Tribulus terrestris Cytotoxicity against Breast Cancer MCF-7 and Lung Cancer A549 Cell Lines Is Mediated via Activation of Apoptosis, Caspase-3, DNA Degradation, and Suppressing Bcl-2 Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection, Extraction, and Phytochemical Analysis
2.2. Chemicals and Reagents
2.3. Estimation of Total Phenolic Content
2.4. Estimation of Total Flavonoid Content
2.5. In Vitro Cytotoxicity of T. terrestris Extracts
2.6. Determination of Apoptosis (Annexin-V Assay)
2.7. Caspase Activation Assay
2.8. Anti-Bcl-2 Assay
2.9. DNA Degradation Analysis by TUNEL Assay
2.10. GC–MS Analysis
2.11. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Estimation of Total Phenols Content
3.3. Estimation of Total Flavonoid Content
3.4. In Vitro Cytotoxicity of T. terrestris Extracts
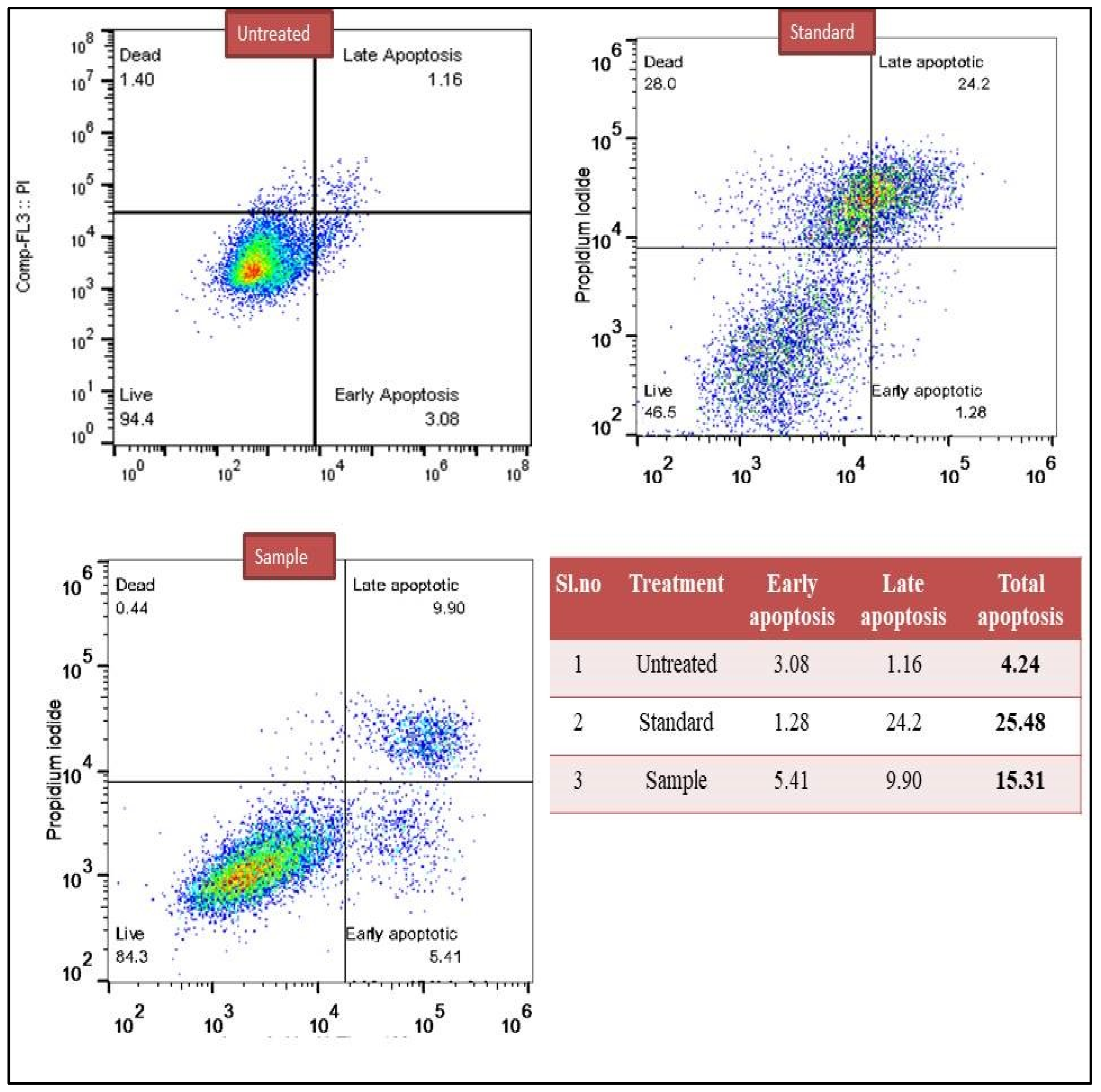
3.5. Determination of Early and Late Apoptosis (Annexin V Assay)
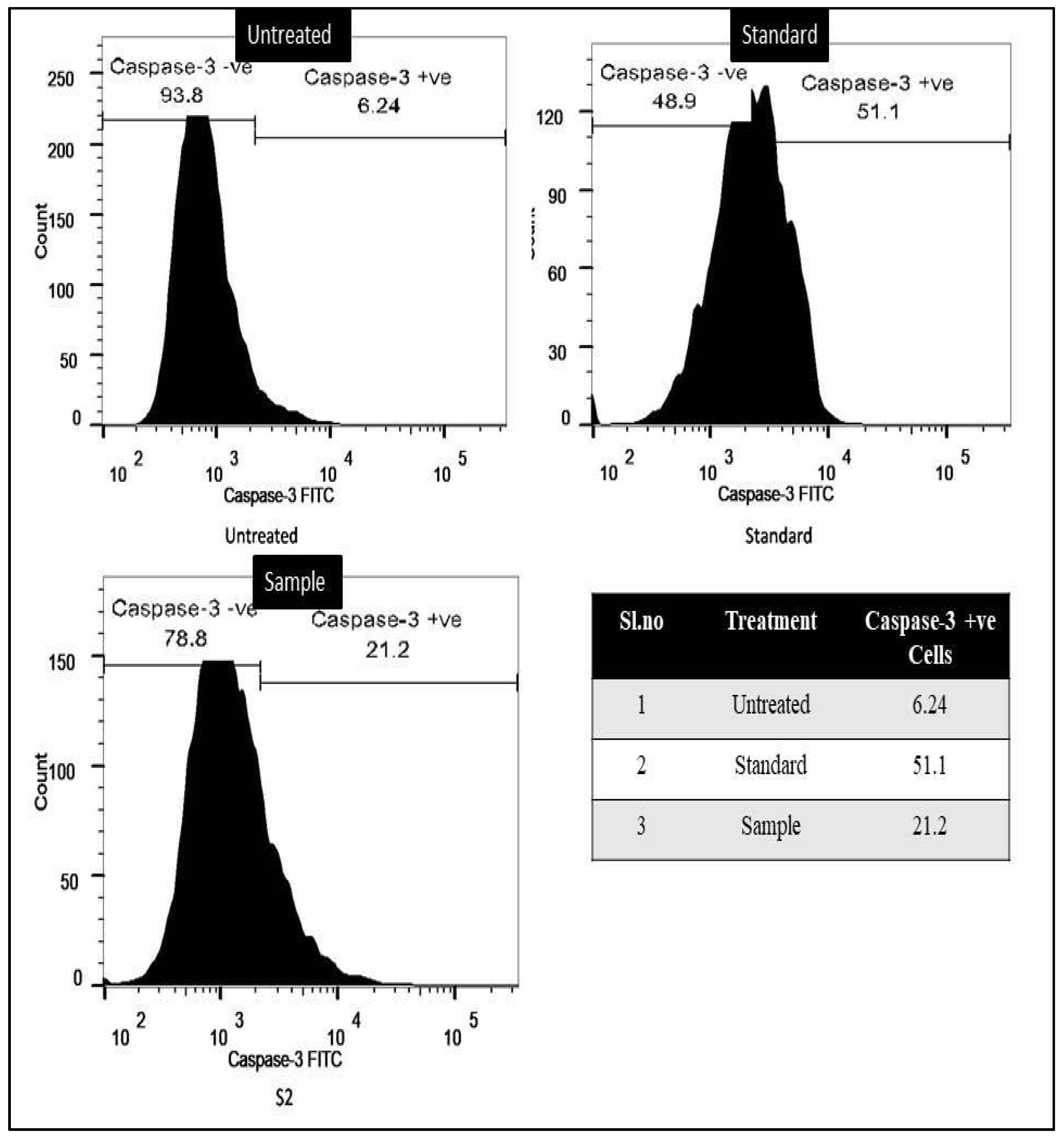
3.6. Caspase-3 Activation Assay
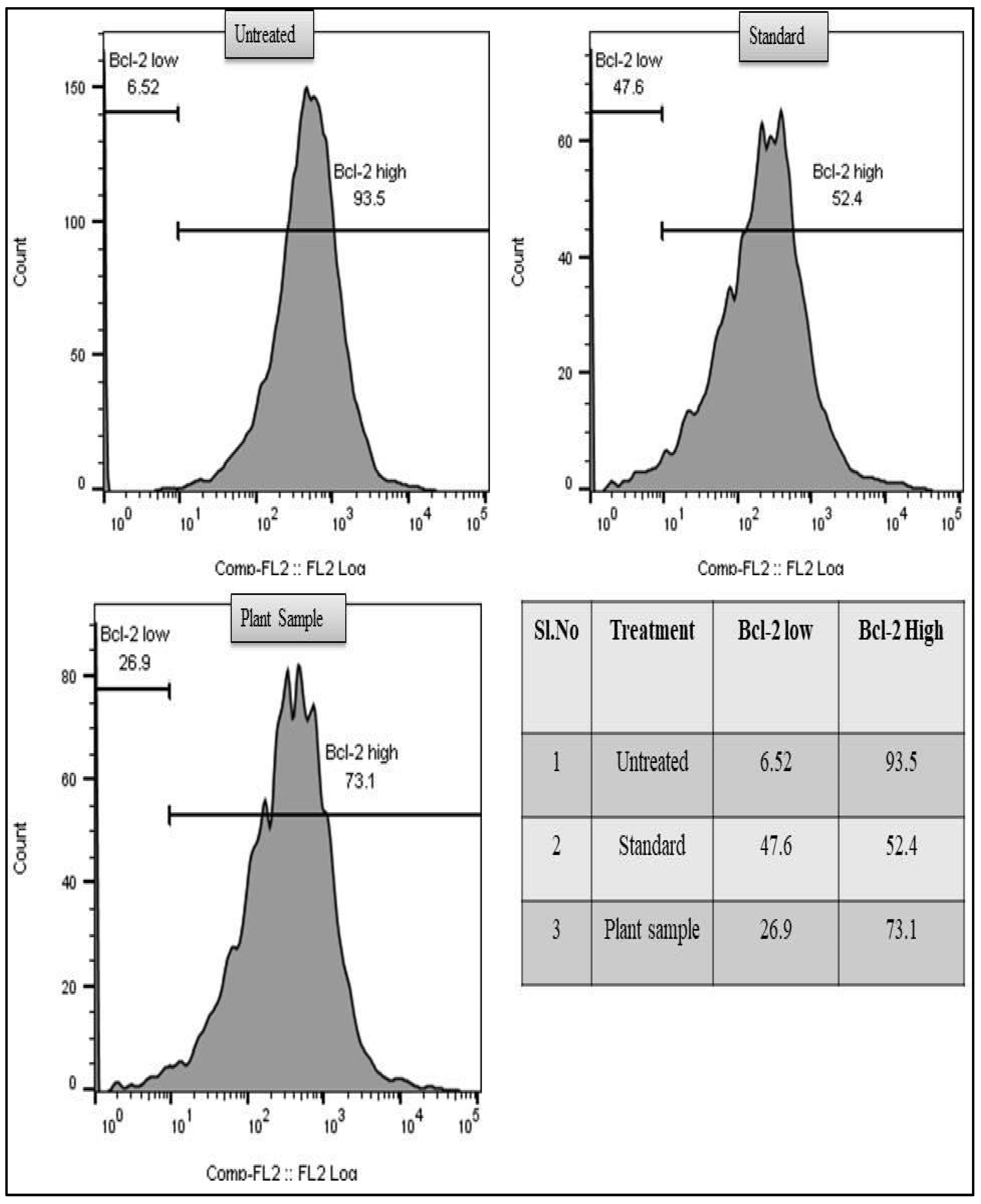
3.7. Anti-Bcl-2 Assay
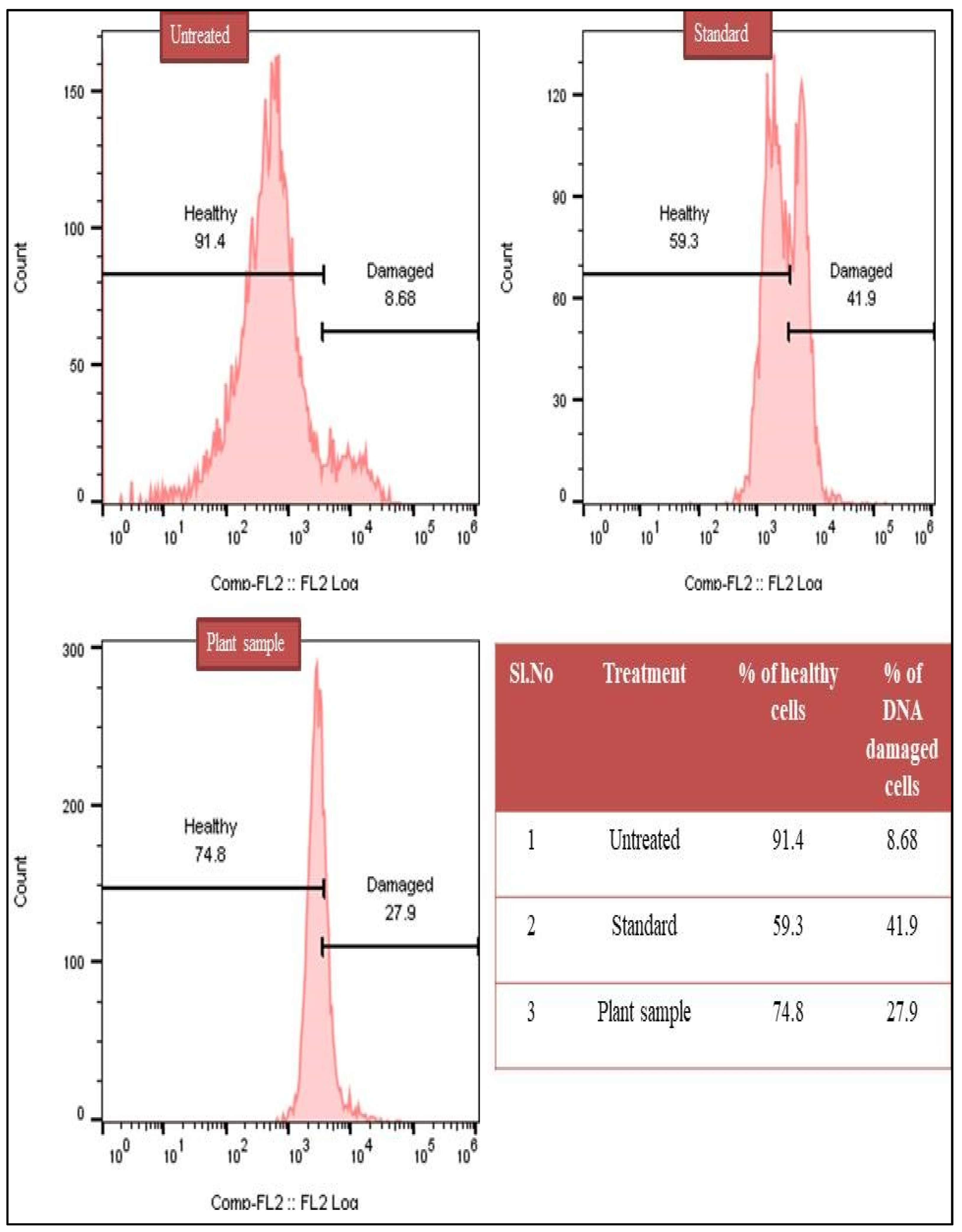
3.8. DNA Degradation Analysis by TUNEL Assay
3.9. GCMS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Althubiti, M.A.; Nour Eldein, M.M. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med. J. 2018, 39, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Algehyne, E.A.; Jibril, M.L.; Algehainy, N.A.; Alamri, O.A.; Alzahrani, A.K. Fuzzy Neural Network Expert System with an Improved Gini Index Random Forest-Based Feature Importance Measure Algorithm for Early Diagnosis of Breast Cancer in Saudi Arabia. Big Data Cogn. Comput. 2022, 6, 13. [Google Scholar] [CrossRef]
- Alqahtani, W.S.; Almufareh, N.A.; Domiaty, D.M.; Albasher, G.; Alduwish, M.A.; Alkhalaf, H.; Almuzzaini, B.; Al-Marshidy, S.S.; Alfraihi, R.; Elasbali, A.M.; et al. Epidemiology of cancer in Saudi Arabia thru 2010-2019: A systematic review with constrained meta-analysis. AIMS Public Health 2020, 7, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.A.; Al Sharhan, N.A.; Alharthi, B.; Babu, S.R.; Alsaleh, A.B.; Alasiri, A.M.; Assidi, M.; Buhmeida, A.; Almawi, W.Y. Detection of genetic mutations in patients with breast cancer from Saudi Arabia using Ion AmpliSeq™ Cancer Hotspot Panel v. 2.0. Biomed. Rep. 2022, 16, 26. [Google Scholar] [CrossRef]
