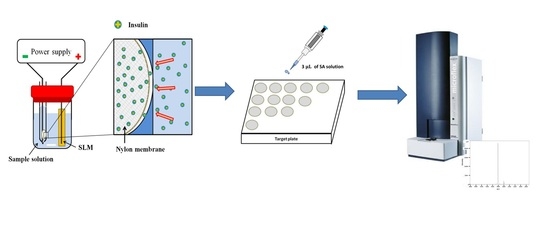
Nylon Membrane-Based Electromembrane Extraction Coupled with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for the Determination of Insulin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
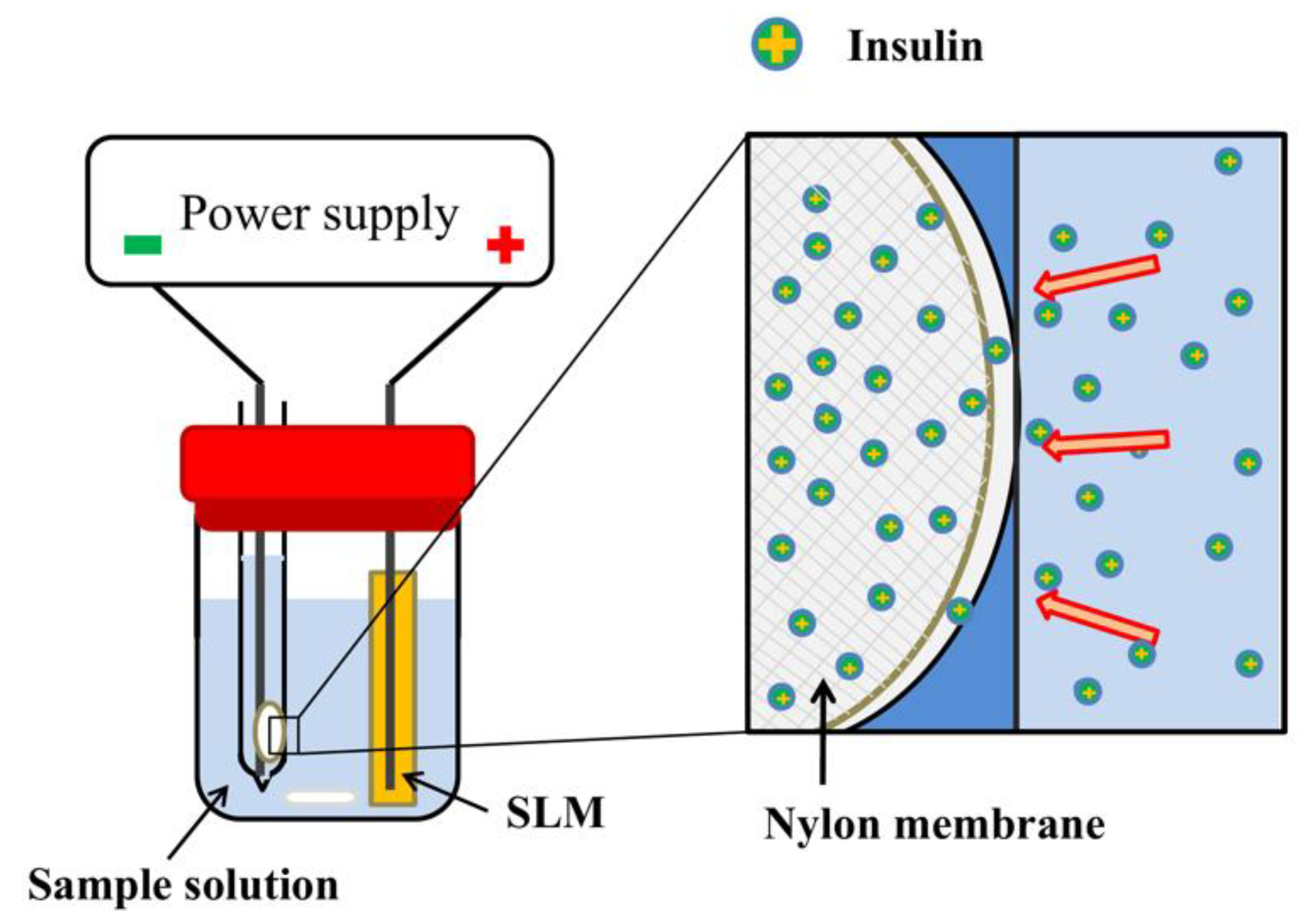
2.2. EME Procedure
2.3. MALDI/MS Measurements
2.4. Preparation of Human Urine Sample
3. Results
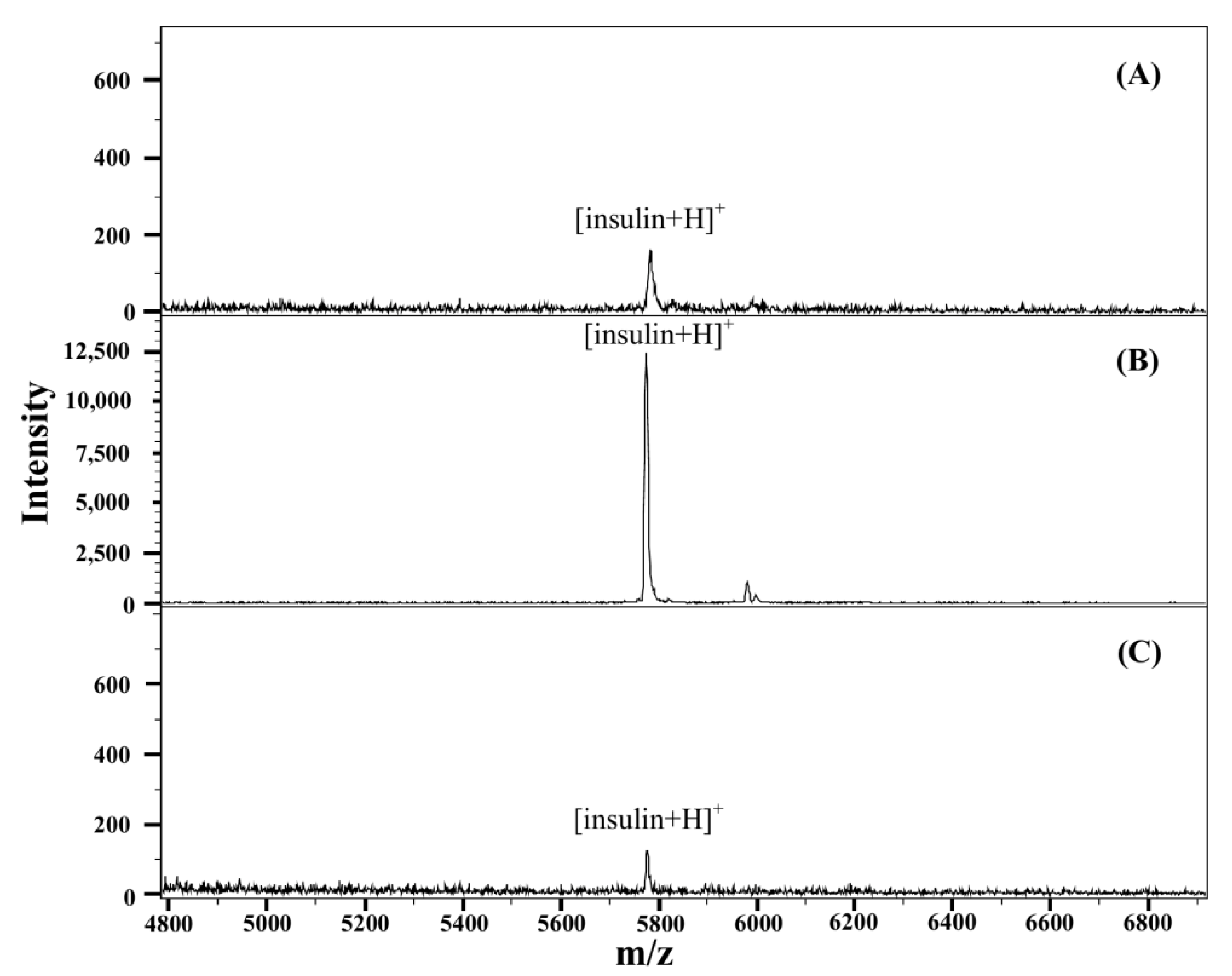
3.1. Detection of Insulin on Nylon Membrane by MALDI/MS
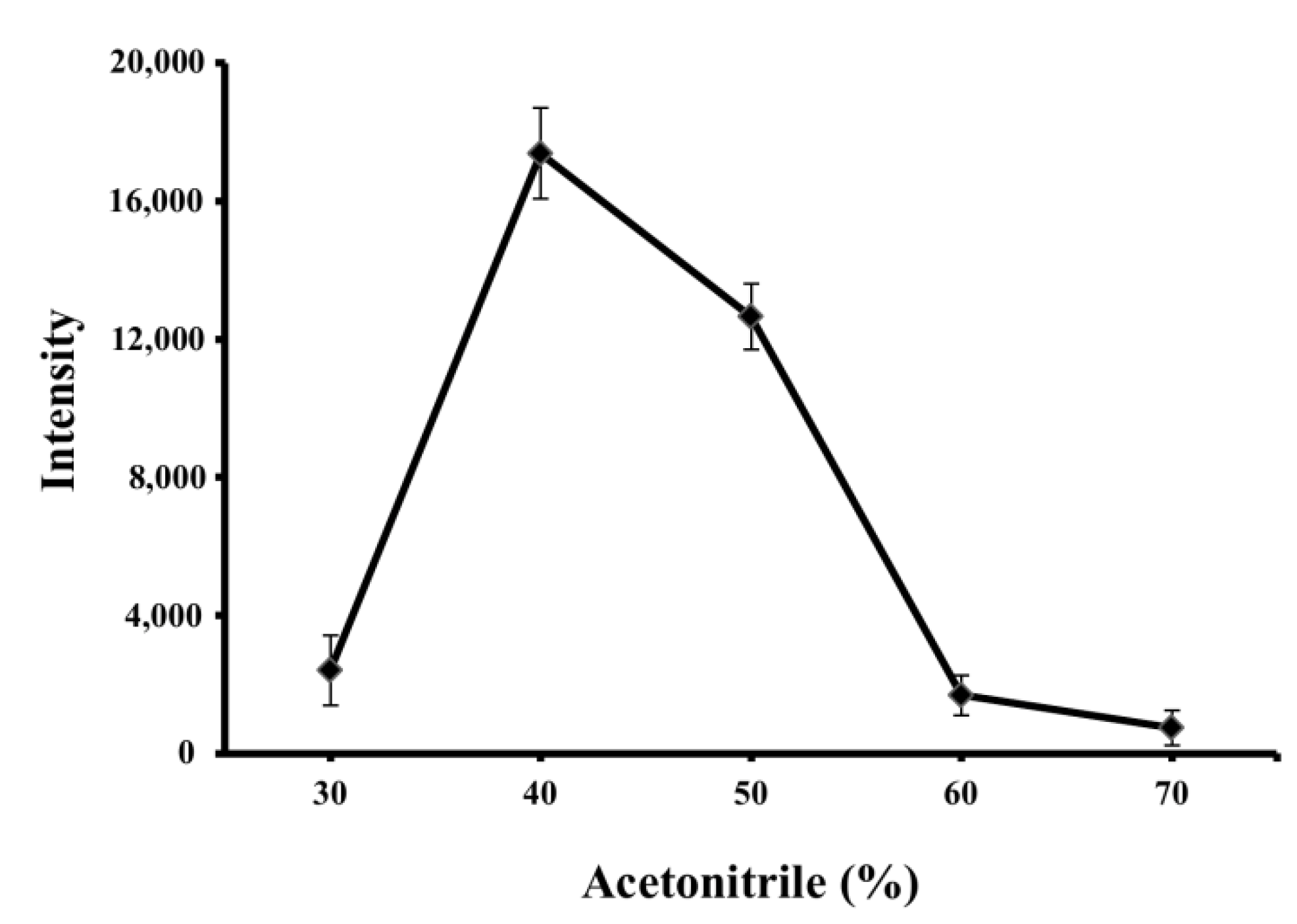
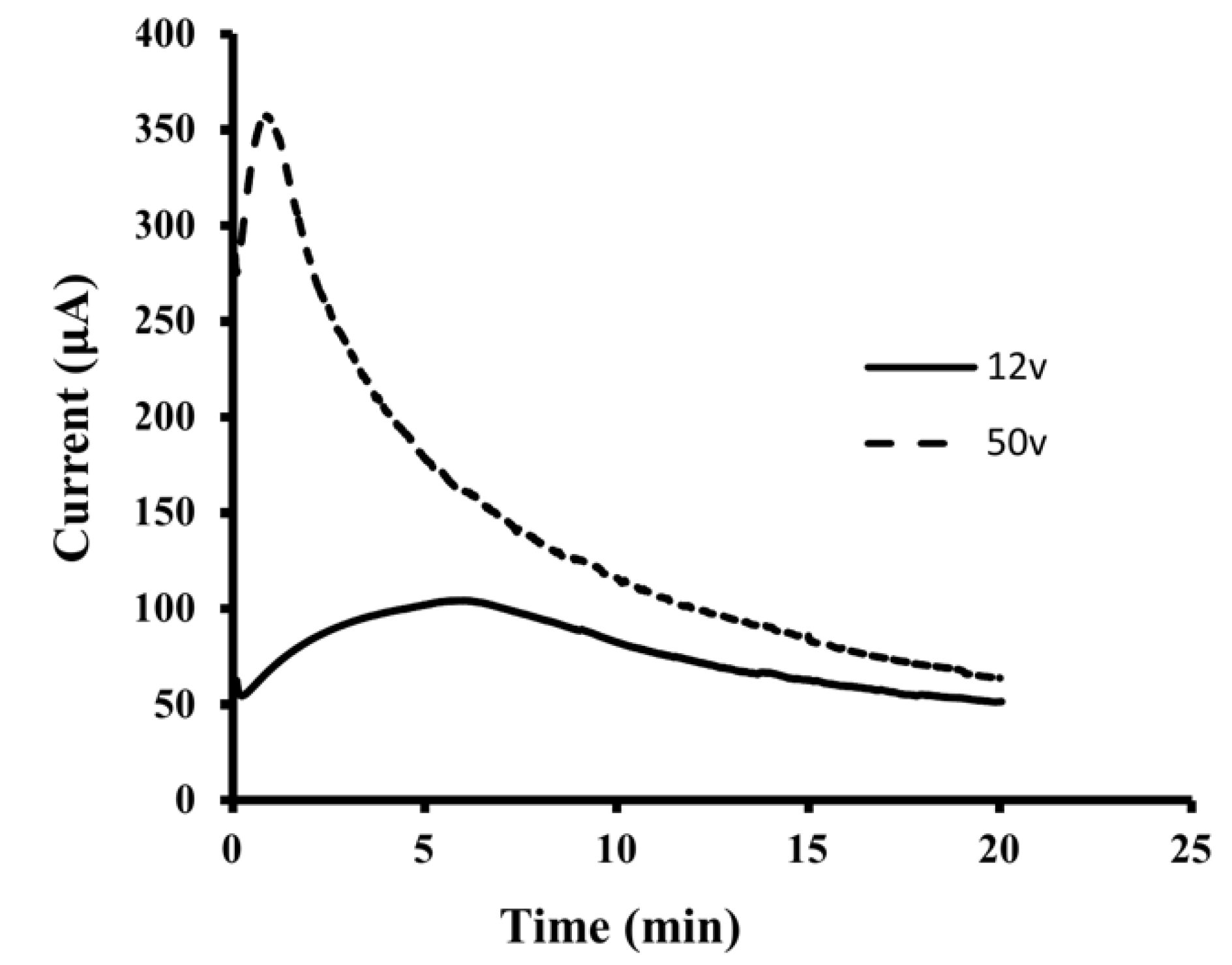
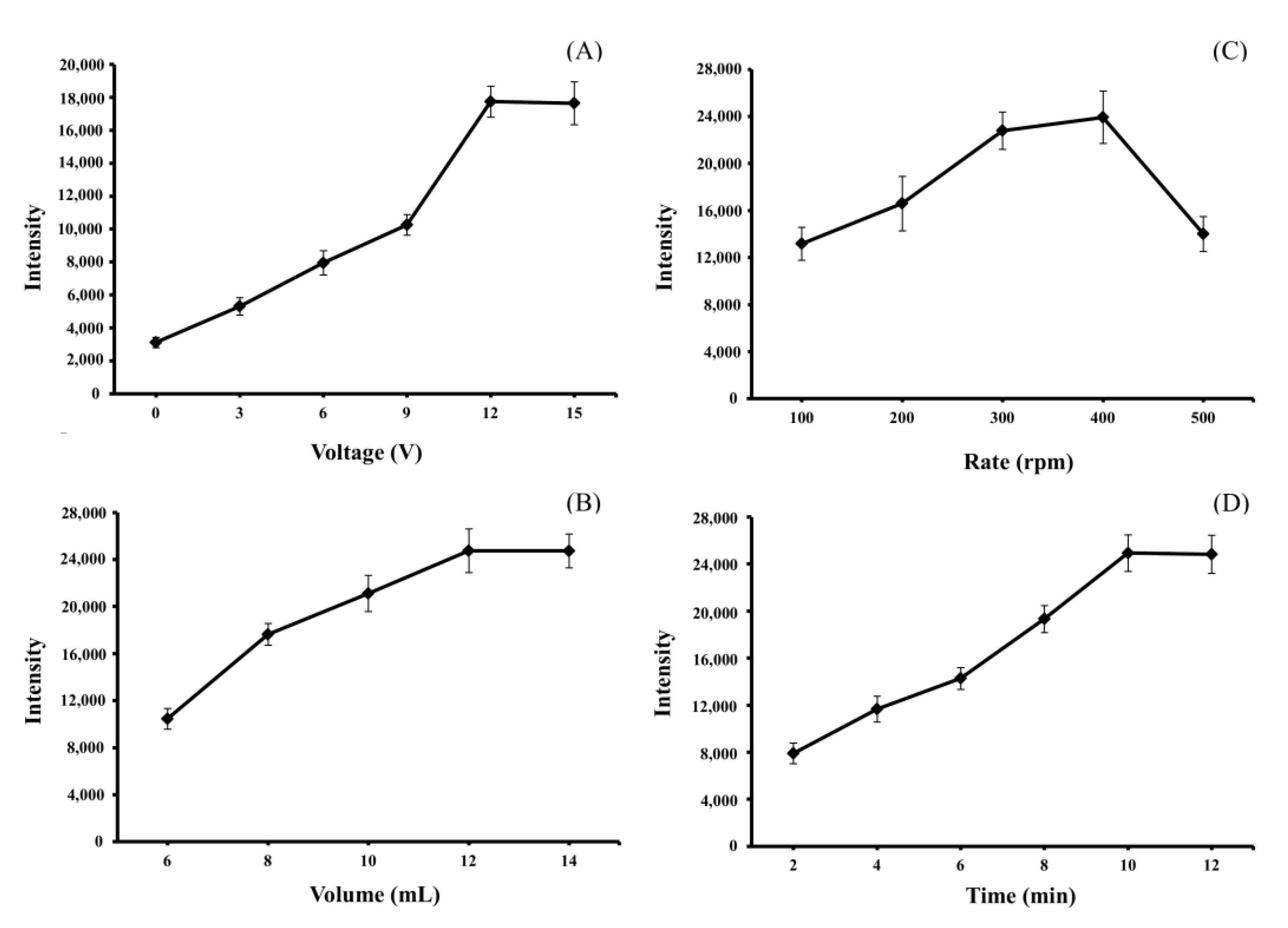
3.2. Optimization of EME Conditions
3.3. Analytical Characteristics
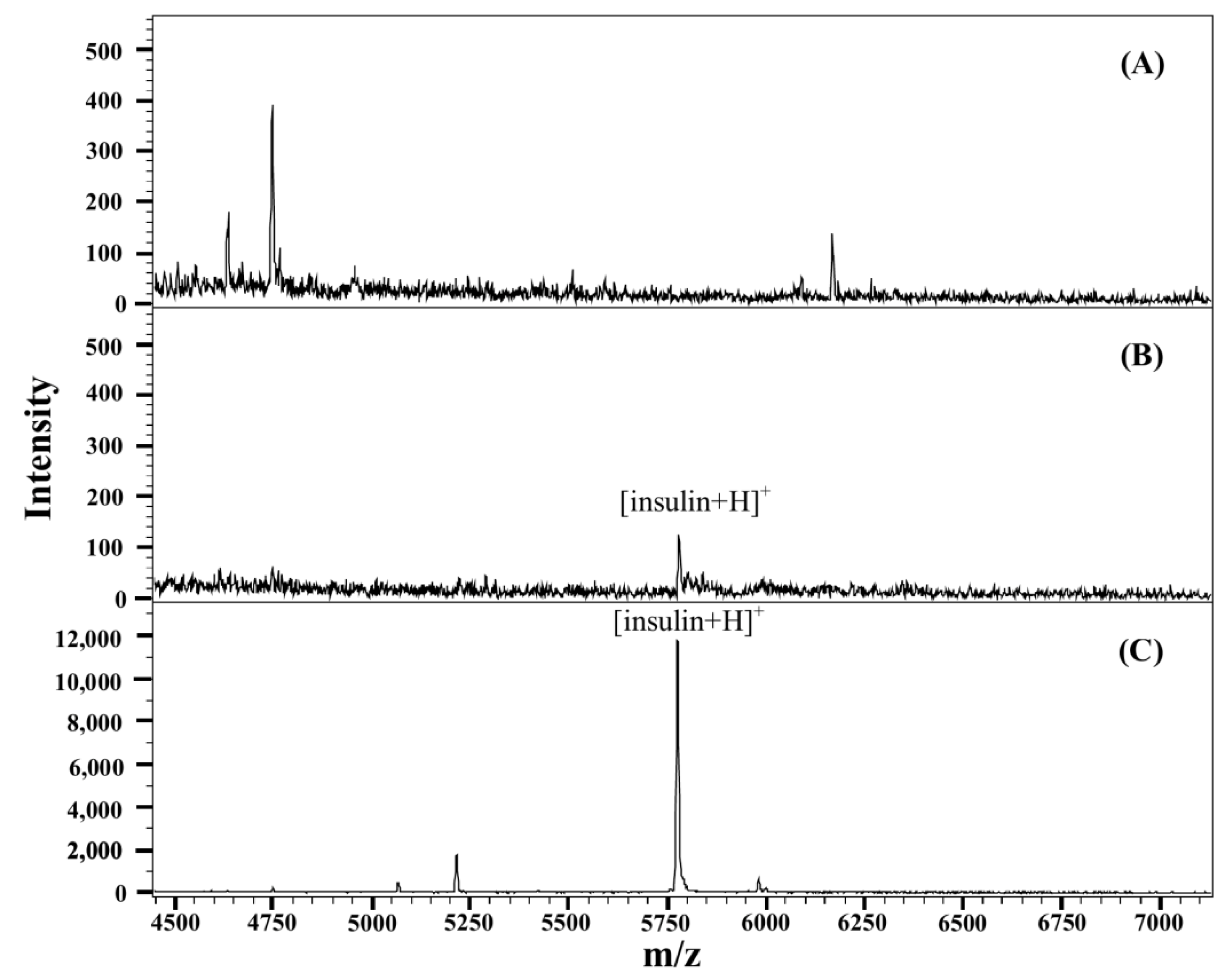
3.4. Analysis of Insulin in Complicated Sample Matrices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen-Bjergaard, S.; Rasmussen, K.E. Electrokinetic migration across artificial liquid membranes new concept for rapid sample preparation of biological fluids. J. Chromatogr. A 2006, 1109, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Balchen, M.; Halvorsen, T.G.; Reubsaet, L.; Pedersen-Bjergaard, S. Rapid isolation of angiotensin peptides from plasma by electromembrane extraction. J. Chromatogr. A 2009, 1216, 6900–6905. [Google Scholar] [CrossRef] [PubMed]
