Antimycobacterial Activity of Rosmarinus officinalis (Rosemary) Extracted by Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. DES Preparation
2.4. Extraction Procedure
2.5. Antimycobacterial Activity
2.5.1. MDR-M. tuberculosis Bacterial Strain
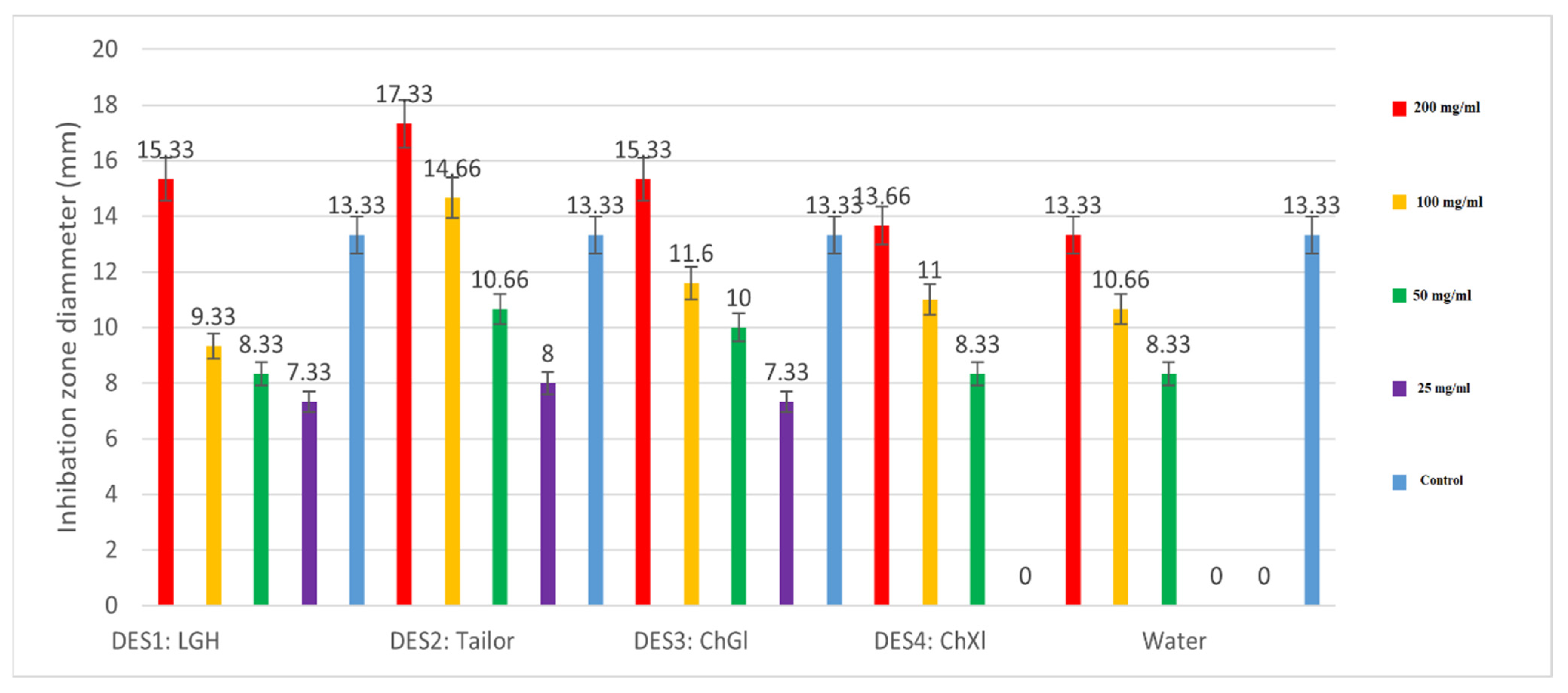
2.5.2. Disk Diffusion Assay for Determining the Inhibitory Zone (DIZ)
2.5.3. Determination of Minimal Inhibitory Concentrations (MICs)
2.5.4. Determination of Minimal Bacterial Concentrations (MBCs)
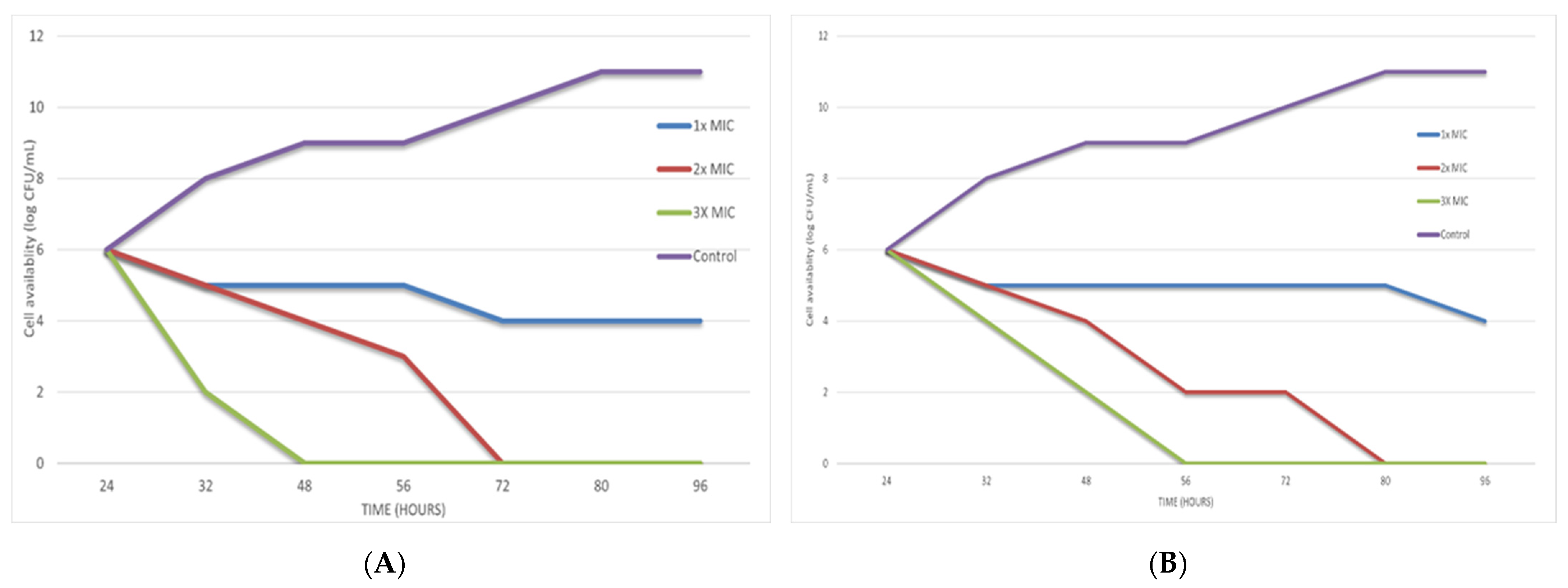
2.6. Time-Kill Assay
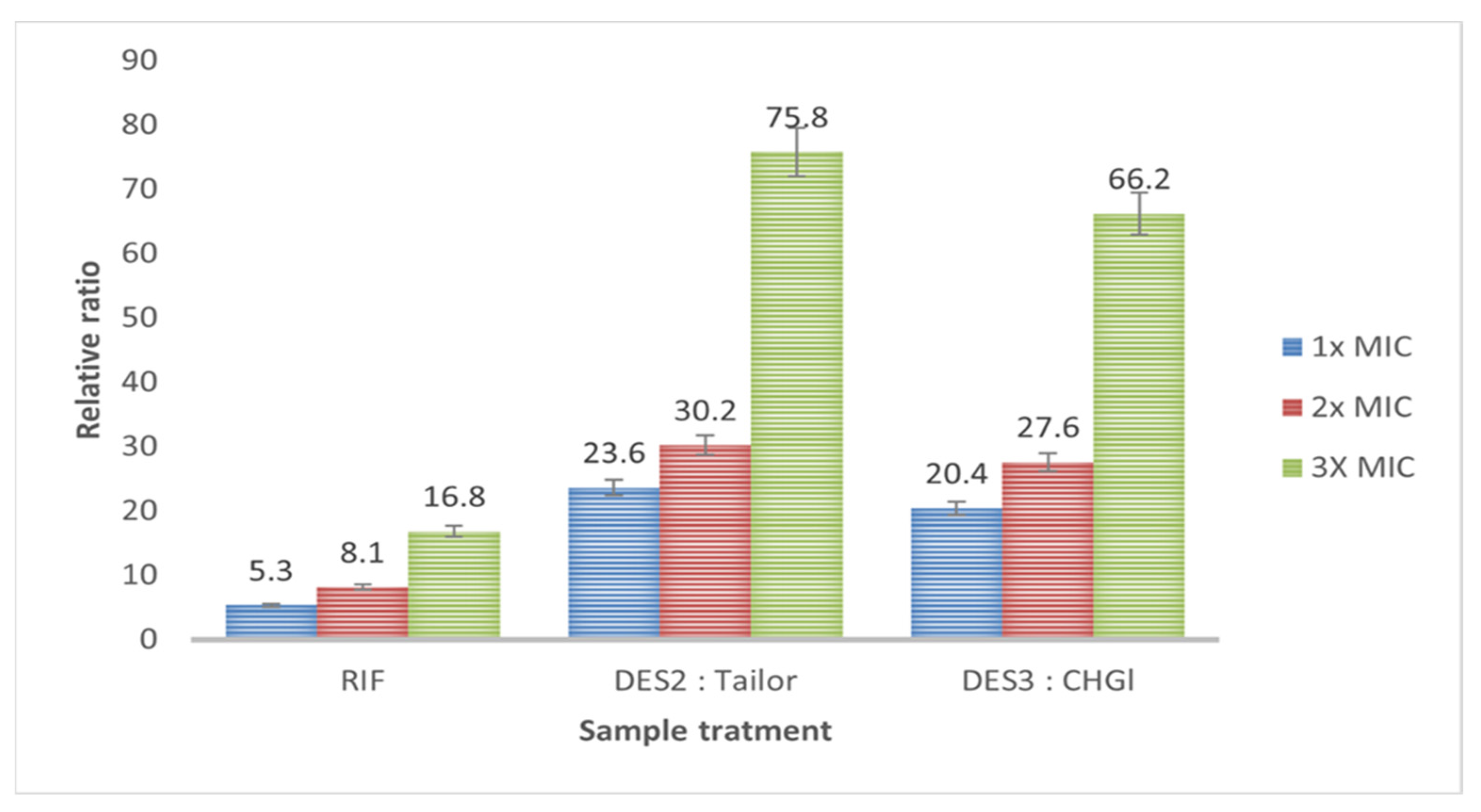
2.7. Cell Wall Integrity
2.8. In Vitro Cytotoxicity Assay
2.8.1. Cell Lines and Cell Cultures
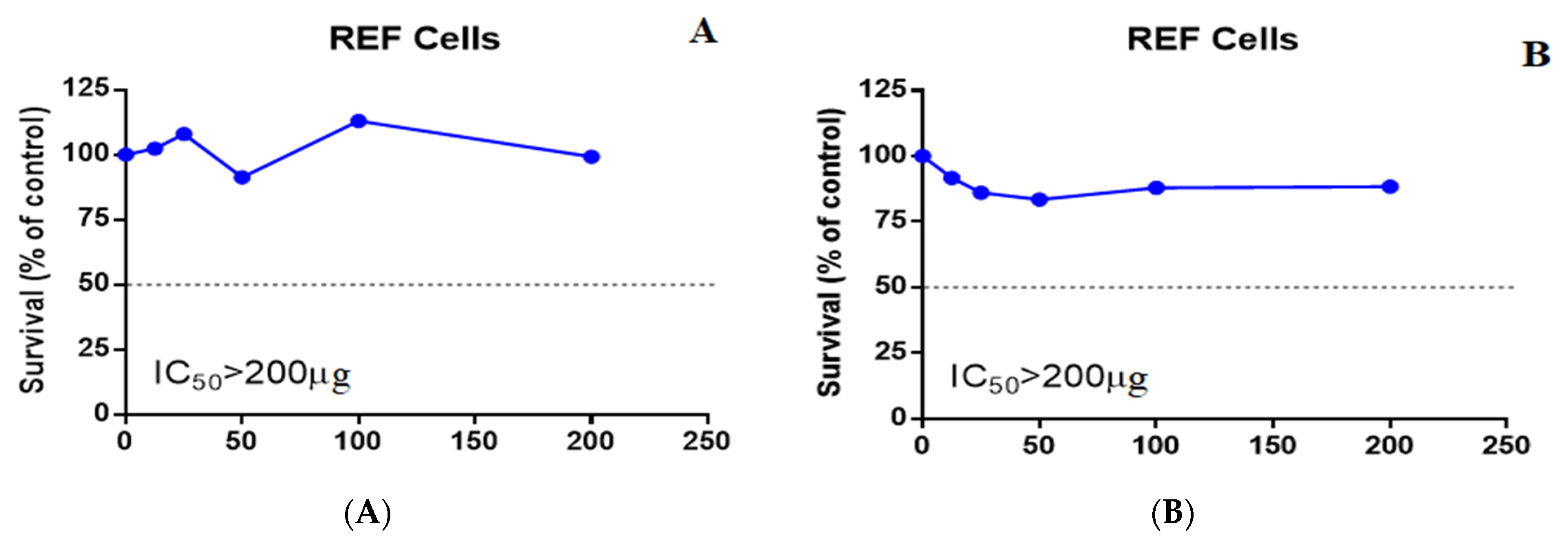
2.8.2. Cytotoxicity Assay
2.9. Phytochemical Analysis
2.9.1. Total Phenolic and Flavonoid Contents. [TPC and TFC]
2.9.2. Identification of Bioactive Constituents Using Liquid Chromatography-Mass Spectroscopy
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehyab, A.S.; Bakar, M.F.A.; AlOmar, M.K.; Sabran, S.F. A review of medicinal plant of Middle East and North Africa (MENA) region as source in tuberculosis drug discovery. Saudi J. Biol. Sci. 2020, 27, 2457. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.-L.; Han, L.-F.; Wang, R.-F.; Yang, Z.-C. Bioassay-guided isolation of an antimycobacterial compound from Leonurus japonicus Houtt. South Afr. J. Bot. 2019, 121, 92–97. [Google Scholar] [CrossRef]
- MacPherson, P.; Lebina, L.; Motsomi, K.; Bosch, Z.; Milovanovic, M.; Ratsela, A.; Lala, S.; Variava, E.; Golub, J.E.; Webb, E.L. Prevalence and risk factors for latent tuberculosis infection among household contacts of index cases in two South African provinces: Analysis of baseline data from a cluster-randomised trial. PLoS ONE 2020, 15, e0230376. [Google Scholar] [CrossRef] [PubMed]
- Mangwani, N.; Singh, P.K.; Kumar, V. Medicinal plants: Adjunct treatment to tuberculosis chemotherapy to prevent hepatic damage. J. Ayurveda Integr. Med. 2020, 11, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Ahmad, H.I.; Jabbar, A.; Tolba, M.M.; AbouZid, S.; Irm, N.; Zulfiqar, F.; Iqbal, M.Z.; Ahmad, S.; Aslam, Z. Use of medicinal plants for respiratory diseases in Bahawalpur, Pakistan. BioMed Res. Int. 2021, 2021, 5578914. