Abstract
This article discusses the use of a sorbent-based microextraction technique employing a capsule device to isolate amphotericin B (AMB) from human serum before analysis by high performance liquid chromatography (HPLC). AMB is a macrocyclic compound used for the treatment of invasive fungal infections. Before determining AMB in human serum by HPLC, a sample preparation step is required. Capsule phase microextraction (CPME) integrates the stirring and filtration mechanisms in a single unit, simplifying the sample preparation procedure. Moreover, it results in fast extraction kinetics and high extraction efficiency, while it has proved to be a powerful tool for bioanalysis. Different sol–gel sorbent encapsulated microextraction capsules were investigated, and sol–gel Carbowax 20 M was finally chosen as the basis for the microextraction device. Accordingly, the sample preparation protocol was investigated using a face-centered central composite design to achieve good extraction performance. The optimum protocol was validated in terms of linearity, selectivity, limit of detection (LOD), limit of quantitation (LOQ), precision, and accuracy. The linear range of the developed approach was 0.10–10.0 μg mL−1. The LOD value was 0.03 μg mL−1, and the LOQ value was 0.10 μg mL−1. Method accuracy (expressed as relative recovery) was 87–113%, while the relative standard deviation of the repeatability (sr) and within-laboratory reproducibility (sR) were <12.4%. The sol–gel sorbent encapsulated microextraction capsules were reusable for at least 10 extraction cycles. All things considered, the proposed method exhibited good overall performance, and it could be used in bioanalysis for quality control, therapeutic drug monitoring and research purposes.
1. Introduction
Amphotericin B (AMB) is a macrocyclic compound that contains both amino and carboxyl groups, and it shows significant biological activity due to its characteristic structure. In particular, AMB has an amphipathic character because of the hydroxyl groups on one side of the macrolide ring and the rigid lipophilic chain with seven conjugated double bonds on the other side [1]. This drug is widely used to treat invasive fungal infections, including coccidiosis, aspergillosis, candidiasis, and cryptococcosis [1,2]. The monitoring of the dosages and the distribution in the human body of AMB during therapy is an important parameter that can play a crucial role in the effectiveness of the treatment. Specifically, the careful monitoring of patients that undergo treatment with AMB is required since the long-term use of this drug is associated with various adverse effects, including infusion-related toxicity and nephrotoxicity [3]. Thus, the development of simple and efficient bioanalytical protocols for the monitoring of AMB is of utmost importance.
High-performance liquid chromatography is typically used for determining AMB in biological samples [1,3]. Sample preparation is considered a critical step of the analytical procedure that aims to remove potentially interfering compounds, preconcentrate the target analyte, and reduce the matrix effect [4]. The traditional sample preparation techniques used in bioanalysis include liquid–liquid extraction, solid-phase extraction, and protein precipitation [5,6]. However, these protocols are characterized by some significant disadvantages, including high consumption of samples and hazardous solvents, which contradict the principles of Green Analytical Chemistry (GAC) [7]. Depending on the required extent of analyte extraction, the required amount of sample and solvent and the extraction device’s geometry, numerous microextraction methods have been proposed and used in bioanalysis. Typical examples of them include microextraction by packed sorbent [8], disposable pipette extraction [9], solid-phase microextraction [8], liquid-phase microextraction [10], stir-bar sorptive extraction [11], fabric phase sorptive extraction [12] and capsule phase microextraction (CPME) [13].
CPME was proposed by Kabir and Furton in 2017 [14]. In CPME, extraction of the target analytes from the sample matrix is performed by using specially developed microextraction capsules. These capsules are prepared by combining two porous polypropylene capillary tubes. The first tube contains the sol–gel sorbent, and the second includes a magnetic rod. As such, the stirring and extraction mechanisms are integrated into a versatile “all-in-one” device that is handy and reusable [15]. Moreover, the filtration mechanism is also integrated in the final device because of the inherent porosity of the tubes. The integration of sample preparation steps complies with the recently proposed 10 principles of Green Sample Preparation [16].
The application of CPME in bioanalysis results in rapid and miniaturized sample preparation schemes that are characterized by good performance. This can be attributed to the high efficiency of the sol–gel sorbents that are typically used. These sorbents are either coated in cotton fibers and inserted into the polypropylene tube or prepared in situ within the lumen of the tube [17]. Until now, CPME has been used in different bioanalytical protocols for the extraction of ibuprofen from urine [13], antifungal and anthelmintic drugs from urine [18], doxorubicin and its metabolites from rat plasma [17], and the extraction of bisphenols from human breast milk [15]. In all cases, the sample preparation scheme was characterized by handling simplicity, high sample throughput, reduced consumption of organic solvents, and reduced waste generation.
In this work, CPME was used for the first time for the determination of AMB in human serum samples. The final determination of the drug was conducted by high-performance liquid chromatography–ultraviolet detection (HPLC-UV). All factors that could potentially influence the performance of the bioanalytical protocol were studied, and chemometrics were employed for the optimization of the most critical ones. Following its optimization, the entire methodology was validated and used for the monitoring of AMB in human serum samples.
2. Materials and Methods
2.1. Reagents and Solutions
AMB (>98%), nimesulide (≥98.0%), methyl trimethoxysilane, tetramethyl orthosilicate, and poly(tetrahydrofuran) were obtained from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade solvents, such as methanol (MeOH) and acetonitrile (ACN), were obtained from Honeywell (Charlotte, NC, USA). Milli-Q water was provided by a B30 purification system (Adrona SIA, Riga, Latvia).
Stock standard solutions of AMB at a concentration of 1000 μg mL−1 were prepared in DMSO. Nimesulide (used as internal standard, ISTD) solution was prepared in MeOH by dissolving 25 mg of drug API in a 25 mL volumetric flask. The above solutions were kept at 4 °C. Working standards were made in water from the stock solutions.
Cylindrical magnetic rods (1/4” × 1/16”) were obtained from K&J Magnetics Inc. (Pipersville, PA, USA). Membrane Accurel® porous membranes were obtained from 3M Inc. (St. Paul, MN, USA). Isopropanol, CH2Cl2, NH4OH and HCl were obtained from Fisher Scientific (Milwaukee, WI, USA).
The preparation of the microextraction capsules is described in detail elsewhere [13]. In brief, the general manufacturing procedure involved the following processes:
- (a)
- Preparation of the porous tubular membranes.
- (b)
- Preparation of the sol solution for the herein examined sol–gel sorbents.
- (c)
- In situ creation of a monolithic bed within the membrane.
- (d)
- Aging, conditioning, and cleaning of the obtained CPME media.
2.2. HPLC Instrumentation and Conditions
A Shimadzu 2010A HPLC-UV system (Kyoto, Japan) equipped with a high-pressure quaternary gradient pump, a column compartment, an UV detector, and an autosampler was utilized. LC Solutions software (vs. 1.25 SP4) was used. Separation of AMB and the ISTD was achieved using a Poroshell 120 EC-C18 column (50 × 4.6 mm, 2.7 μm) from Agilent Technologies (Santa Clara, CA, USA). The target analyte was separated from the ISTD and the matrix interferences in gradient mode using citric acid 22 mM (pH 4.2 adjusted with 1 M NaOH solution): MeOH 60:40 v/v (A) and citric acid 22 mM (pH 4.2 adjusted with 1 M NaOH solution): MeOH 20:80 v/v (B). The initial mobile phase composition was 50:50, A/B v/v. The composition was changed to 0:100 A/B v/v at 3 min, and it was kept constant until 5 min. Finally, it changed back to the initial parameters (i.e., 50:50, A/B v/v) at 5.5 min, and the system was equilibrated until 10 min. A flow rate of 0.6 mL min−1 and a column temperature of 30 °C were used. The drugs were monitored at 380 nm, and the injection volume was 10 μL.