- Drouin, N.; Kubáň, P.; Rudaz, S.; Pedersen-Bjergaard, S. Elecromembrane extraction: Overview of the last decade. Trends Anal. Chem. 2019, 113, 357–363. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Z.; Gjestad, A.; Pedersen-Bjergaard, S.; Shen, X. Elecromembrane extraction. Trends Anal. Chem. 2017, 95, 47–56. [Google Scholar] [CrossRef]
- Balchen, M.; Hatterud, A.G.; Reubsaet, L.; Pedersen-Bjergaard, S. Fundamental studies on the electrokinetic transfer of net cationic peptides across supported liquid membranes. J. Sep. Sci. 2011, 34, 186–195. [Google Scholar] [CrossRef]
- Fashi, A.; Khanban, F.; Yaftian, M.R.; Zamani, A. The cooperative effect of reduced grapheme oxide and Triton X-114 on the electromembrane microextraction efficiency of Pramipexole as a model analyte in urine samples. Talanta 2017, 162, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gjelstad, A.; Pedersen-Bjergaard, S. Exhaustive extraction of peptides by electromembrane extraction. Anal. Chim. Acta 2015, 853, 328–334. [Google Scholar] [CrossRef]
- Huang, C.; Gjelstad, A.; Pedersen-Bjergaard, S. Selective electromembrane extraction based on isoelectric point: Fundamental studies with angiotensin II antipeptide as model analyte. J. Membr. Sci. 2015, 481, 115–123. [Google Scholar] [CrossRef]
