Fabrication of Poly(amide-co-ester) Solvent Resistant Nanofiltration Membrane from P-nitrophenol and Trimethyl Chloride via Interfacial Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
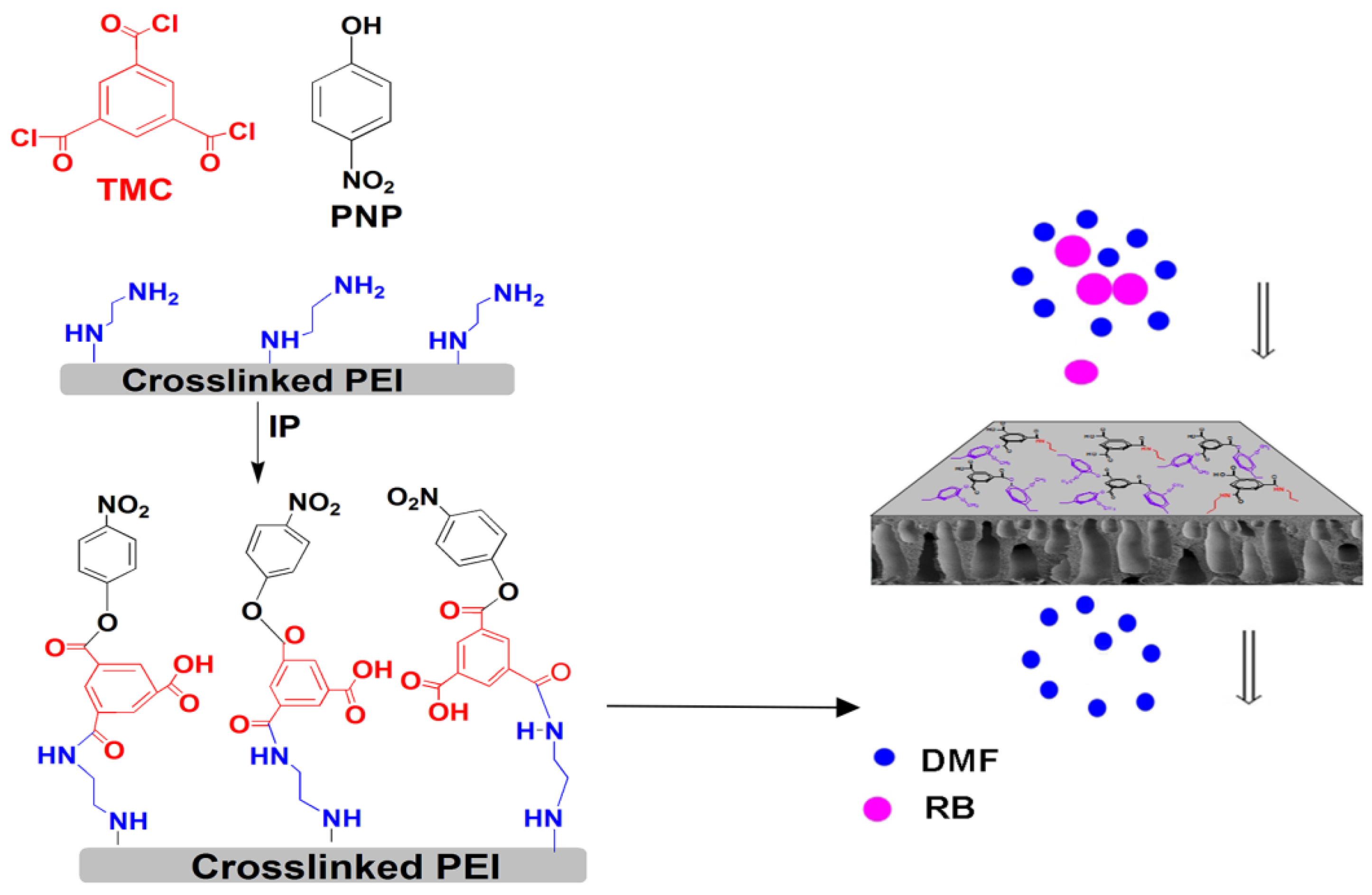
2.2. Membrane Preparation
2.2.1. Preparation PEI Support and EDA-Crosslinked PEI
2.2.2. Preparation of PNP Modified TFC Membranes
2.3. Membrane Structural Characterization
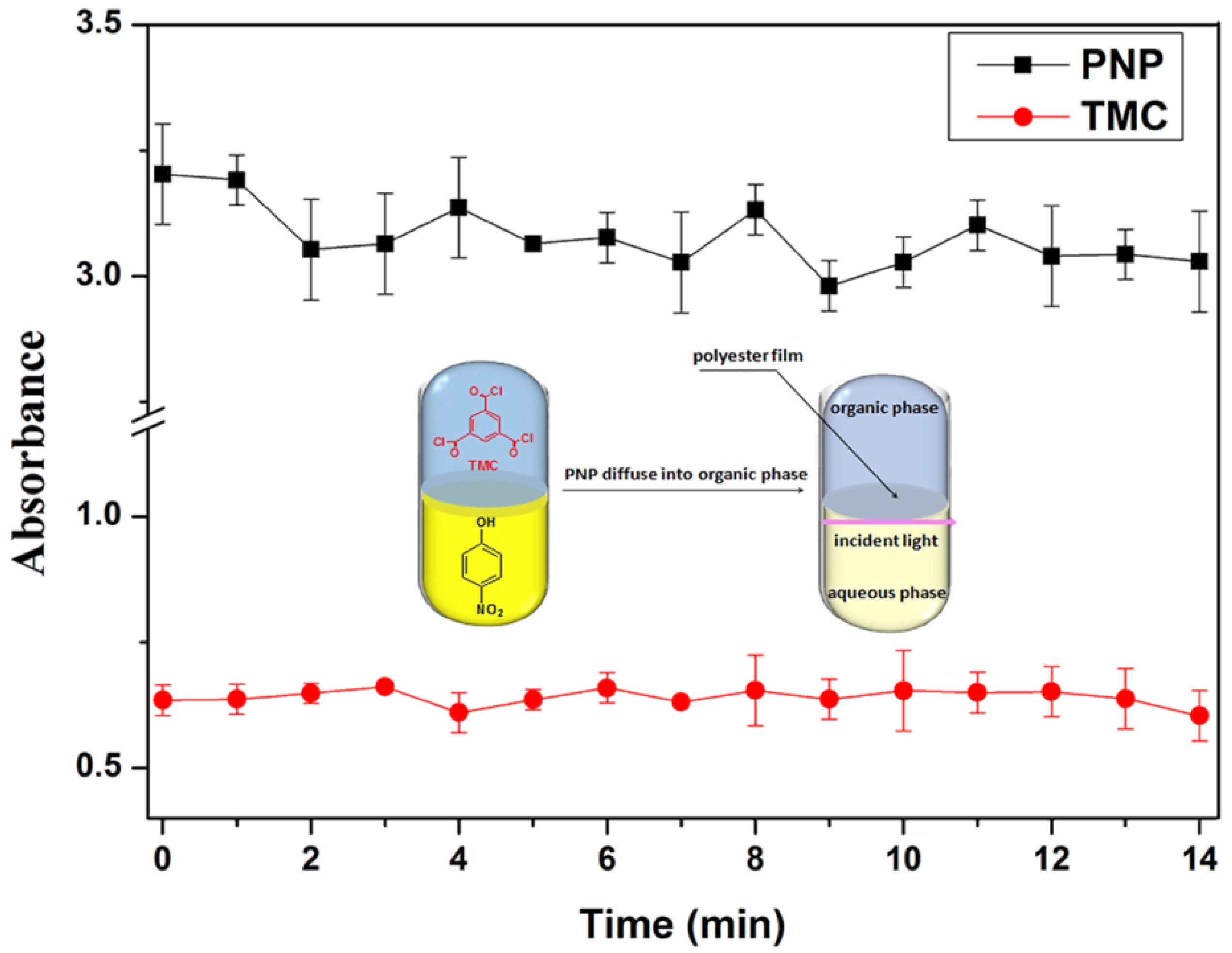
2.4. UV Characterization of PNP Consumption Rate
3. Results
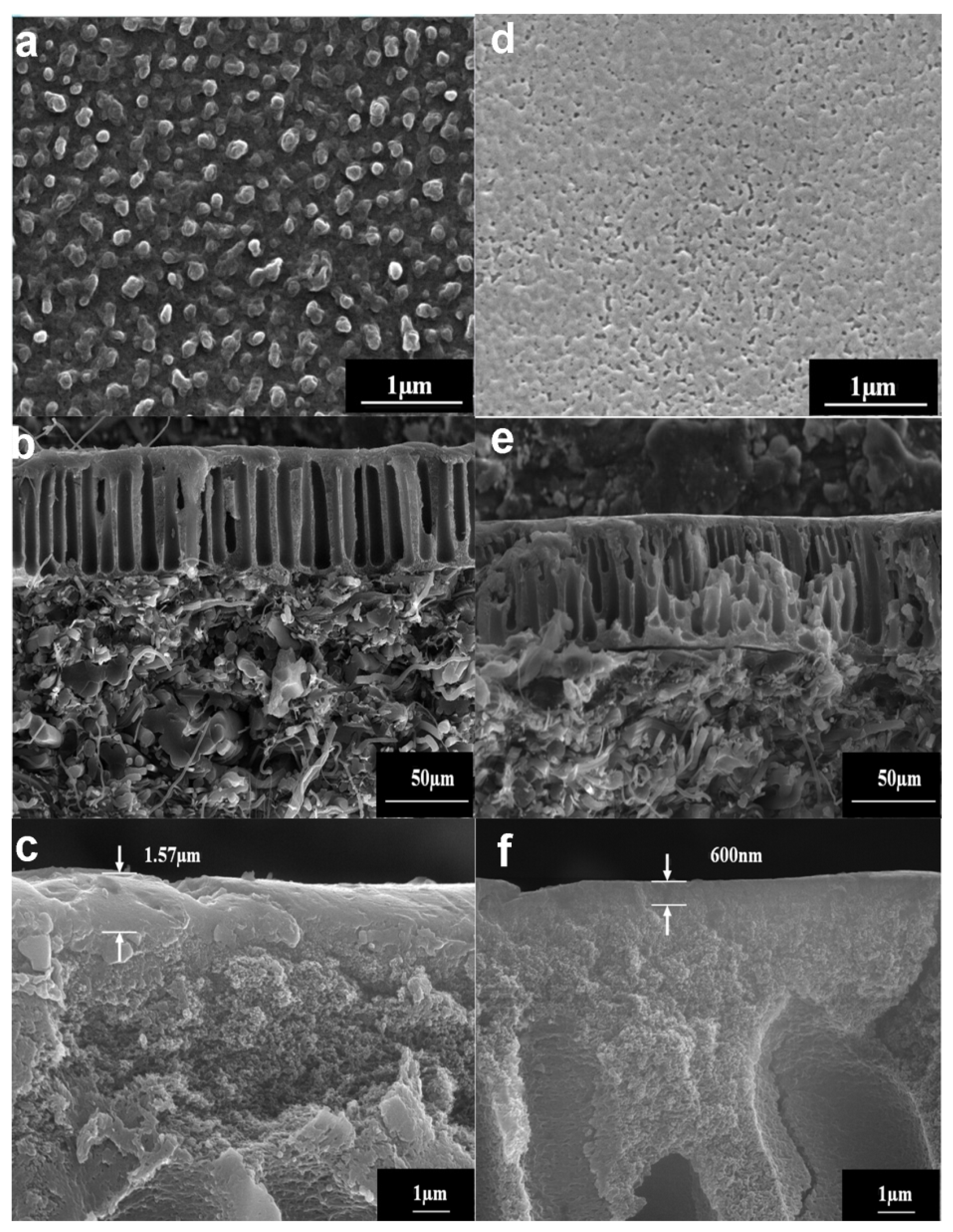
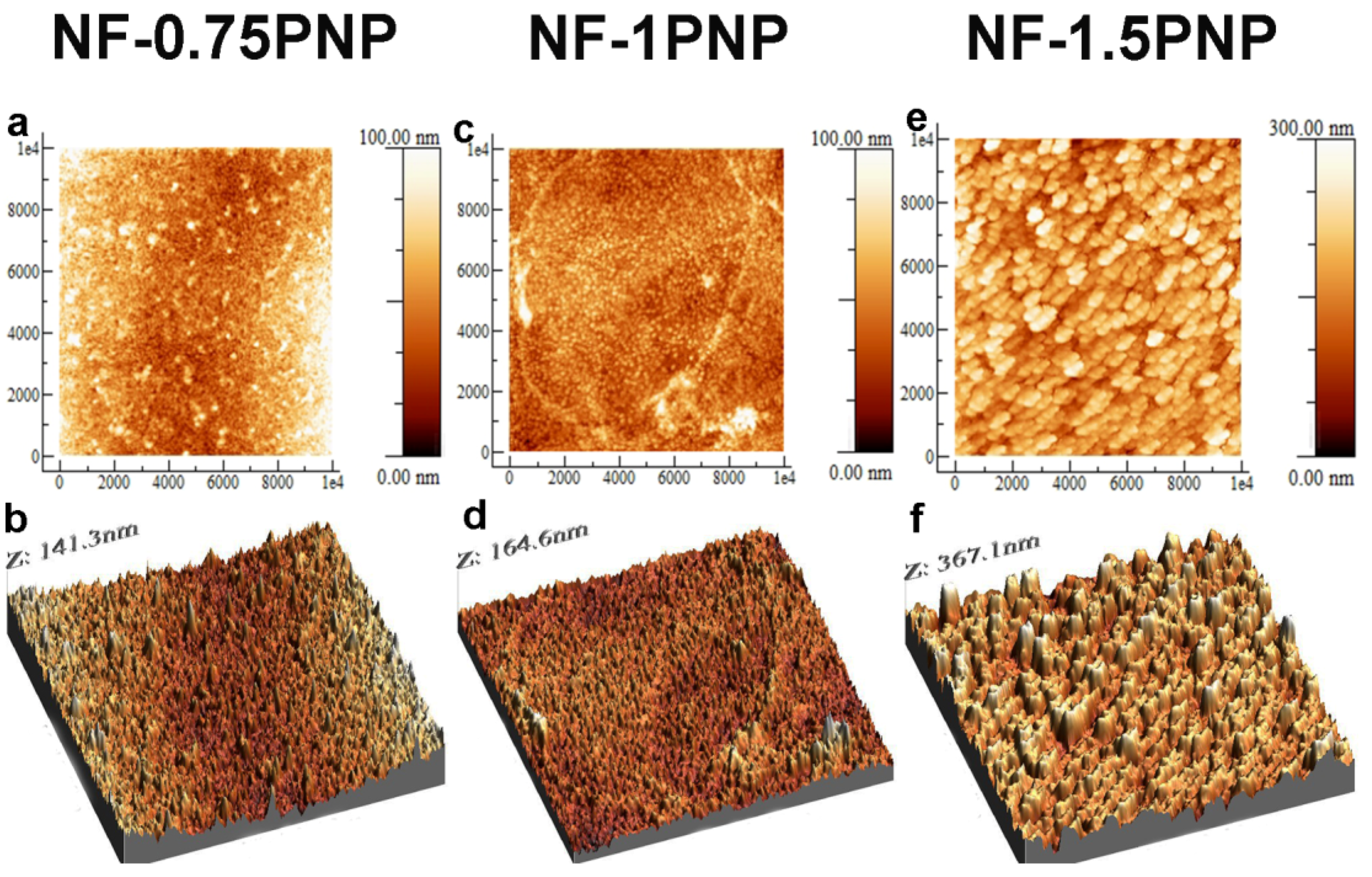
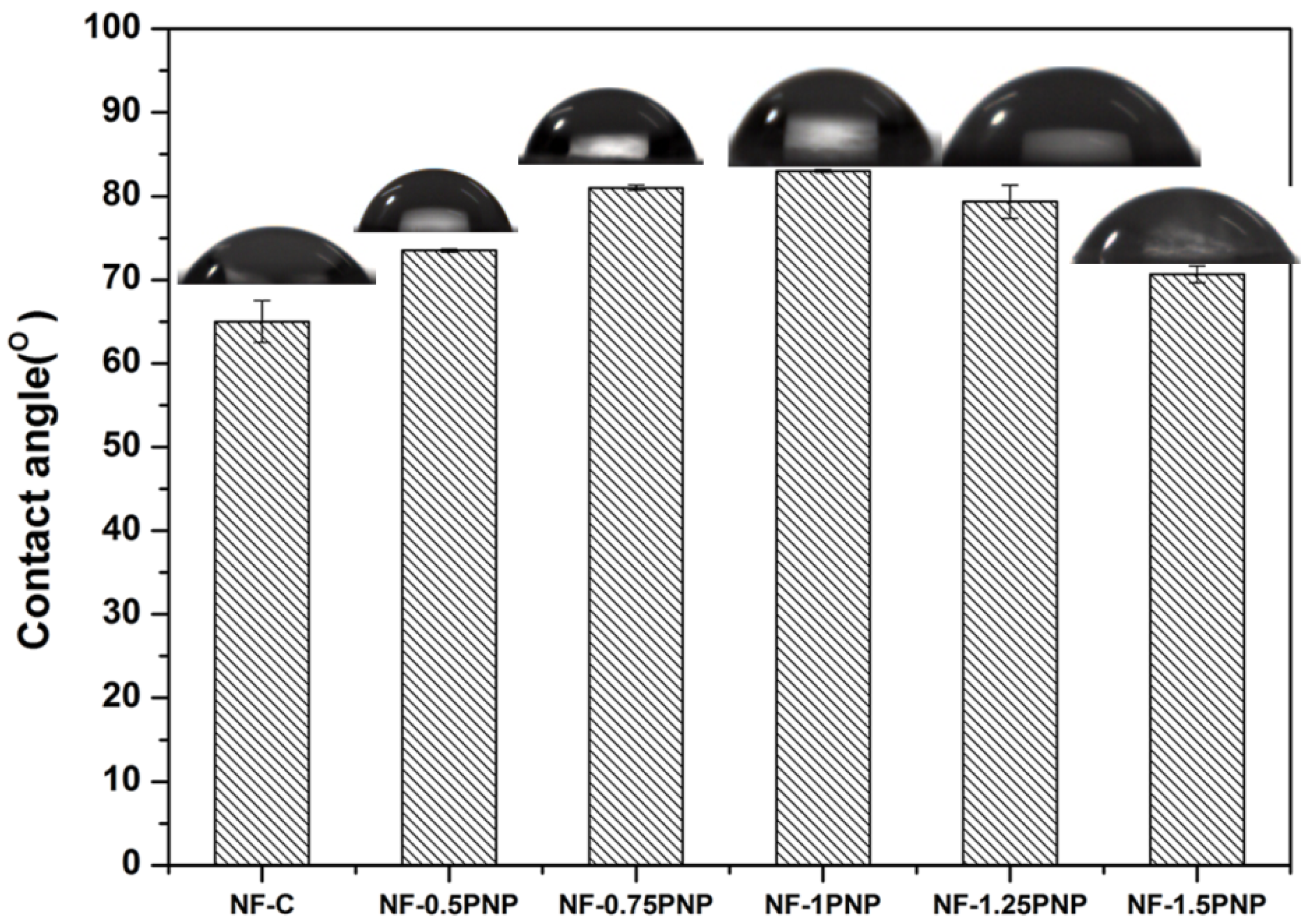
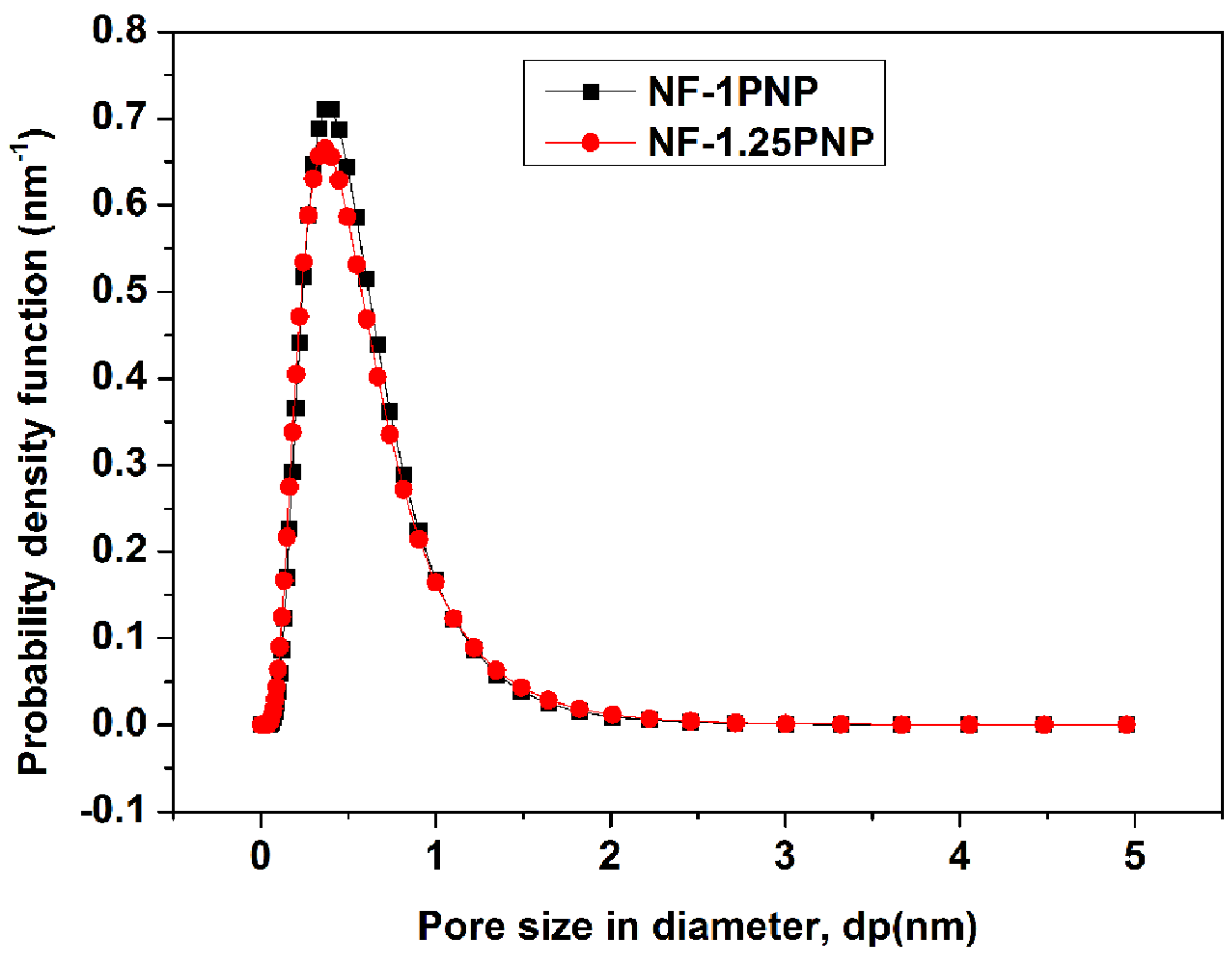
3.1. Morphologies
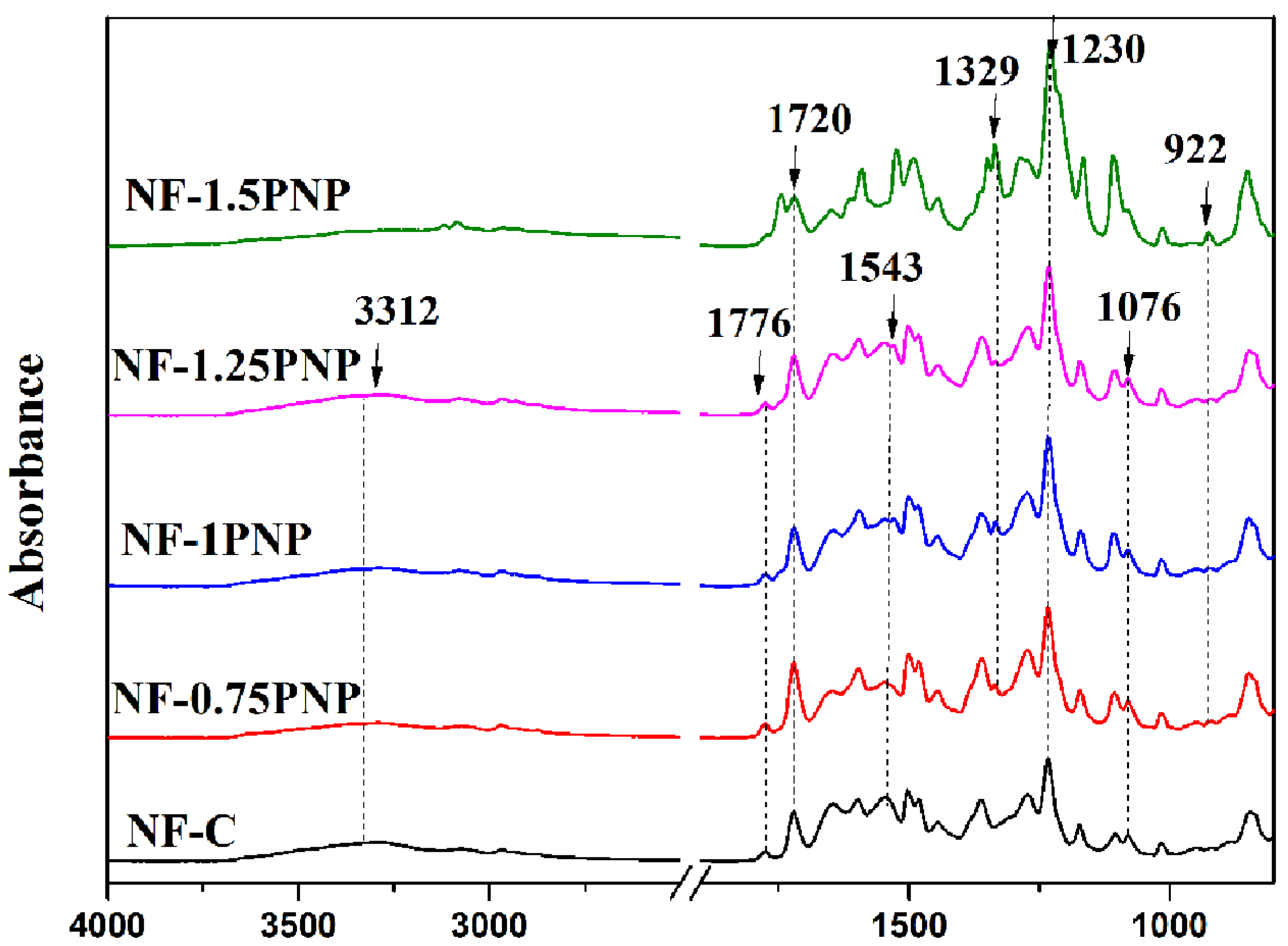
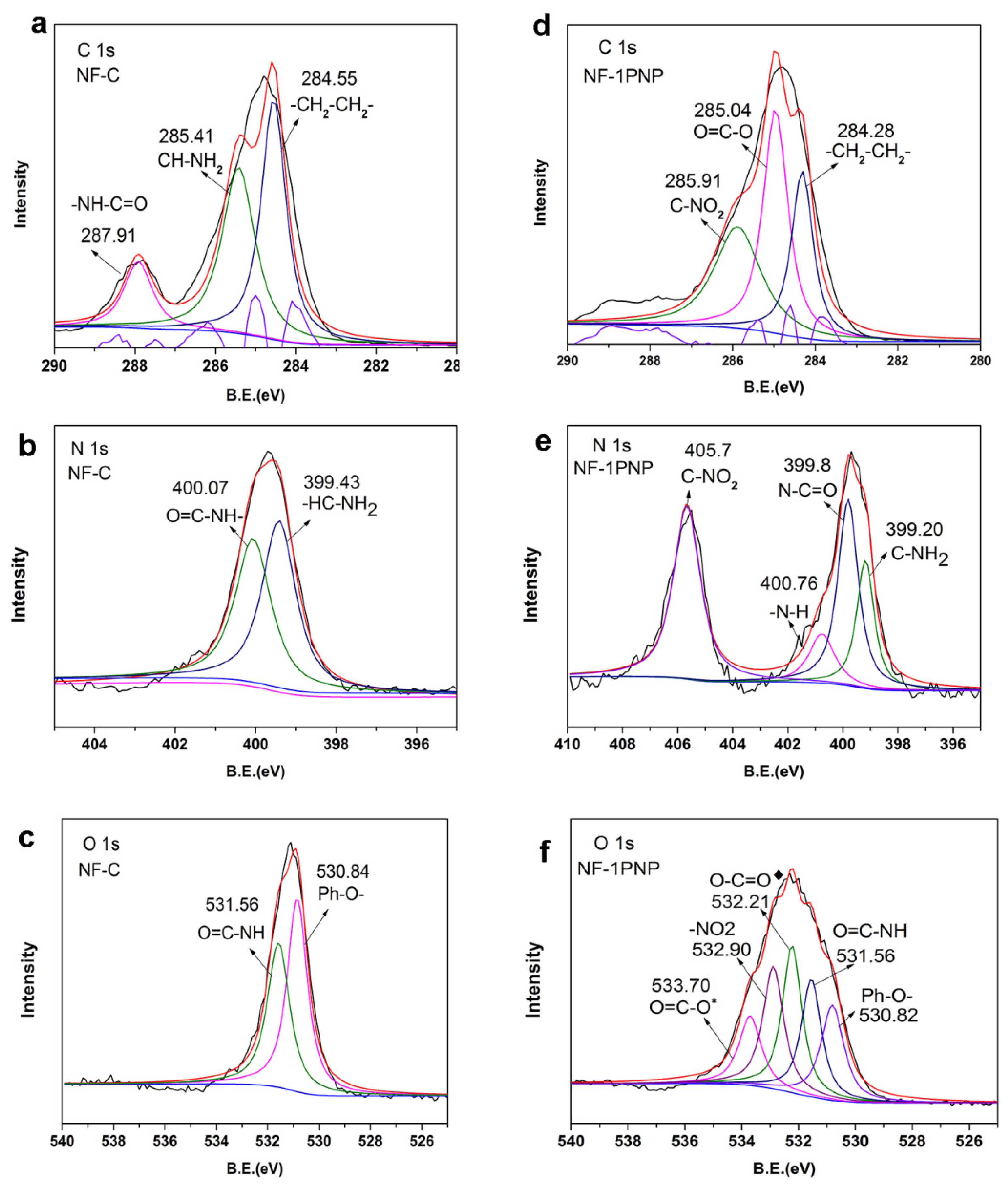
3.2. Chemical Structure Analysis
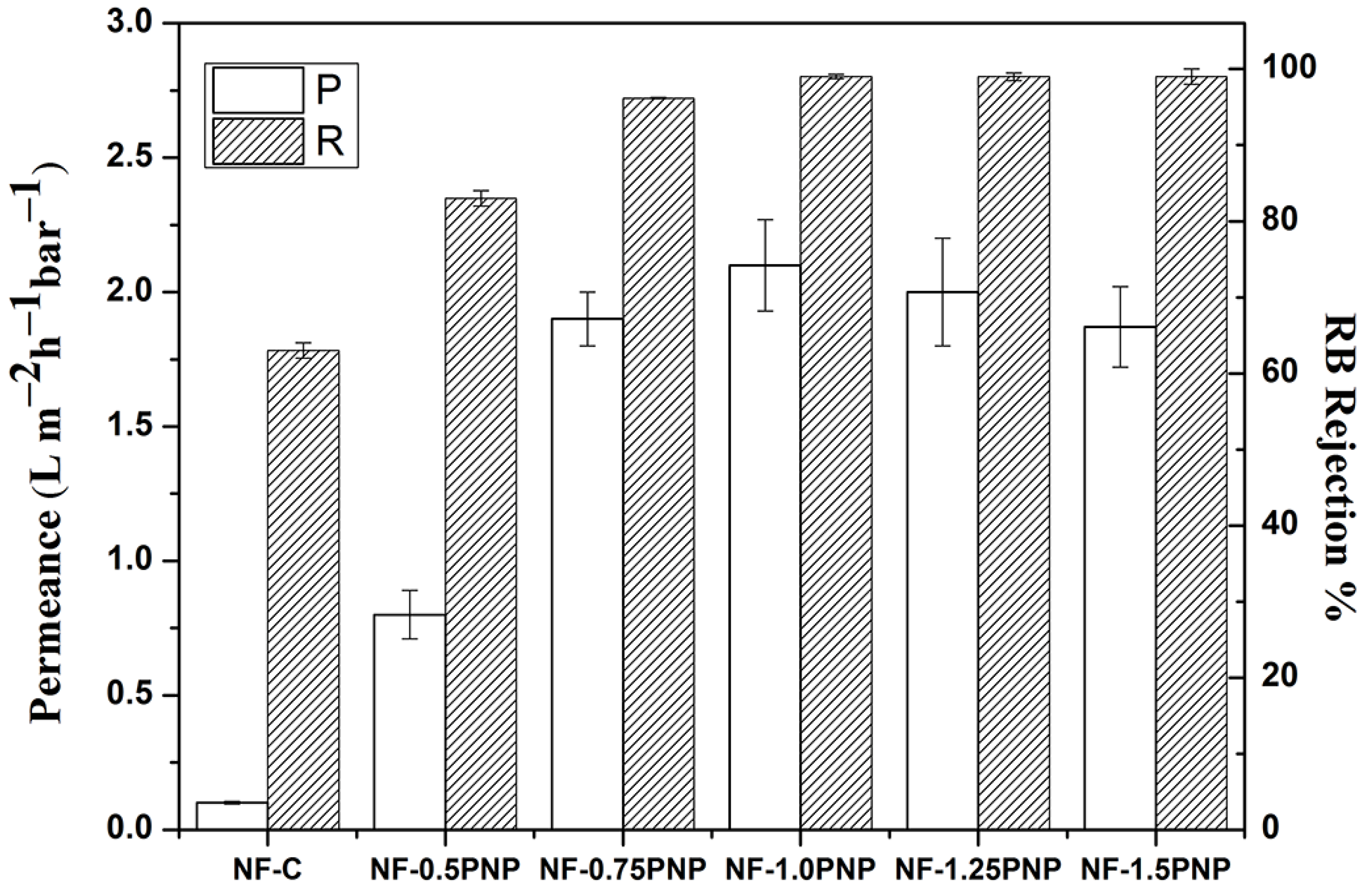
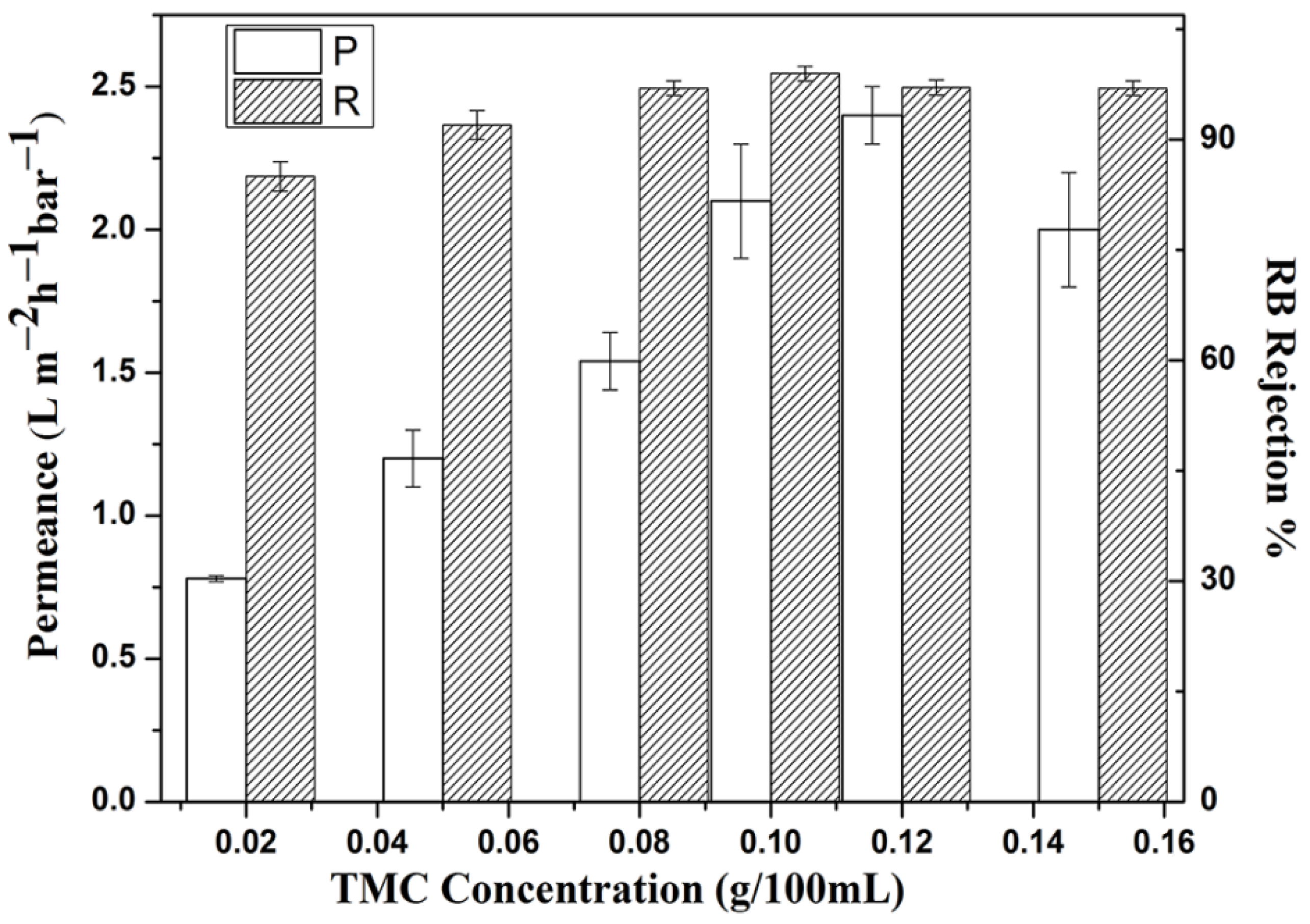
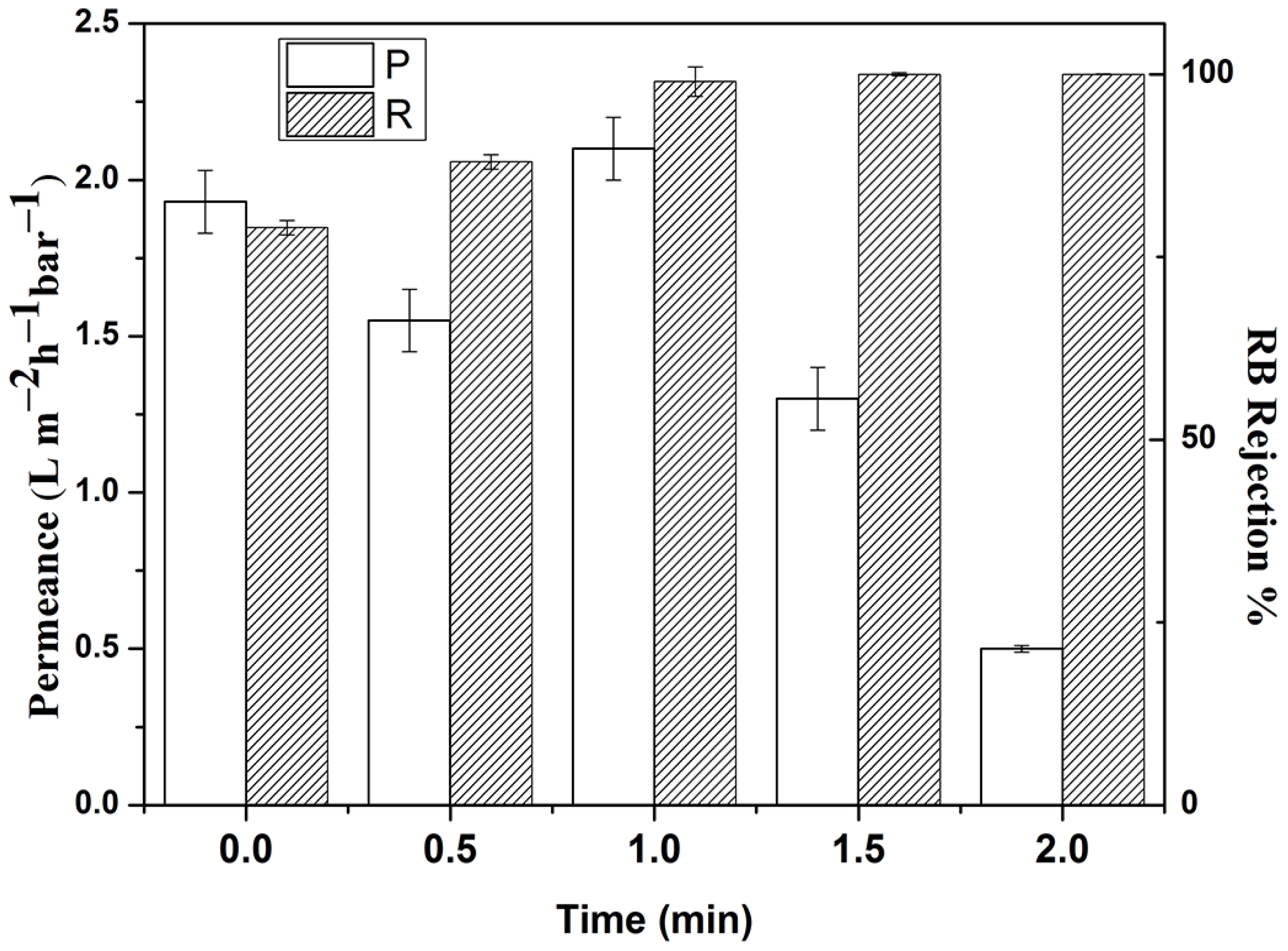
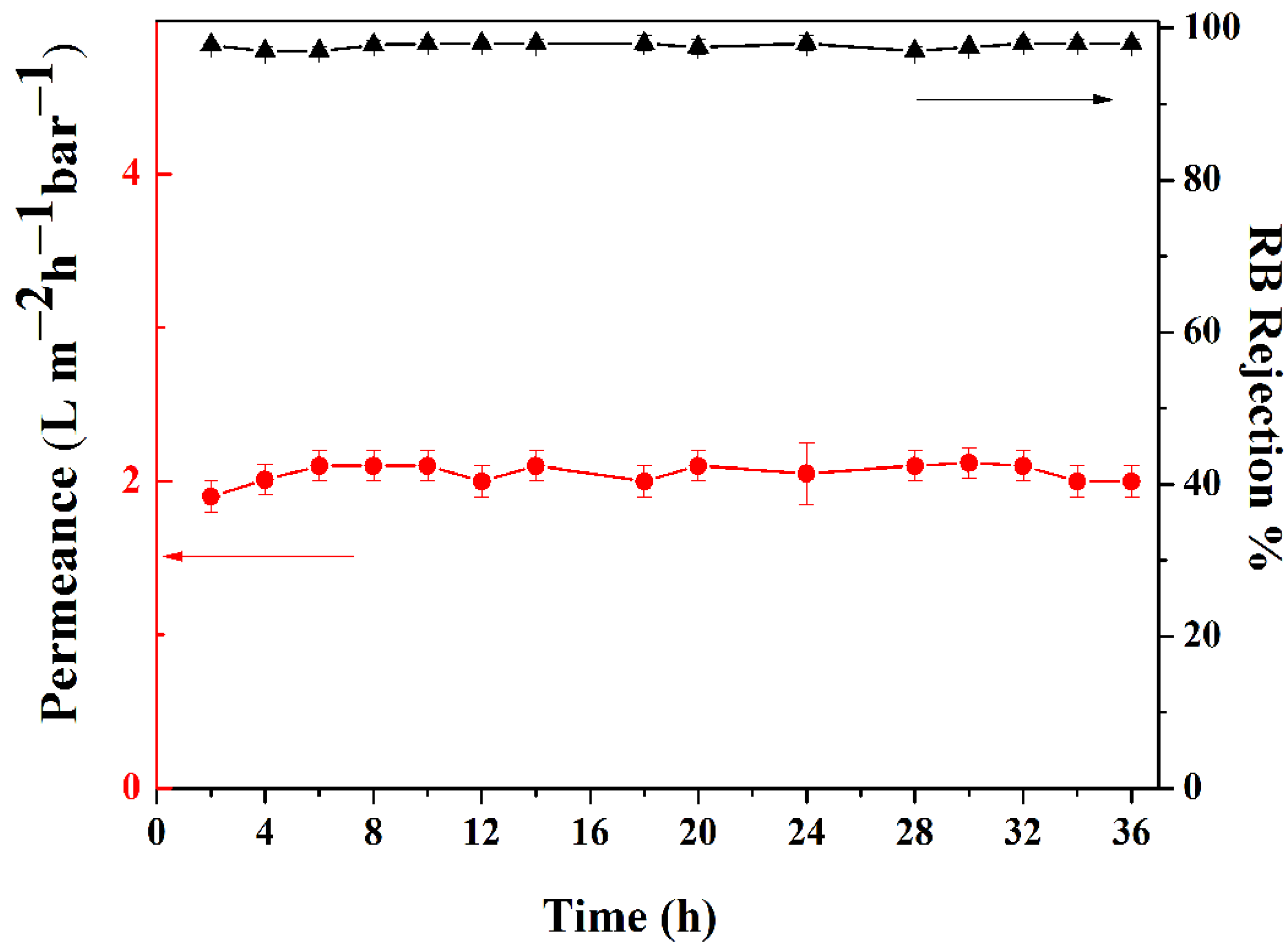
3.3. Membrane Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, G.M.; Feng, Y.; Li, B.; Tham, H.M.; Lai, J.Y.; Chung, T.S. Recent progress of organic solvent nanofiltration membranes. Prog. Polym. Sci. 2021, 12, 101470. [Google Scholar] [CrossRef]
- Scharzec, B.; Holtkötter, J.; Bianga, J.; Dreimann, J.M.; Vogt, D.; Skiborowski, M. Conceptual study of co-product separation from catalyst-rich recycle streams in thermomorphic multiphase systems by OSN. Chem. Eng. Res. Des. 2020, 157, 65–76. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Hadizade, G.; Binaeian, E.; Emami, M.R.S. Preparation and characterization of hexagonal mesoporous silica/polyacrylamide nanocomposite capsule (PAM-HMS) for dye removal from aqueous solutions. J. Mol. Liq. 2017, 238, 499–507. [Google Scholar] [CrossRef]