2.3. CPME Procedure
The collection of the samples was performed by healthy volunteers that did not take any AMB medication. All volunteers provided their written consent after being fully informed of the procedures followed in these experiments. Serum samples were stored at −20 °C. Prior to the CPME procedure, 25 μL of each ISTD solution, 25 μL of analytes working solution (or Milli-Q water in the case of blank samples) and 200 μL of water were added to 250 µL of serum sample. No filtration or centrifugation steps were required.
For the activation of the sol–gel sorbent, the microextraction capsule was immersed in 2 mL of MeOH for 5 min, followed by immersion in 2 mL of water. Accordingly, the capsule was immersed in the sample solution (500 μL) for the adsorption of the analyte. AMB was extracted within 25 min under stirring at 500 rpm. After this step, the capsule was rinsed with H2O and dried with a lint-free tissue. For the elution of AMB, the capsule was placed in an Eppendorf tube, and 250 μL of MeOH was added. The tube was vortexed for 10 s, and elution was completed after 5 min. After each extraction, the capsule was washed with the 2 mL of MeOH used in the activation step, left to dry, and then stored.
2.4. Multivariate Optimization
Response surface methodology associated with the central composite design was employed to highlight the optimized conditions for the sample volume, extraction time and stirring rate parameters. This procedure has been frequently used for the optimization of microextraction techniques, and it combines two-level full or fractional factorial designs with additional axial or star points and at least one point at the center of the experimental region being studied [19,20]. TIBCO Statistica software v. 13.3.0 (TIBCO Software Inc., Palo Alto, CA, USA) was employed for the experimental designs.
2.5. Method Validation
The proposed bioanalytical method was validated according to the Food and Drug Administration (FDA) as per selectivity, linearity, precision, accuracy, limit of detection (LOD) and limit of quantitation (LOQ) [21]. The selectivity was assessed by analyzing different drug-free and spiked serum samples in order to demonstrate the lack of interference from endogenous compounds. The linearity of the method was determined by plotting the ratio of the peak area of the analyte to ISTD against the nominal concentrations of matrix-matched calibration standards. The experimental data were fitted to linear regression analysis without a weighting factor. The precision and accuracy were investigated at three concentration levels on the same working day and on three consecutive days. The studied concentration levels represented the lower limit of quantitation (LLOQ), a medium quality control (MQC) level, and a high quality control (HQC) level. The acceptance criteria for the precision is that the% RSD should be less than 15% and 20% for QC levels and LLOQ, respectively. In terms of accuracy, the% relative recoveries (% RR) should be 85–115% and 80–120% for the QC and LLOQ levels, respectively. The LOD and LOQ were estimated using the S/N = 3 and S/N = 10 criteria, respectively. The AMB was considered stable for the stability test if the% RSD of the replicates was not more than 15% and the RR (%) ranged between 85–115%.
3. Results and Discussion
3.1. Optimization of CPME Parameters
The type of CPME sorbent, sample pH, elution solvent and its volume, elution time and salt concentration were investigated using the one-factor-at-a-time (OFAT) approach to achieve optimal extraction efficiency. For this purpose, each parameter was individually examined, and its optimum value was found and used for further experiments. The initial sample preparation conditions were the following, sample volume: 1 mL, type of eluent: MeOH, eluent volume: 1 mL, extraction time: 30 min, stirring rate: 400 rpm. Following the examination of these factors, response surface methodology was used during the optimization of sample volume, extraction time and stirring rate. A standard solution containing AMB at a concentration of 1.0 μg mL−1 was used throughout the optimization study. For each experiment, the extraction recovery (%ER) of AMB was calculated.
3.1.1. Study of CPME Sorbent and Sample pH
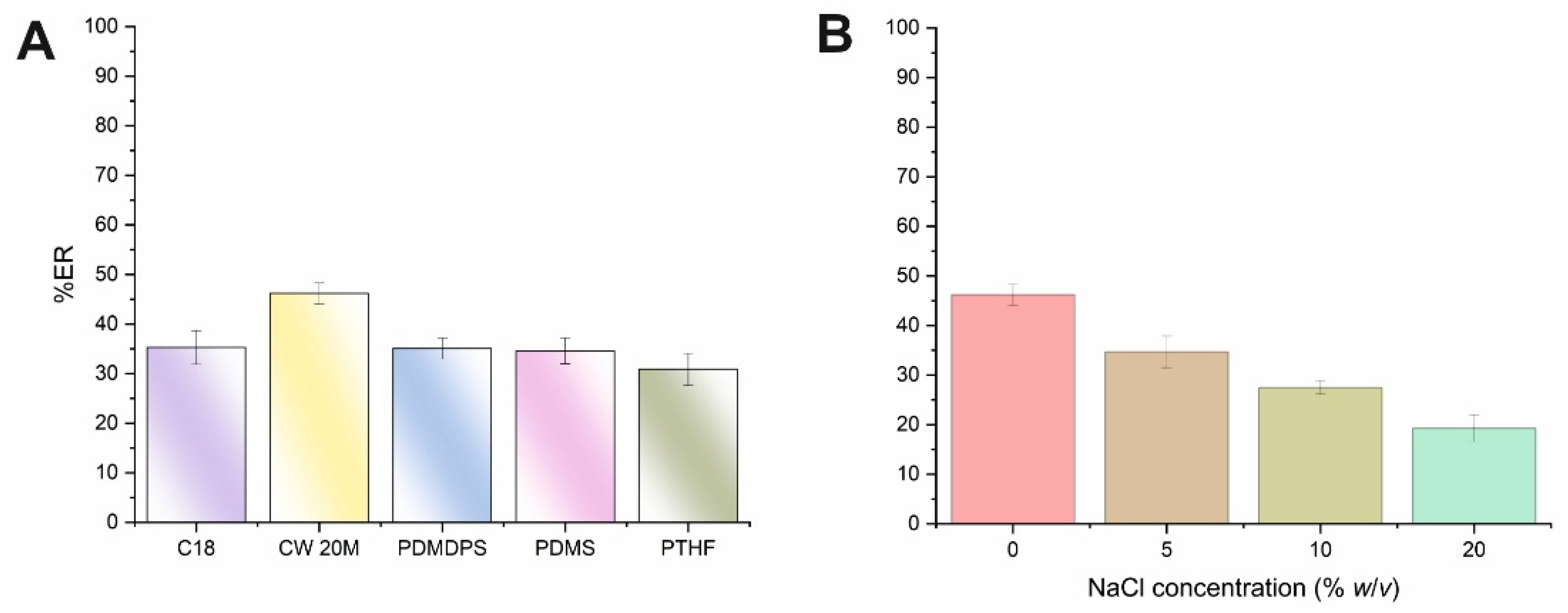
The initial parameter that was optimized was the type of monolithic CPME sorbent using microextraction capsules of 1 cm in length, since the volume of biological fluid is rather limited. On this basis, five different types of microextraction capsules with different polarities were examined (see supplementary material). AMB is a macrocyclic lactone that contains a carboxylic acid group (pKa 5.7) and a basic mycosamine sugar (pKa 10) [22]. The above monolithic sorbents were evaluated by using a standard aqueous solution of AMB at pH 7. As can be seen in Figure 1A, moderate extraction recoveries were obtained for AMB for the CPME tested. However, the polar monolithic-based sorbent CW 20M provided higher extraction efficiencies compared to the medium polarity PTHF and the non-polar sorbents (i.e., C18, PDMDPS, PDMS), and thus this membrane was selected for further experiments. Based on these findings, the predominant interactions between the analyte and the polyethylene glycol sorbent may be attributed to hydrogen bonding. Moreover, at neutral pH media, weak hydrophilic and physical adsorption between AMB and the substantiate portion of the monolithic silica backbone can take place.
