Enhancement of the Green Extraction of Bioactive Molecules from Olea europaea Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
2.1.2. Chemical
2.2. Methods
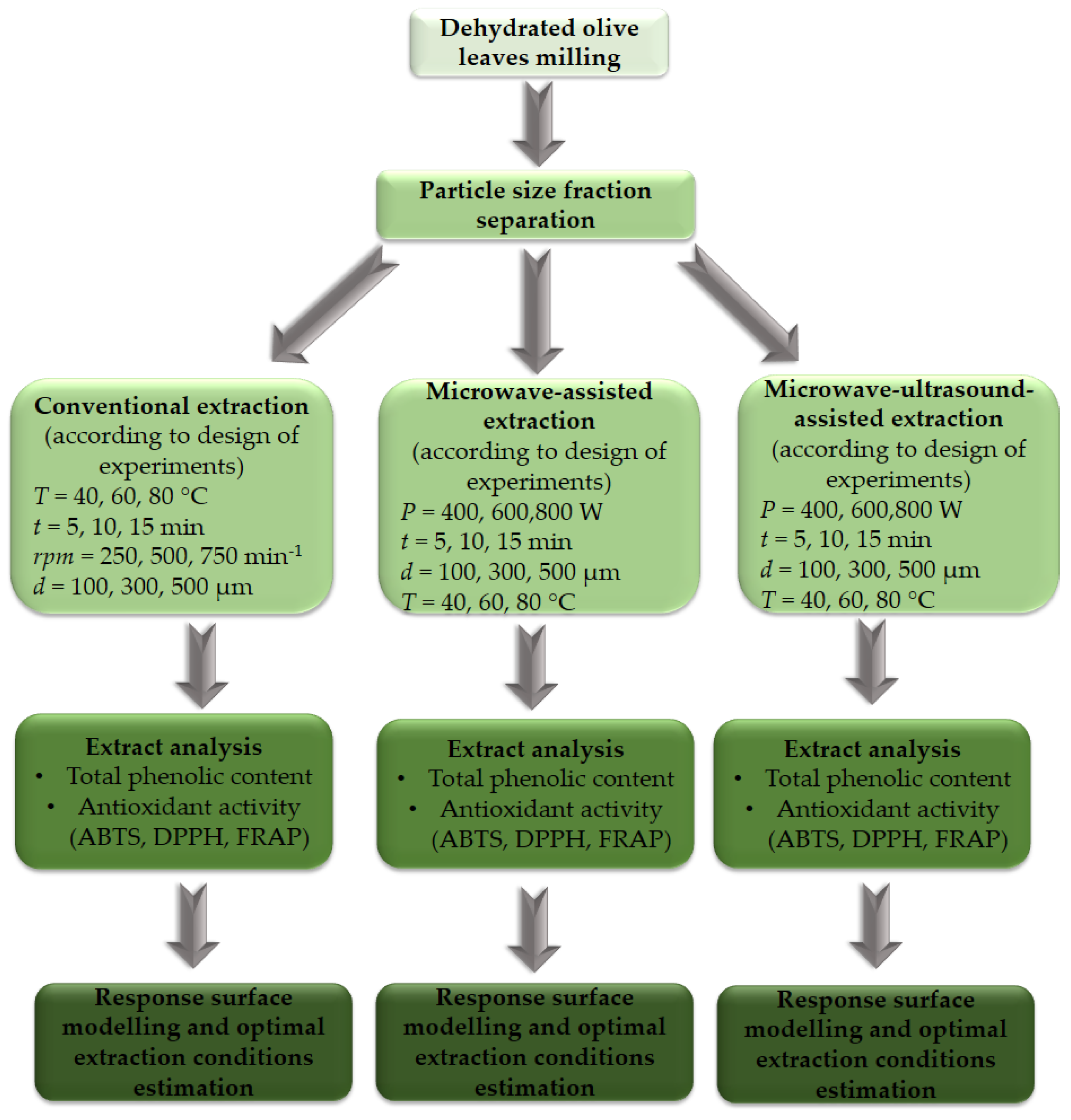
2.2.1. Milling and Separation of Particle Size Fractions
2.2.2. Conventional Extraction (CE) Procedure
2.2.3. Microwave-Assisted Extraction (MWE) and Microwave–Ultrasound-Assisted Extraction (MWUE) Procedure
2.2.4. Total Polyphenolic Content (TPC) and Antioxidant Activities (AA) Measurement
2.2.5. Design of the Experiments and Response Surface Modeling
2.2.6. Statistical Analysis
3. Results
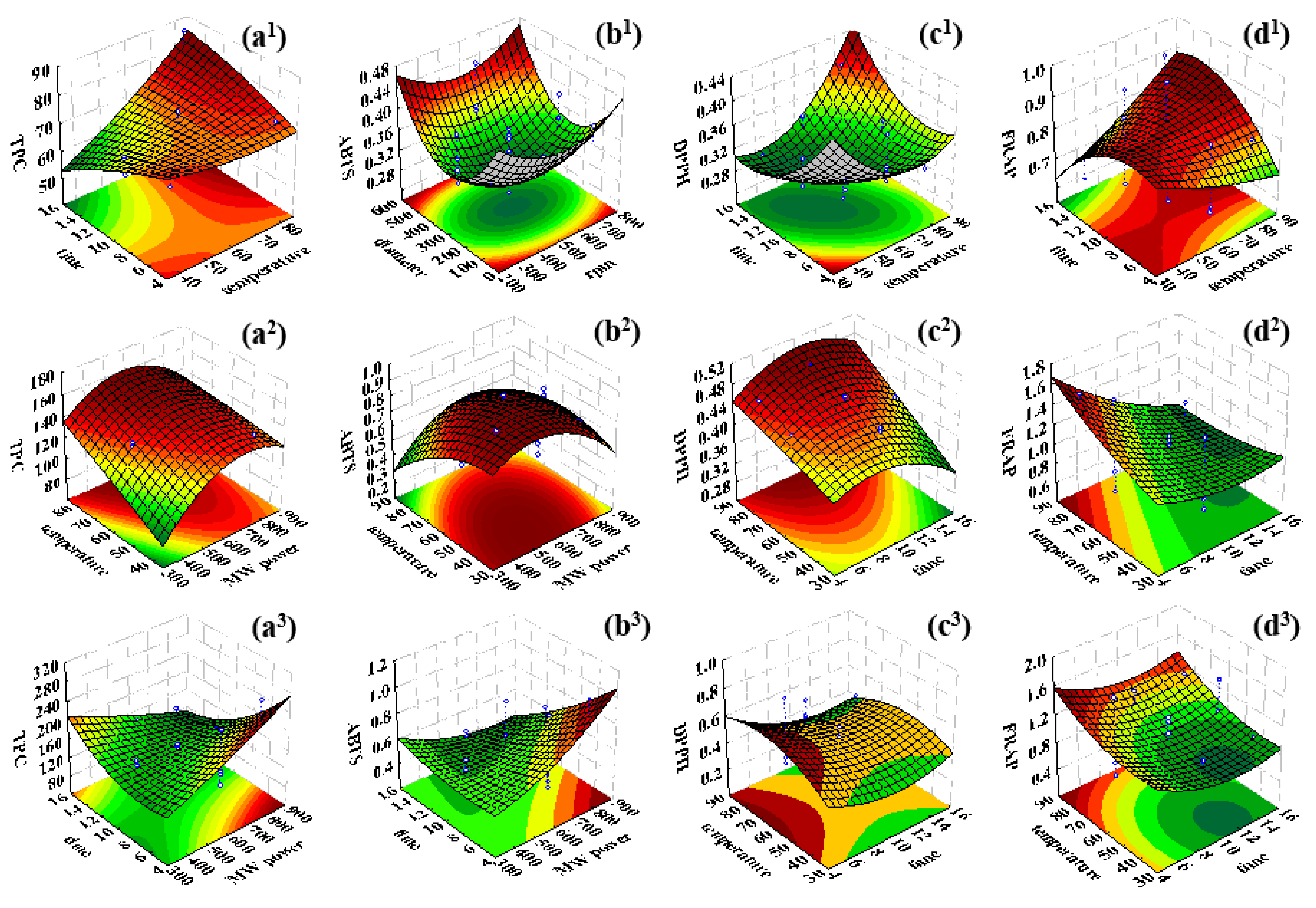
3.1. Response Surface Analysis of the Total Phenolic Content and Antioxidant Activity of the Olive Leaves Aqueous Extracts Produced by Conventional Extraction
3.2. Response Surface Analysis of the Total Phenolic Content and Antioxidant Activity of the Olive Leaves Aqueous Extracts Produced by Microwave-Assisted Extraction and Microwave–Ultrasound-Assisted Extraction
3.3. Optimization and Model Validation for CE, MWE and MWUE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, N.; Pinho, T.; Casal, S.; Pere, A.M.; Batista, P.; Pereira, J.A. Chemical characterization of oleaster, Olea europaea var. sylvestris (Mill.) Lehr., oils from different locations of Northeast Portugal. Appl. Sci. 2020, 10, 6414. [Google Scholar] [CrossRef]
- Ozturk, M.; Altay, V.; Gönenç Unal, B.T.; Efe, R.; Akçiçek, E.; Bukhari, A. An overview of olive cultivation in Turkey: Botanical features, eco-physiology and phytochemical aspects. Agronomy 2021, 11, 295. [Google Scholar] [CrossRef]
- Salah, M.B.; Abdelmelek, H.; Abderraba, M. Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Med. Chem. 2012, 2, 107–111. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Reale, L.; Caruso, T.; Ferranti, F. Productive and vegetative behavior of olive cultivars in super high-density olive grove. Sci. Argic. 2015, 72, 20–27. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of aqueous extraction of phenolic compounds from olive leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Barba, F.J.; Španić, I.; Zorić, Z.; Dragović-Uzelac, V.; Bursać Kovačević, D. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea). Food Bioprod. Process. 2017, 107, 19–28. [Google Scholar] [CrossRef]
- Sifaoui, I.; Chammem, N.; Abderrabba, M.; Mejri, M. Optimization of phenolic compounds extraction from olive leaves using experimental design methodology. J. Mater. Environ. Sci. 2016, 7, 1119–1127. [Google Scholar]
- Vogel, P.; Kasper Machado, I.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Morelo Dal Bosco, S. Polyphenols benefits of olive leaf (Olea europaea L.) to human health. Nutr. Hosp. 2015, 31, 1427–1433. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J. State of the art on functional virgin olive oils enriched with bioactive compounds and their properties. Int. J. Mol. Sci. 2017, 18, 668. [Google Scholar] [CrossRef]