- Alghamdi, I.G.; Hussain, I.I.; Alghamdi, M.S.; El-Sheemy, M.A. The incidence rate of female breast cancer in Saudi Arabia: An observational descriptive epidemiological analysis of data from Saudi Cancer Registry 2001–2008. Breast Cancer (Dove Med. Press) 2013, 5, 103–109. [Google Scholar] [CrossRef][Green Version]
- WHO. International Agency for Research in Cancer (IARC) Saudi Arabia. Source: Globocan. 2018. Available online: https://gco.iarc.fr/today/data/factsheets/populations/682-saudi-arabia-fact-sheets.pdf (accessed on 30 August 2022).
- Kuruppu, A.I.; Paranagama, P.; Goonasekara, C.L. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm. J. 2019, 27, 565–573. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Chhatre, S.; Nesari, T.; Somani, G.; Kanchan, D.; Sathaye, S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Dinchev, D. Saponins in Tribulus terrestris—Chemistry and Bioactivity. Phytochem. Rev. 2005, 4, 111–137. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.E. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. [Google Scholar] [CrossRef]
- Tounekti, T.; Mahdhi, M.; Khemira, H. Ethnobotanical Study of Indigenous Medicinal Plants of Jazan Region, Saudi Arabia. Evid. Based Complement. Alternat. Med. 2019, 2019, 3190670. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Kolesárová, A. Puncture vine (Tribulus terrestris L.) in control of health and reproduction. Physiol. Res. 2021, 70 (Suppl. S4), S657–S667. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Riaz, M.; Talpur, M.M.; Pirzada, T. Phytopharmacology of Tribulus terrestris. J. Biol. Regul. Homeost. Agents 2016, 30, 785–788. [Google Scholar] [PubMed]
