Development of an HPLC-DAD Method for the Extraction and Quantification of 5-Fluorouracil, Uracil, and 5-Fluorodeoxyuridin Monophosphate in Cells and Culture Media of Lactococcus lactis
Abstract
:1. Introduction
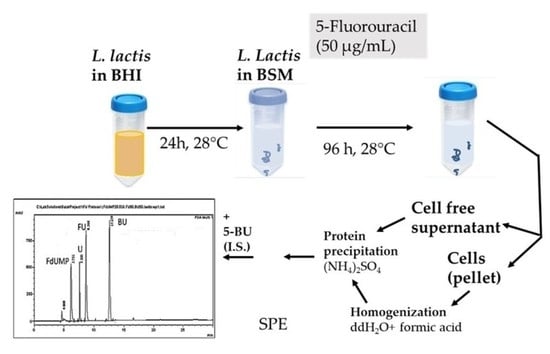
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard Preparation
2.3. Sample Preparation
2.4. Sample Analysis Using HPLC-DAD
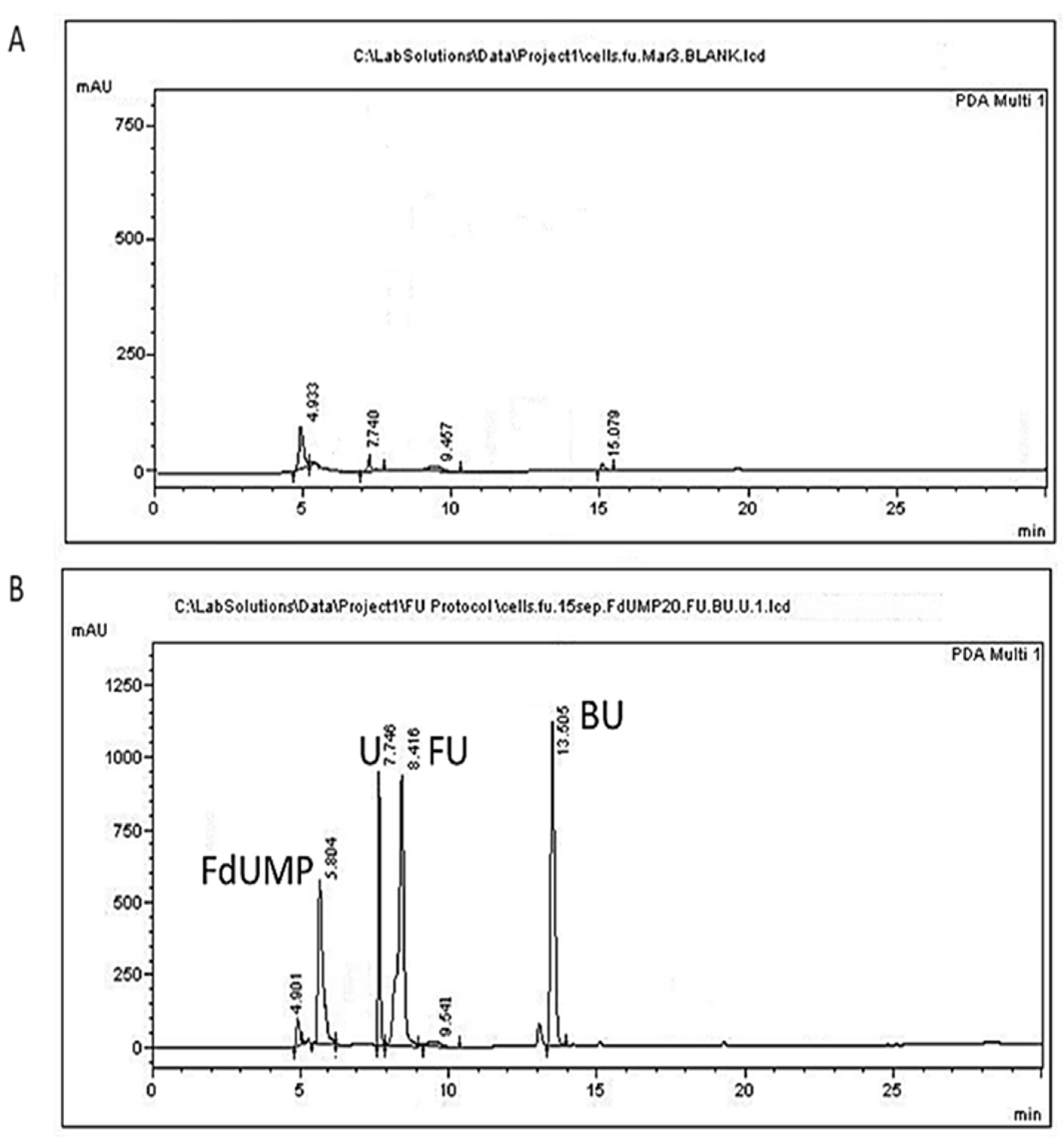
2.5. Peak Identification
2.6. Photodegradation Assay
2.7. FU Administration
3. Results and Discussion
3.1. Optimization of Sample Preparation
3.2. Method Validation
3.3. Photodegradation of 5-FU
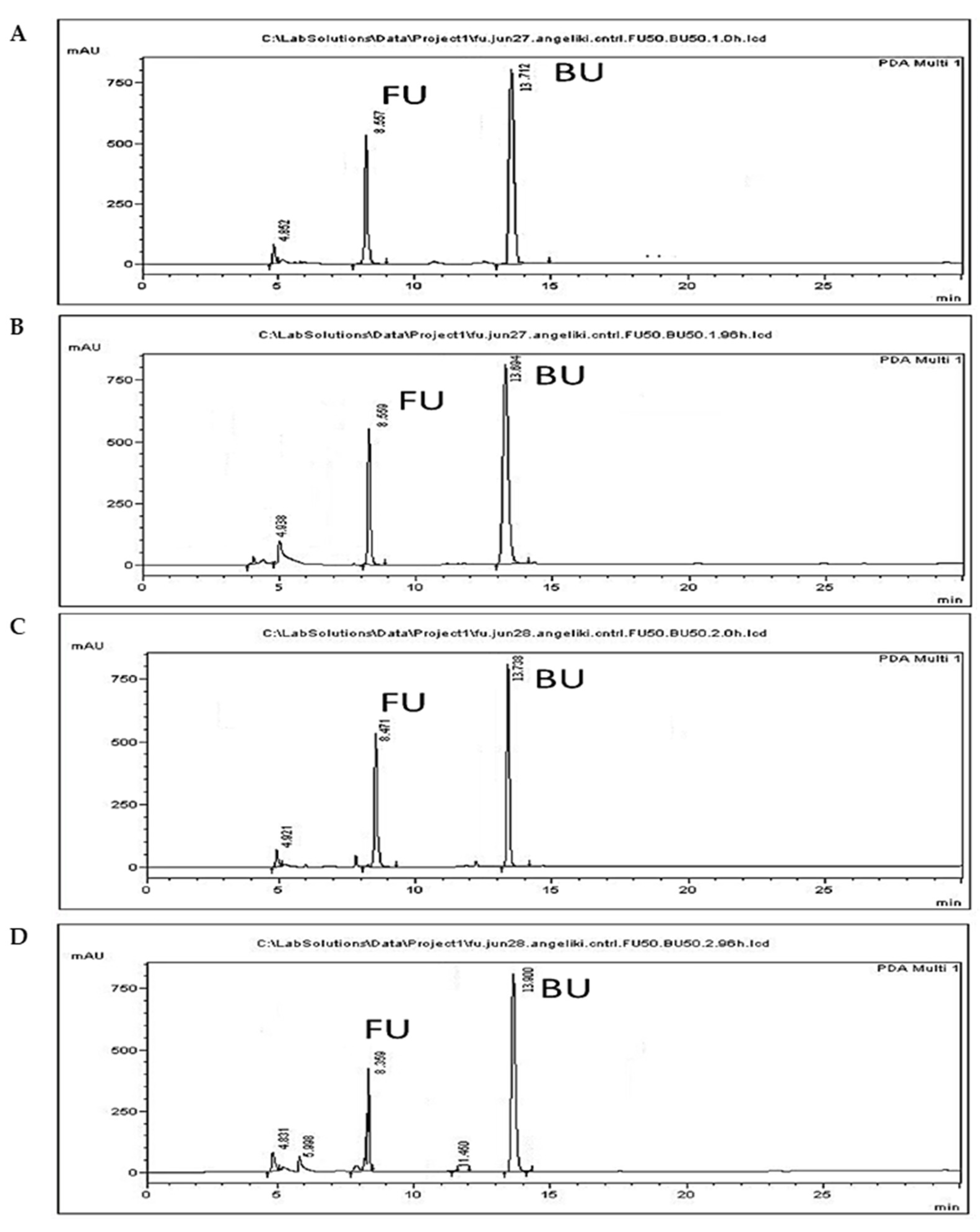
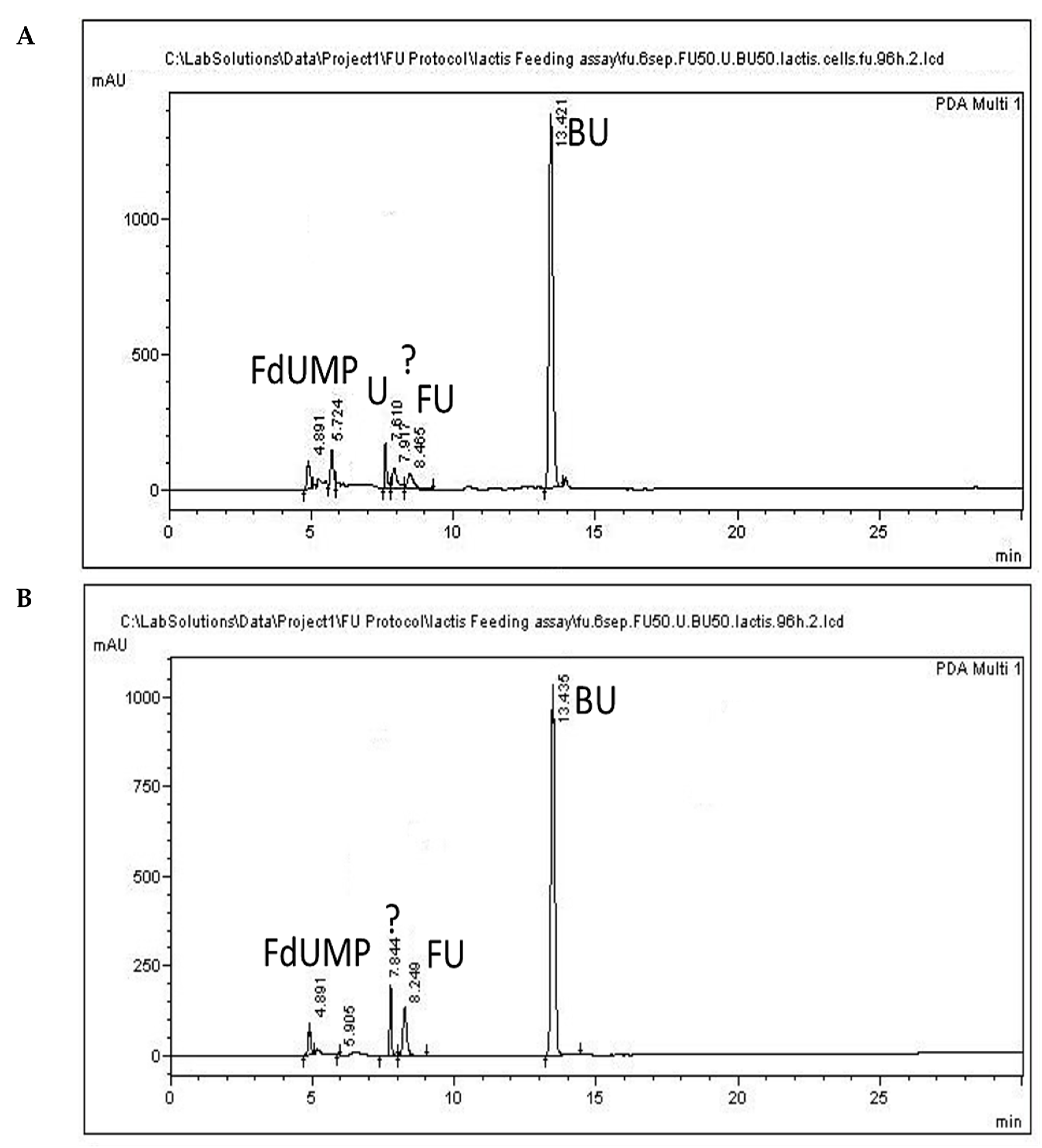
3.4. 5-FU Administration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dushinsky, R.; Pleven, E.; Heidelberger, C. The synthesis of 5-fluoropyrimidines. J. Am. Chem. Soc. 1957, 79, 4559–4560. [Google Scholar] [CrossRef]
- Shirasaka, T. Development history and concept of an oral anticancer agent S-1 (TS-1): Its clinical usefulness and future vistas. Jpn. J. Clin. Oncol. 