- Altıok, E.; Baycın, D.; Bayraktar, O.; Ülkü, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Dragović Uzelac, V. Optimizing acidity and extraction time for polyphenolic recovery and antioxidant capacity in grape pomace skin extracts with response surface methodology approach. J. Food Process. Preserv. 2016, 40, 1256–1263. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovnikovic, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Lovrić, V.; Putnik, P.; Bursać Kovačević, D.; Jukić, M.; Dragović-Uzelac, V. Effect of microwave-assisted extraction on the phenolic compounds and antioxidant capacity of blackthorn flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-assisted extraction as a green technology approach to recover polyphenols from Castanea sativa shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S. Optimized microwave assisted extraction (MAE) of alkaloids and polyphenols from Berberis roots using multiple-component analysis. Sci. Rep. 2020, 10, 917. [Google Scholar] [CrossRef]
- Azaroual, L.; Liazid, A.; Mansouri, F.E.; Brigui, J.; Ruíz-Rodriguez, A.; Barbero, G.F.; Palma, M. Optimization of the microwave-assisted extraction of simple phenolic compounds from grape skins and seeds. Agronomy 2021, 11, 1527. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Valorisation of black carrot pomace: Microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Evaluation of enzyme and microwave-assisted conditions on extraction of anthocyanins and total phenolics from black soybean (Glycine max L.) seed coat. Int. J. Biol. Macromol. 2019, 135, 1070–1081. [Google Scholar] [CrossRef]
- Guan, X.; Li, L.; Liu, J.; Li, S. Effects of ultrasonic-microwave-assisted technology on hordein extraction from barley and optimization of process parameters using response surface methodology. J. Food Qual. 2018, 2018, 9280241. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Ni, Y.; Yao, L.; Jiang, H.; Ren, X.; Fu, Y.; Zhao, C. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019, 206, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, C.; Duan, Z.; Liu, B.; Duan, W.; Shang, F. Ultrasonic microwave-assisted extraction of polyphenols, flavonoids, triterpenoids, and vitamin C from Clinacanthus nutans. Czech J. Food Sci. 2017, 35, 89–94. [Google Scholar] [CrossRef]
- Chamutpong, S.; Chen, C.-J.; Chaiprateep, E. Optimization ultrasonic-microwave-assisted extraction of phenolic compounds from Clinacanthus nutans using response surface methodology. J. Adv. Pharm. Technol. Res. 2021, 12, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Q.; Zheng, B.; Zhao, C. The optimization of ultrasonic-microwave assisted synergistic extraction of Lotus plumule extract rich in flavonoids and its hypoglycemic activity. Food Prod. Process. Nutr. 2021, 3, 23. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Benković, M.; Valinger, D.; Jurina, T.; Belščak-Cvitanović, A.; Gajdoš Kljusurić, J. Optimizing bioactive compounds extraction from different medicinal plants and prediction through nonlinear and linear models. Ind. Crop. Prod. 2018, 126, 449–458. [Google Scholar] [CrossRef]
- Valinger, D.; Jurina, T.; Benković, M.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Mathematical Modelling and Optimization in Solid-Liquid Extractions of Bioactives from Medicinal Plants. In Medicinal Plants: Properties, Uses and Production; Semwal, D.K., Ed.; Nova Science Publisher, Inc.: New York, NY, USA, 2021; pp. 273–305. [Google Scholar]
