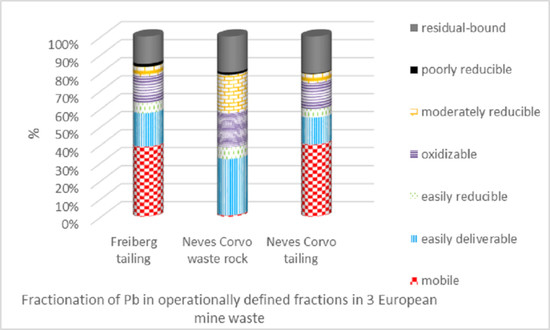
Fractionation of Metal(loid)s in Three European Mine Wastes by Sequential Extraction
Abstract
:1. Introduction
2. Materials and Methods
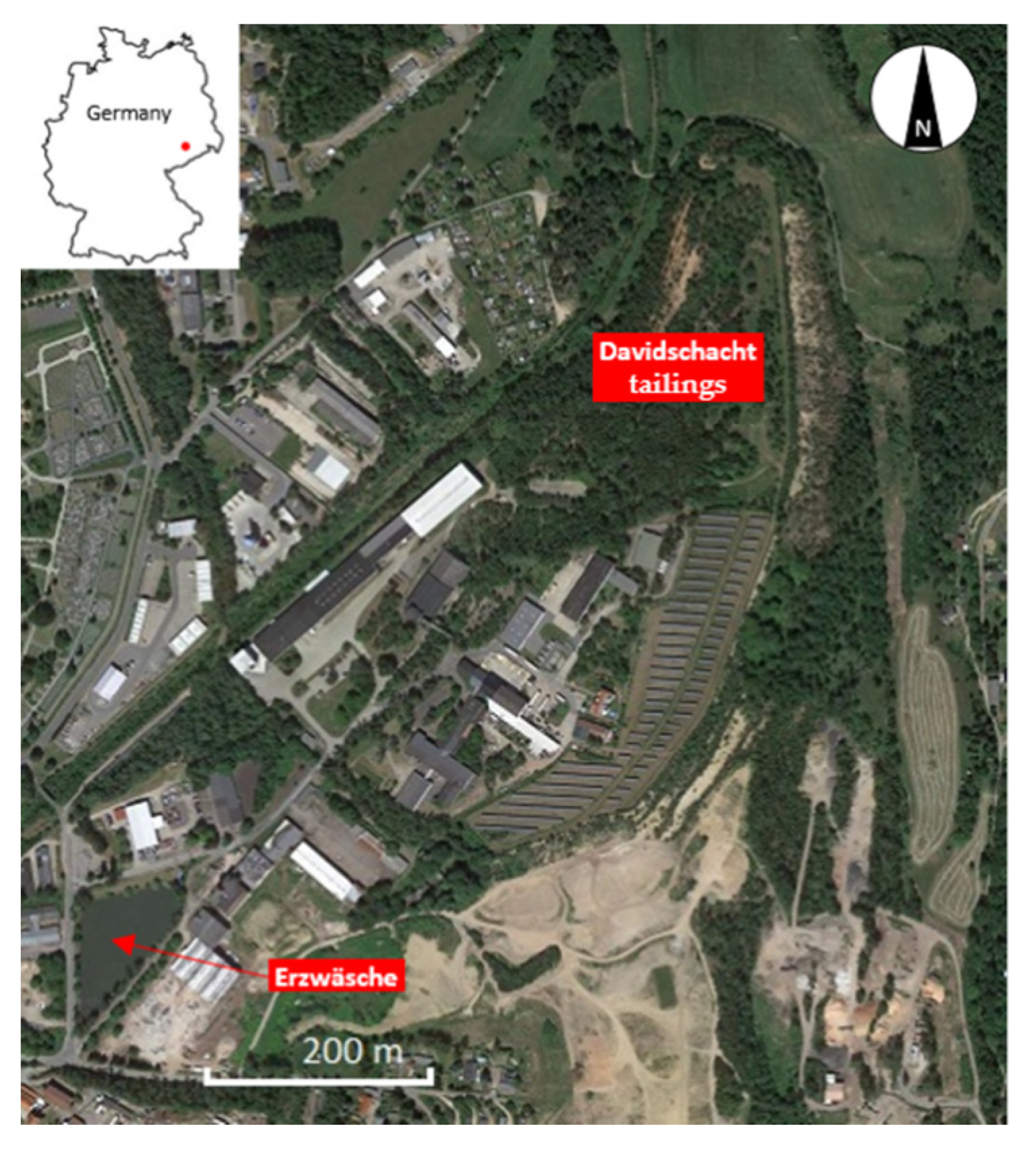
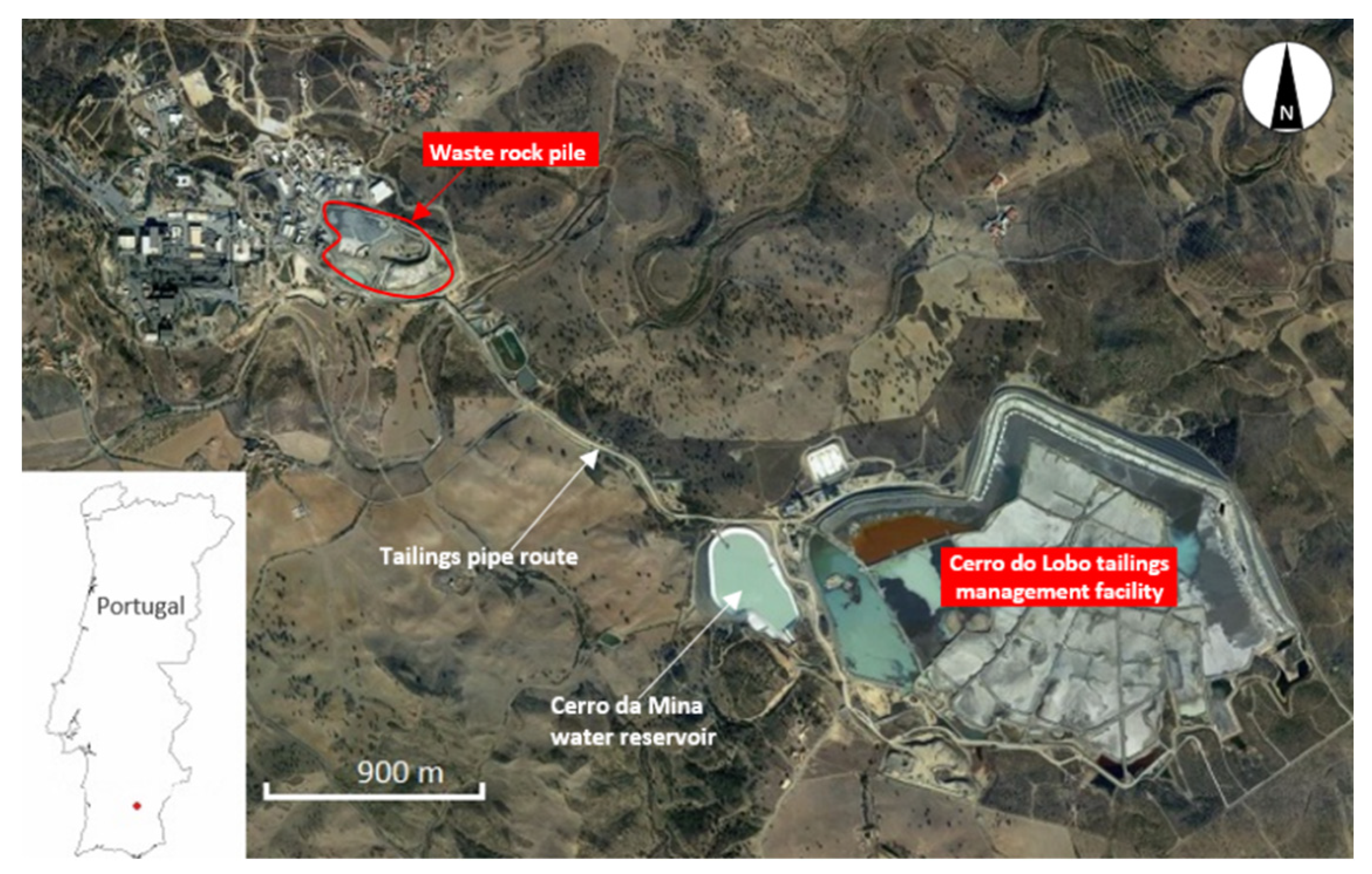
2.1. The Mine Waste Samples
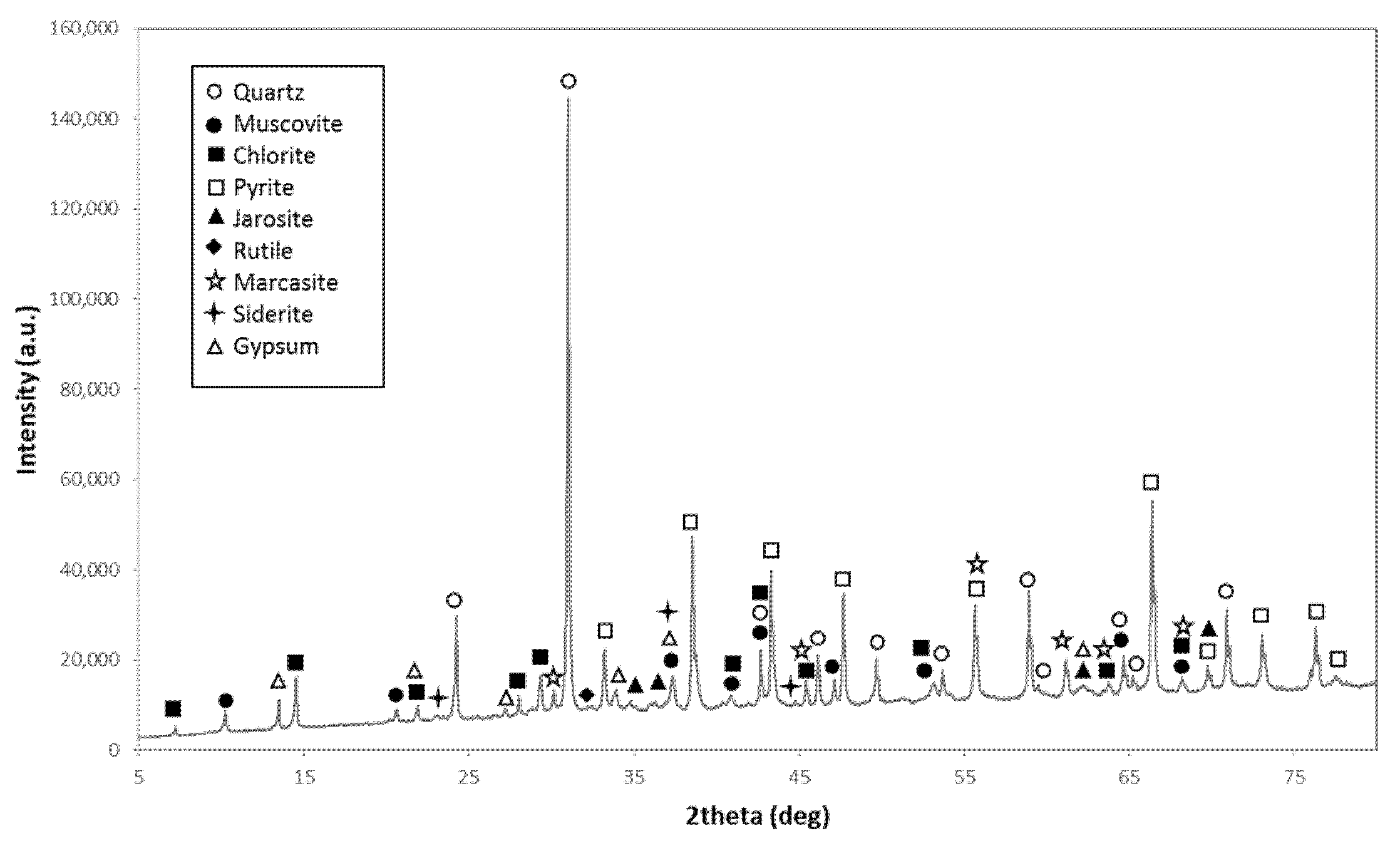
2.2. Sample Preparation and Characterization