2009, 39, 2–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Cho, Y.H.; Ro, E.J.; Yoon, J.S.; Mizutani, T.; Kang, D.W.; Park, J.C.; Kim, T.I.; Clevers, H.; Choi, K.Y. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat. Commun. 2020, 11, 5321. [Google Scholar] [CrossRef]
- Houghton, J.A.; Harwood, F.G.; Tillman, D.M. Thymineless death in colon carcinoma cells is mediated via fas signaling. Proc. Natl. Acad. Sci. USA 1997, 94, 8144–8149. [Google Scholar] [CrossRef] [Green Version]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- Di Paolo, A.; Danesi, R.; Ciofi, L.; Vannozzi, F.; Bocci, G.; Lastella, M.; Amatori, F.; Martelloni, B.M.; Ibrahim, T.; Amadori, D.; et al. Improved analysis of 5-Fluorouracil and 5,6-dihydro-5-Fluorouracil by HPLC with diode array detection for determination of cellular dihydropyrimidine dehydrogenase activity and pharmacokinetic profiling. Ther. Drug Monit. 2005, 27, 362–368. [Google Scholar] [CrossRef]
- Lampropoulou, D.I.; Laschos, K.; Amylidi, A.L.; Angelaki, A.; Soupos, N.; Boumpoucheropoulos, S.; Papadopoulou, E.; Nanou, E.; Zidianakis, V.; Nasioulas, G.; et al. Fluoropyrimidine-induced toxicity and DPD deficiency. A