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Steele, B.; Bryant, J.; Fouad, E.; Toyang, N.; Ngwa, W. Antiviral activity of Jamaican medicinal plants and isolated bioactive compounds. Molecules 2021, 26, 607. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- Robson, H.; Braund, R.; Glass, M.; Ashton, J.; Tatley, M. Synthetic cannabis: Adverse events reported to the New Zealand Pharmacovigilance Centre. Clin. Toxicol. 2021, 59, 472–479. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Pourbagher-Shahri, A.M. Hypolipidemic effects of Rosmarinus officinalis L. J. Cell. Physiol. 2019, 234, 14680–14688. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Babakir-Mina, M. Investigation of rosemary herbal extracts (Rosmarinus officinalis) and their potential effects on immunity. Phytother. Res. 2020, 34, 1829–1837. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L.(rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 1–22. [Google Scholar] [CrossRef]
- Gonçalves, C.; Fernandes, D.; Silva, I.; Mateus, V. Potential Anti-Inflammatory Effect of Rosmarinus officinalis in Preclinical In Vivo Models of Inflammation. Molecules 2022, 27, 609. [Google Scholar] [CrossRef]
- Dheyab, A.S.; Abu Bakar, M.F.; AlOmar, M.; Sabran, S.F.; Hanafi, A.F.M.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8, 176. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Cavalheiro, F.B.; Toci, A.T.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Namieśnik, J. Ionic liquids and deep eutectic mixtures: Sustainable solvents for extraction processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef]
- Abdul Hadi, N.m.; Ng, M.H.; Choo, Y.M.; Hashim, M.A.; Jayakumar, N.S. Performance of choline-based deep eutectic solvents in the extraction of tocols from crude palm oil. J. Am. Oil Chem. Soc. 2015, 92, 1709–1716. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Deep eutectic solvents microbial toxicity: Current state of art and critical evaluation of testing methods. J. Hazard. Mater. 2022, 425, 127963. [Google Scholar] [CrossRef]
