Anti-Allergic and Antioxidant Potential of Polyphenol-Enriched Fractions from Cyclopia subternata (Honeybush) Produced by a Scalable Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Resins
2.2. Preparation of C. subternata Hot Water Extract
2.3. Static Adsorption and Desorption
2.4. Small-Column Dynamic Adsorption and Desorption
2.5. Large-Column Fractionation
2.6. Identification and Quantification of Phenolic Compounds
2.7. Radical Scavenging Assays
2.7.1. Superoxide Anion Radical () Scavenging Assay
2.7.2. 2,2-Diphenyl-1-picrylhydrazyl Radical (DPPH•) Scavenging Assay
2.7.3. Oxygen Radical Absorbance Capacity Assay (ORAC)
2.8. Xanthine Oxidase (XO) Inhibition Assay
2.9. β-Hexosaminidase Release Assay (Anti-Allergy Potential)
2.10. Data Analysis
3. Results
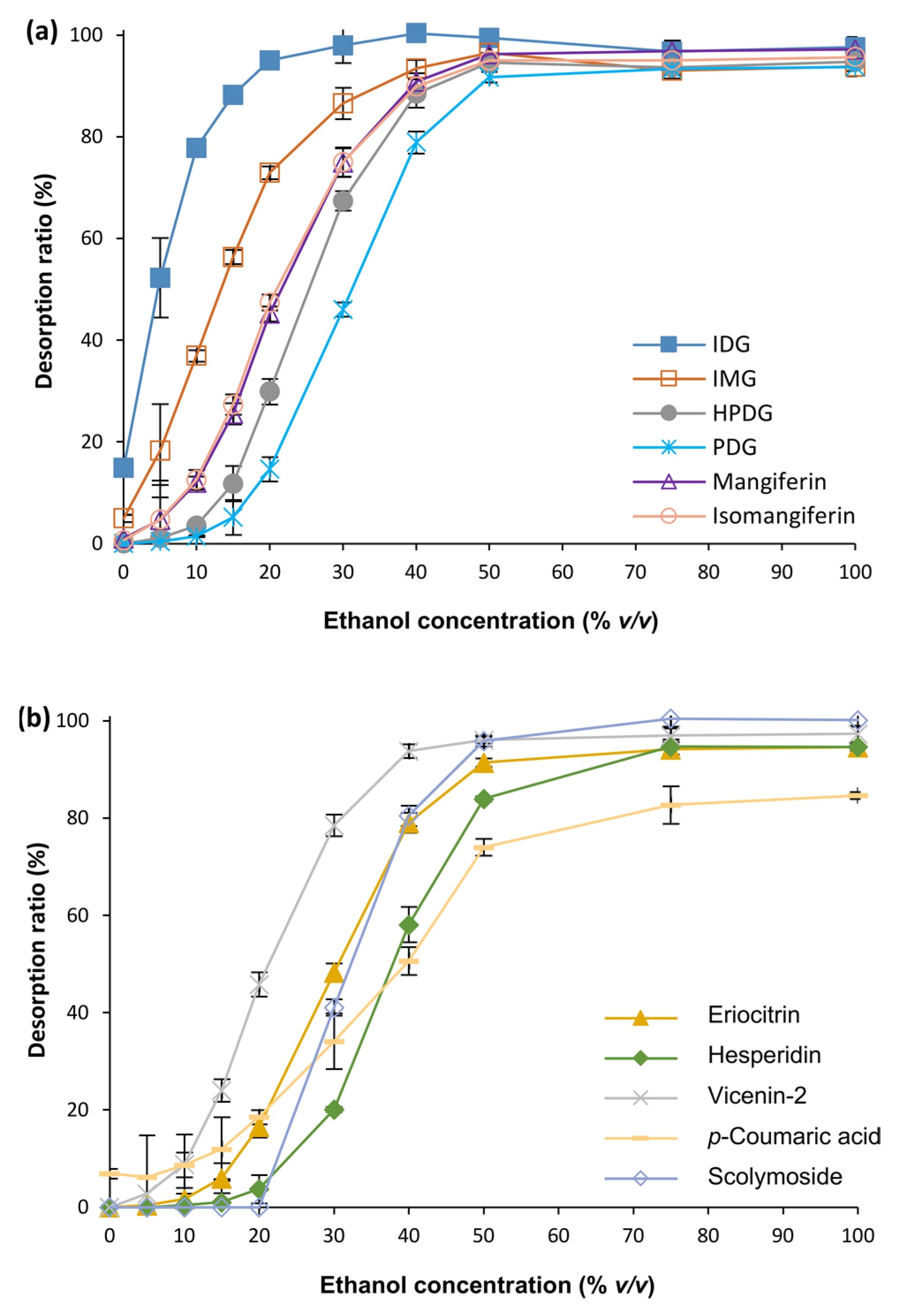
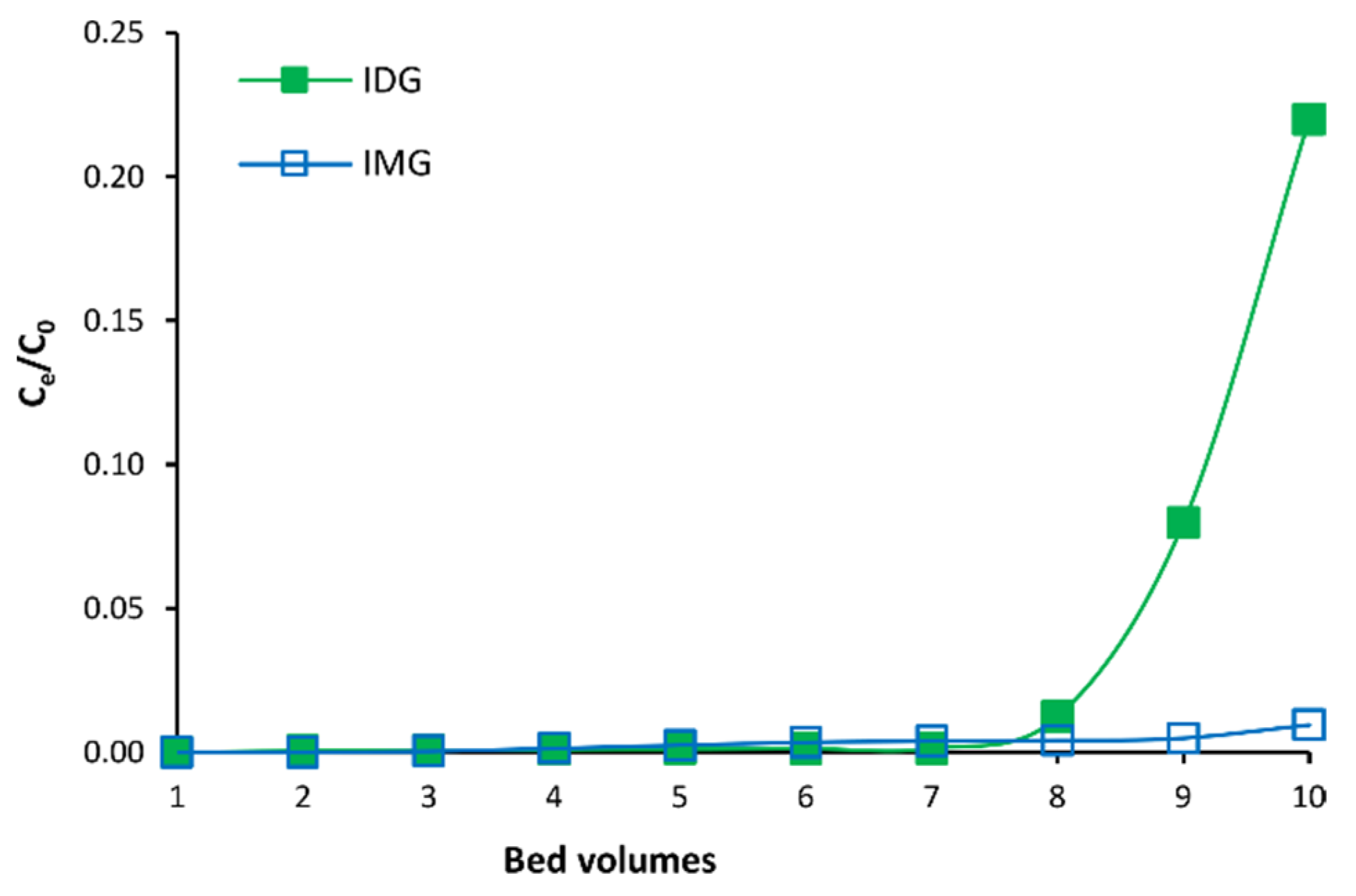
3.1. Optimization of MARC Parameters
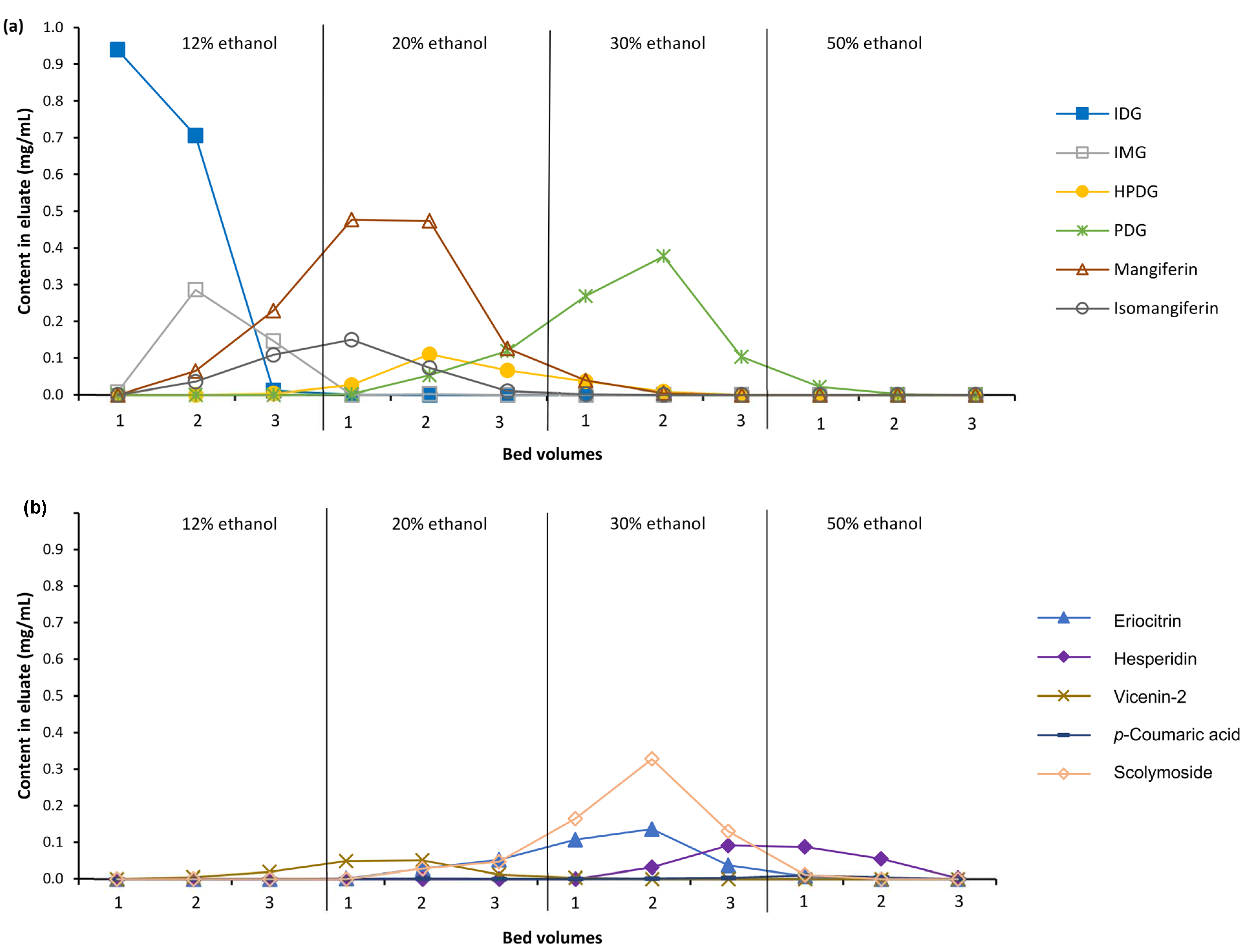
3.2. Fractionation of C. subternata Extract Using MARC
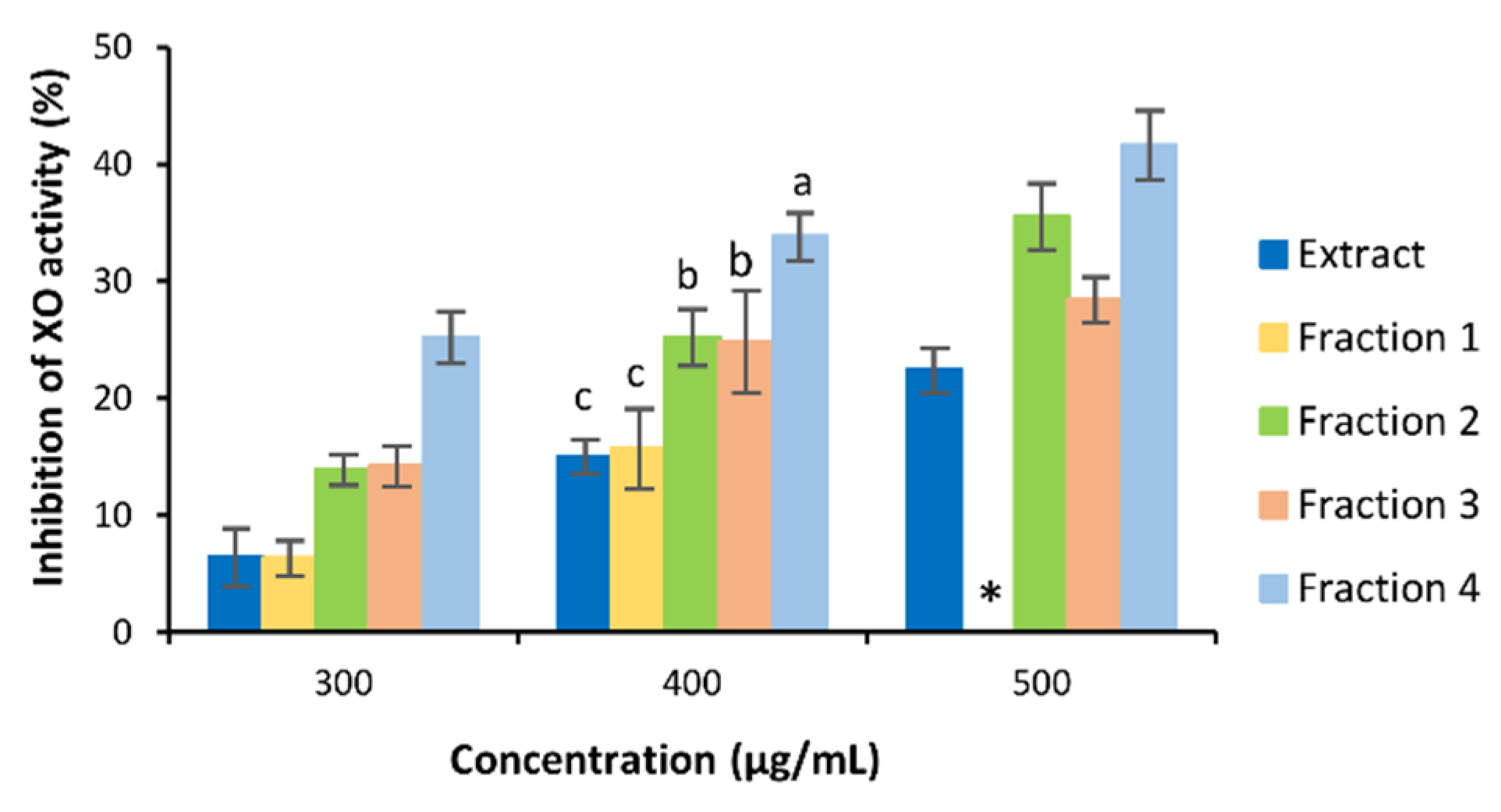
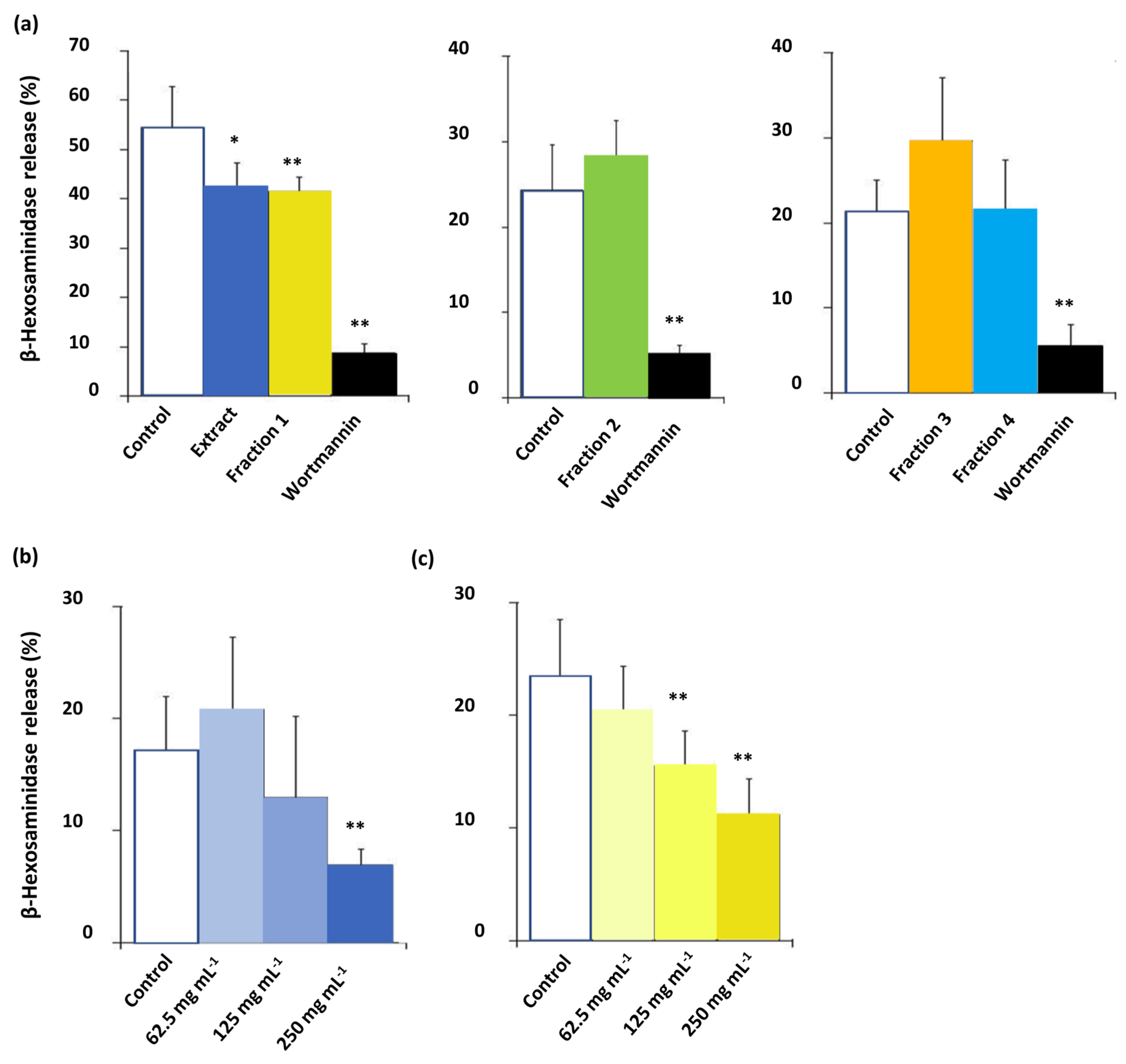
3.3. Bioactivity of C. subternata Extract and Fractions
4. Discussion
4.1. Production of Polyphenol-Enriched Fractions of C. subternata
4.2. Bioactivity of C. subternata Extract and Fractions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bessa, C.; Francisco, T.; Dias, R.; Mateus, N.; de Freitas, V.; Pérez-Gregorio, R. Use of Polyphenols as Modulators of Food Allergies. From Chemistry to Biological Implications. Front. Sustain. Food Syst. 2021, 5, 623611. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, A.H. Food Allergies and Asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 249–254. [Google Scholar] [CrossRef]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary Polyphenols in the Prevention and Treatment of Allergic Diseases: Polyphenols Alleviate Allergic Inflammation. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, K.; Gandhe, R.; Boldogh, I.; Sur, S. Reactive Oxygen Species (ROS) and Allergic Responses. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3239–3266. ISBN 978-3-642-30018-9. [Google Scholar]
- Qu, J.; Li, Y.; Zhong, W.; Gao, P.; Hu, C. Recent Developments in the Role of Reactive Oxygen Species in Allergic Asthma. J. Thorac. Dis. 2017, 9, E32–E43. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Schulze, A.E.; Joubert, E.; De Villiers, A.; Malherbe, C.J.; Stander, M.A. Food Ingredient Extracts of Cyclopia subternata (Honeybush): Variation in Phenolic Composition and Antioxidant Capacity. Molecules 2012, 17, 14602–14624. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Miura, Y.; Hattori, M.; Matsuda, H.; Malherbe, C.J.; Muller, C.J.F.; Joubert, E.; Yoshida, T. Cyclopia Extracts Enhance Th1-, Th2-, and Th17-type T Cell Responses and Induce Foxp3+ Cells in Murine Cell Culture. Planta Med. 2018, 84, 311–319. [Google Scholar] [CrossRef]
- Yoshida, T.; Malherbe, C.J.; Okon, K.; Miura, Y.; Hattori, M.; Matsuda, H.; Muller, C.J.; Joubert, E. Enhanced Production of Th1- and Th2-Type Antibodies and Induction of Regulatory T Cells in Mice by Oral Administration of Cyclopia Extracts with Similar Phenolic Composition to Honeybush Herbal Tea. J. Funct. Foods 2020, 64, 103704. [Google Scholar] [CrossRef]