case report of early onset, lethal capecitabine-induced toxicity and mini review of the literature. Uridine triacetate: Efficacy and safety as an antidote. Is it accessible outside USA? J. Oncol. Pharm. Pract. 2020, 26, 747–753. [Google Scholar] [CrossRef]
- Ezzeldin, H.; Diasio, R. Dihydropyrimidine dehydrogenase deficiency, a pharmacogenetic syndrome associated with potentially life-threatening toxicity following 5-fluorouracil administration. Clin. Color. Cancer 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Saif, M.W.; Choma, A.; Salamone, S.J.; Chu, E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. J. Natl. Cancer Inst. 2009, 101, 1543–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Chen, H.; Zhang, R.; Liu, Y.; Kong, N.; Guo, Y.; Xu, M. 5-Fluorouracil induced dysregulation of the microbiome-gut-brain axis manifesting as depressive like behaviors in rats. Biochim. Et Biophys. Acta Mol. Basis Dis. 2020, 1866, 165884. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Folch Ayora, A.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef]
- Feng, J.; Gao, M.; Zhao, C.; Yang, J.; Gao, H.; Lu, X.; Ju, R.; Zhang, X.; Zhang, Y. Oral Administration of Probiotics Reduces Chemotherapy-Induced Diarrhea and Oral Mucositis: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 823288. [Google Scholar] [CrossRef]
- Baldwin, C.; Millette, M.; Oth, D.; Ruiz, M.T.; Luquet, F.-M.; Lacroix, M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr. Cancer 2010, 62, 371–378. [Google Scholar] [CrossRef]
- Darbandi, A.; Mirshekar, M.; Shariati, A.; Moghadam, M.T.; Lohrasbi, V.; Asadolahi, P.; Talebi, M. The effects of probiotics on reducing the colorectal cancer surgery complications: A periodic review during 2007–2017. Clin. Nutr. 2020, 39, 2358–2367. [Google Scholar] [CrossRef]
- Agraib, L.M.A.-S.A.; Salah, S.; Abu-hijlih, R.; Abuhijla, F. The effect of probiotics supplementation on the side effects of chemo radiotherapy for colorectal cancer: A literature review. Oncol. Radiother. 2020, 1, 1–9. Available online: https://www.oncologyradiotherapy.com/articles/the-effect-of-probiotics-supplementation-on-the-side-effects-of-chemo-radiotherapy-for-colorectal-cancer-a-literature-review-54551.htmL (accessed on 31 March 2022).