- Kang, L.P.; Wu, K.L.; Yu, H.S.; Pang, X.; Liu, J.; Han, L.F.; Zhang, J.; Zhao, Y.; Xiong, C.Q.; Song, X.B.; et al. Steroidal saponins from Tribulus terrestris. Phytochemistry 2014, 107, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Dinchev, D.; Janda, B.; Evstatieva, L.; Oleszek, W.; Aslani, M.R.; Kostova, I. Distribution of steroidal saponins in Tribulus terrestris from different geographical regions. Phytochemistry 2008, 69, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Mendrick, D.L.; Paine, M.F.; Roe, A.L.; Yeung, C.K. “Natural” is not synonymous with “Safe”: Toxicity of natural products alone and in combination with pharmaceutical agents. Regul. Toxicol. Pharmacol. 2020, 113, 104642. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, S.A.; Alshabi, A.M.; Shaikh, I.A.; Orabi, M.A.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Shettar, A.K.; Hoskeri, J.H. In Vitro Cytotoxicity and Spectral Analysis-Based Phytochemical Profiling of Methanol Extract of Barleria hochstetteri, and Molecular Mechanisms Underlying Its Apoptosis-Inducing Effect on Breast and Lung Cancer Cell Lines. Separations 2022, 9, 298. [Google Scholar] [CrossRef]
- Islam, S.; Koly, S.; Zaman, S.; Sukorno, F.; Ahammed, S.; Munira, S.; Hridoy, R. Estimation of phytochemical, antioxidant screening profile and thrombolytic activities of methanolic extract of Antidesma bunius L. leaf. Int. J. Hortic. Sci. Technol. 2018, 6, 358–363. [Google Scholar] [CrossRef]
- Erdenechimeg, C.; Guiqide, A.; Dejidmaa, B.; Chimedragchaa, C.; Purevsuren, S. Total phenolic, flavonoid, alkaloid and iridoid content and preventive effect of Lider-7-tang on lipopolysaccharide-induced acute lung injury in rats. Braz. J. Med. Biol. Res. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Adedapo, A.A.; Jimoh, F.O.; Koduru, S.; Masika, P.J.; Afolayan, A.J. Assessment of the medicinal potentials of the methanol extracts of the leaves and stems of Buddleja saligna. BMC Complement. Altern. Med. 2009, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Orabi, M.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Hoskeri, J.H.; Shettar, A.K.; et al. Phytochemicals from Corchorus olitorius methanolic extract induce apoptotic cell death via activation of caspase 3, anti-Bcl-2 activity, and DNA degradation in breast and lung cancer cell lines. J. King Saud Univ. Sci. 2022, 34, 102238. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytom. J. Int. Soc. Anal. Cytol. 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Faridha Begum, I.; Mohankumar, R.; Jeevan, M.; Ramani, K. GC–MS analysis of bio-active molecules derived from Paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian J. Microbiol. 2016, 56, 426–432. [Google Scholar] [CrossRef]
- Thekkangil, A.; Suchithra, T.V. Antidermatophytic lead compounds from Streptomycetes albidoflavus STV1572a against Tinea infections by Tricophyton mentagrophytes. Microb. Pathog. 2020, 142, 104037. [Google Scholar] [CrossRef]
- Dills, W.L., Jr.; Meyer, W.L. 1976. Studies on 1-deoxy-D-fructose, 1-deoxy-D-glucitol, and 1-deoxy-D-mannitol as antimetabolites. Biochemistry 1976, 15, 4506–4512. [Google Scholar] [CrossRef]
- Roopa, M.S.; Shubharani, R.; Rhetso, T.; Sivaram, V. Comparative analysis of phytochemical constituents, free radical scavenging activity and GC-MS analysis of leaf and flower extract of Tithonia diversifolia (Hemsl.) A. Gray. Int. J. Pharm. Sci. Res. 2020, 11, 5081–5090. [Google Scholar]
- Gomathi, S.; Velayutham, P.; Karthi, C.; Santhoshkumar, S. In vitro callus induction and phytochemical screening of Corbichonia decumbens (Forssk.) Exell through GC MS analysis. J. Pharmacogn. Phytochem. 2019, 8, 566–571. [Google Scholar]
- Mohammed, D.Y.; Dwaish, A.S.; Jawad, A.L.M. Anti-phytopathogenic fungi activities of Cladophora glomerata extract. World J. Pharm. Res. 2013, 2, 1868–1877. [Google Scholar]
- Arora, S.; Meena, S. Pharmacological Studies on Flowers of Ceropegia bulbosa Roxb. var. bulbosa and lushii (Grah.) Hook. F from Thar Desert of Rajasthan, India. Res. J. Pharmacogn. Phytochem. 2018, 10, 226–232. [Google Scholar] [CrossRef]
- Pinto, M.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Cienc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- El-Demerdash, E. Anti-inflammatory and antifibrotic effects of methyl palmitate. Toxicol. Appl. Pharmacol. 2011, 254, 238–244. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef]