- Werth, K.; Kaupenjohann, P.; Skiborowski, M. The potential of organic solvent nanofiltration processes for oleochemical industry. Sep. Purif. Technol. 2017, 182, 185–196. [Google Scholar] [CrossRef]
- White, L.S.; Nitsch, A.R. Solvent recovery from lube oil filtrates with a polyimide membrane. J. Membr. Sci. 2000, 179, 267–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Q.; Liang, X.; Wang, J.; Jiang, Y.; Liu, J. High-flux, high-selectivity loose nanofiltration membrane mixed with zwitterionic functionalized silica for dye/salt separation. Appl. Surf. Sci. 2020, 515, 146005. [Google Scholar] [CrossRef]
- Feng, W.; Li, J.; Fang, C.; Zhang, L.; Zhu, L. Controllable thermal annealing of polyimide membranes for highly-precise organic solvent nanofiltration. J. Membr. Sci. 2022, 643, 120013. [Google Scholar] [CrossRef]
- Loh, X.X.; Sairam, M.; Bismarck, A.; Steinke, J.H.G.; Livingston, A.G.; Li, K. Crosslinked integrally skinned asymmetric polyaniline membranes for use in organic solvents. J. Membr. Sci. 2009, 326, 635–642. [Google Scholar] [CrossRef]
- Matthias, M.; Cédric, V.; Marloes, T.; Guy, K.; Vankelecom, I. Crosslinked PVDF-membranes for Solvent Resistant Nanofiltration. J. Membr. Sci. 2018, 566, 223–230. [Google Scholar]