- Jyoti, M.A.; Nam, K.-W.; Jang, W.S.; Kim, Y.-H.; Kim, S.-K.; Lee, B.-E.; Song, H.-Y. Antimycobacterial activity of methanolic plant extract of Artemisia capillaris containing ursolic acid and hydroquinone against Mycobacterium tuberculosis. J. Infect. Chemother. 2016, 22, 200–208. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Singh, R.; Hussain, S.; Verma, R.; Sharma, P. Anti-mycobacterial screening of five Indian medicinal plants and partial purification of active extracts of Cassia sophera and Urtica dioica. Asian Pac. J. Trop. Med. 2013, 6, 366–371. [Google Scholar] [CrossRef]
- Foongladda, S.; Roengsanthia, D.; Arjrattanakool, W.; Chuchottaworn, C.; Chaiprasert, A.; Franzblau, S. Rapid and simple MTT method for rifampicin and isoniazid susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2002, 6, 1118–1122. [Google Scholar]
- Silva, F.; Lourenço, O.; Queiroz, J.A.; Domingues, F.C. Bacteriostatic versus bactericidal activity of ciprofloxacin in Escherichia coli assessed by flow cytometry using a novel far-red dye. J. Antibiot. 2011, 64, 321–325. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Nace, G.W.; Irwin, P.L. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods 2003, 55, 475–479. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lee, S.; Yoon, Y.; Choi, K.-H. Antimicrobial action of oleanolic acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis. PLoS ONE 2015, 10, e0118800. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.F.; Ahmad, N.E.; Suleiman, M.; Rahmat, A.; Isha, A. Garcinia dulcis fruit extract induced cytotoxicity and apoptosis in HepG2 liver cancer cell line. BioMed Res. Int. 2015, 2015, 916902. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.-N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Zakaria, F.; Ibrahim, W.N.W.; Ismail, I.S.; Ahmad, H.; Manshoor, N.; Ismail, N.; Zainal, Z.; Shaari, K. LCMS/MS Metabolite Profling and Analysis of Acute Toxicity Effect of the Ethanolic Extract of Centella asiatica on Zebrafsh Model. Pertanika J. Sci. Technol. 2019, 27, 985–1003. [Google Scholar]
- Abu Bakar, F.I.; Abu Bakar, M.F.; Abdullah, N.; Endrini, S.; Fatmawati, S. Optimization of extraction conditions of phytochemical compounds and anti-gout activity of Euphorbia hirta L. (Ara Tanah) using response surface methodology and liquid chromatography-mass spectrometry (LC-MS) Analysis. Evid.-Based Complement. Altern. Med. 2020, 2020, 4501261. [Google Scholar] [CrossRef]