- Abbasi, S.; Haeri, S.A. Enrichment of psychotropic drugs using rhamnolipid bioaggregates after electromembrane extraction based on an agarose gel using a rotating electrode as a green and organic solvent-free strategy. J. Chromatogr. A 2021, 1655, 462500. [Google Scholar] [CrossRef]
- Pourahadi, A.; Nojavan, S.; Davarani, S.S.H. Gel-electromembrane extraction of peptides: Determination of five hypothalamic agents in human plasma samples. Talanta 2020, 217, 121025. [Google Scholar] [CrossRef]
- Sedehi, S.; Tabani, H.; Nojavan, S. Electro-driven extraction of polar compounds using agarose gel as a new membrane: Determination of amino acids in fruit juice and human plasma samples. Talanta 2018, 179, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Tabani, H.; Nojavan, S. Application of polyacrylamide gel as a new membrane in electromembrane extraction for the quantification of basic drugs in breast milk and wasterwater samples. J. Pharm. Biomed. Anal. 2018, 151, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, S.; Farhadi, K.; Molaei, R.; Forough, M. Silver nanoparticles-tragacanth gel as a green membrane for effective extraction and determination of capecitabine. J. Sep. Sci. 2020, 43, 2666–2674. [Google Scholar] [CrossRef]
- Román-Hidalgo, C.; Martín-Valero, M.J.; Fernández-Torres, R.; Callejón-Mochón, M.; Bello-López, M.A. New nanostructured support for carrier-mediated electromembrane extraction of high polar compounds. Talanta 2017, 162, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Cristina, R.-H.; Jesús, M.-V.M.; Rut, F.-T.; Ángel, B.-L.M. Use of polymer inclusion membranes (PIMs) as support for electromembrane extraction of non-steroidal anti-inflammatory drugs and highly polar acidic drugs. Talanta 2018, 179, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Mamat, N.A.; See, H.H. Simultaneous electromembrane extraction of cationic and anionic herbicides across hollow polymer inclusion membranes with a bubbleless electrode. J. Chromatogr. A 2017, 1504, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mamat, N.A.; See, H.H. Development and evaluation of electromembrane extraction across a hollow polymer inclusion membrane. J. Chromatogr. A 2015, 1406, 34–39. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Elyasi, Y.; Rastegari, M. An improvement of electrospun membrane reusability via titanium dioxide nanoparticles and silane compounds for the electromembrane extraction. Anal. Chim. Acta 2019, 1088, 168–177. [Google Scholar] [CrossRef]
- Román-Hidalgo, C.; López-Perez, G.; Martín-Valero, M.J.; Bello-López, M.A. Chitosan tailor-made membranes as biopolymeric support for electromembrane extraction. Talanta 2019, 199, 290–295. [Google Scholar] [CrossRef]