- Bakar, F.I.A.; Bakara, M.F.A.B.; Abdullah, N.; Endrini, S.; Fatmawati, S. Optimization of extraction conditions of phytochemical compounds and anti-gout activity of Euphorbia hirta L. (Ara Tanah) using response surface methodology and Liquid Chromatography-Mass Spectrometry (LC-MS) analysis. Evid.-Base Complement. Altern. Med. 2020, 2020, 4501261. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization method for phenolic compounds extraction from medicinal plant (Juniperus procera) and phytochemicals screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef]
- AOAC (1995) Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, MA, USA. Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 10 May 2021).
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Romero, C.; García, P.; Brense, M. Characterization of bioactive compounds in commercial olive leaf extracts, and olive leaves and their infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of phenolic compounds in olive leaves by different drying and storage methods. Separations 2021, 8, 156. [Google Scholar] [CrossRef]
- Lins, P.G.; Pugine, S.M.P.; Scatolini, A.M. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Topuz, S.; Bayram, M. Oleuropein extraction from leaves of three olive varieties (Olea europaea L.): Antioxidant and antimicrobial properties of purified oleuropein and oleuropein extracts. J. Food Process. Preserv. 2021, in press. [Google Scholar] [CrossRef]
- Nicolí, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellisi, L. Evaluation of phytochemical and antioxidant properties of 15 Italian Olea europaea L. cultivar leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Benković, M.; Belščak-Cvitanović, A.; Valinger, D.; Jurina, T.; Gajdoš Kljusurić, J. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols, antioxidants and extraction yield from Asteraceae plants. Ind. Crops Prod. 2016, 91, 205–214. [Google Scholar] [CrossRef]
- Perušić, Ž.; Saftić, L.; Kisović, D.; Kraljić Pavelić, S. Polyphenol-based design of functional olive leaf infusion. Food Technol. Biotechnol. 2019, 57, 171–182. [Google Scholar] [CrossRef]
- Martiny, T.R.; Dotto, G.L.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S. Freezing effect on the oleuropein content of olive leaves extracts obtained from microwave-assisted extraction. Int. J. Environ. Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. valorisation of Olea europaea L. Olive leaves through the evaluation of their extracts: Antioxidant and antimicrobial activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Phar. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Al-Marazeeq, K.; Haddadin, M.S.Y.; Abdulla, B.; Haddadin, J.S. Biological activities of olive leaves extract from nabali baladi variety against lipid and protein oxidation. Int. J. Biol. Biotechnol. 2016, 13, 283–291. [Google Scholar]
- Anne, R.; Nithyanandam, R. Optimization of extraction of bioactive compounds from medicinal herbs using response surface methodology. Int. Proc. Chem. Biol. Environ. Eng. 2016, 99, 76–85. [Google Scholar] [CrossRef]