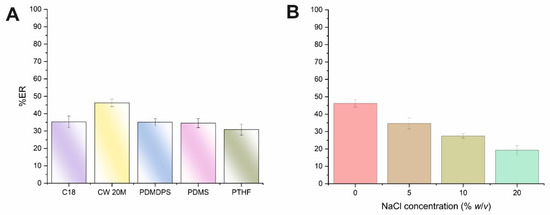
Figure 1.
Effect of the (A) CPME sorbent type and (B) ionic strength (expressed as NaCl concentration,% w/v) on the ER% of AMB.
Another parameter included the study of the%ER of the analyte at two pH values (i.e., 3 and 7). Almost similar extraction efficiency was recorded at both studied pH values indicating that the protonated form of AMB did not significantly affect its interactions with the CW 20M sorbent. Taking into consideration the above-mentioned issue and sample handling simplicity, the neutral pH value (unadjusted pH) was selected and adopted for further experiments.
3.1.2. Study of Ionic Strength
The addition of salt can decrease the solubility of compounds with intermediate polarity, resulting in their enhanced transference to the sorbent. This phenomenon is known as the salting-out effect. However, the addition of salt can also result in an enhancement of the solution’s viscosity, and thus it can diminish the salting-out effect and reduce the mass transfer. The effect of the salt addition on the ER% of AMB was assessed under variable concentrations of NaCl (i.e., 0–20% w/v). As shown in Figure 1B, the ER% values of AMB were progressively decreased by enhancing sample ionic strength. As a result, no addition of salt was chosen for the CPME protocol.
3.1.3. Study of Sample Volume, Extraction Time, and Stirring Rate
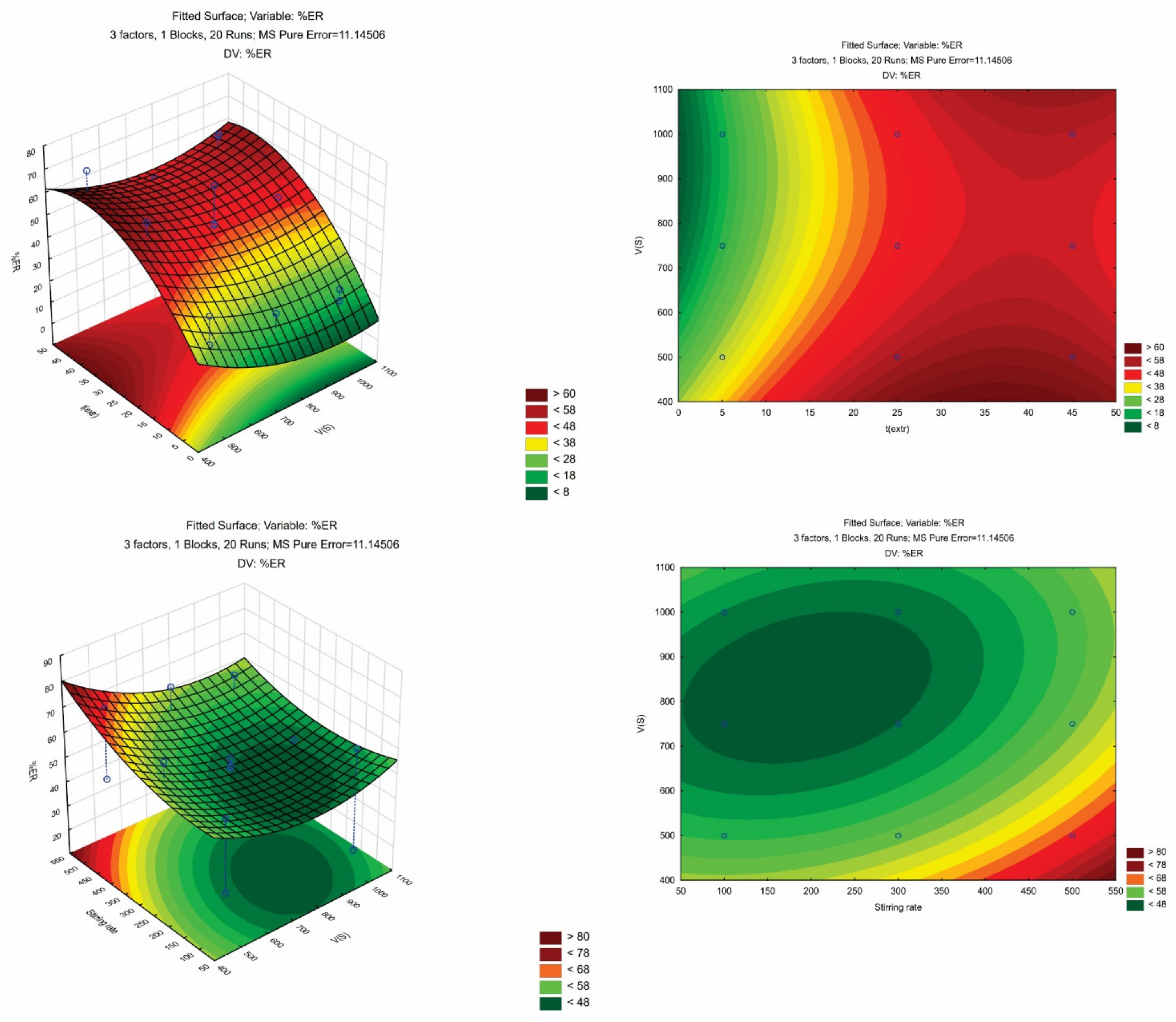
The next step included the optimization of the three parameters, namely, sample volume (Vs), extraction time (text.) and stirring rate using response surface methodology through the face-centered central composite design (FC-CCD). For the experimental design, a set of 20 experiments were carried out, and they included six center point runs. In this set of experiments, the pH and the ionic strength of the sample were 7 and 0% w/v, respectively. Table 1 shows the factorial design points. The CPME experiments were randomized, aiming to eliminate any potential systematic errors. A fitted, second-order polynomial quadratic model was constructed using multivariate regression analysis.

Table 1.
Experimental FC-CCD domain for the optimization of CPME parameters.