- Cattaneo, L.; Cicconi, R.; Mignogna, G.; Giorgi, A.; Mattei, M.; Graziani, G.; Ferracane, R.; Grosso, A.; Aducci, P.; Schininà, M.E. Anti-proliferative effect of Rosmarinus officinalis L. extract on human melanoma A375 cells. PLoS ONE 2015, 10, e0132439. [Google Scholar]
- Newton, S.M.; Lau, C.; Wright, C.W. A review of antimycobacterial natural products. Phytother. Res. 2000, 14, 303–322. [Google Scholar] [CrossRef]
- Okunade, A.L.; Elvin-Lewis, M.P.; Lewis, W.H. Natural antimycobacterial metabolites: Current status. Phytochemistry 2004, 65, 1017–1032. [Google Scholar] [CrossRef]
- Luo, X.; Pires, D.; Aínsa, J.A.; Gracia, B.; Mulhovo, S.; Duarte, A.; Anes, E.; Ferreira, M.-J.U. Antimycobacterial evaluation and preliminary phytochemical investigation of selected medicinal plants traditionally used in Mozambique. J. Ethnopharmacol. 2011, 137, 114–120. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, J.; Gai, Q.-Y.; Wang, P.; Guo, N.; Niu, L.-L.; Fu, Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, R.; Pal, M.; Pandey, S. How polar are choline chloride-based deep eutectic solvents? Phys. Chem. Chem. Phys. 2014, 16, 1559–1568. [Google Scholar] [CrossRef]
- Alderwick, L.J.; Harrison, J.; Lloyd, G.S.; Birch, H.L. The mycobacterial cell wall—Peptidoglycan and arabinogalactan. Cold Spring Harb. Perspect. Med. 2015, 5, a021113. [Google Scholar] [CrossRef]
- Adibian, F.; Ghaderi, R.S.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Ghazvini, K.; Youssefi, M.; Soleimanpour, S.; Darroudi, M. Green synthesis of selenium nanoparticles using Rosmarinus officinalis and investigated their antimicrobial activity. BioMetals 2022, 35, 147–158. [Google Scholar] [CrossRef]
- Bakker-Woudenberg, I.A.; van Vianen, W.; van Soolingen, D.; Verbrugh, H.A.; van Agtmael, M.A. Antimycobacterial agents differ with respect to their bacteriostatic versus bactericidal activities in relation to time of exposure, mycobacterial growth phase, and their use in combination. Antimicrob. Agents Chemother. 2005, 49, 2387–2398. [Google Scholar] [CrossRef]
- Woods, G. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic Actinomycetes; Tentative standard. NCCLS M24-T2 2000, 20, 1–59. [Google Scholar]