- Chisca, S.; Duong, P.H.H.; Emwas, A.H.; Sougrat, R.; Nunes, S.P. Crosslinked copolyazoles with a zwitterionic structure for organic solvent resistant membranes. Polym. Chem. 2015, 6, 543–554. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Li, X.; An, Q.F. A review of nano-confined composite membranes fabricated inside the porous support. Adv. Membr. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, S.; Wang, J.; Zhang, Y.; Tian, M.B.; Bruggen, B. Microporous organic polymer-based membranes for ultrafast molecular separations. Prog. Polym. Sci. 2020, 110, 101308. [Google Scholar] [CrossRef]
- Feng, Y.; Weber, M.; Maletzko, C.; Chung, T.S. Fabrication of organic solvent nanofiltration membranes via facile bioinspired one-step modification. Chem. Eng. Sci. 2019, 198, 74–84. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, H.; Wang, J.; Ding, R.; Du, Z.; Liu, J.; Cao, S. Mineralization-inspired preparation of composite membranes with Polyethyleneimine–Nanoparticle hybrid active layer for solvent resistant nanofiltration. J. Membr. Sci. 2014, 470, 70–79. [Google Scholar] [CrossRef]
- Peyravi, M.; Jahanshahi, M.; Rahimpour, A.; Javadi, A.; Hajavi, S. Novel thin film nanocomposite membranes incorporated with functionalized TiO2 nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 2014, 241, 155–166. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Hua, D.; Chung, T.S. Cross-linked mixed matrix membranes (MMMs) consisting of amine-functionalized multi-walled carbon nanotubes and P84 polyimide for organic solvent nanofiltration (OSN) with enhanced flux. J. Membr. Sci. 2018, 548, 319–331. [Google Scholar] [CrossRef]