In Table S1, the most important effects and interactions are presented as they were evaluated by ANOVA. The calculated models’ R2 were >0.9225 showing that the predicted models explain the responses adequately. The p-value of the “lack-of-fit” (LOF) was non-significant since it was higher than 0.05. Figure S1 shows the diagnostic plots (i.e., the plot of residuals against the predicted values and the normal probability plot of residuals). A good correlation between the actual and the predicted responses can be observed, since all data were monotonously dispersed around the line. The contour plots of AMB and a set of response surfaces are depicted in Figure 2.
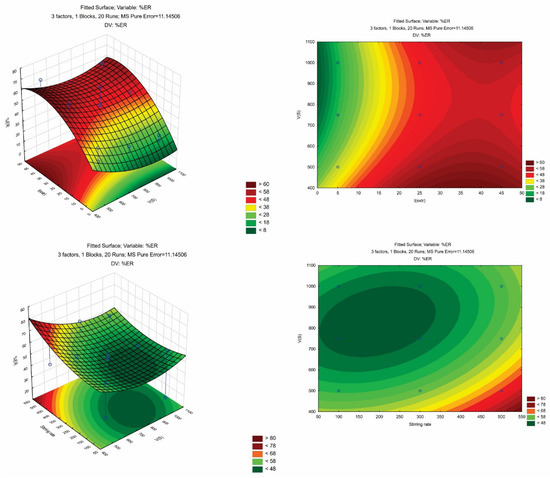
Figure 2.
3D and contour plots illustrating the effects of VS, text., and stirring rate on the%ER of AMB.
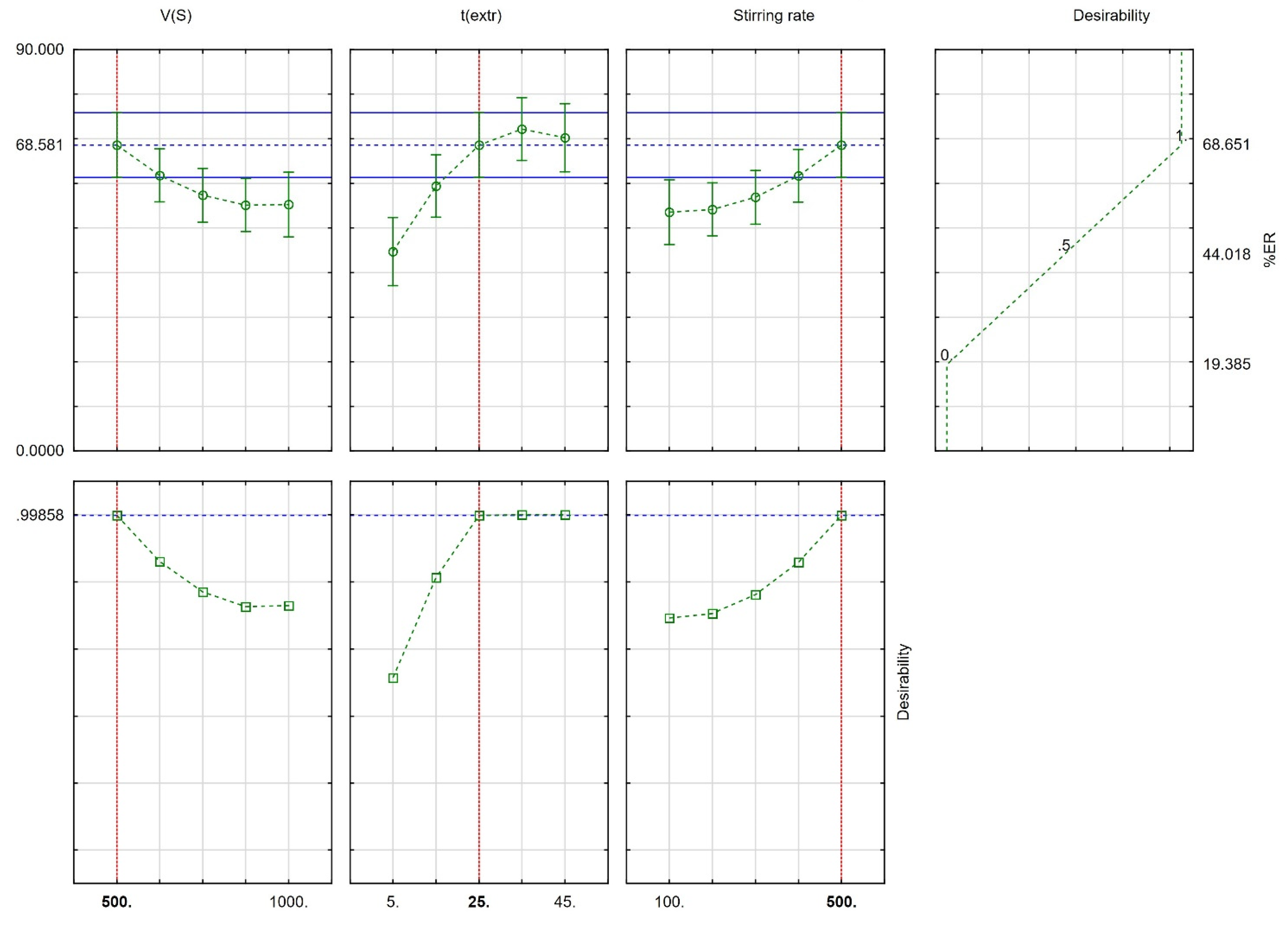
Derringer’s desirability function (D) was used (scaled between 0 to 1) as a fully desirable response, aiming to find the optimal CPME conditions. The desirability graphs and the prediction profiles are shown in Figure 3. The optimum conditions were 500 μL for Vs, 25 min for text., and 500 rpm for the stirring rate. Finally, six repetitive extractions were carried out under optimum conditions for their confirmation. The differences between the predicted values and the obtained values from the set of experiments were <7%.
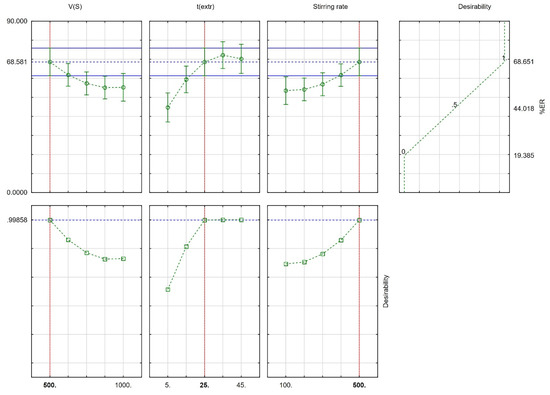
Figure 3.
Profiles for predicted values and desirability function (red-colored lines designate the optimum value).
3.1.4. Study of the Elution Solvent, Volume and Elution Time
To find the optimum desorption conditions of AMB from the CPME device different parameters, including the kind of elution solvent, its volume, and the elution time were examined. The goal of this set of experiments was to provide the highest possible desorption efficiency and to avoid any undesirable carry-over phenomena.