- de Steenwinkel, J.E.; de Knegt, G.J.; ten Kate, M.T.; van Belkum, A.; Verbrugh, H.A.; Kremer, K.; van Soolingen, D.; Bakker-Woudenberg, I.A. Time–kill kinetics of anti-tuberculosis drugs, and emergence of resistance, in relation to metabolic activity of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2010, 65, 2582–2589. [Google Scholar] [CrossRef]
- Mohamad, S.; Ibrahim, P.; Wahab, H.A. Effects of isoniazid on viability, cell morphologies and acid fastness properties of Mycobacterium avium NCTC 8559 during the growth cycle. Chemotherapy 2007, 53, 263–266. [Google Scholar] [CrossRef]
- World Health Organization; Stop TB Initiative. Treatment of tuberculosis: Guidelines; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Rodriguez-Rivera, F.P.; Zhou, X.; Theriot, J.A.; Bertozzi, C.R. Visualization of mycobacterial membrane dynamics in live cells. J. Am. Chem. Soc. 2017, 139, 3488–3495. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chiang, M.-L.; Chou, C.-C. The effect of temperature and length of heat shock treatment on the thermal tolerance and cell leakage of Cronobacter sakazakii BCRC 13988. Int. J. Food Microbiol. 2009, 134, 184–189. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Li, P.; Liu, G.; Zhang, J.; Dai, Y. Mode of action of pentocin 31-1: An antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control 2008, 19, 817–822. [Google Scholar] [CrossRef]
- Sun, T.; Qin, B.; Gao, M.; Yin, Y.; Wang, C.; Zang, S.; Li, X.; Zhang, C.; Xin, Y.; Jiang, T. Effects of epigallocatechin gallate on the cell-wall structure of Mycobacterial smegmatis mc2155. Nat. Prod. Res. 2015, 29, 2122–2124. [Google Scholar] [CrossRef]
- Fukuda, T.; Matsumura, T.; Ato, M.; Hamasaki, M.; Nishiuchi, Y.; Murakami, Y.; Maeda, Y.; Yoshimori, T.; Matsumoto, S.; Kobayashi, K. Critical roles for lipomannan and lipoarabinomannan in cell wall integrity of mycobacteria and pathogenesis of tuberculosis. MBio 2013, 4, e00472-00412. [Google Scholar] [CrossRef]
- Bero, J.; Kpoviessi, S.; Quetin-Leclercq, J. Anti-parasitic activity of essential oils and their active constituents against Plasmodium, Trypanosoma and Leishmania. Nov. Plant Bioresour. Appl. Food Med. Cosmet. 2014, 455–469. [Google Scholar] [CrossRef]
- Cilla, A.; Diego Quintaes, K.; Barberá, R.; Alegria, A. Phospholipids in human milk and infant formulas: Benefits and needs for correct infant nutrition. Crit. Rev. Food Sci. Nutr. 2016, 56, 1880–1892. [Google Scholar] [CrossRef]