- Singh, V.; Brecik, M.; Mukherjee, R.; Evans, J.C.; Svetlíková, Z.; Blaško, J.; Surade, S.; Blackburn, J.; Warner, D.F.; Mikušová, K.; et al. The complex mechanism of antimycobacterial action of 5-fluorouracil. Chem. Biol. 2015, 22, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Kyrila, G.; Katsoulas, A.; Schoretsaniti, V.; Rigopoulos, A.; Rizou, E.; Doulgeridou, S.; Sarli, V.; Samanidou, V.; Touraki, M. Bisphenol A removal and degradation pathways in microorganisms with probiotic properties. J. Hazard. Mater. 2021, 413, 125363. [Google Scholar] [CrossRef]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 2022, 20, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- García-González, A.P.; Ritter, A.D.; Shrestha, S.; Andersen, E.C.; Yilmaz, L.S.; Walhout, A. Bacterial Metabolism Affects the C. elegans Response to Cancer Chemotherapeutics. Cell 2017, 169, 431–441.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinussen, J.; Hammer, K. Cloning and characterization of upp, a gene encoding uracil phosphoribosyltransferase from Lactococcus lactis. J. Bacteriol. 1994, 176, 6457–6463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björnberg, O.; Rowland, P.; Larsen, S.; Jensen, K.F. Active site of dihydroorotate dehydrogenase A from Lactococcus lactis investigated by chemical modification and mutagenesis. Biochemistry 1997, 36, 16197–16205. [Google Scholar] [CrossRef]
- Dobritzsch, D.; Schneider, G.; Schnackerz, K.D.; Lindqvist, Y. Crystal structure of dihydropyrimidine dehydrogenase, a major determinant of the pharmacokinetics of the anti-cancer drug 5-fluorouracil. EMBO J. 2001, 20, 650–660. [Google Scholar] [CrossRef] [Green Version]
- Breda, M.; Barattè, S. A review of analytical methods for the determination of 5-fluorouracil in biological matrices. Anal. Bioanal. Chem. 2010, 397, 1191–1201. [Google Scholar] [CrossRef]
- Escoriaza, J.; Aldaz, A.; Calvo, E.; Giráldez, J. Simple and sensitive determination of 5-fluorouracil in plasma by high-performance liquid chromatography. Application to clinical pharmacokinetic studies. J. Chromatogr. B Biomed. Sci. Appl. 1999, 736, 97–102. [Google Scholar] [CrossRef]
- Casale, F.; Canaparo, R.; Muntoni, E.; Serpe, L.; Zara, G.P.; Della Pepa, C.; Berno, E.; Costa, M.; Eandi, M. Simultaneous HPLC determination of 5-fluorouracil and its metabolites in plasma of cancer patients. Biomedical chromatography: BMC 2002, 16, 446–452. [Google Scholar] [CrossRef]
- Ackland, S.P.; Garg, M.B.; Dunstan, R.H. Simultaneous Determination of Dihydrofluorouracil and 5-Fluorouracil in Plasma by High-Performance Liquid Chromatography. Anal. Biochem. 1997, 246, 79–85. [Google Scholar] [CrossRef]
- Maring, J.G.; Schouten, L.; Greijdanus, B.; de Vries, E.G.; Uges, D.R. A simple and sensitive fully validated HPLC-UV method for the determination of 5-fluorouracil and its metabolite 5,6-dihydrofluorouracil in plasma. Ther. Drug Monit. 2005, 27, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Barberi-Heyob, M.; Merlin, J.L.; Weber, B. Determination of 5-Fluorouracil and Its Main Metabolites in Plasma by High-Performance Liquid Chromatography. J. Chromatogr. 1992, 573, 241–252. [Google Scholar] [CrossRef]
- Joulia, J.M.; Pinguet, F.; Grosse, P.Y.; Astre, C.; Bressolle, F. Determination of 5-fluorouracil and its main metabolites in plasma by high-performance liquid chromatography: Application to a pharmacokinetic study. J. Chromatogr. B Biomed. Sci. Appl. 1997, 692, 427–435. [Google Scholar] [CrossRef]