- Hansen, F.A.; Pedersen-Bjergaard, S. Electromembrane extraction of streptomycin from biological fluids. J. Chromatogr. A 2021, 1639, 461915. [Google Scholar] [CrossRef]
- Yeh, C.-S.; Cheng, P.-S.; Chang, S.Y. Solvent-free electromembrane extraction: A new concept in electro-driven extraction. Talanta 2019, 199, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Ho, K.C.; Lin, Y.S.; Chen, Y.C. Gold Nanoparticles as Selective and Concentrating Probes for Samples in MALDI MS Analysis. Anal. Chem. 2004, 76, 4337–4342. [Google Scholar]
- Kong, X.L.; Huang, L.C.L.; Hsu, C.M.; Chen, W.H.; Han, C.C.; Chang, H.C. High-Affinity Capture of Proteins by Diamond Nanoparticles for Mass Spectrometric Analysis. Anal. Chem. 2005, 77, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.; Haslam, C.; Hardy, N.; Leveridge, M.; Marshall, P. A systematic investigation of the best buffers for use in screening by MALDI-MASS spectromstry. Slas Discov. Adv. Life Sci. RD 2017, 22, 1262–1269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.S.; Chen, W.C.; Chang, S.Y. Electromembrane extraction of posaconazole for matrix-assisted laser desorption/ionization mass spectrometric detection. Membranes 