- Pérez-Larrán, P.; Díaz-Reinoso, B.; Moure, A.; Alonso, J.L.; Domínguez, H. Adsorption Technologies to Recover and Concentrate Food Polyphenols. Curr. Opin. Food Sci. 2018, 23, 165–172. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, Concentration and Purification of Phenolic Compounds by Adsorption: A Review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Mastuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural Requirements of Flavonoids for Inhibition of Antigen-Induced Degranulation, TNF-α and IL-4 Production from RBL-2H3 Cells. Bioorg. Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- Miller, N.; Malherbe, C.J.; Joubert, E. Xanthone- and Benzophenone-Enriched Nutraceutical: Development of a Scalable Fractionation Process and Effect of Batch-to-Batch Variation of the Raw Material (Cyclopia genistoides). Sep. Purif. Technol. 2020, 237, 116465. [Google Scholar] [CrossRef]
- Wang, W.; Ma, C.; Chen, S.; Zhu, S.; Lou, Z.; Wang, H. Preparative Purification of Epigallocatechin-3-Gallate (EGCG) from Tea Polyphenols by Adsorption Column Chromatography. Chromatographia 2014, 77, 1643–1652. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z.; Gomes, A.; Fernandes, E.; Lima, J.; Bragagnolo, N. In Vitro Scavenging Capacity of Annatto Seed Extracts Against Reactive Oxygen and Nitrogen Species. Food Chem. 2011, 127, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.; Joubert, E.; De Beer, D.; Malherbe, C.J.; Witthuhn, C. Phenylethanoid Glycosides as Major Antioxidants in Lippia multiflora Herbal Infusion and Their Stability During Steam Pasteurisation of Plant Material. Food Chem. 2011, 127, 581–588. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.M.; Álvarez, E.; Arranz, J.A.; Siso, I.G.; Orallo, F. In Vitro Effects of Mangiferin on Superoxide Concentrations and Expression of the Inducible Nitric Oxide Synthase, Tumour Necrosis Factor-α and Transforming Growth Factor-β Genes. Biochem. Pharmacol. 2003, 65, 1361–1371. [Google Scholar] [CrossRef]