- Guerrieri, A.; Palmisano, F.; Zambonin, P.G.; De Lena, M.; Lorusso, V. Solid-phase extraction of fluoropyrimidine derivatives on a copper-modified strong cation exchanger: Determination of doxifluridine, 5-fluorouracil and its main metabolites in serum by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. 1993, 617, 71–77. [Google Scholar] [CrossRef]
- Nassim, M.A.; Shirazi, F.H.; Cripps, C.M.; Veerasinghan, S.; Molepo, M.J.; Obrocea, M.; Redmond, D.; Bates, S.; Fry, D.; Stewart, D.J.; et al. An HPLC method for the measurement of 5-fluorouracil in human plasma with a low detection limit and a high extraction yield. Int. J. Mol. Med. 2002, 10, 513–516. [Google Scholar] [CrossRef]
- Wickremsinhe, E.R.; Lee, L.B.; Schmalz, C.A.; Torchia, J.; Ruterbories, K.J. High sensitive assay employing column switching chromatography to enable simultaneous quantification of an amide prodrug of gemcitabine (LY2334737), gemcitabine, and its metabolite dFdU in human plasma by LC-MS/MS. J. Chromatogr. B Biomed. Sci. Appl. 2013, 932, 117–122. [Google Scholar] [CrossRef]
- Salvador, A.; Millérioux, L.; Renou, A. Simultaneous LC-MS-MS Analysis of Capecitabine and its Metabolites (5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil) After Off-Line SPE from Human Plasma. Chromatographia 2006, 63, 609–615. [Google Scholar] [CrossRef]
- Pandey, K.; Dubey, R.S.; Prasad, B.B. A Critical Review on Clinical Application of Separation Techniques for Selective Recognition of Uracil and 5-Fluorouracil. Indian J. Clin. Biochem. IJCB 2016, 31, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Semail, N.F.; Abdul Keyon, A.S.; Saad, B.; Noordin, S.S.; Nik Mohamed Kamal, N.N.S.; Mohamad Zain, N.N.; Azizi, J.; Kamaruzaman, S.; Yahaya, N. Analytical method development and validation of anticancer agent, 5-fluorouracil, and its metabolites in biological matrices: An updated review. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 562–579. [Google Scholar] [CrossRef]
- Anderson, L.W.; Parker, R.J.; Collins, J.M.; Ahlgren, J.D.; Wilkinson, D.; Strong, J.M. Gas Chromatographic—Mass Spectrometric Method for Routine Monitoring of 5-Fluorouracil in Plasma of Patients Receiving Low-Level Protracted Infusions. J. Chromatogr. B Biomed. Sci. Appl. 1992, 581, 195–201. [Google Scholar] [CrossRef]
- Marunaka, T.; Umeno, Y. Determination of 5-Fluorouracil and Pyrimidine Bases in Plasma by Gas Chromatography-Chemical Ionization-Mass Fragmentography. J. Chromatogr. B Biomed. Sci. Appl. 1980, 221, 382–386. [Google Scholar] [CrossRef]
- Remaud, G.; Boisdron-Celle, M.; Morel, A.; Gamelin, A. Sensitive MS/MS-liquid chromatography assay for simultaneous determination of tegafur, 5-fluorouracil and 5-fluorodihydrouracil in plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 824, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Licea-Perez, H.; Wang, S.; Bowen, C. Development of a sensitive and selective LC-MS/MS method for the determination of alpha-fluoro-beta-alanine, 5-fluorouracil and capecitabine in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 2009, 877, 1040–1046. [Google Scholar] [CrossRef]
- Holleran, J.L.; Eiseman, J.L.; Parise, R.A.; Kummar, S.; Beumer, J.H. LC–MS/MS Assay for the Quantitation of FdCyd and Its Metabolites FdUrd and FU in Human Plasma. J. Pharm. Biomed. Anal. 2016, 129, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Del Nozal, M.; Bernal, J.; Marenero, P.; Pampliega, A. Extraction Procedures for the HPLC determination of 5-fluorouracil in biological samples. J. Liq. Chromatogr. Relat. Technol. 1994, 17, 1621–1636. [Google Scholar] [CrossRef]