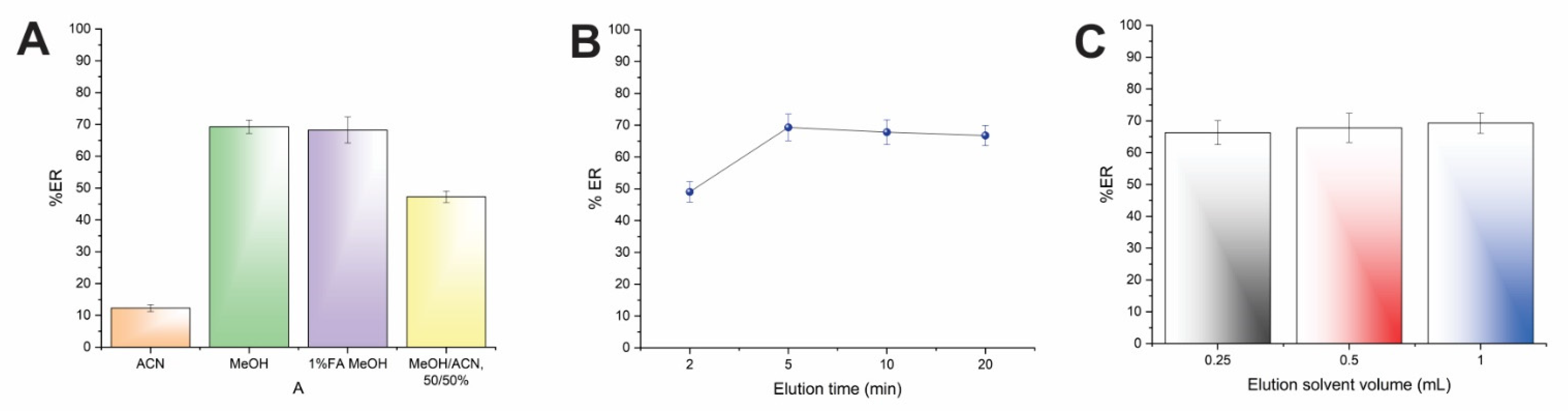
Four eluents (i.e., MeOH, ACN, MeOH: ACN, 50:50 v/v, and 1% v/v formic acid in MeOH) with different polarities were investigated, and the results of the study are illustrated in Figure 4A. As can be seen, the elution efficiency was limited when ACN was utilized, while the usage of MeOH resulted in the highest recoveries. The 1% formic acid in MeOH did not improve the desorption performance of AMB in comparison with MeOH. Thus, MeOH was chosen as eluent. It has to be highlighted that the selection of MeOH complies with the Pfizer solvent selection guide, taking into consideration solvent toxicity [23].
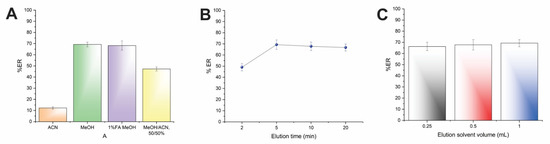
Figure 4.
Effect of the (A) elution solvent type, (B) elution time, and (C) elution solvent volume on the%ER of AMB.
Subsequently, the desorption time of the CPME procedure was studied between 2 and 20 min to guarantee sufficient contact time between MeOH and AMB. Generally, the desorption time must be as low as possible to obtain rapid protocols with high sample throughput. As shown in Figure 4B, the complete elution of AMB was achieved within 5 min. Therefore, this time span was used in further experiments.
As the last step, the volume of MeOH was studied to obtain quantitative elution of AMB, as well as reduced solvent consumption in accordance with the principles of GAC [15]. Different volumes between 250–1000 μL were investigated. Figure 4C shows that efficient desorption of AMB can be achieved by 250 μL of MeOH. Thus, an organic solvent volume of 250 μL was chosen.
3.2. Method Validation
The proposed CPME-HPLC-UV protocol for the determination of AMB in human serum matrixes was validated in terms of linearity, selectivity, LOD, LOQ, precision and accuracy.
Human serum samples spiked with AMB at seven different concentration levels, between 0.10 to 10.0 μg mL−1, were prepared. All samples were subjected to CPME-HPLC-UV analysis, and the peak area of AMB and ISTD were recorded. The unweighted regression equation was y = 0.5005x − 0.0127. The r2 value was greater than 0.9965, showing good linearity within the examined range. The LLOQ was set at 0.10 μg mL−1, and the LOD was estimated to be 0.03 μg mL−1, based on the S/N = 10 criterion and S/N = 3 criterion, respectively.
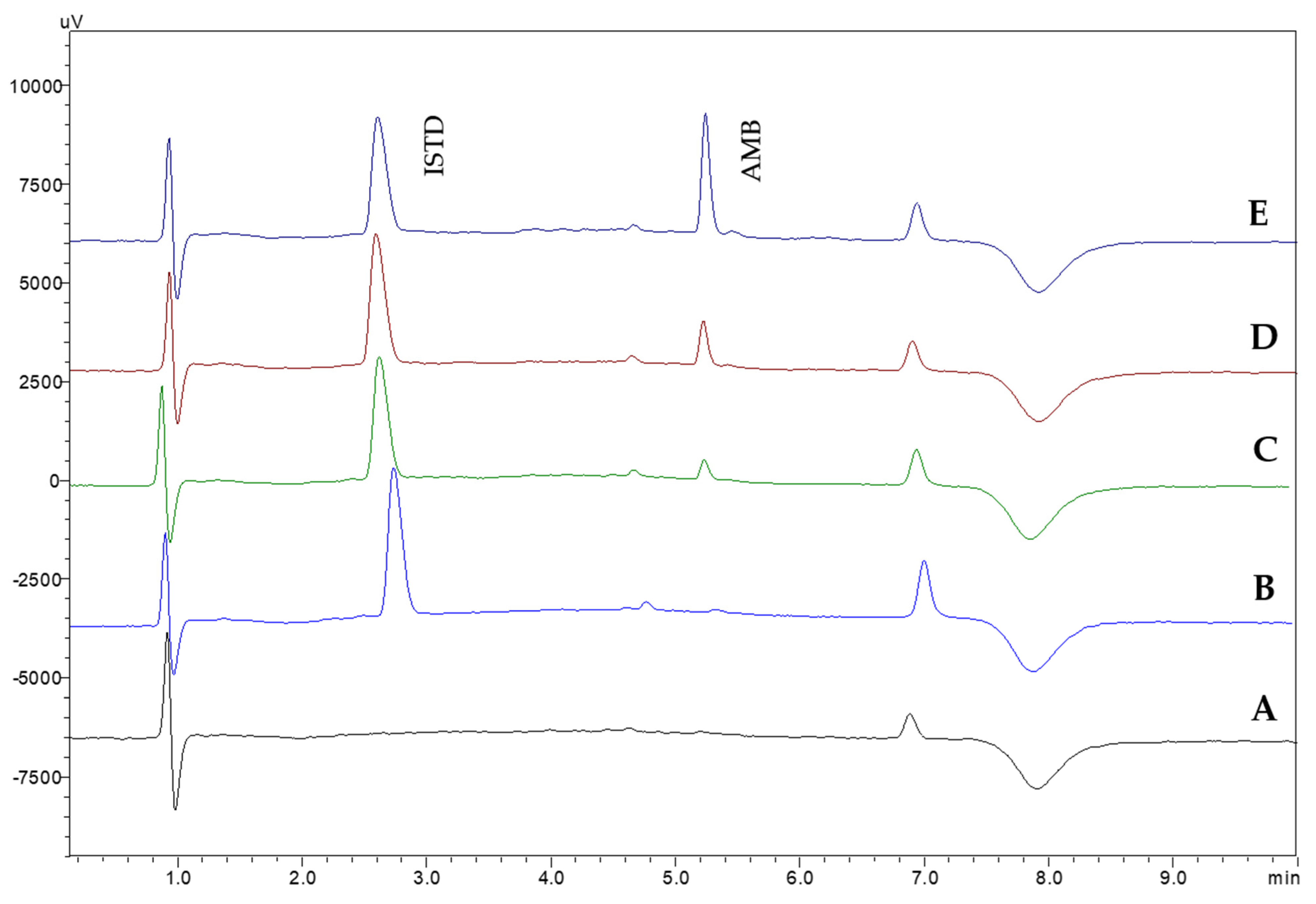
Method selectivity was investigated by analyzing six different blank human serum samples. The absence of interfering peaks on the retention time of AMB and the ISTD demonstrates the selectivity of the proposed CPME-HPLC-UV method. In Figure 5, representative chromatograms from the analysis of blank and spiked samples are shown.