- Kammerer, J.; Carle, R.; Kammerer, D.R. Adsorption and Ion Exchange: Basic Principles and Their Application in Food Processing. J. Agric. Food Chem. 2011, 59, 22–42. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Batch and Fixed Bed Column Studies on Phenolic Adsorption from Wine Vinasses by Polymeric Resins. J. Food Eng. 2017, 209, 52–60. [Google Scholar] [CrossRef]
- Putnik, G.; Sluga, A.; ElMaraghy, H.; Teti, R.; Koren, Y.; Tolio, T.; Hon, B. Scalability in Manufacturing Systems Design and Operation: State-of-the-Art and Future Developments Roadmap. CIRP Ann. 2013, 62, 751–774. [Google Scholar] [CrossRef]
- Prentice, J.; Evans, S.T.; Robbins, D.; Ferreira, G. Pressure-Flow Experiments, Packing, and Modeling for Scale-Up of a Mixed Mode Chromatography Column for Biopharmaceutical Manufacturing. J. Chromatogr. A 2020, 1625, 461117. [Google Scholar] [CrossRef] [PubMed]
- Beelders, T.; De Beer, D.; Stander, M.A.; Joubert, E. Comprehensive Phenolic Profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and -MS/MS Reveals Novel Xanthone and Benzophenone Constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef]
- Schulze, A.E.; Beelders, T.; Koch, I.S.; Erasmus, L.M.; De Beer, D.; Joubert, E. Honeybush Herbal Teas (Cyclopia spp.) Contribute to High Levels of Dietary Exposure to Xanthones, Benzophenones, Dihydrochalcones and Other Bioactive Phenolics. J. Food Compos. Anal. 2015, 44, 139–148. [Google Scholar] [CrossRef]