2022, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Askari, N.; Beheshti-Marnani, A.; Askari, M.B.; Rohani, T. Detection of ultra-trace levels of insulin by Fe3O4@MoS2/γGO-GCE a sensor based on isoelectric points. J. Mater. Sci. Mater. Electron. 2019, 30, 9652–9662. [Google Scholar] [CrossRef]
- Rahmani, T.; Rahimi, A.; Nojavan, S. Study on electrical current variations in electromembrane extraction process: Relation between extraction recovery and magnitude of electrical current. Anal. Chim. Acta 2016, 903, 81–90. [Google Scholar] [CrossRef]
- Ara, K.M.; Raofie, F. Low-voltage electrochemically stimulated stir membrane liquid-liquid microextraction as a novel technique for the determination of methadone. Talanta 2017, 168, 105–112. [Google Scholar] [CrossRef]
- Liang, K.; Wu, H.; Li, Y. Immune-enrichment of insulin in bio-fluids on gold-nanoparticle decorated target plate and in situ detection by MALDI MS. Clin. Proteom. 2017, 14, 5. [Google Scholar] [CrossRef]
- Chang, S.Y.; Zhang, N.Y.; Chen, C.S.; Chen, C.D.; Chen, Y.Y.; Wang, C.R.C. Analysis of peptides and proteins affinity-bound to iron oxide nanoparticles by MALDI MS. J. Am. Soc. Mass Spectrom. 2007, 18, 910–918. [Google Scholar] [CrossRef][Green Version]