2.3. Sequential Chemical Extraction
3. Results and Discussion
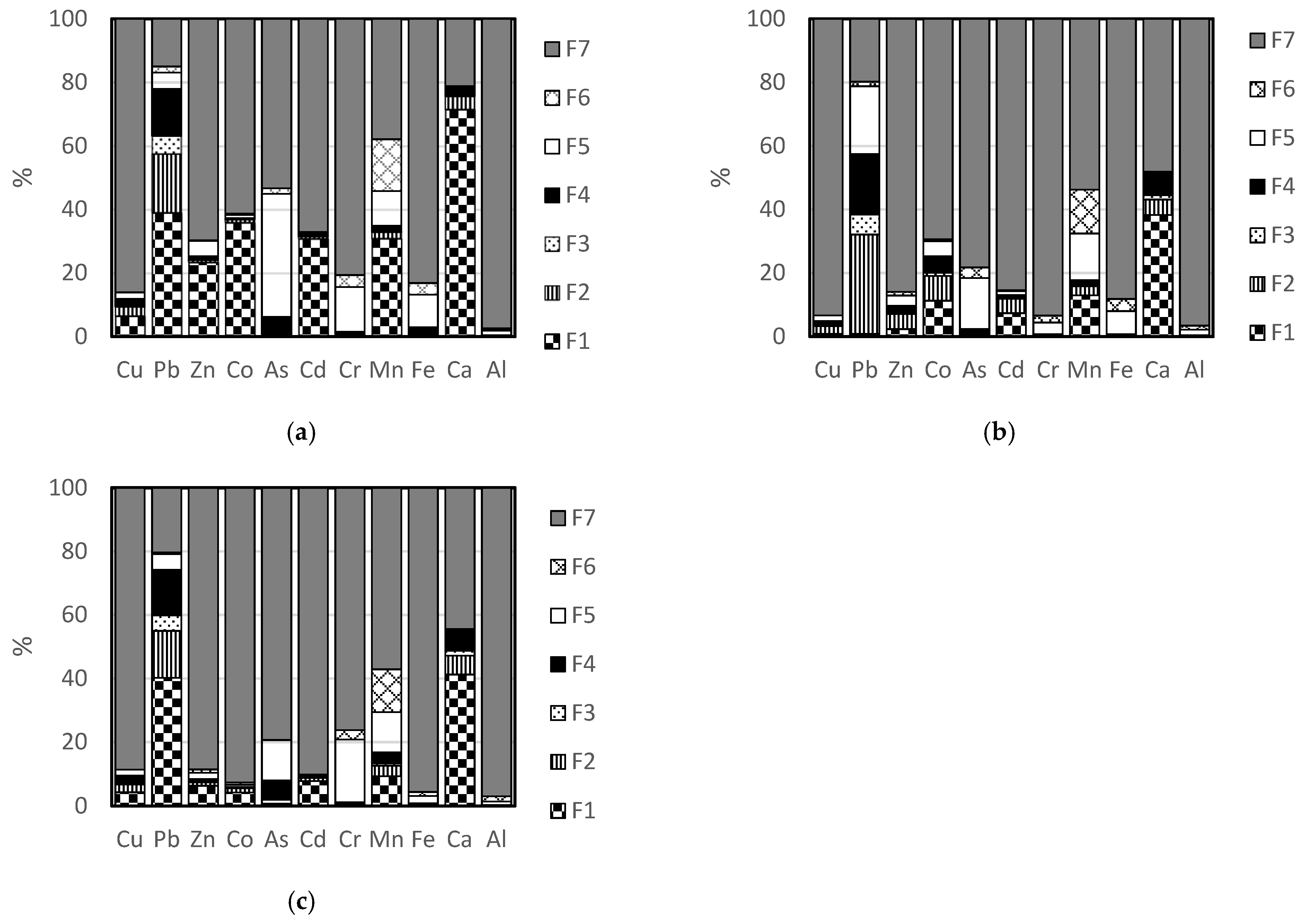
3.1. Total Composition and Distribution of Elements in the Freiberg Tailing
3.2. Total Composition and Distribution of Elements in the Neves Corvo Waste Rock
3.3. Total Composition and Distribution of Elements in the Neves Corvo Tailing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blight, G. Chapter 5—Mine Waste: A Brief Overview of Origins, Quantities, and Methods of Storage. In Waste; Letcher, T.M., Vallero, D.A., Eds.; Academic Press: Boston, MA, USA, 2011; pp. 77–88. [Google Scholar] [CrossRef]
- European Commission. Mining Waste. 2018. Available online: https://ec.europa.eu/environment/topics/waste-and-recycling/mining-waste_en#ecl-inpage-497 (accessed on 14 January 2022).