- Laforenza, U.; Bottino, C.; Gastaldi, G. Mammalian aquaglyceroporin function in metabolism. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2016, 1858, 1–11. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. SpringerPlus 2016, 5, 1–12. [Google Scholar] [CrossRef]
- Roldos, V.; Nakayama, H.; Rolón, M.; Montero-Torres, A.; Trucco, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G. Activity of a hydroxybibenzyl bryophyte constituent against Leishmania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M. Antimicrobial and antibiotic potentiating activity of essential oils from tropical medicinal herbs and spices. Antibiot. Resist. 2016, 271. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Espino, M.; Solari, M.; de los Ángeles Fernández, M.; Boiteux, J.; Gómez, M.R.; Silva, M.F. NADES-mediated folk plant extracts as novel antifungal agents against Candida albicans. J. Pharm. Biomed. Anal. 2019, 167, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Samarth, R.; Samarth, M.; Matsumoto, Y. Medicinally important aromatic plants with radioprotective activity. Futur. Sci. OA 2017, 3, FSO247. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Chanaj-Kaczmarek, J.; Derda, M.; Budzianowska, A.; Thiem, B.; Ekiert, H.; Szopa, A. The Evaluation of Phenolic Acids and Flavonoids Content and Antiprotozoal Activity of Eryngium Species Biomass Produced by Biotechnological Methods. Molecules 2022, 27, 363. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Elias, G. Rosmarinus officinalis (Rosemary): A novel therapeutic agent for antioxidant, antimicrobial, anticancer, antidiabetic, antidepressant, neuroprotective, anti-inflammatory, and anti-obesity treatment. Biomed J. Sci. Technol. Res. 2017, 1, 1–6. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R. The effect of drying of the composition of volatile organic compounds in Rosmarinus officinalis, Laurus nobilis, Salvia officinalis and Thymus serpyllum. A HS-SPME-GC-MS study. J. Essent. Oil Bear. Plants 2015, 18, 1209–1223. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
| Names | % | Supplier Company | Originated |
|---|---|---|---|
| L(+) lactic acid | 85 | Chem-lab NV | Belgium |
| Choline chloride | 98% | Xi’an Geekee Biotech | China |