- Santos, J.S.; Deolindo, C.T.P.; Esmerino, L.A.; Genovese, M.I.; Fujita, A.; Marques, M.B.; Rosso, N.D.; Daguer, H.; Valese, A.C.; Granato, D. Effects of time and extraction temperature on phenolic composition and functional properties of red rooibos (Aspalathus linearis). Food Res. Int. 2016, 89, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, O.; Ferreira, I.C.F.R. Optimization and comparison of maceration and microwave extraction systems for the production of phenolic compounds from Juglans regia L. for the valorization of walnut leaves. Ind. Crops Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon 2020, 6, e03666. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. water as a solvent of election for obtaining oleuropein-rich extracts from olive (Olea europaea) leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
- Le Man, H.; Behera, S.K.; Park, H.S. Optimization of operational parameters for ethanol production from Korean food waste leachate. Int. J. Environ. Sci. Technol. 2010, 7, 157–164. [Google Scholar] [CrossRef]
- Teng, D.; Fang, Y.; Song, X.; Gao, Y. Optimization of enzymatic hydrolysis parameters for antioxidant capacity of peptide from goat placenta. Food Bioprod. Process. 2011, 89, 202–208. [Google Scholar] [CrossRef]
- Kumar, M.; Dahzja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of microwave-assisted extraction of phenolic antioxidants from grape seeds (Vitis vinifera). Food Bioprod. Process. 2013, 6, 441–455. [Google Scholar] [CrossRef]
- Périno, S.; Pierson, J.T.; Ruiz, K.; Cravotto, G.; Chemat, F. Laboratory to pilot scale: Microwave extraction for polyphenols lettuce. Food Chem. 2016, 204, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Punia, S.; Amarowicz, R.; Kaur, C. Evaluation of cellulolytic enzyme-assisted microwave extraction of Punica granatum peel phenolics and antioxidant activity. Plant Foods Hum. Nutr. 2020, 75, 614–620. [Google Scholar] [CrossRef]
- Quoc, L.P.T. Microwave-assisted extraction of phenolic compounds from coffee (Coffea robusta L. Linden) bee pollen. Herba Pol. 2021, 67, 3. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Chanioti, S.; Simandoura, P.; Tzia, C. Evaluation of extracts prepared from olive oil by-products using microwave-assisted enzymatic extraction: Effect of encapsulation on the stability of final products. Waste Biomass Valor. 2016, 7, 831–842. [Google Scholar] [CrossRef]
- Kirbaşlar, Ş.I.; Şahin, S. Recovery of bioactive ingredients from biowaste of olive tree (Olea europaea) using microwave-assisted extraction: A comparative study. Biomass Convers. Biorefin. 2021, in press. [CrossRef]
- Saifullah, M.; McCullum, R.; Vuong, Q.V. Optimization of microwave-assisted extraction of polyphenols from Lemon Myrtle: Comparison of modern and conventional extraction techniques based on bioactivity and total polyphenols in dry extracts. Processes 2021, 9, 2212. [Google Scholar] [CrossRef]
- Chowdhury, A.; Panneerselvam, T.; Suthendran, K.; Bhattachejee, C.; Balasubramanian, S.; Murugesan, S.; Suraj, B.; Selvaraj, K. Optimization of microwave-assisted extraction of bioactive polyphenolic compounds from Marsilea quadrifolia L. using RSM and ANFIS modelling. Indian J. Nat. Prod. Resour. 2018, 9, 204–221. [Google Scholar]
- Nguyen, V.T.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Optimization of microwave-assisted extraction from Phyllanthus amarus for phenolic compounds-enriched extracts and antioxidant capacity. Chem. Pap. 2016, 70, 713–725. [Google Scholar] [CrossRef]