- Shastri, L.; Abdelhamid, H.N.; Nawaz, M.; Wu, H.F. Synthesis, characterization and bifunctional applications of bidentate silver nanoparticle assisted single drop microextraction as a highly sensitive preconcentrating probe for protein analysis. RSC Adv. 2015, 5, 41595–41603. [Google Scholar] [CrossRef]
- Shrivas, K.; Wu, H.F. Rapid and highly sensitive protein extraction via cobalt oxide nanoparticle-based liquid-liquid microextraction coupled with MALDI mass spectrometry. Analyst 2012, 137, 890–895. [Google Scholar] [CrossRef] [PubMed]

| Insulin | Linear Range (nM) | Regression Equation a | R b | LOD c (nM) | EF | Recovery |
|---|---|---|---|---|---|---|
| Standard solution | 1.0–100.0 | y = 253.7x + 5.4 | 0.9991 | 0.3 | 103 | 65.1% |
| Urine samples | 5.0–100.0 | y = 253.2x + 45.6 | 0.9979 | 1.5 | 77 | 57.2% |
| Analyte | Concentration (nM) | Within-Day | Day-to-Day | ||
|---|---|---|---|---|---|
| Precision a (%) | Accuracy b (%) | Precision a (%) | Accuracy b (%) | ||
| Insulin | 8.0 | 2.3 | −5.8 | 5.1 | −5.3 |
| 50.0 | 3.3 | 0.6 | 3.5 | 3.0 | |
| 80.0 | 2.0 | 0.6 | 3.6 | −1.1 | |
| Analytical Method | Sample Preparation | Extraction Time (min) | S/N Ratio Enhancement | LOD (nM) | Reference |
|---|---|---|---|---|---|
| MALDI/MS | NP-LLMS | 10 | 12.5 | 5.0 | [32] |
| MALDI/MS | NP-SDME | 10 | not available | 80.0 | [31] |
| MALDI/MS | NP-based SPE | 60 | 15 | 0.1 | [30] |