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical characterization of mine waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Vassilev, S.; Lihareva, N.; Vassileva, C. Sequential leaching behaviour of some elements during chemical treatment of ceramic cenospheres from coal fly ash. Comptes Rendus L’académie Bulg. Des Sci. 2006, 59, 753–758. [Google Scholar]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Ure, A.M.; Davidson, C. Chemical Speciation in the Environment, 2nd ed.; Blackwell Science: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Kersten, M.; Förstner, U. Chemical Fractionation of Heavy Metals in Anoxic Estuarine and Coastal Sediments. Water Sci. Technol. 1986, 18, 121–130. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR Three Step Sequential Extraction Procedure Prior to the Certification of New Sediment and Soil Reference Materials. J. Environ. Monit. JEM 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of Heavy Metals in Soils and Sediments. An Account of the Improvement and Harmonization of Extraction Techniques Undertaken Under the Auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Kennedy, H.V.; Sanchez, A.L.; Oughton, D.H.; Rowland, A.P. Use of Single and Sequential Chemical Extractants to Assess Radionuclide and Heavy Metal Availability From Soils for Root Uptake. Analyst 1997, 122, 89R–100R. [Google Scholar] [CrossRef]
- Ribbe, N.; Arinaitwe, K.; Dadi, T.; Friese, K.; von Tümpling, W. Trace-element behaviour in sediments of Ugandan part of Lake Victoria: Results from sequential extraction and chemometrical evaluation. Environ. Earth Sci. 2021, 80, 323. [Google Scholar] [CrossRef]
- Xu, F.; Jia, Y.; Wang, Y.; Zhang, F.; Li, L.; Li, Y.; Ren, L.; Wang, D.; Zhang, T. Does sand mining affect the remobilization of copper and zinc in sediments?—A case study of the Jialing River (China). Environ. Res. 2021, 200, 111416. [Google Scholar] [CrossRef]
- Basheeru, K.A.; Okoro, H.K.; Adekola, F.A.; Abdus-Salam, N. Mobility and Sequential Extraction of Potentially Toxic Elements in Sediment of Lagos Lagoon. Chem. Afr. 2021, 4, 411–427. [Google Scholar] [CrossRef]
- Vollprecht, D.; Riegler, C.; Ahr, F.; Stuhlpfarrer, S.; Wellacher, M. Sequential chemical extraction and mineralogical bonding of metals from Styrian soils. Int. J. Environ. Sci. Technol. 2020, 17, 3663–3676. [Google Scholar] [CrossRef] [Green Version]
- Migoni, D.; Papadia, P.; Cannito, F.; Fanizzi, F.P. Sequential Extraction Analysis of Arsenic in Soil Samples Collected in an Agricultural Area of Brindisi, Apulia (Italy), in the Proximity of a Coal-Burning Power Plant. Appl. Sci. 2021, 11, 2115. [Google Scholar] [CrossRef]
- Golui, D.; Datta, S.P.; Dwivedi, B.S.; Meena, M.C.; Trivedi, V.K.; Jaggi, S.; Bandyopadhyay, K.K. Assessing Geoavailability of Zinc, Copper, Nickel, Lead and Cadmium in Polluted Soils Using Short Sequential Extraction Scheme. Soil Sediment Contam. Int. J. 2021, 30, 74–91. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Lim, Y.; Yu, J.; Chen, S.; Woo, S.W.; Yoon, S.; Bae, S.; Kim, H.S. Characterization of rare earth elements present in coal ash by sequential extraction. J. Hazard. Mater. 2021, 402, 123760. [Google Scholar] [CrossRef]
- Lin, R.; Stuckman, M.; Howard, B.H.; Bank, T.L.; Roth, E.A.; Macala, M.K.; Lopano, C.; Soong, Y.; Granite, E.J. Application of sequential extraction and hydrothermal treatment for characterization and enrichment of rare earth elements from coal fly ash. Fuel 2018, 232, 124–133. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Pöykiö, R.; Perämäki, P.; Kuokkanen, T. The use of a sequential leaching procedure for heavy metal fractionation in green liquor dregs from a causticizing process at a pulp mill. Chemosphere 2005, 61, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Lavergren, U.; Åström, M.E.; Bergbäck, B.; Holmström, H. Mobility of trace elements in black shale assessed by leaching tests and sequential chemical extraction. Geochem. Explor. Environ. Anal. 2009, 9, 71–79. [Google Scholar] [CrossRef]
- Pérez-Moreno, S.M.; Gázquez, M.J.; Pérez-López, R.; Vioque, I.; Bolívar, J.P. Assessment of natural radionuclides mobility in a phosphogypsum disposal area. Chemosphere 2018, 211, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Kania, M.; Gautier, M.; Blanc, D.; Lupsea-Toader, M.; Merlot, L.; Quaresima, M.-C.; Gourdon, R. Leaching behavior of major and trace elements from sludge deposits of a French vertical flow constructed wetland. Sci. Total Environ. 2019, 649, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Oleszczuk, P. Sequential extraction of nickel and zinc in sewage sludge- or biochar/sewage sludge-amended soil. Sci. Total Environ. 2018, 636, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Nkinahamira, F.; Suanon, F.; Chi, Q.; Li, Y.; Feng, M.; Huang, X.; Yu, C.-P.; Sun, Q. Occurrence, geochemical fractionation, and environmental risk assessment of major and trace elements in sewage sludge. J. Environ. Manag. 2019, 249, 109427. [Google Scholar] [CrossRef] [PubMed]