- Odagiri, H.; Ichihara, S.; Semura, E.; Utoh, M.; Tateishi, M.; Kuruma, I. Determination of 5-Fluorouracil in Plasma and Liver after Oral Administration of 5’-Deoxy-5-Fluorouridine using Gas Chromatography-Mass Spectrometry. J. Pharmacobio-Dyn. 1988, 11, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, S.; Hamada, M.; Murayama, N.; Nakajima, Y.; Kaniwa, N.; Matsumoto, Y.; Fukuoka, M.; Sawada, J.-I.; Ohno, Y. Cytosolic and microsomal activation of doxifluridine and tegafur to produce 5-fluorouracil in human liver. Cancer Chemother. Pharm. 2002, 50, 454–458. [Google Scholar] [CrossRef]
- Zufía, L.; Aldaz, A.; Castellanos, C.; Giráldez, J. Determination of 5-fluorouracil and its prodrug tegafur in plasma and tissue by high-performance liquid chromatography in a single injection: Validation for application in clinical pharmacokinetic studies. Ther. Drug Monit. 2003, 25, 221–228. [Google Scholar] [CrossRef]
- Wrightson, W.R.; Myers, S.R.; Galandiuk, S. HPLC analysis of 5-FU and FdUMP in tissue and serum. Biochem. Biophys. Res. Commun. 1995, 216, 808–813. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Paschoal, J.; Lopez, R. Quantification of 5-FU in skin samples for the development of new delivery systems for topical cancer treatment. Die Pharm. 2018, 73, 133–138. [Google Scholar] [CrossRef]
- Jochheim, C.; Janning, P.; Marggraf, U.; Löffler, T.M.; Hasse, F.; Linscheid, M. A procedure for the determination of 5-fluorouracil in tissue using microbore HPLC and fluorescence detection. Anal. Biochem. 1994, 217, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, E.; Boisdron-Celle, M.; Larra, F.; Robert, J. A Simple Chromatographic Method for the Analysis of Pyrimidines and their Dihydrogenated Metabolites. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 3155–3172. [Google Scholar] [CrossRef]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Miolo, G.; Marzano, C.; Gandin, V.; Palozzo, A.C.; Dalzoppo, D.; Salvador, A.; Caffieri, S. Photoreactivity of 5-fluorouracil under UVB light: Photolysis and cytotoxicity studies. Chem. Res. Toxicol. 2011, 24, 1319–1326. [Google Scholar] [CrossRef]
- Martinussen, J.; Andersen, P.S.; Hammer, K. Nucleotide metabolism in Lactococcus lactis: Salvage pathways of exogenous pyrimidines. J. Bacteriol. 1994, 176, 1514–1516. [Google Scholar] [CrossRef] [Green Version]
- Martinussen, J.; Glaser, P.; Andersen, P.S.; Saxild, H.H. Two genes encoding uracil phosphoribosyltransferase are present in Bacillus subtilis. J. Bacteriol. 1995, 177, 271–274. [Google Scholar] [CrossRef] [Green Version]
- Deenen, M.J.; Rosing, H.; Hillebrand, M.J.; Schellens, J.H.; Beijnen, J.H. Quantitative determination of capecitabine and its six metabolites in human plasma using liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 913–914, 30–40. [Google Scholar] [CrossRef]
- Botticelli, A.; Borro, M.; Onesti, C.E.; Strigari, L.; Gentile, G.; Cerbelli, B.; Romiti, A.; Occhipinti, M.; Sebastiani, C.; Lionetto, L.; et al. Degradation Rate of 5-Fluorouracil in Metastatic Colorectal Cancer: A New Predictive Outcome Biomarker? PLoS ONE 2016, 11, e0163105. [Google Scholar] [CrossRef]


| Analyte in Medium/Std. Curve Equation/R2 (F, Fcritical, p) | Nominal Conc. (μg/mL) | Mean Calculated Concentration (μg/mL) (Mean ± SD) | Relative Bias (%) | Precision | Recovery (Mean ± SD) | |
|---|---|---|---|---|---|---|
| Intraday (RSD %) | Interday (RSD %) | |||||
| 5-FU in BSM y = 0.0054x + 0.104 0.9999 (34,170.8, 6.4, 2.6 × 10−9) | 5 | 5.3 ± 1.0 | 7.0 | 3.5 | 4.5 | 107.0 ± 19.4 |
| 25 | 24.8 ± 3.8 | −0.7 | 2.7 | 3.3 | 99.2 ± 15.3 | |
| 50 | 50.1 ± 10.7 | 0.3 | 2.8 | 3.3 | 100.2 ± 21.4 | |
| 75 | 74.6 ± 0.8 | −0.5 | 0.9 | 1.0 | 99.5 ±1.1 | |
| 100 | 100.6 ± 7.9 | 0.6 | 6.4 | 7.8 | 100.6 ± 7.9 | |
| 5-FU in L. lactis cells y = 0.0051x + 0.079 0.9999 (37,773, 6.4, 2.1 × 10−9) | 5 | 4.7 ± 0.8 | −5.3 | 0.1 | 0.2 | 94.6 ± 1.3 |