- Xu, S.J.; Luo, L.H.; Tong, Y.H.; Shen, Q.; Xu, Z.L.; Wu, Y.Z.; Yang, H. Organic solvent nanofiltration (OSN) membrane with polyamantadinamide active layer for reducing separation performance inconformity. Sep. Purif. Technol. 2022, 278, 119582. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Anjum, D.H.; Peinemann, K.V.; Nunes, S.P. Thin porphyrin composite membranes with enhanced organic solvent transport. J. Membr. Sci. 2018, 563, 684–693. [Google Scholar] [CrossRef]
- Huang, S.H.; Hsu, C.J.; Liaw, D.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of chemical structures of amines on physicochemical properties of active layers and dehydration of isopropanol through interfacially polymerized thin-film composite membranes. J. Membr. Sci. 2008, 307, 73–81. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Jeong, B.H.; Huang, X.; Hoek, E.M.V. Impacts of reaction and curing conditions on polyamide composite reverse osmosis membrane properties. J. Membr. Sci. 2008, 311, 34–45. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, J.; Shi, C.; Zhou, A.; Bian, C.; Li, W. Thin film composite nanofiltration membrane prepared by the interfacial polymerization of 1,2,4,5-benzene tetracarbonyl chloride on the mixed amines cross-linked poly(ether imide) support. J. Membr. Sci. 2016, 520, 19–28. [Google Scholar] [CrossRef]
- Zhou, A.; Shi, C.; He, X.; Fu, Y.; Anjum, A.W.; Zhang, J.; Li, W. Polyarylester nanofiltration membrane prepared from monomers of vanillic alcohol and trimesoyl chloride. Sep. Purif. Technol. 2018, 193, 58–68. [Google Scholar] [CrossRef]