| Glycerol | ≥99% | Panreac | Spain |
| Xylitol | 98% | Xi’an Geekee Biotech | China |
| Glucose anhydrous | 99% | HiMedia | India |
| D(-) fructose | 99% | HiMedia | India |
| Middlebrook 7H10 agar | HiMedia | India | |
| Middlebrook 7H9 broth medium | HiMedia | India | |
| Gallic acid | 99% | Sigma-Aldrich Chemicals | USA |
| Rutin | 99% | Sigma-Aldrich Chemicals | USA |
| DES Types | Full Name | Molar Ratio |
|---|---|---|
| DES1: LGH | Lactic acid, glucose and water | 5:1 |
| DES2: Tailor | Glycerol, xylitol and D-(-)-fructose | 3:3:3 |
| DES3: ChGl | Choline chloride: glycerol | 1:2 |
| DES4: ChXl | Choline chloride: xylitol | 1:1 |
| Type of DESs | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| DES1: LGH | 12.5 mg | 100 mg |
| DES2: Tailor | 3.12 mg | 12.5 mg |
| DES3: ChGl | 6.25 mg | 25 mg |
| DES4: ChXl | 25 mg | NA |
| Water | 50 mg | NA |
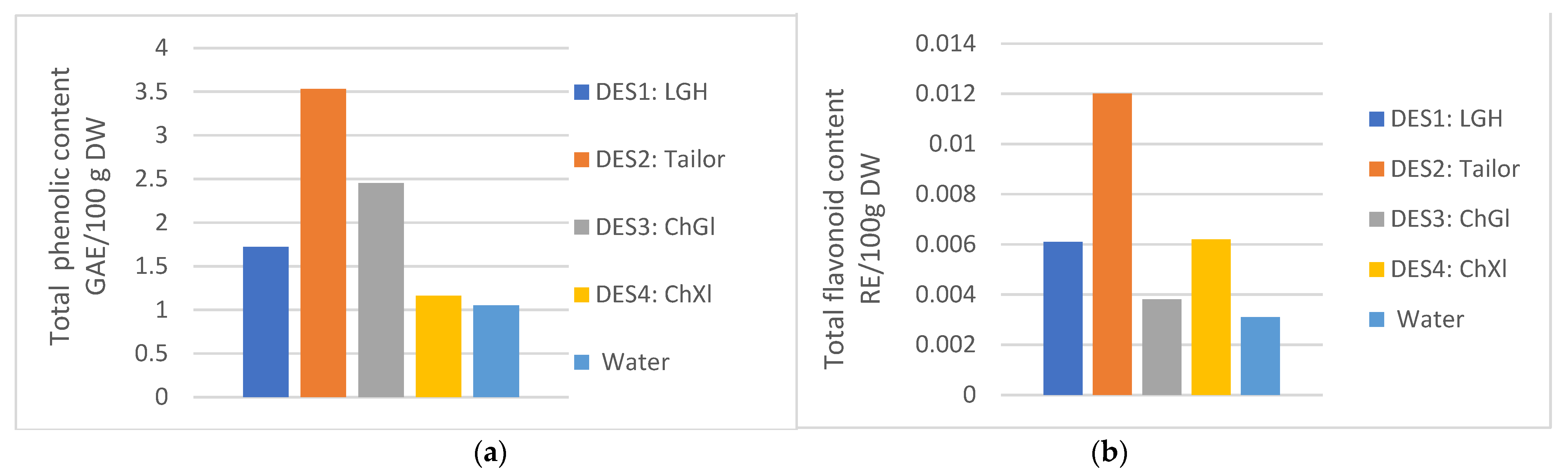
| Type of DESs | TPC mg GAE/100 g DW | TFC mg RE/100 g DW |
|---|---|---|
| DES1: LGH | 1.720 ± 0.096 | 0.0061 ± 0.0003 |
| DES2: Tailor | 3.530 ± 0.251 | 0.012 ± 0.0017 |
| DES3: ChGl | 2.450 ± 0.200 | 0.0038 ± 0.001 |
| DES4: ChXl | 1.160 ± 0.076 | 0.0062 ± 0.0009 |
| Water | 1.050 ± 0.132 | 0.0031 ± 0.0016 |
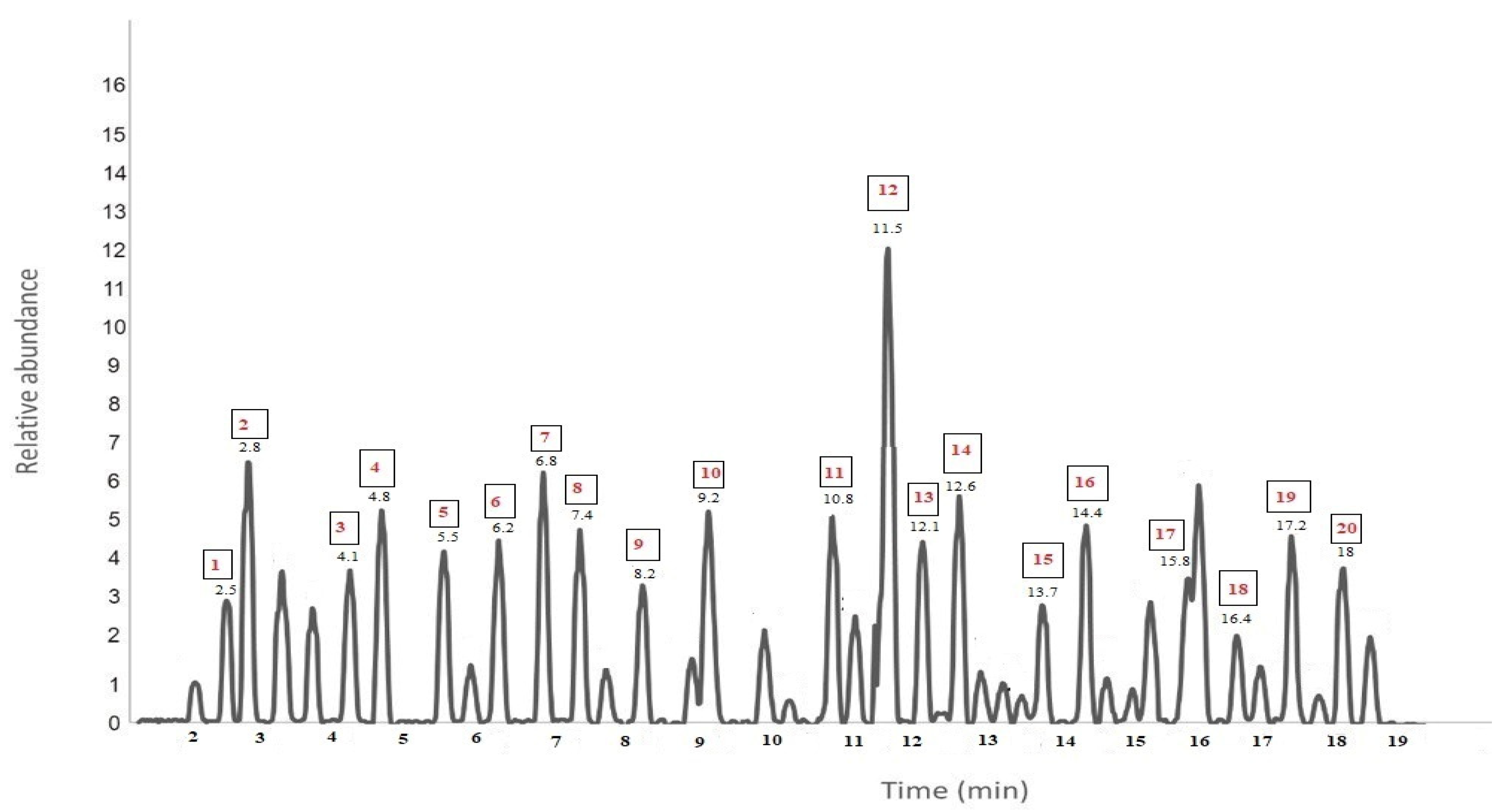
| No | Chemical Compound | R.T (min) | % | Molecular Weight (g/mol) | Molecular Formula |
|---|---|---|---|---|---|
| 1 | Rosmarinic acid | 2.5 | 1.6 | 360.3 | C18H16O8 |
| 2 | Camphene | 2.8 | 10.3 | 136.23 | C10H16 |
| 3 | Thymol | 4.1 | 1.6 | 150.22 | C10H14O |
| 4 | Camphenilol | 4.8 | 5.3 | 140.22 | C9H16O |
| 5 | Diosmin | 5.5 | 1.6 | 608.5 | C28H32O15 |
| 6 | Cirsimaritin | 6.2 | 1.7 | 314.29 | C17H14O6 |
| 7 | α-pinen | 6.8 | 9.6 | 136.23 | C10H16 |
| 8 | Betulinic acid | 7.4 | 1.7 | 456.7 | C30H48O3 |
| 9 | Oleanolic acid | 8.2 | 1.5 | 456.7 | C30H48O3 |
| 10 | Limonene | 9.2 | 3.4 | 136.23 | C10H16 |
| 11 | Apigenin | 10.8 | 4.8 | 270.24 | C15H10O5 |
| 12 | Camphor | 11.5 | 11.2 | 152.23 | C10H16O |
| 13 | Carnosol | 12.1 | 4.2 | 330.4 | C20H20O4 |
| 14 | Linalool | 12.6 | 8.2 | 154.24 | C10H18O |
| 15 | Carnosic acid | 13.7 | 1.5 | 332.4 | C20H28O4 |
| 16 | Myrecne | 14.4 | 6.3 | 136.23 | C10H16 |
| 17 | Luteolin 3-O-beta-D-glucuronide | 15.8 | 1.6 | 462.4 | C21H18O12 |
| 18 | Limonene | 16.4 | 7.5 | 136.23 | C10H16 |