- Khorasanipour, M.; Rashidi, S. Geochemical fractionation pattern and environmental behaviour of rare earth elements (REEs) in mine wastes and mining contaminated sediments; Sarcheshmeh mine, SE of Iran. J. Geochem. Explor. 2020, 210, 106450. [Google Scholar] [CrossRef]
- Khelifi, F.; Melki, A.; Hamed, Y.; Adamo, P.; Caporale, A.G. Environmental and human health risk assessment of potentially toxic elements in soil, sediments, and ore-processing wastes from a mining area of southwestern Tunisia. Environ. Geochem. Health 2020, 42, 4125–4139. [Google Scholar] [CrossRef]
- Jelenová, H.; Majzlan, J.; Amoako, F.Y.; Drahota, P. Geochemical and mineralogical characterization of the arsenic-, iron-, and sulfur-rich mining waste dumps near Kaňk, Czech Republic. Appl. Geochem. 2018, 97, 247–255. [Google Scholar] [CrossRef]
- Gitari, M.W.; Akinyemi, S.A.; Ramugondo, L.; Matidza, M.; Mhlongo, S.E. Geochemical fractionation of metals and metalloids in tailings and appraisal of environmental pollution in the abandoned Musina Copper Mine, South Africa. Environ. Geochem. Health 2018, 40, 2421–2439. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Valero, A.M.; Sáez, R.; Pérez-López, R.; Delgado, J.; Nieto, J.M. Evaluation of heavy metal bio-availability from Almagrera pyrite-rich tailings dam (Iberian Pyrite Belt, SW Spain) based on a sequential extraction procedure. J. Geochem. Explor. 2009, 102, 87–94. [Google Scholar] [CrossRef]
- Davidson, C.M.; Urquhart, G.J.; Ajmone-Marsan, F.; Biasioli, M.; da Costa Duarte, A.; Díaz-Barrientos, E.; Grčman, H.; Hossack, I.; Hursthouse, A.S.; Madrid, L.; et al. Fractionation of potentially toxic elements in urban soils from five European cities by means of a harmonised sequential extraction procedure. Anal. Chim. Acta 2006, 565, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Anju, M.; Banerjee, D.K. Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 2010, 78, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Zeien, H.; Brummer, G.W. Mitteilungen Deutsche Bodenkundliehe Gesellschaft. In Proceedings of the Tagung der Deutschen Bodenkundlichen Gesellschaft, Münster, Germany, 3–10 September 1989; Volume 59, pp. 1–1268. [Google Scholar]
- Li, X.; Thornton, I. Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Appl. Geochem. 2001, 16, 1693–1706. [Google Scholar] [CrossRef]
- Sultan, European Commission. European Training Network for the Remediation and Reprocessing of Sulfidic Mining Waste Sites; Grant Agreement No 812580; European Commission Horizon 2020. European Commission, 2018. Available online: https://etn-sultan.eu/ (accessed on 14 January 2022).
- Caraballo, M.A.; Serna, A.; Macías, F.; Pérez-López, R.; Ruiz-Cánovas, C.; Richter, P.; Becerra-Herrera, M. Uncertainty in the measurement of toxic metals mobility in mining/mineral wastes by standardized BCR® SEP. J. Hazard. Mater. 2018, 360, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, J.; Henjes-Kunst, F.; Seifert, T.; Gutzmer, J. Age and genesis of polymetallic veins in the Freiberg district, Erzgebirge, Germany: Constraints from radiogenic isotopes. Miner. Depos. 2019, 54, 217–236. [Google Scholar] [CrossRef]
- Unesco World Heritage: Freiberg Mining Area. Available online: https://www.montanregion-erzgebirge.de/welterbe/freiberg.html (accessed on 14 January 2022).
- Seifert, T.; Sandmann, D. Mineralogy and geochemistry of indium-bearing polymetallic vein-type deposits: Implications for host minerals from the Freiberg district, Eastern Erzgebirge, Germany. Ore Geol. Rev. 2006, 28, 1–31. [Google Scholar] [CrossRef]
- Fritz, E.; Jahns, C. Die Spülhalde Davidschacht in Freiberg—Geschichte, Umweltproblematik und geplante Sanierung. Freib. Ecol. Online 2017, 2, 4–17. [Google Scholar]
- Richert, E.; Bernstein, C.; Funke, L.; Schulze, C. Vegetation der Spülhalde Davidschacht in Freiberg—Offenlandgesellschaften und Transektanalysen. Freib. Ecol. Online 2017, 2, 52–65. [Google Scholar]
- Lundin-Mining. Operations: Neves-Corvo. 2021. Available online: https://www.lundinmining.com/operations/neves-corvo/ (accessed on 12 January 2022).