- Breeta, R.D.I.E.; Grace, V.M.B.; Wilson, D.D. Methyl Palmitate—A suitable adjuvant for Sorafenib therapy to reduce in vivo toxicity and to enhance anti-cancer effects on hepatocellular carcinoma cells. Basic Clin. Pharmacol. Toxicol. 2021, 128, 366–378. [Google Scholar] [CrossRef]
- Olowofolahan, A.O.; Oyebode, O.T.; Olorunsogo, O.O. Methyl palmitate reversed estradiol benzoate-induced endometrial hyperplasia in female rats. Toxicol. Mech. Methods 2021, 31, 43–52. [Google Scholar] [CrossRef]
- Ouf, S.A.; Ali, M.I.; Haggag, M.G.; Elsafty, D.O.; Faraag, A.H. Enhancement of antidermatophytic activities of Citrullus colocynthis Schrad collected from different ecological habitats in Egypt using fluconazole. Phytomedicine Plus 2022, 2, 100178. [Google Scholar] [CrossRef]
- Mu’nisa, A.; Pagarra, H.; Maulana, Z. Active compounds extraction of cocoa pod husk (Thebroma cacao L.) and potential as fungicides. J. Phys. Conf. Ser. 2018, 1028, 012013. [Google Scholar]
- Hentati, F.; El-Euch, G.; Bouhlal, Y.; Amouri, R. Ataxia with vitamin E deficiency and abetalipoproteinemia. Handb. Clin. Neurol. 2011, 103, 295–305. [Google Scholar]
- Joshi, S.; Mishra, D.; Bisht, G.; Khetwal, K.S. Essential oil composition and antimicrobial activity of Lobelia pyramidalis Wall. EXCLI J. 2011, 10, 274–279. [Google Scholar] [PubMed]
- Adekoyeni, O.O.; Ajayi, F.; Adegoke, A. GC-MS analysis and identification of pharmacological Components of doum palm nuts. Niger. J. Sci. Res. 2019, 18, 571–578. [Google Scholar]
- To’bungan, N.; Jati, W.N.; Zahida, F. Acute toxicity and anticancer potential of knob weed (Hyptis capitata) ethanolic leaf extract and fraction. Plant Sci. Today 2022, 9, 955–962. [Google Scholar]
- Ibrahim, N.; Fairus, S.; Zulfarina, M.S.; Naina Mohamed, I. The Efficacy of Squalene in Cardiovascular Disease Risk-A Systematic Review. Nutrients 2020, 12, 414. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- WHO. Non-Communicable Diseases. 2019. Available online: https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 21 August 2022).