- Stander, M.A.; Redelinghuys, H.; Masike, K.; Long, H.; Van Wyk, B.-E. Patterns of Variation and Chemosystematic Significance of Phenolic Compounds in the Genus Cyclopia (Fabaceae, Podalyrieae). Molecules 2019, 24, 2352. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F.; Álvarez, E.; Basaran, H.; Lugnier, C. Comparative Study of the Vasorelaxant Activity, Superoxide-Scavenging Ability and Cyclic Nucleotide Phosphodiesterase-Inhibitory Effects of Hesperetin and Hesperidin. Naunyn-Schmiedebergs Arch. Pharmacol. 2004, 370, 452–463. [Google Scholar] [CrossRef]
- Malherbe, C.J.; Willenburg, E.; de Beer, D.; Bonnet, S.L.; van der Westhuizen, J.H.; Joubert, E. Iriflophenone-3-C-Glucoside From Cyclopia genistoides: Isolation and Quantitative Comparison of Antioxidant Capacity with Mangiferin and Isomangiferin Using On-Line HPLC Antioxidant Assays. J. Chromatogr. B 2014, 951–952, 164–171. [Google Scholar] [CrossRef]
- Beelders, T.; de Beer, D.; Joubert, E. Thermal Degradation Kinetics Modeling of Benzophenones and Xanthones during High-Temperature Oxidation of Cyclopia genistoides (L.) Vent. Plant Material. J. Agric. Food Chem. 2015, 63, 5518–5527. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-Evaluation of the 2,2-Diphenyl-1-Picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Vyas, A.; Syeda, K.; Ahmad, A.; Padhye, S.; Sarkar, F.H. Perspectives on Medicinal Properties of Mangiferin. Mini-Rev. Med. Chem. 2012, 12, 412–425. [Google Scholar] [CrossRef]