- Zhai, Z.; Jiang, C.; Zhao, N.; Dong, W.; Li, P.; Sun, H.; Niu, Q.J. Polyarylate membrane constructed from porous organic cage for high-performance organic solvent nanofiltration. J. Membr. Sci. 2020, 595, 117505. [Google Scholar] [CrossRef]
- Zhou, A.; Almijbilee, M.M.A.; Zheng, J.; Wang, L. A thin film composite membrane prepared from monomers of vanillin and trimesoyl chloride for organic solvent nanofiltration. Sep. Purif. Technol. 2021, 263, 118394. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J.; Zhou, A.; Kong, A.; Almijbilee, M.M.A.; Zheng, X.; Zhang, J.; Li, W. Poly[acrylate-co-amide] network composite via photopolymerization for organic solvent nanofiltration separation. Sep.Purif. Technol. 2020, 246, 116855. [Google Scholar] [CrossRef]
- Park, S.H.; Yang, C.; Ayaril, N.; Szekely, G. Solvent-Resistant Thin-Film Composite Membranes from Biomass-Derived Building Blocks: Chitosan and 2,5-Furandicarboxaldehyde. ACS Sustain. Chem. Eng. 2021, 10, 998–1007. [Google Scholar] [CrossRef]
- Weng, R.; Huang, X.; Liao, D.; Xu, S.; Peng, L.; Liu, X. A novel cellulose/chitosan composite nanofiltration membrane prepared with piperazine and trimesoyl chloride by interfacial polymerization. RSC Adv. 2020, 10, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic thin film composite nanofiltration membranes derived solely from sustainable sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Li, W.; Bian, C.; Fu, C.; Zhou, A.; Shi, C.; Zhang, J. A poly(amide-co-ester) nanofiltration membrane using monomers of glucose and trimesoyl chloride. J. Membr. Sci. 2016, 504, 185–195. [Google Scholar] [CrossRef]