| 25 | 25.6 ± 2.7 | 2.7 | 2.2 | 3.1 | 102.6 ± 9.5 | |
| 50 | 50.4 ± 3.3 | 0.8 | 3.8 | 5.4 | 102.5 ± 3.9 | |
| 75 | 75.8 ± 6.2 | 1.2 | 3.1 | 4.3 | 101.1 ± 3.4 | |
| 100 | 98.5 ± 5.6 | −1.4 | 3.9 | 4.8 | 100.6 ± 5.6 | |
| FdUMP in BSM y = 0.003 + 0.010 0.9999 (128,406.1, 9.3, 3.7 × 10−8) | 5 | 5.1 ± 0.4 | 2.6 | 2.8 | 7.1 | 102.6 ± 7.2 |
| 10 | 9.9 ± 0.4 | −1.3 | 3.1 | 4.0 | 98.7 ± 4.0 | |
| 15 | 14.7 ± 1.2 | −2.0 | 6.8 | 8.3 | 97.9 ± 8.2 | |
| 20 | 20.2 ± 1.3 | 1.3 | 5.3 | 6.6 | 101.3 ± 12.2 | |
| FdUMP in L. lactis cells y = 0.004x + 0.012 0.9998 (70,690.9, 6.4, 6 × 10−10) | 5 | 4.8 ± 0.2 | −3.5 | 0.9 | 3.9 | 96.4 ± 3.8 |
| 10 | 10.1 ± 0.4 | 0.7 | 2.3 | 4.5 | 100.7 ± 4.5 | |
| 15 | 14.8 ± 1.2 | −1.4 | 5.7 | 6.8 | 98.5 ± 7.9 | |
| 20 | 19.7 ± 1.0 | −1.4 | 4.6 | 5.2 | 98.6 ± 5.2 | |
| Uracil in BSM y = 0.014x + 0.041 0.9993 (5197, 9, 4.5 × 10−6) | 5 | 5.3 ± 0.6 | 6.8 | 0.3 | 10.6 | 106.8 ± 11.1 |
| 10 | 10.1 ± 1.1 | 0.8 | 7.3 | 10.9 | 101.1 ± 11.5 | |
| 15 | 14.8 ± 1.1 | −1.5 | 1.0 | 7.1 | 99.8 ± 7.2 | |
| 20 | 20.0 ± 0.7 | 0.2 | 1.8 | 3.6 | 102.2 ±3.7 | |
| Uracil in L. lactis cells y = 0.13x + 0.046 0.9994 (5649.3, 9.3, 4 × 10−6) | 5 | 4.8 ± 0.3 | −3.03 | 2.9 | 3.5 | 96.9 ± 5.8 |
| 10 | 10.9 ± 0.1 | 9.52 | 0.8 | 1.0 | 109.5 ± 1.5 | |
| 15 | 14.9 ± 0.8 | −0.01 | 3.7 | 4.5 | 99.9 ± 5.5 | |
| 20 | 19.9 ± 0.4 | −0.16 | 1.4 | 1.8 | 99.8 ± 2.1 | |
| Std. Curve | Retention Time (min) Mean ± SD (RSD %) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|
| 5-FU in BSM | 8.12 ± 0.11 | 1.2 | 3.7 |
| 5-FU in L. lactis cells | 8. 37 ± 0.17 | 0.9 | 3.0 |
| FdUMP in BSM | 5.89 ± 0.09 | 0.4 | 1.2 |
| FdUMP in L. lactis cells | 5.81 ± 0.11 | 0.4 | 1.2 |
| Uracil in BSM | 7.25 ± 0.11 | 0.7 | 2.1 |
| Uracil in L. lactis cells | 7.76 ± 0.11 | 0.7 | 2.2 |
| Analyte/Sample | Amount (μg/mL) Mean ± SDp (CI, n = 3, df = 2) | Amount in Total Culture (0.25 g Cells/30 mL) (μg) |
|---|---|---|
| 5-FU/medium | 5.2 ± 0.8 (2.0) | 156 |
| 5-FU/cells | 10.5 ± 0.63 (1.6) | 26.2 |
| F-dUMP/medium | nq | - |
| F-dUMP/cells | 21.2 ± 1.2 (2.55) | 47.2 |
| Uracil/medium | nd | - |
| Uracil cells | nq | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavromatis, P.; Stampouli, K.; Vliora, A.; Mayilyan, A.; Samanidou, V.; Touraki, M. Development of an HPLC-DAD Method for the Extraction and Quantification of 5-Fluorouracil, Uracil, and 5-Fluorodeoxyuridin Monophosphate in Cells and Culture Media of Lactococcus lactis. Separations 2022, 9, 376. https://doi.org/10.3390/separations9110376
Mavromatis P, Stampouli K, Vliora A, Mayilyan A, Samanidou V, Touraki M. Development of an HPLC-DAD Method for the Extraction and Quantification of 5-Fluorouracil, Uracil, and 5-Fluorodeoxyuridin Monophosphate in Cells and Culture Media of Lactococcus lactis. Separations. 2022; 9(11):376. https://doi.org/10.3390/separations9110376
Chicago/Turabian StyleMavromatis, Petros, Kyriaki Stampouli, Angeliki Vliora, Anna Mayilyan, Victoria Samanidou, and Maria Touraki. 2022. "Development of an HPLC-DAD Method for the Extraction and Quantification of 5-Fluorouracil, Uracil, and 5-Fluorodeoxyuridin Monophosphate in Cells and Culture Media of Lactococcus lactis" Separations 9, no. 11: 376. https://doi.org/10.3390/separations9110376
APA StyleMavromatis, P., Stampouli, K., Vliora, A., Mayilyan, A., Samanidou, V., & Touraki, M. (2022). Development of an HPLC-DAD Method for the Extraction and Quantification of 5-Fluorouracil, Uracil, and 5-Fluorodeoxyuridin Monophosphate in Cells and Culture Media of Lactococcus lactis. Separations, 9(11), 376. https://doi.org/10.3390/separations9110376