| 19 | Rosmanol | 17.2 | 3.1 | 346.4 | C20H26O5 |
| 20 | Ursolic acid | 18 | 2.9 | 456.7 | C30H48O3 |
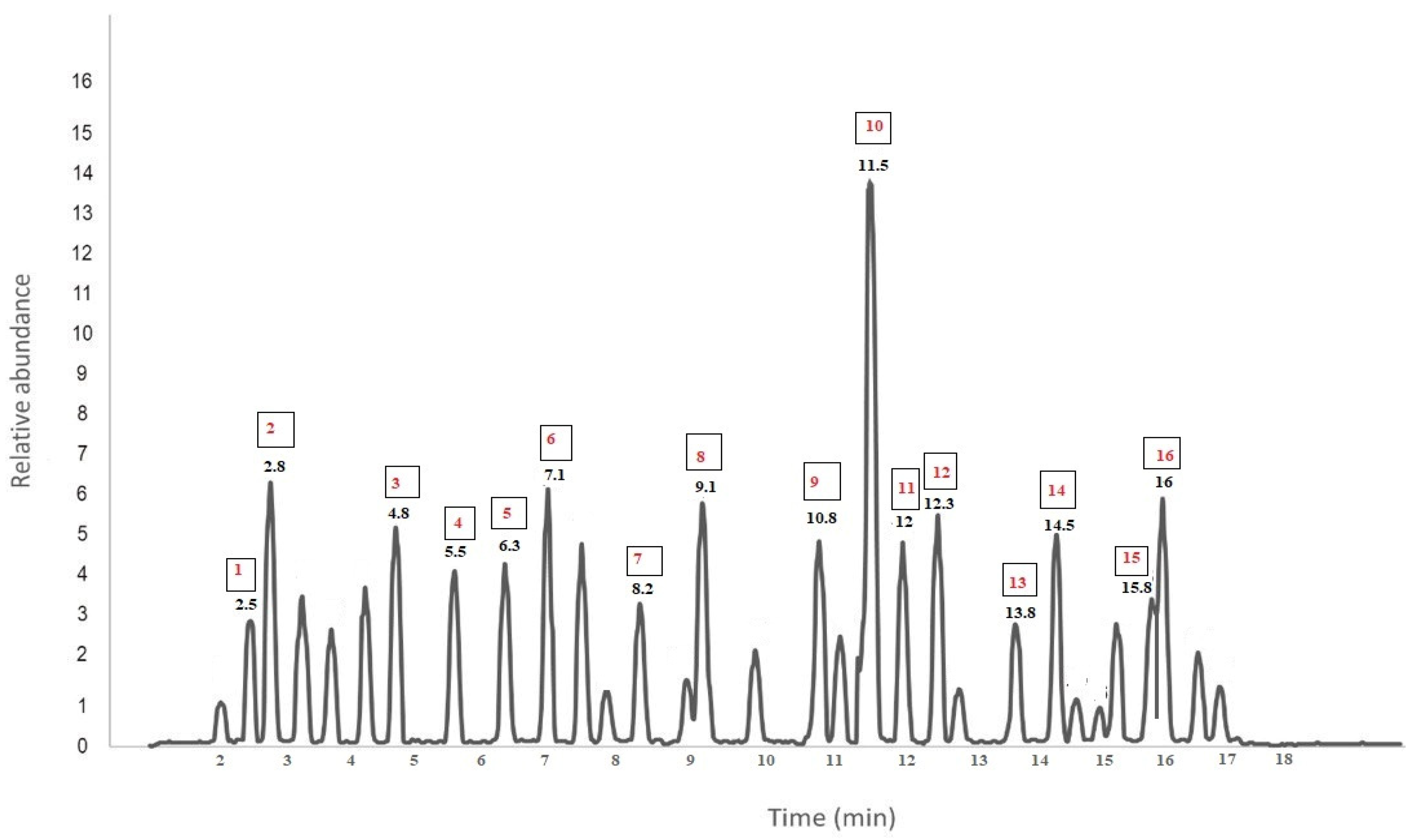
| No | Chemical Compound | R.T (min) | % | Molecular Weight (g/mol) | Molecular Formula |
|---|---|---|---|---|---|
| 1 | Cirsimaritin | 2.5 | 0.7 | 314.29 | C17H14O6 |
| 2 | α-pinen | 2.8 | 11 | 136.23 | C10H16 |
| 3 | Carnosol | 4.8 | 6.9 | 330.4 | C20H20O4 |
| 4 | Luteolin 3-O-beta-D-glucuronide | 5.5 | 1.5 | 462.4 | C21H18O12 |
| 5 | Rosmadial | 6.3 | 1.5 | 334.4 | C20H24O5 |
| 6 | Linalool | 7.1 | 9.6 | 154.24 | C10H18O |
| 7 | Hesperidin | 8.2 | 1.1 | 610.6 | C28H34O15 |
| 8 | Limonene | 9.1 | 8.4 | 136.23 | C10H16 |
| 9 | Oleanolic acid | 10.8 | 6.2 | 456.7 | C10H18O |
| 10 | Camphor | 11.5 | 12.2 | 152.23 | C10H16O |
| 11 | Ursolic acid | 12 | 6 | 456.7 | C30H48O3 |
| 12 | Camphene | 12.3 | 9.1 | 136.23 | C10H16 |
| 13 | Methyl carnosate | 13.8 | 0.8 | 346.5 | C21H30O4 |
| 14 | Myrecne | 14.5 | 8.1 | 136.23 | C10H16 |
| 15 | Rosmarinic acid | 15.8 | 1.1 | 360.3 | C18H16O8 |
| 16 | Camphenilol | 16 | 7.5 | 140.22 | C9H16O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dheyab, A.S.; Kanaan, M.Q.; Hussein, N.A.; AlOmar, M.K.; Sabran, S.F.; Abu Bakar, M.F. Antimycobacterial Activity of Rosmarinus officinalis (Rosemary) Extracted by Deep Eutectic Solvents. Separations 2022, 9, 271. https://doi.org/10.3390/separations9100271
Dheyab AS, Kanaan MQ, Hussein NA, AlOmar MK, Sabran SF, Abu Bakar MF. Antimycobacterial Activity of Rosmarinus officinalis (Rosemary) Extracted by Deep Eutectic Solvents. Separations. 2022; 9(10):271. https://doi.org/10.3390/separations9100271
Chicago/Turabian StyleDheyab, Ali Sami, Mohammed Qahtan Kanaan, Nabeel Abood Hussein, Mohamed Khalid AlOmar, Siti Fatimah Sabran, and Mohd Fadzelly Abu Bakar. 2022. "Antimycobacterial Activity of Rosmarinus officinalis (Rosemary) Extracted by Deep Eutectic Solvents" Separations 9, no. 10: 271. https://doi.org/10.3390/separations9100271
APA StyleDheyab, A. S., Kanaan, M. Q., Hussein, N. A., AlOmar, M. K., Sabran, S. F., & Abu Bakar, M. F. (2022). Antimycobacterial Activity of Rosmarinus officinalis (Rosemary) Extracted by Deep Eutectic Solvents. Separations, 9(10), 271. https://doi.org/10.3390/separations9100271