- Liu, J.; Mu, T.; Sun, H.; Fauconnier, M.L. Optimization of ultrasonic–microwave synergistic extraction of flavonoids from sweet potato leaves by response surface methodology. J. Food Process. Preserv. 2019, 43, e13928. [Google Scholar] [CrossRef]
| Run | T/°C | t/min | rpm/min−1 | d/µm | TPC/mgGAE gd.m.−1 | ABTS/mmolTrolox gd.m.−1 | DPPH/mmolTrolox gd.m.−1 | FRAP/mmolFeSO4·7H2O gd.m.−1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 (−1) | 5 (−1) | 500 (0) | 300 (0) | 69.551 ± 7.417 | 0.371 ± 0.059 | 0.337 ± 0.056 | 0.741 ± 0.005 |
| 2 | 40 (−1) | 15 (1) | 500 (0) | 300 (0) | 57.725 ± 6.277 | 0.312 ± 0.039 | 0.302 ± 0.002 | 0.618 ± 0.004 |
| 3 | 80 (1) | 5 (−1) | 500 (0) | 300 (0) | 73.172 ± 5.876 | 0.361 ± 0.043 | 0.307 ± 0.009 | 0.812 ± 0.041 |
| 4 | 80 (1) | 15 (1) | 500 (0) | 300 (0) | 88.305 ± 3.271 | 0.353 ± 0.012 | 0.401 ± 0.014 | 1.049 ± 0.010 |
| 5 | 60 (0) | 10 (0) | 250 (−1) | 100 (−1) | 66.131 ± 10.256 | 0.376 ± 0.104 | 0.293 ± 0.002 | 0.718 ± 0.076 |
| 6 | 60 (0) | 10 (0) | 750 (1) | 100 (−1) | 69.115 ± 4.069 | 0.377 ± 0.023 | 0.306 ± 0.026 | 0.743 ± 0.041 |
| 7 | 60 (0) | 10 (0) | 250 (−1) | 500 (1) | 68.832 ± 1.358 | 0.384 ± 0.048 | 0.278 ± 0.017 | 0.717 ± 0.004 |
| 8 | 60 (0) | 10 (0) | 750 (1) | 500 (1) | 69.178 ± 5.755 | 0.329 ± 0.038 | 0.242 ± 0.097 | 0.726 ± 0.288 |
| 9 | 60 (0) | 10 (0) | 500 (0) | 300 (0) | 64.598 ± 2.119 | 0.352 ± 0.002 | 0.305 ± 0.009 | 0.819 ± 0.133 |
| 10 | 60(0) | 5 (−1) | 500 (0) | 100 (−1) | 69.753 ± 4.095 | 0.355 ± 0.024 | 0.319 ± 0.038 | 0.637 ± 0.105 |
| 11 | 60 (0) | 15 (1) | 500 (0) | 100 (−1) | 61.326 ± 1.930 | 0.362 ± 0.084 | 0.281 ± 0.002 | 0.647 ± 0.099 |
| 12 | 60 (0) | 5 (−1) | 500 (0) | 500 (1) | 70.063 ± 4.095 | 0.457 ± 0.061 | 0.343 ± 0.031 | 0.654 ± 0.117 |
| 13 | 60 (0) | 15 (1) | 500 (0) | 500 (1) | 60.499 ± 4.748 | 0.368 ± 0.067 | 0.265 ± 0.019 | 0.622 ± 0.006 |
| 14 | 40 (−1) | 10 (0) | 250 (−1) | 300 (0) | 63.366 ± 13.121 | 0.405 ± 0.085 | 0.317 ± 0.048 | 0.699 ± 0.063 |
| 15 | 80 (1) | 10 (0) | 250 (−1) | 300 (0) | 75.649 ± 1.047 | 0.313 ± 0.067 | 0.334 ± 0.015 | 0.676 ± 0.150 |
| 16 | 40 (−1) | 10 (0) | 750 (1) | 300 (0) | 68.543 ± 0.332 | 0.351 ± 0.012 | 0.294 ± 0.024 | 0.821 ± 0.100 |
| 17 | 80 (1) | 10 (0) | 750 (1) | 300 (0) | 72.827 ± 1.388 | 0.396 ± 0.126 | 0.346 ± 0.039 | 0.778 ± 0.017 |
| 18 | 60 (0) | 10 (0) | 500 (0) | 300 (0) | 66.460 ± 4.331 | 0.282 ± 0.009 | 0.301 ± 0.029 | 0.795 ± 0.003 |
| 19 | 60 (0) | 5 (−1) | 250 (−1) | 300 (0) | 70.615 ± 3.014 | 0.338 ± 0.016 | 0.396 ± 0.007 | 0.858 ± 0.074 |
| 20 | 60 (0) | 15 (1) | 250 (−1) | 300 (0) | 66.189 ± 7.616 | 0.358 ± 0.086 | 0.337 ± 0.004 | 0.845 ± 0.045 |
| 21 | 60 (0) | 5 (−1) | 750 (1) | 300 (0) | 69.644 ± 6.191 | 0.312 ± 0.040 | 0.338 ± 0.005 | 0.745 ± 0.002 |
| 22 | 60 (0) | 15 (1) | 750 (1) | 300 (0) | 67.193 ± 1.988 | 0.331 ± 0.037 | 0.338 ± 0.004 | 0.762 ± 0.033 |
| 23 | 40 (−1) | 10 (0) | 500 (0) | 100 (−1) | 61.944 ± 2.955 | 0.351 ± 0.019 | 0.341 ± 0.002 | 0.815 ± 0.002 |
| 24 | 80 (1) | 10 (0) | 500 (0) | 100 (−1) | 69.271 ± 4.108 | 0.357 ± 0.102 | 0.328 ± 0.022 | 0.820 ± 0.106 |
| 25 | 40 (−1) | 10 (0) | 500 (0) | 500 (1) | 67.634 ± 8.389 | 0.358 ± 0.012 | 0.311 ± 0.017 | 0.913 ± 0.086 |
| 26 | 80 (1) | 10 (0) | 500 (0) | 500 (1) | 73.958 ± 3.064 | 0.296 ± 0.026 | 0.304 ± 0.013 | 0.813 ± 0.044 |
| 27 | 60 (0) | 10 (0) | 500 (0) | 300 (0) | 76.379 ± 3.121 | 0.245 ± 0.047 | 0.313 ± 0.038 | 0.965 ± 0.095 |