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Cheema, S.; Maisonneuve, P.; Lowenfels, A.B.; Abraham, A.; Doraiswamy, S.; Mamtani, R. Influence of Age on 2040 Cancer Burden in the Older Population of the Gulf Cooperation Council (GCC) Countries: Public Health Implications. Cancer Control 2021, 28, 10732748211027158. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23. [Google Scholar] [CrossRef]
- Srivastava, A.; Jalink, M.; de Moraes, F.Y.; Booth, C.M.; Berry, S.R.; Rubagumya, F.; Roitberg, F.; Sengar, M.; Hammad, N. Tracking the workforce 2020–2030: Making the case for a cancer workforce registry. JCO Glob. Oncol. 2021, 7, 925–933. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015.
- Tran, K.B.; Lang, J.J.; Compton, K.; Xu, R.; Acheson, A.R.; Henrikson, H.J.; Kocarnik, J.M.; Penberthy, L.; Aali, A.; Abbas, Q.; et al. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef]
- World-Health-Organization. Cancer: Fact Sheet No. 297. 2015. Available online: http://www.who.int (accessed on 2 October 2016).
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nature reviews. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Derakhshandeh, K.; Jalalizadeh, A.; Mostafaie, A.; Hosseinzadeh, L. Encapsulation in PLGA-PEG enhances 9-nitro-camptothecin cytotoxicity to human ovarian carcinoma cell line through apoptosis pathway. Res. Pharm. Sci. 2015, 10, 161–168. [Google Scholar] [PubMed]
- Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review. Nutrients 2018, 10, 36. [Google Scholar] [CrossRef]
- Burda, N.Y.; Zhuravel, I.O.; Dababneh, M.F.; Fedchenkova, Y.A. Analysis of diosgenin and phenol compounds in Tribulus terrestris L. Pharmacia 2019, 66, 41. [Google Scholar] [CrossRef]
- Mathur, M.; Sundaramoorthy, S. Ethnopharmacological studies of Tribulus terrestris (Linn). in relation to its aphrodisiac properties. Afr. J. Tradit. Complement. Altern. Med. 2012, 10, 83–94. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, characterization, and health benefits. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Gul, S.; Maqbool, M.F.; Zheng, D.; Li, Y.; Khan, M.; Ma, T. Alpinetin: A Dietary Flavonoid with Diverse Anticancer Effects. Appl. Biochem. Biotechnol. 2022, 14, 1–24. [Google Scholar] [CrossRef]
- Kumar, R.; Vijayalakshmi, S.; Nadanasabapathi, S. Health Benefits of Quercetin. Def. Life Sci. J. 2017, 2, 142. [Google Scholar] [CrossRef]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Kuete, V.; Omosa, L.K.; Midiwo, J.O.; Karaosmanoğlu, O.; Sivas, H. Cytotoxicity of naturally occurring phenolics and terpenoids from Kenyan flora towards human carcinoma cells. J. Ayurveda Integr. Med. 2019, 10, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorganic Chem. 2022, 124, 105817. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General cytotoxicity assessment by means of the MTT assay. In Protocols In Vitro Hepatocyte Research; Humana Press: New York, NY, USA, 2015; pp. 333–348. [Google Scholar]
- Tan, A.S.; Berridge, M.V. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: A simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J. Immunol. Methods 2000, 238, 59–68. [Google Scholar] [CrossRef]
- Pintor, A.V.B.; Queiroz, L.D.; Barcelos, R.; Primo, L.S.G.; Maia, L.C.; Alves, G.G. MTT versus other cell viability assays to evaluate the biocompatibility of root canal filling materials: A systematic review. Int. Endod. J. 2020, 53, 1348–1373. [Google Scholar] [CrossRef]
- Maqbul, M.S.; Alshabi, A.M.; Khan, A.A.; Iqubal, S.M.S.; Mohammed, T.; Shaikh, I.A.; Dawoud, A.; Muddapur, U.M.; Hussain, M.S.; Singh, S.K. Comparison of E-Test Values for Standard Antibiotics and Conventional Antimicrobial Assay Values for Ethanoic Acids against Nosocomial Multidrug-Resistant Pseudomonas Aeruginosa. J. Pure Appl. Microbiol. 2020, 14, 255–260. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.C.; Min, J.S.; Kim, M.J.; Kim, J.A.; Kor, M.H.; Yoo, H.S.; Ahn, J.K. Aqueous extract of Tribulus terrestris Linn induces cell growth arrest and apoptosis by down-regulating NF-κB signaling in liver cancer cells. J. Ethnopharmacol. 2011, 136, 197–203. [Google Scholar] [CrossRef]