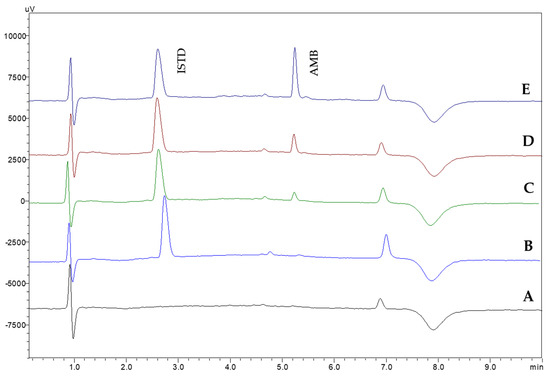
Figure 5.
Representative chromatograms of the analysis of (A) blank serum and spiked with (B) ISTD, (C) ISTD and AMB (0.25 μg mL−1), (D) ISTD and AMB (0.50 μg mL−1), and (E) ISTD and AMB (1.0 μg mL−1) using the proposed CPME-HPLC-UV method. c(ISTD): 20 μg mL−1.
The intra-day method repeatability and the inter-day method accuracy and precision were examined by performing five individual extractions (n = 5) on the same day and by performing three extractions daily for three consecutive days (n = 3 × 4) at three different concentration levels (i.e., 0.1, 1.0 and 10.0 μg mL−1). The RSD values were between 4.8–12.4% for intra-day repeatability and between 3.2–9.7% for inter-day precision.
Finally, the method’s intra-day and inter-day accuracy were expressed as relative recovery%, and they were evaluated by performing the different extractions that were mentioned above. All relative recoveries were acceptable, ranging between 87.0 and 113.0%, demonstrating good method accuracy.
3.3. Microextraction Capsule Reusability
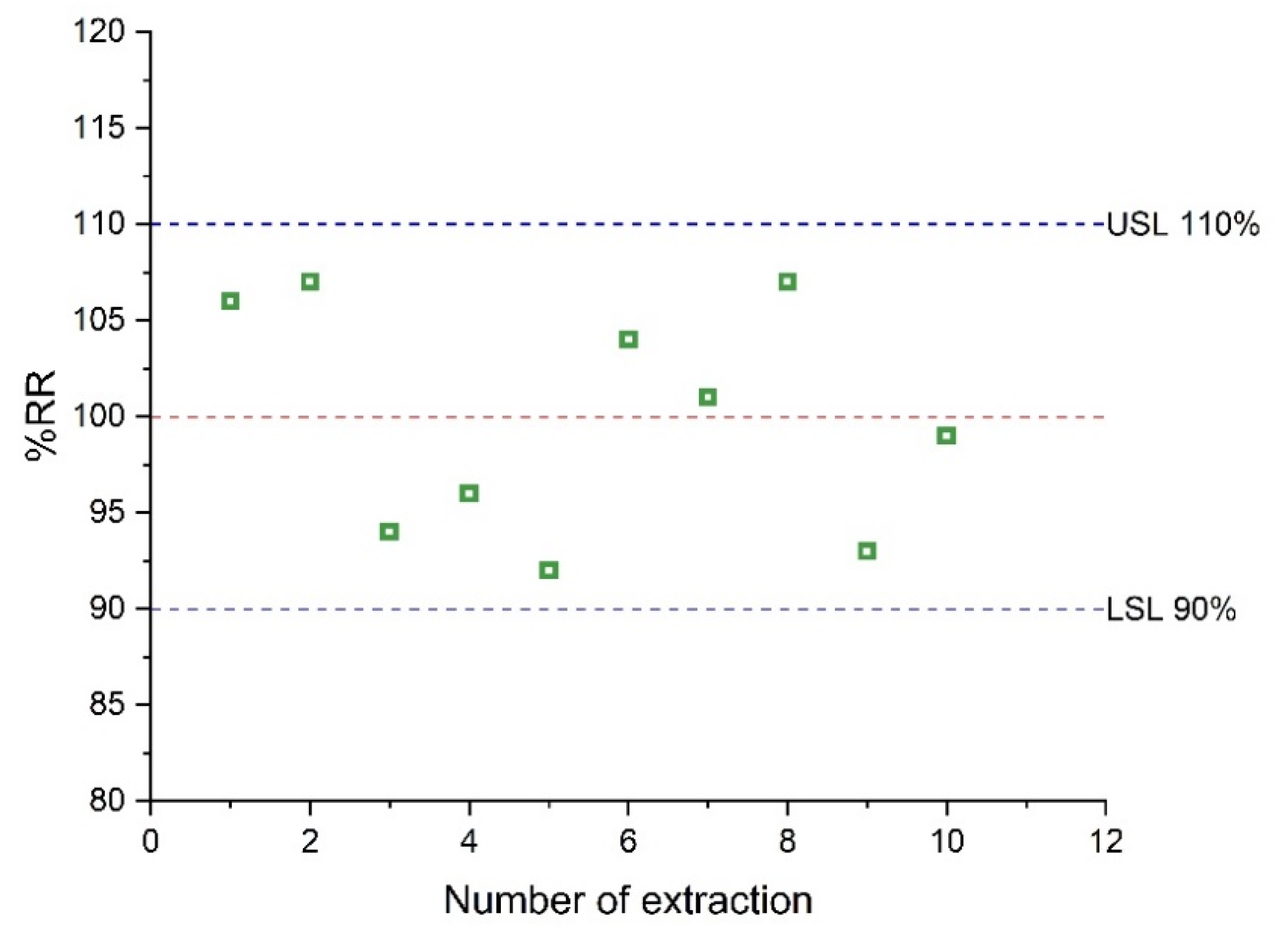
The reusability of the sol–gel CW 20M microextraction capsule was studied in continuous extraction cycles. For these experiments, the same capsule was used for the extraction of AMB from a spiked serum sample at a level of 1.0 μg mL−1. A criterion of 10% of extraction performance loss was used to evaluate the reusability. The results are shown in Figure 6. After the completion of 10 cycles, the loss of the extraction efficiency was not significant, demonstrating that the microextraction capsule can be used at least 10 times.