| Variable | Model Equation | R2 | |
|---|---|---|---|
| conventional extraction | TPC | Y = 68.175 + 11.197·X1 − 3.720·X2 + 0.848·X3 + 2.093·X4 − 1.203·X12 + 1.036·X22 + 0.733·X32 + 3.180·X42 + 12.097·X1X2 − 3.999·X1X3 − 0.503·X1X4 + 0.987·X2X3 − 0.567·X2X4 − 1.633·X3X4 | 0.827 |
| ABTS | Y = 0.385 − 0.0148·X1 − 0.018·X2 + 0.003·X3 − 0.013·X4 − 0.028·X12−0.032·X22 − 0.037·X32 − 0.052·X42 + 0.026·X1X2 + 0.069·X1X3 − 0.043·X1X4 − 0.001·X2X3 − 0.048·X2X4 − 0.031·X3X4 | 0.778 | |
| DPPH | Y = 0.3341 + 0.020·X1 − 0.019·X2 − 0.014 ·X3 − 0.010·X4 − 0.026·X12 − 0.032·X22 − 0.021·X32 − 0.005·X42 + 0.064·X1X2 + 0.017·X1X3 − 0.003·X1X4 + 0.030·X2X3 − 0.019·X2X4 − 0.020·X3X4 | 0.871 | |
| FRAP | Y = 0.731 + 0.033·X1 − 0.005·X2 + 0.002·X3 + 0.014·X4 − 0.007·X12 + 0.061·X22 − 0.029·X32 − 0.064·X42 + 0.111·X1X2 − 0.009·X1X3 − 0.049·X1X4 + 0.084·X2X3 − 0.021·X2X4 − 0.026·X3X4 | 0.754 | |
| microwave-assisted extraction | TPC | Y = 124.059 + 13.197·Z1 − 10.988·Z2 + 6.355·Z3 + 25.868·Z4 − 11.958·Z12 + 8.607·Z22 + 10.672·Z32 + 0.668·Z42 + 16.824·Z1Z2 − 0.108·Z1Z3 − 11.263·Z1Z4 + 0.297·Z2Z3 + 8.179·Z2Z4 − 4.045·Z3Z4 | 0.768 |
| ABTS | Y = 0.593 − 0.017·Z1 + 0.050·Z2 + 0.081·Z3 − 0.151·Z4 + 0.086·Z12 + 0.101·Z22 + 0.067·Z32 + 0.046·Z42 + 0.028·Z1Z2 + 0.157·Z1Z3 + 0.095·Z1Z4 − 0.029·Z2Z3 − 0.118·Z2Z4 − 0.107·Z3Z4 | 0.849 | |
| DPPH | Y = 0.408 + 0.009·Z1 − 0.018·Z2 + 0.023·Z3 + 0.067·Z4 + 0.005·Z12 + 0.031·Z22 + 0.025·Z32 + 0.001·Z42 − 0.031·Z1Z2 + 0.022·Z1Z3 + 0.001·Z1Z4 + 0.017·Z2Z3 + 0.024·Z2Z4 + 0.012·Z3Z4 | 0.858 | |
| FRAP | Y = 0.977 − 0.025·Z1 − 0.401·Z2 − 0.075·Z3 + 0.140·Z4 + 0.050·Z12 − 0.074·Z22 + 0.074·Z32 − 0.028·Z42 − 0.209·Z1Z2 + 0.346·Z1Z3 + 0.284·Z1Z4 + 0.384·Z2Z3 − 0.189·Z2Z4 + 0.087·Z3Z4 | 0.817 | |
| ultrasound-microwave-assisted extraction | TPC | Y = 159.091 + 11.247·Z1 − 39.523·Z2 − 10.311·Z3 + 26.713·Z4 − 2.106·Z12 − 23.453·Z22 − 7.442·Z32 + 11.836·Z42 − 64.399·Z1Z2 − 14.828·Z1Z3 + 19.174·Z1Z4 + 48.693·Z2Z3 − 4.796·Z2Z4 − 2.249·Z3Z4 | 0.875 |
| ABTS | Y = 0.579 + 0.107·Z1 − 0.249·Z2 − 0.045·Z3 − 0.132·Z4 − 0.0370·Z12 − 0.034·Z22 − 0.057·Z32 + 0.030·Z42 − 0.213·Z1Z2 − 0.133·Z1Z3 − 0.143·Z1Z4 − 0.272·Z2Z3 + 0.006·Z2Z4 + 0.234·Z3Z4 | 0.898 | |
| DPPH | Y = 0.472 + 0.015·Z1 − 0.126·Z2 + 0.053·Z3 + 0.021·Z4 − 0.052·Z12 − 0.077·Z22 + 0.054·Z32 + 0.054·Z42 − 0.247·Z1Z2 − 0.064·Z1Z3 + 0.002·Z1Z4 + 0.032·Z2Z3 − 0.059·Z2Z4 − 0.208·Z3Z4 | 0.799 | |
| FRAP | Y = 1.067- 0.052·Z1 − 0.084·Z2 + 0.052·Z3 + 0.145·Z4 + 0.095·Z12+0.168·Z22 − 0.007 ·Z32 − 0.061·Z42 − 0.489·Z1Z2 − 0.049·Z1Z3 − 0.009 ·Z1Z4 − 0.011·Z2Z3 + 0.009·Z2Z4 + 0.023·Z3Z4 | 0.863 |
| Microwave-Assisted Extraction | Microwave–Ultrasound-Assisted Extraction Process | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | P/W | t/min | d/µm | T/°C | TPC | ABTS | DPPH | FRAP | TPC | ABTS | DPPH | FRAP |
| 1 | 400 (−1) | 5 (−1) | 300 (0) | 60 (0) | 133.279 ± 3.223 | 0.503 ± 0.021 | 0.389 ± 0.022 | 0.829 ± 0.083 | 146.858 ± 2.417 | 0.558 ± 0.143 | 0.469 ± 0.009 | 0.770 ± 0.168 |
| 2 | 800 (1) | 5 (−1) | 300 (0) | 60 (0) | 140.329 ± 7.659 | 0.552 ± 0.044 | 0.438 ± 0.017 | 1.181 ± 0.517 | 273.779 ± 4.968 | 1.014 ± 0.041 | 0.933 ± 0.042 | 1.837 ± 0.026 |
| 3 | 400 (−1) | 15 (1) | 300 (0) | 60 (0) | 90.209 ± 10.542 | 0.619 ± 0.005 | 0.420 ± 0.031 | 0.815 ± 0.075 | 147.805 ± 3.628 | 0.386 ± 0.047 | 0.456 ± 0.005 | 1.452 ± 0.874 |