- Pourali, M.; Yaghoobi, M.M.; Salehi Sormaghi, M.H. Cytotoxic, Anti-Proliferative and Apoptotic Effects of Tribulus terrestris L. Fruit Extract on Human Prostate Cancer Lncap and Colon Cancer HT-29 Cell Lines. Jundishapur J. Nat. Pharm. Prod. 2017, 12, e33561. [Google Scholar] [CrossRef]
- Neychev, V.K.; Nikolova, E.; Zhelev, N.; Mitev, V.I. Saponins from Tribulus terrestris L. are less toxic for normal human fibroblasts than for many cancer lines: Influence on apoptosis and proliferation. Exp. Biol. Med. (Maywood, N.J.) 2007, 232, 126–133. [Google Scholar]
- Basaiyye, S.S.; Naoghare, P.K.; Kanojiya, S.; Bafana, A.; Arrigo, P.; Krishnamurthi, K.; Sivanesan, S. Molecular mechanism of apoptosis induction in Jurkat E6-1 cells by Tribulus terrestris alkaloids extract. J. Tradit. Complement. Med. 2018, 8, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Soni, A.; Siddiqi, N.J.; Sharma, P. An insight into the anticancer mechanism of Tribulus terrestris extracts on human breast cancer cells. 3 Biotech 2019, 9, 58. [Google Scholar] [CrossRef]
- Patel, A.; Bhatt, M.; Soni, A.; Sharma, P. Identification of steroidal saponins from Tribulus terrestris and their in silico docking studies. J. Cell. Biochem. 2021, 122, 665–1685. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Qu, W.J.; Zhang, X.L.; Yang, H.J.; Zhuang, X.Y.; Zhang, P. Investigation on inhibitory and apoptosis-inducing effects of saponins from Tribulus terrestris on hepatoma cell line BEL-7402. China J. Chin. Mater. Med. 2004, 29, 681–684. [Google Scholar]
- Abirami, P.; Rajendran, A. GC-MS analysis of Tribulus terrestris. l. Asian J. Plant Sci. Res. 2011, 1, 13–16. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009, 8. [Google Scholar]
- Sun, B.; Qu, W.; Bai, Z. The inhibitory effect of saponins from Tribulus terrestris on Bcap-37 breast cancer cell line in vitro. J. Chin. Med. Mater. 2003, 26, 104–106. [Google Scholar]
- Su, L.; Chen, G.; Feng, S.G.; Wang, W.; Li, Z.F.; Chen, H.; Liu, Y.X.; Pei, Y.H. Steroidal saponins from Tribulus terrestris. Steroids 2009, 74, 399–403. [Google Scholar] [CrossRef]
| Concentration (µg/mL) | Percentage of Cell Viability | |||
|---|---|---|---|---|
| Hexane | Ethyl Acetate | Methanol | Aqueous | |
| 50 | 91.84 ± 0.017 * | 98.35 ± 0.017 | 88.62 ± 0.002 * | 95.86 ± 0.008 |
| 100 | 87.56 ± 0.008 * | 80.51 ± 0.021 * | 78.31 ± 0.002 * | 80.40 ± 0.011 * |
| 150 | 70.82 ± 0.007 * | 66.80 ± 0.008 * | 66.43 ± 0.005 * | 70.49 ± 0.001 * |
| 200 | 61.75 ± 0.018 * | 45.44 ± 0.001 * | 52.87 ± 0.022 * | 60.91 ± 0.018 * |
| 250 | 47.05 ± 0.010 * | 32.76 ± 0.008 * | 32.76 ± 0.022 * | 53.23 ± 0.012 * |
| Concentration (µg/mL) | Percentage of Cell Viability | |||
|---|---|---|---|---|
| Hexane | Ethyl Acetate | Methanol | Aqueous | |
| 50 | 96.38 ± 0.008 | 90.99 ± 0.001 * | 43.57 ± 0.003 * | 49.39 ± 0.012 * |
| 100 | 88.35 ± 0.001 * | 79.95 ± 0.021 * | 27.13 ± 0.002 * | 44.71 ± 0.005 * |
| 150 | 78.45 ± 0.013 * | 71.96 ± 0.012 * | 22.80 ± 0.015 * | 36.01 ± 0.010 * |
| 200 | 67.94 ± 0.014 * | 63.38 ± 0.023 * | 17.52 ± 0.009 * | 29.71 ± 0.014 * |
| 250 | 53.60 ± 0.018 * | 49.51 ± 0.011 * | 9.90 ± 0.006 * | 18.72 ± 0.026 * |
| Concentration (µg/mL) | Percentage of Cell Viability | |||
|---|---|---|---|---|
| Hexane | Ethyl Acetate | Methanol | Aqueous | |
| 50 | 97.43 ± 0.018 | 91.14 ± 0.009 * | 98.30 ± 0.003 | 91.31 ± 0.008 * |
| 100 | 90.21 ± 0.006 * | 83.98 ± 0.006 * | 89.94 ± 0.014 * | 82.34 ± 0.009 * |
| 150 | 82.13 ± 0.008 * | 70.98 ± 0.011 * | 72.45 ± 0.014 * | 71.31 ± 0.027 * |
| 200 | 68.85 ± 0.009 * | 61.47 ± 0.018 * | 51.25 ± 0.007 * | 60.87 ± 0.014 * |
| 250 | 57.21 ± 0.017 * | 44.48 ± 0.006 * | 41.63 ± 0.005 * | 55.90 ± 0.019 * |
| IC50 (µg/mL) | |||
|---|---|---|---|
| Test Sample | L929 Cell Line | MCF-7 Cell Line | A549 Cell Line |