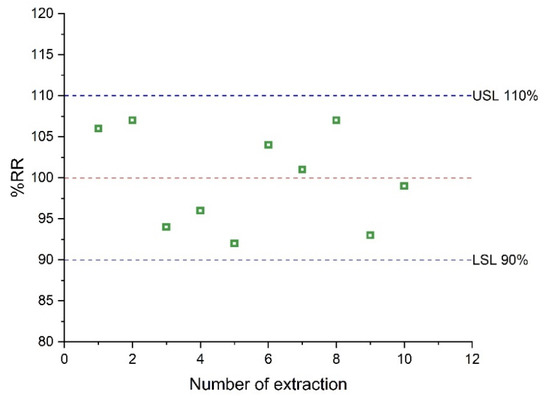
Figure 6.
Reusability of CW 20M CPME membrane.
3.4. Sample Stability
The stability of the target analyte in the serum sample was examined at 0, 4, and 8 h, stored at 25 °C and at 0, 8, and 24 h stored at +4 °C. For this purpose, blank human serum samples were spiked with AMB at a concentration of 1.0 μg mL−1, and they were stored under the studied conditions. After this time span, the samples were subjected to CPME-HPLC-UV analysis. A criterion of ±15% as a deviation from the nominal value was set to assess the stability. In accordance with previous studies, no degradation of AMB was observed under these conditions.
3.5. Application to Human Serum Samples
In order to prove the applicability of the CPME-HPLC-UV protocol, different individual human serum samples were analyzed. For this purpose, the authentic samples were spiked at three different concentration levels (i.e., 0.1, 1.0, and 10.0 μg mL−1) and they were subjected to the developed method. As shown in Table 2, the relative recovery was 83.9–114.6%, while the%RSD was 4.3–9.8%, showing good method applicability.

Table 2.
Relative recoveries of AMB in human serum samples.
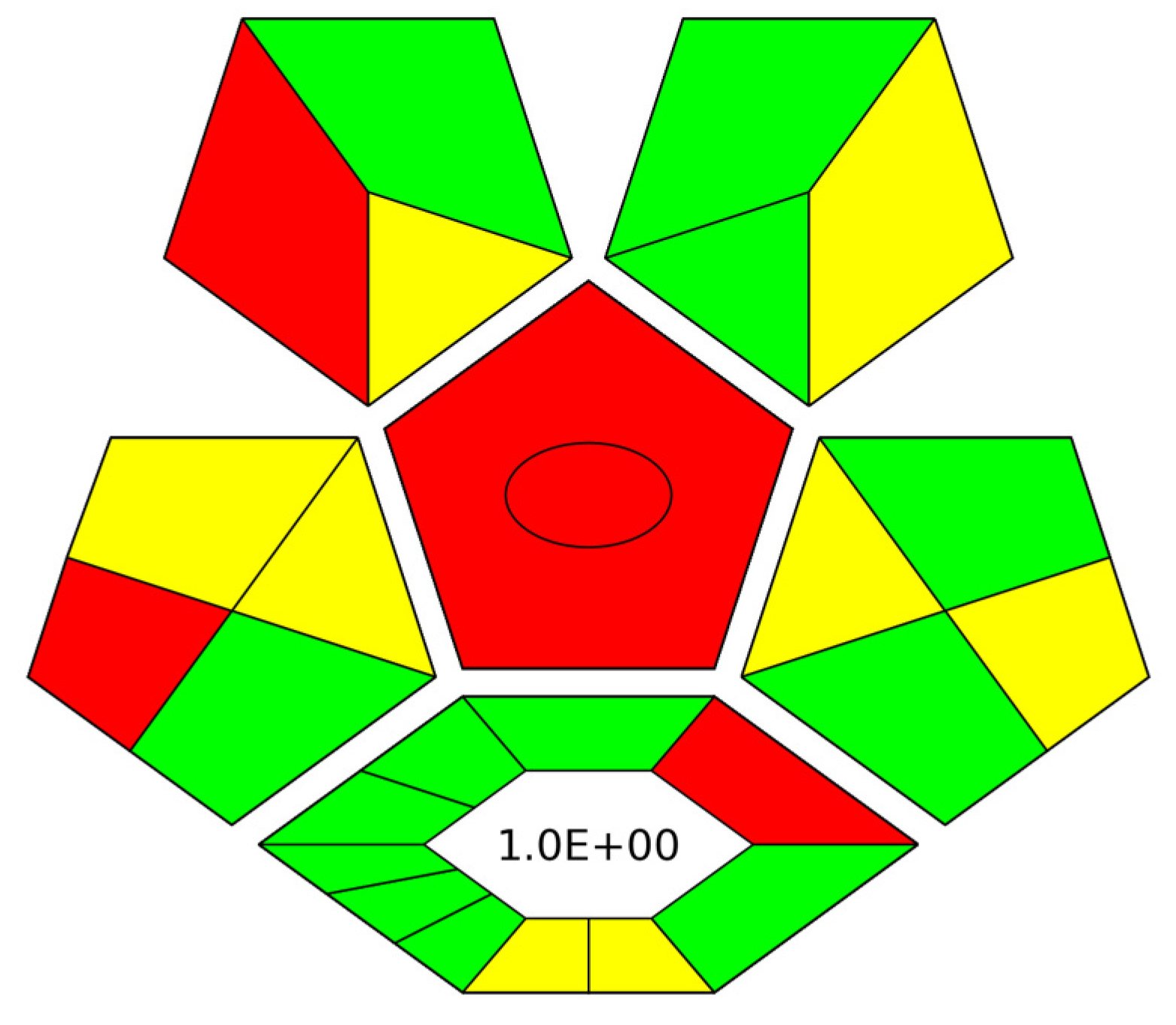
3.6. Evaluation of the Green Character of the Proposed Method by ComplexGAPI Index
For the complete evaluation of the herein proposed method, the complexGAPI index [24] was used to assess its green character. As such, the environmental friendliness of the CPME-HPLC-UV protocol was examined from the collection of the sample to the final step of the analytical determination [25], while the synthetical route for the microextraction capsules was also taken into consideration. Figure 7 shows the obtained pictogram for the proposed method. The preparation of the microextraction devices meets most of the requirements of the index (green color), resulting in an environmentally friendly procedure. Regarding the sample collection/sample preparation and analytical determination of AMB, many of the criteria are also met (e.g., low organic solvent consumption and reduced waste generation) in terms of the green elements of the final protocol.
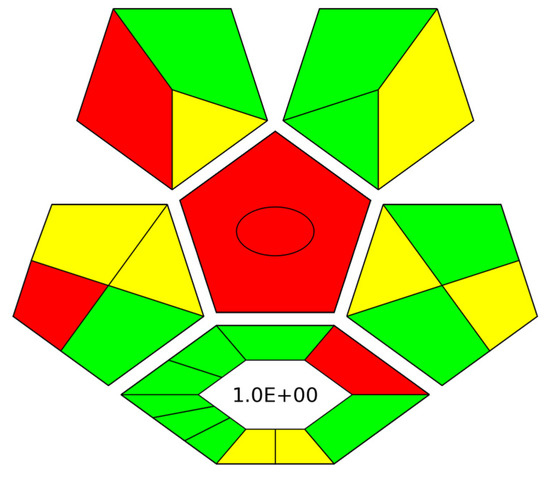
Figure 7.
ComplexGAPI pictogram for the CPME-HPLC-UV protocol for the determination of AMB in human serum.