| 4 | 800 (1) | 15 (1) | 300 (0) | 60 (0) | 130.909 ± 1.674 | 0.724 ± 0.005 | 0.407 ± 0.022 | 0.748 ± 0.088 | 145.928 ± 11.687 | 0.416 ± 0.017 | 0.425 ± 0.013 | 0.314 ± 0.791 |
| 5 | 600 (0) | 10 (0) | 100 (−1) | 40 (−1) | 114.747 ± 2.412 | 0.668 ± 0.012 | 0.374 ± 0.017 | 0.741 ± 0.071 | 139.686 ± 1.229 | 0.934 ± 0.005 | 0.422 ± 0.030 | 0.836 ± 0.029 |
| 6 | 600 (0) | 10 (0) | 500 (1) | 40 (−1) | 122.573 ± 3.673 | 0.779 ± 0.067 | 0.374 ± 0.032 | 0.637 ± 0.002 | 138.947 ± 4.328 | 0.458 ± 0.012 | 0.404 ± 0.007 | 0.772 ± 0.011 |
| 7 | 600 (0) | 10 (0) | 100 (−1) | 80 (1) | 147.286 ± 11.975 | 0.669 ± 0.098 | 0.461 ± 0.009 | 0.919 ± 0.134 | 160.582 ± 0.560 | 0.409 ± 0.061 | 0.474 ± 0.014 | 1.070 ± 0.018 |
| 8 | 600 (0) | 10 (0) | 500 (1) | 80 (1) | 147.023 ± 2.903 | 0.577 ± 0.019 | 0.486 ± 0.002 | 0.990 ± 0.128 | 155.346 ± 2.296 | 0.400 ± 0.009 | 0.039 ± 0.031 | 1.099 ± 0.023 |
| 9 | 600 (0) | 10 (0) | 300 (0) | 60 (0) | 144.724 ± 10.899 | 0.742 ± 0.078 | 0.433 ± 0.005 | 0.933 ± 0.226 | 191.953 ± 1.044 | 0.871 ± 0.013 | 0.656 ± 0.011 | 0.466 ± 0.028 |
| 10 | 400 (−1) | 10 (0) | 300 (0) | 40 (−1) | 103.274 ± 1.872 | 0.873 ± 0.011 | 0.442 ± 0.027 | 1.358 ± 0.967 | 136.212 ± 1.675 | 0.491 ± 0.019 | 0.441 ± 0.012 | 0.958 ± 0.044 |
| 11 | 800 (1) | 10 (0) | 300 (0) | 40 (−1) | 134.746 ± 0.718 | 0.692 ± 0.164 | 0.451 ± 0.018 | 0.879 ± 0.007 | 98.924 ± 7.168 | 0.609 ± 0.016 | 0.323 ± 0.002 | 0.659 ± 0.033 |
| 12 | 400 (−1) | 10 (0) | 300 (0) | 80 (1) | 135.748 ± 9.539 | 0.499 ± 0.063 | 0.458 ± 0.000 | 0.915 ± 0.037 | 154.739 ± 6.687 | 0.615 ± 0.205 | 0.601 ± 0.007 | 1.299 ± 0.191 |
| 13 | 800 (1) | 10 (0) | 300 (0) | 80 (1) | 144.695 ± 10.278 | 0.510 ± 0.039 | 0.469 ± 0.013 | 1.005 ± 0.035 | 155.800 ± 10.381 | 0.446 ± 0.095 | 0.486 ± 0.002 | 0.962 ± 0.048 |
| 14 | 600 (0) | 5 (−1) | 100 (−1) | 60 (0) | 126.139 ± 7.506 | 0.570 ± 0.013 | 0.416 ± 0.009 | 1.735 ± 1.252 | 248.462 ± 4.706 | 0.521 ± 0.002 | 0.544 ± 0.011 | 0.949 ± 0.055 |
| 15 | 600 (0) | 15 (1) | 100 (−1) | 60 (0) | 120.603 ± 2.890 | 0.497 ± 0.012 | 0.386 ± 0.008 | 0.710 ± 0.110 | 147.407 ± 6.897 | 0.499 ± 0.024 | 0.438 ± 0.016 | 0.876 ± 0.059 |
| 16 | 600 (0) | 5 (−1) | 500 (1) | 60 (0) | 125.021 ± 0.533 | 0.748 ± 0.045 | 0.405 ± 0.020 | 1.030 ± 0.352 | 157.989 ± 8.071 | 1.024 ± 0.370 | 0.506 ± 0.012 | 1.096 ± 0.059 |
| 17 | 600 (0) | 15 (1) | 500 (1) | 60 (0) | 120.082 ± 2.415 | 0.616 ± 0.177 | 0.409 ± 0.037 | 0.774 ± 0.093 | 154.320 ± 2.694 | 0.457 ± 0.011 | 0.464 ± 0.018 | 0.978 ± 0.032 |
| 18 | 600 (0) | 10 (0) | 300 (0) | 60 (0) | 145.619 ± 6.261 | 0.803 ± 0.012 | 0.467 ± 0.019 | 0.945 ± 0.103 | 192.185 ± 4.097 | 0.875 ± 0.269 | 0.691 ± 0.013 | 0.907 ± 0.019 |
| 19 | 400 (−1) | 10 (0) | 100 (−1) | 60 (0) | 121.011 ± 9.311 | 0.722 ± 0.119 | 0.394 ± 0.044 | 1.049 ± 0.248 | 136.310 ± 5.320 | 0.524 ± 0.018 | 0.425 ± 0.025 | 0.885 ± 0.016 |
| 20 | 800 (1) | 10 (0) | 100 (-1) | 60 (0) | 116.626 ± 2.051 | 0.522 ± 0.150 | 0.374 ± 0.006 | 0.681 ± 0.092 | 140.470 ± 1.098 | 0.759 ± 0.282 | 0.435 ± 0.002 | 0.894 ± 0.026 |
| 21 | 400 (−1) | 10 (0) | 500 (1) | 60 (0) | 137.222 ± 6.833 | 0.651 ± 0.226 | 0.422 ± 0.027 | 0.815 ± 0.081 | 164.974 ± 13.254 | 0.534 ± 0.049 | 0.563 ± 0.003 | 1.178 ± 0.041 |