- Carvalho, J.R.S.; Relvas, J.M.R.S.; Pinto, A.M.M.; Frenzel, M.; Krause, J.; Gutzmer, J.; Pacheco, N.; Fonseca, R.; Santos, S.; Caetano, P.; et al. Indium and selenium distribution in the Neves-Corvo deposit, Iberian Pyrite Belt, Portugal. Mineral. Mag. 2018, 82 (Suppl. S1), S5–S41. [Google Scholar] [CrossRef] [Green Version]
- Relvas, J.M.R.S.; Barriga, F.J.A.S.; Pinto, Á.; Ferreira, A.; Pacheco, N.; Noiva, P.; Barriga, G.; Baptista, R.; de Carvalho, D.; Oliveira, V.; et al. The Neves-Corvo Deposit, Iberian Pyrite Belt, Portugal: Impacts and Future, 25 Years after the Discovery. Integrated Methods for Discovery: Global Exploration in the Twenty-First Century. Soc. Econ. Geol. 2002, 9, 155–176. [Google Scholar] [CrossRef]
- Escobar, A.G.; Relvas, J.M.R.S.; Pinto, Á.M.M.; Oliveira, M. Physical–Chemical Characterization of the Neves Corvo Extractive Mine Residues: A Perspective Towards Future Mining and Reprocessing of Sulfidic Tailings. J. Sustain. Metall. 2021, 7, 1483–1505. [Google Scholar] [CrossRef]
- Hu, Z.; Qi, L. 15.5—Sample Digestion Methods. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 87–109. [Google Scholar] [CrossRef]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Pérez-López, R.; Álvarez-Valero, A.M.; Nieto, J.M.; Sáez, R.; Matos, J.X. Use of sequential extraction procedure for assessing the environmental impact at regional scale of the São Domingos Mine (Iberian Pyrite Belt). Appl. Geochem. 2008, 23, 3452–3463. [Google Scholar] [CrossRef]
- Coetzee, P.P. Determination and speciation of heavy metals in sediments of the Hartbeespoort Dam by sequential chemical extraction. Water SA 1993, 19, 291–300. [Google Scholar] [CrossRef]
- Marin, B.; Valladon, M.; Polve, M.; Monaco, A. Reproducibility testing of a sequential extraction scheme for the determination of trace metal speciation in a marine reference sediment by inductively coupled plasma-mass spectrometry. Anal. Chim. Acta 1997, 342, 91–112. [Google Scholar] [CrossRef]
- Gauthreaux, K.; Noble, C.O.; Falgoust, T.; Beck, M.J.; Sneddon, J.; Beck, J.N. Reliability and Reproducibility of a Sequential Extraction Procedure for Trace Metal Determination in Marsh Sediments in Southwest Louisiana. Microchem. J. 1998, 60, 175–183. [Google Scholar] [CrossRef]
- Li, X.; Shen, Z.; Wai, O.W.H.; Li, Y.-S. Chemical Forms of Pb, Zn and Cu in the Sediment Profiles of the Pearl River Estuary. Mar. Pollut. Bull. 2001, 42, 215–223. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef] [PubMed]
- Buykx, S.E.J.; Bleijenberg, M.; van den Hoop, M.A.G.T.; Gustav Loch, J.P. The effect of oxidation and acidification on the speciation of heavy metals in sulfide-rich freshwater sediments using a sequential extraction procedure. J. Environ. Monit. 2000, 2, 23–27. [Google Scholar] [CrossRef]
- Ahnstrom, Z.S.; Parker, D.R. Development and Assessment of a Sequential Extraction Procedure for the Fractionation of Soil Cadmium. Soil Sci. Soc. Am. J. 1999, 63, 1650–1658. [Google Scholar] [CrossRef]
- Lindsay, M.B.J.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Cappuyns, V.; Rudy, S.; Niclaes, M. Application of the BCR Sequential Extraction Scheme to Dredged Pond Sediments Contaminated by Pb-Zn Mining: A Combined Geochemical and Mineralogical Approach. J. Geochem. Explor. 2007, 93, 78–90. [Google Scholar] [CrossRef]
