | 288.0634 | 287.0561 | 287.0562 | 259.0618; 243.0665; 201.0554; 151.0034; 125.0242 |
| 3,7-dimethyl quercetin | C17H14O7 | 12.21 | 330.0740 | 329.0667 | 329.0668 | 314.03860; 299.01480; 285.0354; 271.02044; 243.0267 |
| afromosin (castanin) | C17H14O5 | 11.35 | 298.0841 | 297.0769 | 297.0769 | 282.05362; 283.06802; 267.03021; 253.04797; 167.04965 |
| dihydro genistein | C15H12O5 | 10.57 | 272.0685 | 271.0612 | 271.0613 | 209.0559; 177.0117; 151.0017; 119.0505; 93.0336 |
| (+,−)-dalbergioidin/eriodictyol | C15H12O6 | 8.08/9.54 | 288.0634 | 287.0561 | 287.0562 | 135.0757; 151.0350; 255.0274; 287.0719; 227.0318 |
| genistein | C15H10O5 | 12.61 | 270.05282 | 269.0456 | 269.0457 | 159.04420; 133.02835; 201.05527; 181.06546; 107.01257 |
| isoquercetin (quercetin-3-glucoside) | C21H20O12 | 7.72 | 464.0954 | 463.0881 | 463.0883 | 300.02771; 355.02985; 271.02491; 243.02969; 178.99773; 151.00262 |
| quercitrin (quercetin-3-rhamnoside) | C21H20O11 | 8.40 | 448.1005 | 447.0932 | 447.0933 | 151.0051; 179.0007; 243.0323; 271.0276; 301.03852; 284.0353 |
| hyperoside (quercetin-3-galactoside) | C21H20O12 | 8.68 | 464.0954 | 463.0881 | 463.0887 | 300.02771; 355.02985; 271.02491; 243.02969; 178.99773; 151.00262 |
| Pelargonidin 3-O-(6-caffeoyl-beta-d-glucoside) | C30 H26 O13 | 5.60 | 594.13734 | 593.1301 | 593.1304 | 145.0282; 255.0293; 284.0313; 285.0390 |
| phloretin | C15H14O5 | 11.40 | 274.0841 | 273.0769 | 273.0770 | 179.03509; 167.03498; 125.0244; 123.04515 |
| phlorizin | C21H24O10 | 8.69 | 436.1370 | 435.1297 | 435.1298 | 167.0459; 273.0846; 274.0563; 122.0333; |
| Other Phytochemical Compounds | ||||||
| Gingerol | C17H26O4 | 15.71 | 294.1831 | 293.1759 | 293.1791 | 178.0657; 193.0906; 99.0809; 293.1751; 137.0009 |
| 5-hydroxyconiferylalcohol | C10H12O4 | 7.45 | 196.0736 | 195.0663 | 195.0665 | 195.0665; 179.0665; 193.0657 |
| azelaic acid | C9H16O4 | 7.64 | 188.1049 | 187.0976 | 187.0967 | 97.1001; 123.1002; 125.1008 |
| Dihydro caffeic acid/ hydroxyphenyl lactic acid | C9H10O4 | 5.42/5.88 | 182.0579 | 181.0507 | 181.0498 | 59.0001; 109.3005; 118.9004; 121.1001; 135.3025; |
| 3,4-dihydroxymandelate | C8H8O5 | 6.32 | 184.0372 | 183.0299 | 183.0290 | 137.0242; 139.0401; 183.0294; |
| cucurbitacin F | C30H46O7 | 12.00 | 518.3244 | 517.3171 | 517.3170 | 517.3170; 499.3065; 385.2386 |
| ursolic and oleanolic acids | C30H48O3 | 16.10 | 456.3604 | 455.3531 | 455.3531 | 455.3521; 456.3550 |
| (S)-abscisic acid | C15H20O4 | 8.13 | 264.1362 | 263.1289 | 263.1289 | 152.8462; 219.6015; 203.8293; 263.0201 |
| geranyl acetate | C12H20O2 | 11.15 | 196.1463 | 195.1391 | 195.1384 | 1195.1384; 136.1387; 121.2860; 93.2864 |
| Sebacic acid | C10H18O4 | 8.47 | 202.1205 | 201.1133 | 201.1124 | 110.9001; 139.1134; 183.1038; 201.1127; |
| Gibberellin Compounds—Plant Hormones (Phytohormones) | ||||||
| gibberellin A2-O-beta-d-glucoside | C25H34O11 | 8.43 | 510.2101 | 509.2029 | 509.2029 | 328.2029, 179.2541 |