| 22 | 800 (1) | 10 (0) | 500 (1) | 60 (0) | 132.621 ± 12.457 | 0.764 ± 0.032 | 0.446 ± 0.010 | 1.139 ± 0.018 | 139.479 ± 10.821 | 0.504 ± 0.028 | 0.445 ± 0.016 | 1.007 ± 0.002 |
| 23 | 600 (0) | 5 (−1) | 300 (0) | 40 (−1) | 131.107 ± 0.689 | 0.595 ± 0.039 | 0.398 ± 0.002 | 1.066 ± 0.372 | 134.462 ± 7.030 | 0.571 ± 0.068 | 0.455 ± 0.002 | 1.061 ± 0.004 |
| 24 | 600 (0) | 15 (1) | 300 (0) | 40 (−1) | 121.445 ± 4.840 | 0.822 ± 0.019 | 0.333 ± 0.024 | 0.916 ± 0.094 | 136.503 ± 0.349 | 0.495 ± 0.039 | 0.469 ± 0.045 | 0.935 ± 0.013 |
| 25 | 600 (0) | 5 (−1) | 300 (0) | 80 (1) | 150.826 ± 0.152 | 0.639 ± 0.062 | 0.460 ± 0.007 | 1.569 ± 0.572 | 163.048 ± 0.160 | 0.479 ± 0.002 | 0.574 ± 0.009 | 1.315 ± 0.028 |
| 26 | 600 (0) | 15 (1) | 300 (0) | 80 (1) | 157.523 ± 2.336 | 0.632 ± 0.006 | 0.441 ± 0.003 | 1.040 ± 0.035 | 155.496 ± 1.004 | 0.415 ± 0.048 | 0.469 ± 0.025 | 1.222 ± 0.013 |
| 27 | 600 (0) | 10 (0) | 300 (0) | 60(0) | 144.644 ± 8.239 | 0.837 ± 0.005 | 0.448 ± 0.002 | 1.098 ± 0.089 | 190.806 ± 8.900 | 0.897 ± 0.069 | 0.629 ± 0.024 | 1.233 ± 0.004 |
| Response Variable | Optimum CE Conditions | Optimum Value | ||||
| Temperature/°C | Time/min | Magnetic Stirrer Rotational Rate/min−1 | Particle Diameter/µm | Predicted | Experimental | |
| TPC | 80 | 15 | 750 | 200 | 78.170 | 79.547 ± 2.680 |
| ABTS | 0.443 | 0.431 ± 0.072 | ||||
| DPPH | 0.425 | 0.416 ± 0.014 | ||||
| FRAP | 0.777 | 0.742 ± 0.119 | ||||
| Response Variable | Optimum MWE Conditions | Optimum Value | ||||
| Microwave Power/W | Time/min | Particle Diameter/µm | Temperature/°C | Predicted | Experimental | |
| TPC | 700 | 7.5 | 300 | 80 | 151.540 | 152.405 ± 7.237 |
| ABTS | 0.659 | 0.651 ± 0.026 | ||||
| DPPH | 0.479 | 0.451 ± 0.014 | ||||
| FRAP | 1.335 | 1.424 ± 0.283 | ||||
| Response Variable | Optimum MWUE Conditions | Optimum Value | ||||
| Microwave Power/W | Time/min | Particle Diameter/µm | Temperature/°C | Predicted | Experimental | |
| TPC | 800 | 5 | 100 | 60 | 272.480 | 273.129 ± 5.339 |
| ABTS | 0.879 | 0.881 ± 0.014 | ||||
| DPPH | 0.803 | 0.778 ± 0.023 | ||||
| FRAP | 1.659 | 1.598 ± 0.308 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valinger, D.; Kušen, M.; Benković, M.; Jurina, T.; Panić, M.; Radojčić Redovniković, I.; Kljusurić, J.G.; Tušek, A.J. Enhancement of the Green Extraction of Bioactive Molecules from Olea europaea Leaves. Separations 2022, 9, 33. https://doi.org/10.3390/separations9020033
Valinger D, Kušen M, Benković M, Jurina T, Panić M, Radojčić Redovniković I, Kljusurić JG, Tušek AJ. Enhancement of the Green Extraction of Bioactive Molecules from Olea europaea Leaves. Separations. 2022; 9(2):33. https://doi.org/10.3390/separations9020033
Chicago/Turabian StyleValinger, Davor, Matea Kušen, Maja Benković, Tamara Jurina, Manuela Panić, Ivana Radojčić Redovniković, Jasenka Gajdoš Kljusurić, and Ana Jurinjak Tušek. 2022. "Enhancement of the Green Extraction of Bioactive Molecules from Olea europaea Leaves" Separations 9, no. 2: 33. https://doi.org/10.3390/separations9020033
APA StyleValinger, D., Kušen, M., Benković, M., Jurina, T., Panić, M., Radojčić Redovniković, I., Kljusurić, J. G., & Tušek, A. J. (2022). Enhancement of the Green Extraction of Bioactive Molecules from Olea europaea Leaves. Separations, 9(2), 33. https://doi.org/10.3390/separations9020033