- Soyekwo, F.; Zhang, Q.; Gao, R.; Qu, Y.; Lin, C.; Huang, X.; Zhu, A.; Liu, Q. Cellulose nanofiber intermediary to fabricate highly-permeable ultrathin nanofiltration membranes for fast water purification. J. Membr. Sci. 2017, 524, 174–185. [Google Scholar] [CrossRef]
- Tarboush, B.J.A.; Rana, D.; Matsuura, T.; Arafat, H.A.; Narbaitz, R.M. Preparation of thin-film-composite polyamide membranes for desalination using novel hydrophilic surface modifying macromolecules. J. Membr. Sci. 2009, 325, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Sanadhya, S.G.; Oswal, S.; Parmar, K.C. Synthesis and characterization of aliphatic-aromatic polyesters using interfacial polycondensation technique. J. Chem. Pharm. Res. 2014, 6, 705–714. [Google Scholar]
- Albrecht, W.; Seifert, B.; Weigel, T.; Schossig, M.; Holländer, A.; Groth, T.; Hilke, R. Amination of Poly(ether imide) Membranes Using Di- and Multivalent Amines. Macromol. Chem. Phys. 2003, 204, 510–521. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.Y.; Venkatraman, S.S.; Ma, L.L. Low-temperature (below Tg) thermal bonding of COC microfluidic devices using UV photografted HEMA-modified substrates: High strength, stable hydrophilic, biocompatible surfaces. J. Mater. Chem. 2011, 21, 15031–15040. [Google Scholar] [CrossRef]
- Li, Y.; Cao, B.; Li, P. Effects of dope compositions on morphologies and separation performances of PMDA-ODA polyimide hollow fiber membranes in aqueous and organic solvent systems. Appl. Surf. Sci. 2019, 473, 1038–1048. [Google Scholar] [CrossRef]
- Toh, Y.H.S.; Lim, F.W.; Livingston, A.G. Polymeric membranes for nanofiltration in polar aprotic solvents. J. Membr. Sci. 2007, 301, 3–10. [Google Scholar]