| gibberellin A8 | C19H24O7 | 8.86 | 364.1522 | 363.1449 | 363.1450 | 275.0321; 118.6022; 160.6709; |
| gibberellin A19/gibberellin A36 | C20H26O6 | 12.08/15.71 | 362.1729 | 361.1657 | 361.1666 | 203.1055; 229.1291; 273.0125; 360.8283 |
| gibberellin A53/gibberellin A14 | C20H28O5 | 10.36/10.74 | 348.1937 | 347.1864 | 347.1862/ 347.1864 | 189.0372; 329.0731; 347.2285;/ 303.1956; 347.1837 |
| gibberellin A1 (gibberellic acid)/gibberellin A29/gibberellin A34 | C19H24O6 | 7.80/8.91/9.23 | 348.1573 | 347.1500 | 347.1500/ 347.1499 347.1501 | 228.5631; 229.2383; 273.1972;/ 259.3782; 303.0891; 347.0805;/ |
| gibberellin A3 | C19H22O6 | 7.35 | 346.1416 | 345.1338 | 345.1344 | 71.0489; 143.0855; 221.1330 |
| Gibberellin A12 | C20H28O4 | 10.01 | 332.1988 | 331.1915 | 331.1914 | 241.1956; 259.2058; 287.2003 |
| Organic Acids | ||||||
| Quinic acid | C7H12O6 | 3.41 | 192.0634 | 191.0561 | 191.0553 | 85.0301; 93.0035; 127.0001; 191.0556 |
| Citric acid | C6H8O7 | 2.14 | 192.027 | 191.0197 | 191.0188 | 173.0091; 129.0193; 111.0088; 87.00877 |
| Malic acid | C4H6O5 | 1.47 | 134.0215 | 133.0142 | 133.0130 | 133.0142; 115.0036; 89.0244; 71.0138 |
| Amino Acids | ||||||
| N-acetyl tryptophan | C13H14N2O3 | 7.43 | 246.1004 | 245.0932 | 245.0929 | 202.0929, 159.0926 |
| N-Acetyl-l-tyrosine | C11H13NO4 | 6.03 | 223.0845 | 222.0772 | 222.0768 | 179.0772, 136.1912 |
| N-Acetyl-l-phenylalanine | C11H13NO3 | 6.86 | 207.0895 | 206.0823 | 206.0815 | 91.1012; 103.0024; 147.2015;164.2021; |
| l-Tyrosine methyl ester | C10H13NO3 | 13.49 | 195.0895 | 194.0823 | 194.0815 | 133.0657; 194.0817 |
| d-Tryptophan | C11H12N2O2 | 6.26 | 204.0899 | 203.0826 | 203.0820 | 158.0820, 130.0820 |
| l-Tryptophan | C11H12N2O2 | 7.42 | 204.0899 | 203.0826 | 203.0820 | 74.0255; 116.0504; 142.0658; 159.0926; 203.0821 |
| d-Tyrosine | C9H11NO3 | 2.55 | 181.0739 | 180.0666 | 180.0658 | 119.0498; 163.0387; 180.0684 |
| l-Glutamic acid | C5H9NO4 | 1.26 | 147.0532 | 146.0459 | 146.0466 | 102.0559; 128.0353; |
| Other bioactive compounds/metabolites | ||||||
| Pantothenic acid (Vitamin B5) | C9H17NO5 | 4.97 | 219.1107 | 218.1034 | 218.1028 | 71.0512; 88.0407; 146.0816; 218.1029; |
| vitamin C | C6H8O6 | 2.64 | 176.0321 | 175.0248 | 175.0238 | 127.0036; 115.0037; 87.0087; 59.0138 |
| Uridine | C9H12N2O6 | 2.44 | 244.0695 | 243.0623 | 243.0619 | 109.9196; 200.0670; 140.0354; 152.0355; 243.0621; |
| Thymidine | C10H14N2O5 | 5.16 | 242.0903 | 241.0830 | 241.0828 | 42.2001; 124.9002; 151.0504; 241.0822; |
| Guanosine | C10H13N5O5 | 4.18 | 283.0917 | 282.0844 | 282.0845 | 108.0201; 133.0154; 150.0421; 282.0844; |
| Compound Name | Formula | R.T. (min) | Exact Mass | Accurate Mass [M − H]- | Experimental Adduct Ion (m/z) | MS2 Fragments (m/z) |
|---|---|---|---|---|---|---|
| Saturated Fatty Acids and Derivates | ||||||
| Lignoceric acid | C24H48O2 | 18.86 | 368.3654 | 367.3582 | 367.3582 | 367.3590; 368.3625; |
| Phytanic acid | C20H40O2 | 17.12 | 312.3028 | 311.2956 | 311.2956 | 311.2956, 267.2956 |