4. Conclusions
CPME serves as a useful bioanalytical technique that efficiently simplifies the sample preparation of complex biological fluids by integrating the stirring and filtration mechanism in one capsule. The utilization of this technique results in high sample throughput, good extraction performance and compliance with many of the principles of GAC. Under optimum adsorption and desorption conditions, good figures of merit were obtained. The sol–gel CW 20M microextraction capsules were found to be reusable for at least 10 times. In the final step, the proposed methodology was successfully applied for the determination of AMB in real human serum samples obtained from different volunteers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9120433/s1. Preparation of capsule device, Figure S1: Plot of observed and predicted values for FC-CCD for AMB.; Table S1: Analysis of variance (ANOVA) for FC-CCD for ER% of AMB.
Author Contributions
Conceptualization, C.K.Z.; methodology, C.K.Z., A.K. (Abuzar Kabir), and N.M.; software, C.K.Z.; validation, N.M.; formal analysis, N.M., A.K. (Anastasia Korpeti) and A.K. (Abuzar Kabir); investigation, N.M., A.K. (Anastasia Korpeti), and A.K. (Abuzar Kabir); data curation, N.M. and A.K. (Anastasia Korpeti); writing—original draft preparation, C.K.Z. and N.M.; writing—review and editing, C.K.Z., A.K. (Abuzar Kabir), and K.G.F.; supervision, C.K.Z.; project administration, C.K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent has been obtained from the volunteers to publish this paper.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Espada, R.; Josa, J.M.; Valdespina, S.; Dea, M.A.; Ballesteros, M.P.; Alunda, J.M.; Torrado, J.J. HPLC Assay for Determination of Amphotericin B in Biological Samples. Biomed. Chromatogr. 2008, 22, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.P.; Vardulaki, K.A.; Conlon, C.; Cooke, J.; Daza-Ramirez, P.; Evans, E.G.V.; Hawkey, P.M.; Herbrecht, R.; Marks, D.I.; Moraleda, J.M.; et al. A Systematic Review of the Antifungal Effectiveness and Tolerability of Amphotericin B Formulations. Clin. Ther. 2003, 25, 1295–1320. [Google Scholar] [CrossRef] [PubMed]
- Balabathula, P.; Janagam, D.R.; Mittal, N.K.; Mandal, B.; Thoma, L.A.; Wood, G.C. Rapid Quantitative Evaluation of Amphotericin B in Human Plasma, by Validated HPLC Method. J. Bioequivalence Bioavailab. 2013, 5, 121–124. [Google Scholar] [CrossRef]
- He, Y.; Concheiro-Guisan, M. Microextraction Sample Preparation Techniques in Forensic Analytical Toxicology. Biomed. Chromatogr. 2019, 33, e4444. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Samanidou, V. Green Sample Preparation of Alternative Biosamples in Forensic Toxicology. Sustain. Chem. Pharm. 2021, 20, 100388. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Metaxa, A.S.; Papadoyannis, I.N. Direct Simultaneous Determination of Uremic toxins: Creatine, Creatinine, Uric acid, and Χanthine in Human Biofluids by HPLC. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 43–57. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC—Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Silva, C.; Cavaco, C.; Perestrelo, R.; Pereira, J.; Câmara, J.S. Microextraction by Packed Sorbent (MEPS) and Solid-Phase Microextraction (SPME) as Sample Preparation Procedures for the Metabolomic Profiling of Urine. Metabolites 2014, 4, 71–97. [Google Scholar] [CrossRef]
- Carasek, E.; Morés, L.; Huelsmann, R.D. Disposable Pipette Extraction: A Critical Review of Concepts, Applications, and Directions. Anal Chim Acta 2022, 1192, 339383. [Google Scholar] [CrossRef]
- Pedersen-bjergaard, S.; Rasmussen, K.E. Bioanalysis of Drugs by Liquid-Phase Microextraction Coupled to Separation Techniques. J. Chromatogr. B 2005, 817, 3–12. [Google Scholar] [CrossRef]
- Tienpont, B.; David, F.; Desmet, K.; Sandra, P. Stir Bar Sorptive Extraction-Thermal Desorption-Capillary GC-MS Applied to Biological Fluids. Anal. Bioanal. Chem. 2002, 373, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, A.; Covone, S.; Rosato, E.; Bonelli, M.; Savini, F.; Furton, K.G.; Gazioglu, I.; D’Ovidio, C.; Kabir, A.; Locatelli, M. Fabric Phase Sorptive Extraction (FPSE) as an Efficient Sample Preparation Platform for the Extraction of Antidepressant Drugs from Biological Fluids. Adv. Sample Prep. 2022, 3, 100022. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; Furton, K.G.; Samanidou, V.F.; Zacharis, C.K. Exploiting the Capsule Phase Microextraction Features in Bioanalysis: Extraction of Ibuprofen from Urine Samples. Microchem. J. 2021, 172, 106934. [Google Scholar] [CrossRef]
- US10352833B2—Microextraction Capsules and Method of Making—Google Patents. Available online: https://patents.google.com/patent/US10352833B2/en (accessed on 5 December 2022).
- Janicka, P.; Płotka-Wasylka, J.; Jatkowska, N.; Chabowska, A.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the New Generation of Green Solvents in Extraction Processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC—Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Mamounas, G.; Manousi, N.; Kabir, A.; Furton, K.G.; Mystridis, G.A.; Vizirianakis, I.S.; Zacharis, C.K. Designing an “All-in-One” Microextraction Capsule Device for the Liquid Chromatographic-Fluorescence Determination of Doxorubicin and Its Metabolites in Rat Plasma. J. Chromatogr. A 2022, 1680, 463432. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Kabir, A.; Furton, K.G.; Tzanavaras, P.D.; Zacharis, C.K. In Situ Synthesis of Monolithic Sol–Gel Polyethylene Glycol-Based Sorbent Encapsulated in Porous Polypropylene Microextraction Capsules and Its Application for Selective Extraction of Antifungal and Anthelmintic Drugs from Human Urine. Microchem. J. 2022, 180, 107594. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; da Silva, E.G.P.; dos Santos, W.N.L.; Quintella, C.M.; David, J.M.; de Andrade, J.B.; Breitkreitz, M.C.; Jardim, I.C.S.F.; Neto, B.B. Statistical Designs and Response Surface Techniques for the Optimization of Chromatographic Systems. J. Chromatogr. A 2007, 1158, 2–14. [Google Scholar] [CrossRef]
- Manousi, N.; Vlachaki, A.; Kika, F.S.; Markopoulou, C.K.; Tzanavaras, P.D.; Zacharis, C.K. Salting-out Homogeneous Liquid-Liquid Microextraction for the Determination of Azole Drugs in Human Urine: Validation Using Total Error Concept. J. Sep. Sci. 2022, 45, 1240–1251. [Google Scholar] [CrossRef]
- FDA, Bioanalytical Method Validation Guidance, Food Drug Adm. 2018. Available online: https://www.Fda.Gov/Regulatory-Information/Search-Fda-Guidance-Documents/BioanalytiCal-Method-Validation-Guidance-Industry (accessed on 10 December 2022).
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Mcelroy, C.R.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary Green Analytical Procedure Index (ComplexGAPI) and Software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).