| MALDI/MS | NP-decorated target plate | 30 | not available | 0.8 | [29] |
| MALDI/MS | EME | 10 | 103 | 0.3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-K.; Hsiao, Y.-W.; Chen, W.-C.; Chang, S.Y. Nylon Membrane-Based Electromembrane Extraction Coupled with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for the Determination of Insulin. Separations 2022, 9, 286. https://doi.org/10.3390/separations9100286
Huang J-K, Hsiao Y-W, Chen W-C, Chang SY. Nylon Membrane-Based Electromembrane Extraction Coupled with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for the Determination of Insulin. Separations. 2022; 9(10):286. https://doi.org/10.3390/separations9100286
Chicago/Turabian StyleHuang, Jun-Kai, Yi-Wen Hsiao, Wen-Chi Chen, and Sarah Y. Chang. 2022. "Nylon Membrane-Based Electromembrane Extraction Coupled with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for the Determination of Insulin" Separations 9, no. 10: 286. https://doi.org/10.3390/separations9100286
APA StyleHuang, J.-K., Hsiao, Y.-W., Chen, W.-C., & Chang, S. Y. (2022). Nylon Membrane-Based Electromembrane Extraction Coupled with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry for the Determination of Insulin. Separations, 9(10), 286. https://doi.org/10.3390/separations9100286