- Tokalioglu, S.; Kartal, S.; Elçi, L. Determination of Heavy Metals and Their Speciation in Lake Sediments by Flame Atomic Absorption Spectrometry after a Four Stage Sequential Extraction Procedure. Anal. Chim. Acta 2000, 413, 30–44. [Google Scholar] [CrossRef]
| Metals (mg/kg) | FR_01 | NC_01 | NC_02 |
|---|---|---|---|
| Cu | 828.30 | 1840.00 | 3726.70 |
| Pb | 4420.00 | 826.30 | 3986.70 |
| Zn | 13,100.00 | 3560.00 | 10,666.70 |
| Co | 40.97 | 84.70 | 184.50 |
| As | 5590.00 | 766.70 | 4986.70 |
| Cd | 136.00 | 9.00 | 27.90 |
| Cr | 37.17 | 64.97 | 42.63 |
| Mn | 3393.30 | 724.30 | 735.00 |
| Fe | 165,666.70 | 122,333.30 | 283,000.00 |
| Ca | 11,776.82 | 5250.00 | 7106.70 |
| Al | 28,000.00 | 55,933.30 | 25,933.30 |
| Mineral Groups (wt%) | FR_01 | NC_01 | NC_02 |
|---|---|---|---|
| Quartz | 42 | 45 | 23 |
| Other silicates | 17 | 39 | 18 |
| Carbonates | 5 | 3 | 2 |
| Pyrite | 22 | 12 | 53 |
| Other sulfides | 8 | <1 | <1 |
| Oxides | <1 | 1 | <1 |
| Sulfates | 5 | <1 | 4 |
| Other minerals | <1 | <1 | <1 |
| Fraction | Extraction Agent/Solvents | Reaction Times and Conditions | |
|---|---|---|---|
| F1. | Mobile | 1 M NH4NO3 | 24 h |
| F2. | Easily deliverable | 1 M CH3COONH4 (pH 6.0) | 24 h |
| F3. | Easily reducible | 0.1 M NH2OH-HCL + 1 M CH3COONH4 (pH 6.0) | 30 min |
| F4. | Oxidizable | 0.025 M NH4-EDTA (pH 4.6) | 90 min |
| F5. | Moderately reducible | 0.2 M NH4-Oxalate (pH 3.25) | 4 h (in the dark) |
| F6. | Poorly reducible | 0.1 M Ascorbic acid + 0.2M NH4-Oxalate | 30 min (96 °C) |
| F7. | Residual fraction | 65% HNO3 + 30% HCl + ~48% HF, 1:3:1 | 10 min (240 °C, 800 W, microwave) |
| FR_01 (Freiberg tailing) | Elements (mg/kg) | F1 Mobile | F2 Easily Deliverable | F3 Easily Reducible | F4 Oxidizable | F5 Moderately Reducible | F6 Poorly Reducible | F6 Residual Bound | Recovery (%) |
| Cu | 56.8 ± 5.6 | 25.1 ± 2.4 | <0.1 | 21.4 ± 1.9 | 18.3 ± 2.4 | <0.1 | 751.3 ± 1 | 105 | |
| Pb | 1910.8 ± 260.1 | 906.7 ± 38.8 | 280.8 ± 6.3 | 724.2 ± 43 | 251.6 ± 7.8 | 92.6 ± 19.5 | 736.3 ± 1 | 111 | |
| Zn | 3716.7 ± 212.6 | 98.5 ± 1.6 | 22.0 ± 1 | 179.3 ± 11.4 | 760.0 ± 60.1 | 48.9 ± 3.7 | 11066.7 ± 153 | 121 | |
| Co | 18.0 ± 0.4 | 0.5 ± 0.03 | 0.1 ± 0.005 | 0.2 ± 0.005 | 0.5 ± 0.05 | 0.2 ± 0.02 | 30.7 ± 0 | 122 | |
| As | 7.1 ± 0.7 | 28.2 ± 3.7 | 12.2 ± 0.7 | 351.7 ± 11.3 | 2493.3 ± 77.5 | 111.8 ± 16.2 | 3436.7 ± 15 | 115 | |
| Cd | 54.3 ± 5.2 | 1.1 ± 0.08 | 0.4 ± 0.02 | 1.3 ± 0.05 | 0.7 ± 0.14 | 0.1 ± 0.004 | 118.3 ± 1 | 130 | |
| Cr | 0.1 ± 0.008 | 0.1 ± 0.02 | 0.2 ± 0.008 | 0.3 ± 0.02 | 6.7 ± 0.4 | 1.8 ± 0.1 | 38.5 ± 1 | 129 | |
| Mn | 990.8 ± 18.8 | 63.3 ± 0.4 | 3.9 ± 0.1 | 60.4 ± 0.5 | 351.7 ± 9.5 | 522.5 ± 58.5 | 1215.5 ± 6 | 95 | |
| Fe | 36.9 ± 3.4 | 28.3 ± 8.8 | 315.0 ± 9.0 | 4466.7 ± 38.2 | 17,033.3 ± 398.7 | 5975.0 ± 894.8 | 137,744.8 ± 1000 | 100 | |
| Ca | 8941.7 ± 152.8 | 520.8 ± 6.3 | 6.0 ± 0.5 | 353.3 ± 9.5 | 15.0 ± 2 | 20.2 ± 2.4 | 2656.7 ± 15 | 106 | |
| Al | 77.3 ± 10.6 | 17.1 ± 2.3 | 5.3 ± 1.3 | 65.2 ± 7.2 | 551.3 ± 48.2 | 248.2 ± 22.5 | 36,433.3 ± 404 | 134 | |
| NC_01 (Neves Corvo waste rock) | Cu | 16.1 ± 0.3 | 47.5 ± 1.5 | <0.1 | 29.8 ± 1.2 | 34.3 ± 1.2 | <0.1 | 1790.0 ± 10 | 104 |