- Rezk, B.M.; Haenen, G.; van der Vijgh, W.J.; Bast, A. The Antioxidant Activity of Phloretin: The Disclosure of a New Antioxidant Pharmacophore in Flavonoids. Biochem. Biophys. Res. Commun. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, S.; Miyake, N.; Kohno, A.H.; Osawa, T. Dihydrochalcones: Evaluation as Novel Radical Scavenging Antioxidants. J. Agric. Food Chem. 2003, 51, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant Activity of the Flavonoid Hesperidin in Chemical and Biological Systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.A.J.; Berghe, D.V. Structure−Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of Xanthine Oxidase by Flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Da Silva, A.; Honório, K.M.; Marangoni, S.; Toyama, M.H.; Da Silva, A.B.F. The Influence of Electronic, Steric and Hydrophobic Properties of Flavonoid Compounds in the Inhibition of the Xanthine Oxidase. J. Mol. Struct. THEOCHEM 2004, 684, 1–7. [Google Scholar] [CrossRef]
- Van Hoorn, D.E.C.; Nijveldt, R.J.; Van Leeuwen, P.A.M.; Hofman, Z.; M’Rabet, L.; De Bont, D.B.A.; Van Norren, K. Accurate Prediction of Xanthine Oxidase Inhibition Based on the Structure of Flavonoids. Eur. J. Pharmacol. 2002, 451, 111–118. [Google Scholar] [CrossRef]
- Suntivich, R.; Songjang, W.; Jiraviriyakul, A.; Ruchirawat, S.; Chatwichien, J. LC-MS/MS Metabolomics-Facilitated Identification of the Active Compounds Responsible for Anti-Allergic Activity of the Ethanol Extract of Xenostegia tridentata. PLoS ONE 2022, 17, e0265505. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.J.; Van Nguyen, T.; Song, C.H.; Chai, O.H. Mangiferin Alleviates Ovalbumin-Induced Allergic Rhinitis via Nrf2/HO-1/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 3415. [Google Scholar] [CrossRef]