- Zhang, Y.; Sun, H.; Sadam, H.; Liu, Y.; Shao, L. Supramolecular chemistry assisted construction of ultra-stable solvent-resistant membranes for angstrom-sized molecular separation. Chem. Eng. J. 2019, 371, 535–543. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Zhang, X.; Cao, B.; Li, P. Formation of Macrovoid-Free PMDA-MDA Polyimide Membranes Using a Gelation/Non-Solvent-Induced Phase Separation Method for Organic Solvent Nanofiltration. Ind. Eng. Chem. Res. 2019, 58, 6712–6720. [Google Scholar] [CrossRef]
- Valtcheva, I.B.; Kumbharkar, S.C.; Kim, J.F.; Bhole, Y.; Livingston, A.G. Beyond polyimide: Crosslinked polybenzimidazole membranes for organic solvent nanofiltration(OSN) in harsh environments. J. Membr. Sci. 2014, 457, 627. [Google Scholar] [CrossRef]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust covalently cross-linked polybenzimidazole/graphene oxide membranes for high-flux organic solvent nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 16140–16147. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Li, C.; Li, S.; Su, B.; Hu, M.Z.; Gao, X. Graphene quantum dots(GQDs)-polyethyleneimine as interlayer for the fabrication of high performanceorganic solvent nanofiltration (OSN) membranes. Chem. Eng. J. 2020, 380, 122462. [Google Scholar] [CrossRef]
| Membrane | Solvent Permeance (L m−2 h−1 bar1) | Solute | Mw of the Solute (g mol−1) | Rejection (%) | Ref. | |
|---|---|---|---|---|---|---|
| PVDF | DMF | 2.16 | Rose Bengal | 1017 | 75 | [10] |
| Crolsslinked-P84 | DMF | 1.67 | Styreneoligomer | 200 | 93 | [37] |