| Hexane extract | 244.16 | 293.27 | 277.14 |
| Ethyl acetate extract | 194.43 | 238.19 | 256.38 |
| Methanol extract | 224.35 | 218.19 | 179.62 |
| Aqueous extract | 255.89 | 271.07 | 189.70 |
| Cisplatin | 9.87 | 2.51 | 11.47 |
| Peak No | Compound Name | Rt | Base m/z | Nature | Uses |
|---|---|---|---|---|---|
| 1 | 9-Octadecene, (E) | 21.194 | 55.05 | Long-chain hydrocarbon | Antimicrobial and antioxidant activity [28,29] |
| 2 | 1-Deoxy-d-mannitol | 23.923 | 73.05 | Sugar alcohol | Antimetabolite [30] |
| 3 | (E)-Phytol | 26.611 | 68.10 | Acyclic hydrogenated diterpene alcohol | Cancer protective, antimicrobial, anti-inflammatory, diuretic action [31] |
| 4 | 8-Pentadecanone | 27.353 | 149.05 | Aliphatic ketone | Anti-cancer [32], Anti-phytopathogenic fungi activities [33], Hepatotoxic, Demyelination [34] |
| 5 | Methyl palmitate | 28.379 | 74.05 | Fatty acid methyl ester | Potent antioxidant property [35] Anti-inflammatory effect, anti-fibrotic effect [36,37] Hepatoprotective, reducing chemotherapy-induced toxicity in vivo, neuroprotective effects, and potent cardio-protective agent [38] Anti-proliferative [39] |
| 6 | Methyl 9,12-octadecadienoate | 31.603 | 67.10 | Fatty acid methyl ester | Hypocholesterolemic hepatoprotective, Antieczemic, anti-histaminic, [34] Antidermatophytic activities [40] Antimicrobial, antioxidant [41] |
| 7 | Methyl linolenate | 31.729 | 79.10 | Polyunsaturated fatty acid methyl ester | cancer-preventive, anti-inflammatory, antieczemic, antihistaminic, hepatoprotective, hypocholesterolemic, nematicide, insectifuge, antiacne, 5-alpha reductase inhibitor- antiandrogenic, anti-arthritic [31], antioxidant and antifungal activity [35] |
| 8 | Isophytol acetate | 31.951 | 71.10 | Monoterpene acetate derivatives | Precursor of vitamin E [42], antimicrobial activity [43] |
| 9 | Methyl isostearate | 32.159 | 74.10 | Fatty acid methyl ester | Recently patented for use in ulcers, antipruritic, keloids, alopecia and anti-inflammatory agents [44] Antioxidant [35], cancer [45] |
| 10 | Squalene | 43.201 | 69.05 | Acyclic polyunsaturated triterpene | Antioxidant, anti-cancer, cardiovascular protective effects [46] Detoxifying agent, emollient and moisturizer [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Orabi, M.A.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Shettar, A.K.; Hoskeri, J.H. Tribulus terrestris Cytotoxicity against Breast Cancer MCF-7 and Lung Cancer A549 Cell Lines Is Mediated via Activation of Apoptosis, Caspase-3, DNA Degradation, and Suppressing Bcl-2 Activity. Separations 2022, 9, 383. https://doi.org/10.3390/separations9110383
Alshabi AM, Alkahtani SA, Shaikh IA, Orabi MAA, Abdel-Wahab BA, Walbi IA, Habeeb MS, Khateeb MM, Shettar AK, Hoskeri JH. Tribulus terrestris Cytotoxicity against Breast Cancer MCF-7 and Lung Cancer A549 Cell Lines Is Mediated via Activation of Apoptosis, Caspase-3, DNA Degradation, and Suppressing Bcl-2 Activity. Separations. 2022; 9(11):383. https://doi.org/10.3390/separations9110383
Chicago/Turabian StyleAlshabi, Ali Mohamed, Saad Ahmed Alkahtani, Ibrahim Ahmed Shaikh, Mohamed A. A. Orabi, Basel A. Abdel-Wahab, Ismail A. Walbi, Mohammed Shafiuddin Habeeb, Masood Medleri Khateeb, Arun K. Shettar, and Joy H. Hoskeri. 2022. "Tribulus terrestris Cytotoxicity against Breast Cancer MCF-7 and Lung Cancer A549 Cell Lines Is Mediated via Activation of Apoptosis, Caspase-3, DNA Degradation, and Suppressing Bcl-2 Activity" Separations 9, no. 11: 383. https://doi.org/10.3390/separations9110383
APA StyleAlshabi, A. M., Alkahtani, S. A., Shaikh, I. A., Orabi, M. A. A., Abdel-Wahab, B. A., Walbi, I. A., Habeeb, M. S., Khateeb, M. M., Shettar, A. K., & Hoskeri, J. H. (2022). Tribulus terrestris Cytotoxicity against Breast Cancer MCF-7 and Lung Cancer A549 Cell Lines Is Mediated via Activation of Apoptosis, Caspase-3, DNA Degradation, and Suppressing Bcl-2 Activity. Separations, 9(11), 383. https://doi.org/10.3390/separations9110383