- Wei, D.; Ci, X.; Chu, X.; Wei, M.; Hua, S.; Deng, X. Hesperidin Suppresses Ovalbumin-Induced Airway Inflammation in a Mouse Allergic Asthma Model. Inflammation 2012, 35, 114–121. [Google Scholar] [CrossRef]
- Kang, H.; Ku, S.-K.; Jung, B.; Bae, J.-S. Anti-Inflammatory Effects of Vicenin-2 and Scolymoside In Vitro and In Vivo. Agents Actions 2015, 64, 1005–1021. [Google Scholar] [CrossRef]
- Kang, B.-C.; Kim, M.-J.; Lee, S.; Choi, Y.-A.; Park, P.-H.; Shin, T.-Y.; Kwon, T.K.; Khang, D.; Kim, S.-H. Nothofagin Suppresses Mast Cell-Mediated Allergic Inflammation. Chem.-Biol. Interact. 2019, 298, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
| Compound | Extract | Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 | Recovery Yield (%) |
|---|---|---|---|---|---|---|
| IDG | 2.128 | 15.68 (7.4) | 0.180 (0.1) | 0.028 (0.01) | nd | 94.9 |
| IMG | 0.702 | 2.555 (3.6) | 1.494 (2.1) | 0.024 (0.03) | nd | 86.9 |
| Mangiferin | 1.921 | 1.403 (0.7) | 8.594 (4.5) | 0.417 (0.2) | 0.016 (0.01) | 99.2 |
| Isomangiferin | 0.507 | 0.545 (1.1) | 2.328 (4.6) | 0.027 (0.1) | nd | 100 |
| HPDG | 0.388 | 0.050 (0.1) | 1.386 (3.6) | 0.228 (0.6) | nd | 84.3 |
| PDG | 1.305 | nd | 1.697 (1.3) | 2.546 (2.0) | 0.333 (0.3) | 78.2 |
| Eriocitrin | 0.464 | nd | 0.737 (1.6) | 0.931 (2.0) | 0.123 (0.3) | 85.2 |
| Hesperidin | 1.130 | nd | nd | 1.069 (0.9) | 5.950 (5.3) | 67.7 |
| Vicenin-2 | 0.231 | 0.151 (0.7) | 1.045 (4.5) | 0.047 (0.2) | nd | 98.7 |
| Scolymoside | 0.977 | nd | 0.998 (1.0) | 2.021 (2.1) | 0.558 (0.6) | 78.6 |
| p-Coumaric acid | 0.031 | nd | 0.019 (0.6) | 0.030 (1.0) | 0.151 (4.8) | 75.9 |
| Yield (g and percentage of extract) | 18.7 (12.6%) | 27.9 (18.8%) | 39.2 (26.5%) | 12.0 (8.1%) |