| Pb | 6.3 ± 0.3 | 235.8 ± 5.4 | 47.4 ± 0.4 | 143.7 ± 2.7 | 160.8 ± 1.9 | 10.6 ± 0.5 | 149.3 ± 1 | 91 | |
| Zn | 83.7 ± 0.6 | 167.8 ± 3 | 21.7 ± 0.6 | 71.4 ± 1.1 | 114.4 ± 1 | 39.8 ± 2.9 | 3046.7 ± 35 | 100 | |
| Co | 9.7 ± 0.1 | 6.7 ± 0.1 | 0.8 ± 0.02 | 4.4 ± 0.1 | 4.1 ± 0.04 | 0.5 ± 0.04 | 59.4 ± 1 | 101 | |
| As | <2.5 | 5.1 ± 0.2 | < 2.5 | 14.4 ± 0.2 | 129.7 ± 1.7 | 27.3 ± 3 | 634.0 ± 5 | 106 | |
| Cd | 0.7 ± 0.006 | 0.4 ± 0.001 | 0.05 ± 0.001 | 0.1 ± 0.0001 | 0.1 ± 0.0004 | 0.02 ± 0.005 | 8.1 ± 0 | 105 | |
| Cr | 0.1 ± 0.003 | 0.1 ± 0.005 | 0.2 ± 0.002 | 0.2 ± 0.01 | 2.7 ± 0.06 | 1.6 ± 0.08 | 69.4 ± 1 | 114 | |
| Mn | 95.8 ± 1 | 21.1 ± 1.3 | 3.8 ± 0.09 | 10.3 ± 0.3 | 107.9 ± 1 | 101.8 ± 6.1 | 396.3 ± 1 | 102 | |
| Fe | <2.5 | 17.1 ± 0.7 | 113.2 ± 3.7 | 941.7 ± 42.6 | 9966.7 ± 152.6 | 5258.3 ± 203.6 | 121,333.3 ± 2309 | 113 | |
| Ca | 2022.8 ± 11.5 | 258.8 ± 0.3 | 70.2 ± 2.3 | 347.3 ± 2.5 | 20.6 ± 0.4 | 25.0 ± 0.1 | 2543.3 ± 42 | 101 | |
| Al | <0.25 | 37.7 ± 0.4 | 12.4 ± 0.4 | 204.1 ± 3.3 | 1043.4 ± 3.9 | 743.1 ± 13.5 | 56,900.0 ± 693 | 105 | |
| NC_02 (Neves Corvo tailing) | Cu | 172.2 ± 3.5 | 98.8 ± 1.4 | <0.1 | 111.3 ± 1.2 | 69.6 ± 2.2 | <0.1 | 3543 ± 3 | 107 |
| Pb | 1541.7 ± 25.2 | 565.8 ± 13.8 | 186.7 ± 4.5 | 550.8 ± 10.1 | 190.7 ± 3.6 | 15.1 ± 3.4 | 781 ± 10 | 96 | |
| Zn | 676.7 ± 16.1 | 115.6 ± 1.9 | 19.9 ± 1.3 | 92.7 ± 2.5 | 208.8 ± 2.1 | 96.3 ± 2.9 | 9440 ± 30 | 100 | |
| Co | 8.6 ± 0.1 | 2.7 ± 0 | 0.3 ± 0.03 | 0.7 ± 0.03 | 1.4 ± 0.02 | 1.5 ± 0.07 | 190 ± 3 | 111 | |
| As | 1.3 ± 0.1 | 38.1 ± 0.5 | 64.3 ± 0.4 | 310.8 ± 1.4 | 650.8 ± 2.9 | 1.7 ± 0.1 | 4093 ± 25 | 103 | |
| Cd | 2.3 ± 0.02 | 0.3 ± 0.001 | 0.1 ± 0.002 | 0.1 ± 0.001 | 0.2 ± 0.002 | 0.03 ± 0.008 | 26.8 ± 0 | 106 | |
| Cr | 0.1 ± 0.02 | 0.1 ± 0.007 | 0.2 ± 0.01 | 0.2 ± 0.02 | 9.6 ± 0.4 | 1.4 ± 0.04 | 37 ± 0 | 115 | |
| Mn | 70.6 ± 2.4 | 24.3 ± 1.4 | 5.2 ± 0.2 | 26.3 ± 1.4 | 95.1 ± 3.8 | 101.8 ± 0.7 | 430 ± 3 | 103 | |
| Fe | 28.6 ± 0.9 | 98.3 ± 0.1 | 556.7 ± 12.6 | 1808.3 ± 30.6 | 7658.3 ± 212.6 | 4133.3 ± 14.4 | 310,666.7 ± 1155 | 115 | |
| Ca | 2850.0 ± 50 | 411.0 ± 5.5 | 107.7 ± 0.5 | 432.8 ± 8.8 | 19.3 ± 0.2 | 19.1 ± 1 | 3066.7 ± 29 | 97 | |
| Al | 5.0 ± 0.1 | 3.2 ± 0.1 | 1.9 ± 0.1 | 54.8 ± 1.1 | 310.6 ± 7.9 | 425.8 ± 17.1 | 25,633.3 ± 58 | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opara, C.B.; Kutschke, S.; Pollmann, K. Fractionation of Metal(loid)s in Three European Mine Wastes by Sequential Extraction. Separations 2022, 9, 67. https://doi.org/10.3390/separations9030067
Opara CB, Kutschke S, Pollmann K. Fractionation of Metal(loid)s in Three European Mine Wastes by Sequential Extraction. Separations. 2022; 9(3):67. https://doi.org/10.3390/separations9030067
Chicago/Turabian StyleOpara, Chiamaka Belsonia, Sabine Kutschke, and Katrin Pollmann. 2022. "Fractionation of Metal(loid)s in Three European Mine Wastes by Sequential Extraction" Separations 9, no. 3: 67. https://doi.org/10.3390/separations9030067
APA StyleOpara, C. B., Kutschke, S., & Pollmann, K. (2022). Fractionation of Metal(loid)s in Three European Mine Wastes by Sequential Extraction. Separations, 9(3), 67. https://doi.org/10.3390/separations9030067