| Stearic acid (Octadecanoic acid) | C18H36O2 | 17.32 | 284.2715 | 283.2643 | 283.2642 | 283.2636; |
| Palmitic acid (Hexadecanoic acid) | C16H32O2 | 16.59 | 256.2402 | 255.2330 | 255.2327 | 255.2317; 256.2350; 237.3001; |
| Behenic acid (Docosanoicacid) | C22H44O2 | 18.40 | 340.3341 | 339.3269 | 339.3267 | 339.3265; 340.3303; |
| Dioxo-hydrox(6,9-dioxo-(11R,15S)-dihydroxy-13E-prostenoic acid (6-keto PGE1) | C20H32O6 | 7.72 | 368.2199 | 367.2126 | 367.2125 | 143.2541; 205.5478; 124.3256; 269.3254;187.1475; |
| Unsaturated Fatty Acids and Derivates | ||||||
| Eicosanoic acid (Arachidic acid) | C20H40O2 | 17.92 | 312.3028 | 311.2956 | 311.2956 | 311.2956; |
| Oleic acid | C18H34O2 | 16.79 | 282.2559 | 281.2486 | 281.2484 | 263.3001; 281.2474; |
| Linolenic acid | C18H30O2 | 15.88 | 278.2246 | 277.2173 | 277.2172 | 233.2001; 259.2002; 277.2188 |
| Farnesoic acid | C15H24O2 | 12.32 | 236.1776 | 235.1704 | 235.1701 | 235.1701, 191.1704 |
| Pinellic acid | C18H34O5 | 10.18 | 330.2406 | 329.2334 | 329.2332 | 329.2332, 285.2332 |
| 9Z,11E-octadecadienoic acid (9Z, 11E-Linoleic acid) | C18H32O2 | 16.30 | 280.2402 | 279.2330 | 279.2328 | 279.2324; 234.2328 |
| (9S,10S)-9,10-Dihydroxyoctadecanoate | C18H36O4 | 13.69 | 316.2614 | 315.2541 | 315.2540 | 315.2540, 271.2541 |
| 9,10-dihydroxy-12Z-octadecenoic acid (9,10-DiHOME)/ 12,13-dihydroxy-9Z-octadecenoic acid | C18H34O4 | 12.89/ 12.41 | 314.2457 | 313.2385 | 313.2385/ 313.2386 | 171.1081; 201.0922; 277.1702; |
| 2-Hydroperoxy-2,4-octadecadienoic acid/ 8R-hydroperoxy-9Z,12Z-octadecadienoic acid (8-HpODE)/ 13S-hydroperoxy-9Z,11E-octadecadienoic acid (13-HpODE)/ 11S-hydroperoxy-9Z,12Z-octadecadienoic acid (11S-HpODE) | C18H32O4 | 13.02/ 13.32/ 13.49/ 12.73 | 312.3028 | 311.2228 | 311.2228/ 311.2228/ 311.2229/ 311.2230 | 223.1041; 205.0112; 211.1208; |
| 11Z-eicosenoic acid (Gondoic acid)/ | C20H38O2 | 17.46 | 310.2872 | 309.2799 | 309.2800 | 309.2794 |
| 9S-hydroperoxy-10E,12Z,15Z-octadecatrienoic acid (9S HpOTrE)/ 13S-hydroperoxy-9Z,11E,15Z-octadecatrienoic acid(13(S)-HpOTrE) | C18H30O4 | 12.11/ 12.62 | 310.2144 | 309.2072 | 309.2073/ 309.2073 | 139.0400; 190.1600; 209.1491; 227.1602; 252.0801; |
| 2-Oxooctadecanoic acid (2-oxostearic acid) | C18H34O3 | 14.51 | 298.2508 | 297.2435 | 297.2433 | 297.2433 |
| 12R,13S-epoxy-9Z-octadecenoic acid (12,13-EODE)/ 9S-hydroxy-10E,12Z-octadecadienoic acid (9S-HODE)/ 13S-hydroxy-9Z,11E-octadecadienoic acid(13S-HODE)/ 9R,10S-epoxy-12Z-octadecenoic acid (Coronaric acid/9,10-EODE) | C18H32O3 | 13.89/ 14.09/ 14.86/ 15.13 | 296.2351 | 295.2279 | 295.2278/ 295.2276/ 295.2277/ 295.2277 | 195.1461; 277.2102; 295.1675; 171.1026; 277.2171; 295.2276; 195.1112; 277.1801 171.0891; 277.1552; |
| 17-Hydroxylinolenic acid/ 9-oxo-10E,12Z-octadecadienoic acid (9-KODE)/ (9Z)-(13S)-12,13-Epoxyoctadeca-9,11-dienoic acid | C18H30O3 | 13.47/ 14.66/ 14.87 | 294.2195 | 293.2122 | 293.2123/ 293.2122/ 293.2122 | 185.0921; 197.1032; 220.1291; 221.1282; 293.8401; |