| PMDA-ODA | DMF | 2.50 | Rose Bengal | 1017 | 96 | [36] |
| PDA/β-CD on P84 | DMF | 6.30 | Rose Bengal | 1017 | 99 | [38] |
| PMDA-MDA | DMF | 6.10 | Rose Bengal | 1017 | 92 | [39] |
| DBX-crosslinkedPBI | DMF | 6.00 | PEG | 2000 | 95 | [40] |
| GO in PBI on PP Support | DMF | 15.00 | Mepenzolate- -Bromide | 420 | 99 | [41] |
| PEI-TMC-GQD | DMF | 1.83 | Rose Bengal | 1017 | 99 | [42] |
| NF-1PNP | DMF | 2.20 | Rose Bengal | 1017 | 98 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.; Li, L.; Li, M.; Chen, Q. Fabrication of Poly(amide-co-ester) Solvent Resistant Nanofiltration Membrane from P-nitrophenol and Trimethyl Chloride via Interfacial Polymerization. Separations 2022, 9, 28. https://doi.org/10.3390/separations9020028
Zhou A, Li L, Li M, Chen Q. Fabrication of Poly(amide-co-ester) Solvent Resistant Nanofiltration Membrane from P-nitrophenol and Trimethyl Chloride via Interfacial Polymerization. Separations. 2022; 9(2):28. https://doi.org/10.3390/separations9020028
Chicago/Turabian StyleZhou, Ayang, Lin Li, Mengying Li, and Qi Chen. 2022. "Fabrication of Poly(amide-co-ester) Solvent Resistant Nanofiltration Membrane from P-nitrophenol and Trimethyl Chloride via Interfacial Polymerization" Separations 9, no. 2: 28. https://doi.org/10.3390/separations9020028
APA StyleZhou, A., Li, L., Li, M., & Chen, Q. (2022). Fabrication of Poly(amide-co-ester) Solvent Resistant Nanofiltration Membrane from P-nitrophenol and Trimethyl Chloride via Interfacial Polymerization. Separations, 9(2), 28. https://doi.org/10.3390/separations9020028