| Sample | IC50 (SOA) a | IC50 (DPPH) b | TACORAC c |
|---|---|---|---|
| Extract | 141c ± 5 | 13.7b ± 0.4 | 2877b ± 167 |
| Fraction 1 | 305a ± 14 | 19.3a ± 1.0 | 4237a ± 1 |
| Fraction 2 | 119c ± 4 | 8.28e ± 0.28 | 4224a ± 965 |
| Fraction 3 | 115c ± 19 | 10.2d ± 0.4 | 2740b ± 105 |
| Fraction 4 | 182b ± 26 | 12.1c ± 1.3 | 3199ab ± 434 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippenaar, C.; Shimbo, H.; Okon, K.; Miller, N.; Joubert, E.; Yoshida, T.; de Beer, D. Anti-Allergic and Antioxidant Potential of Polyphenol-Enriched Fractions from Cyclopia subternata (Honeybush) Produced by a Scalable Process. Separations 2022, 9, 278. https://doi.org/10.3390/separations9100278
Dippenaar C, Shimbo H, Okon K, Miller N, Joubert E, Yoshida T, de Beer D. Anti-Allergic and Antioxidant Potential of Polyphenol-Enriched Fractions from Cyclopia subternata (Honeybush) Produced by a Scalable Process. Separations. 2022; 9(10):278. https://doi.org/10.3390/separations9100278
Chicago/Turabian StyleDippenaar, Carla, Hitoshi Shimbo, Kazunobu Okon, Neil Miller, Elizabeth Joubert, Tadashi Yoshida, and Dalene de Beer. 2022. "Anti-Allergic and Antioxidant Potential of Polyphenol-Enriched Fractions from Cyclopia subternata (Honeybush) Produced by a Scalable Process" Separations 9, no. 10: 278. https://doi.org/10.3390/separations9100278
APA StyleDippenaar, C., Shimbo, H., Okon, K., Miller, N., Joubert, E., Yoshida, T., & de Beer, D. (2022). Anti-Allergic and Antioxidant Potential of Polyphenol-Enriched Fractions from Cyclopia subternata (Honeybush) Produced by a Scalable Process. Separations, 9(10), 278. https://doi.org/10.3390/separations9100278