| 9,12-Dioxododecanoic acid | C12H20O4 | 6.85 | 228.1362 | 227.1289 | 227.1284 | 227.1284, 183.1284 |
| Chestnut Cultivar | Code | Fructose | Glucose | Sucrose | Maltose |
|---|---|---|---|---|---|
| ‘Bournette’ (B) | B 2017 | 5.052 d | 9.536 b | 154.942 a | 2.429 c |
| B 2016 | 2.645 e | 2.99 e | 51.967 g | 2.268 d | |
| ‘Précoce Migoule’ (PM) | PM 2017 | 14.346 a | 14.456 a | 51.461 h | 2.299 d |
| PM 2016 | 2.026 f | 2.099 g | 126.968 c | 3.159 a | |
| ‘Marigoule’ (MG) | MG 2017 | 6.85 c | 7.42 c | 58.85 f | 2.400 c |
| MG 2016 | 1.809 g | 1.795 h | 153.496 b | 2.885 b | |
| ‘Marsol’ (MS) | MS 2017 | 1.900 g | 2.14 g | 63.915 d | 2.000 e |
| MS 2016 | 1.550 h | 1.556 i | 62.705 e | 2.086 e | |
| ‘Marissard’ (MRS) | MRS 2017 | 2.055 f | 2.308 f | 41.047 i | 2.792 b |
| ‘Maraval’ (MV) | MV 2017 | 11.894 b | 5.581 d | 20.343 j | 1.766 f |
| R2 | 0.9999 | 0.9999 | 1.0000 | 0.9941 | |
| F | 16979 | 11345 | 1073511 | 186 | |
| Pr > F | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Significant | Yes | Yes | Yes | Yes |
| Ca | Mg | Na | K | Fe | Ni | Cu | |
|---|---|---|---|---|---|---|---|
| PM 2017 | 29.58 a | 77.19 c | 30.65 e | 2166.46 b | 3.09 b | 0.90 d | 0.60 e |
| PM 2016 | 16.87 i | 104.81 a | 26.39 g | 2652.11 a | 2.51 c | 1.47 a | 0.86 b |
| B 2016 | 26.08 b | 102.88 b | 30.79 d | 860.02 f | 2.15 e | 1.03 c | 0.64 d |
| MG 2016 | 22.92 c | 54.68 i | 22.65 h | 974.86 c | 2.42 d | 1.38 b | 0.98 a |
| MS 2016 | 21.90 d | 65.56 h | 26.57 f | 867.16 e | 2.10 g | 0.71 f | 0.75 c |
| B 2017 | 17.80 g | 77.15 d | 172.03 a | 710.36 i | 2.14 e | 0.63 g | 0.47 g |
| MRS 2017 | 17.15 h | 66.42 g | 157.21 b | 884.42 d | 2.12 f | 0.51 i | 0.60 e |
| MS 2017 | 14.01 j | 48.62 j | 33.17 c | 403.56 j | 3.43 a | 0.21 j | 0.54 f |
| MV 2017 | 19.81 e | 68.45 f | 22.48 i | 747.02 h | 2.09 g | 0.81 e | 0.46 h |
| MG 2017 | 19.70 f | 68.69 e | 4.63 j | 793.65 g | 1.89 h | 0.57 h | 0.31 i |
| R2 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.999 |
| F | 779,671 | 8,429,549 | 121,574,044 | 14,113,376,066 | 14,976 | 14,441 | 2102 |
| Pr > F | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Significant | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciucure, C.T.; Geana, E.-I.; Sandru, C.; Tita, O.; Botu, M. Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania. Separations 2022, 9, 66. https://doi.org/10.3390/separations9030066
Ciucure CT, Geana E-I, Sandru C, Tita O, Botu M. Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania. Separations. 2022; 9(3):66. https://doi.org/10.3390/separations9030066
Chicago/Turabian StyleCiucure, Corina Teodora, Elisabeta-Irina Geana, Claudia Sandru, Ovidiu Tita, and Mihai Botu. 2022. "Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania" Separations 9, no. 3: 66. https://doi.org/10.3390/separations9030066
APA StyleCiucure, C. T., Geana, E.-I., Sandru, C., Tita, O., & Botu, M. (2022). Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania. Separations, 9(3), 66. https://doi.org/10.3390/separations9030066









