Currently Applied Extraction Processes for Secondary Metabolites from Lippia turbinata and Turnera diffusa and Future Perspectives
Abstract
:1. Introduction
2. Traditional Extraction Methods
2.1. Hydrodistillation
2.2. Infusion
2.3. Reflux
2.4. Soxhlet Extractor
2.5. Maceration
| Extract | Method | Solvent | Conditions | Yield | Main Results | Ref. |
|---|---|---|---|---|---|---|
| Turnera diffusa | ||||||
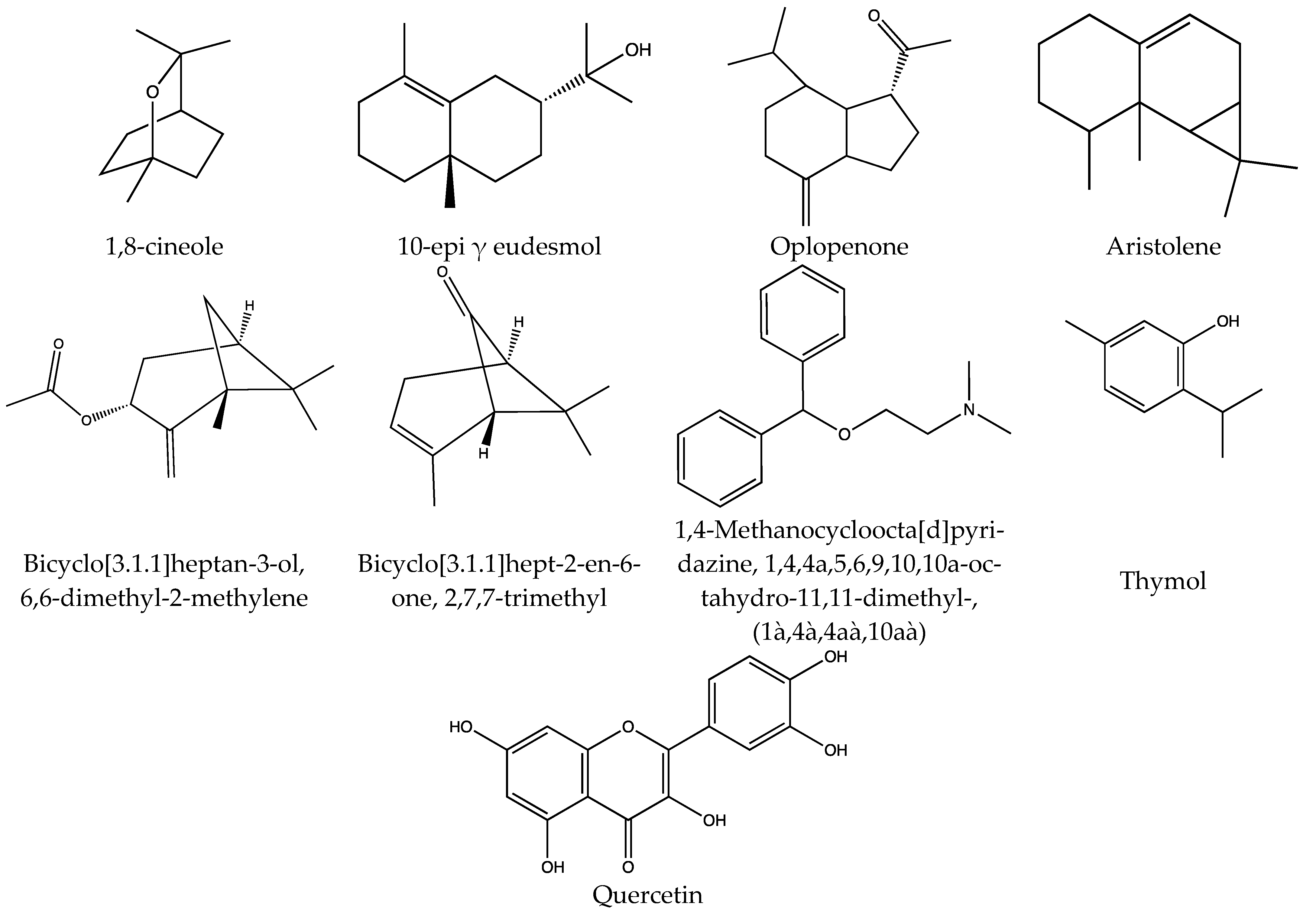
| Oil | Distillation | H2O | 1:15 w/v, 1.5 h in a CTA | 10.9 ± 6.0 μL/g of plant | 1,8-cineole (17.20 ± 8.56%), 10-epi γ eudesmol (4.54 ± 0.49%), oplopenone (3.63 ± 0.37%) and aristolene (3.47 ±1.17%) | [56] |
| 1:3 w/v ratio, 1.5 L/leaves and stems in a CTA | 0.158 mL/g of plant | 1,8-cineole; Bicyclo[3.1.1]heptan-3-ol, 6,6-dimethyl-2-methylene; Bicyclo[3.1.1]hept-2-en-6-one, 2,7,7-trimethyl and 1,4-Methanocycloocta[d]pyridazine, 1,4,4a,5,6,9,10,10a-octahydro-11,11-dimethyl-, (1à,4à,4aà,10aà) | [57] | |||
| 500 g leaves/1 h in a CTA | Approximately 0.002 mL/g of plant | 1,8-cineole (7.1%) and thymol (5.1%) | [58] | |||
| TPP | Infusion | MetOH | 1:10 w/v ratio,28 °C/24 h/ 250 rpm | 0.410 ± 0.0039 mg/g of plant | 0.0080 mg/g of TFC. SRSA and ion chelation were higher in MetOH extract, and the phagocytosis activity increased in those leukocytes stimulated | [59] |
| H2O | 1.22.5 w/v ratio,60 °C/1 h stirred | 33.85 mg/g of plant | 72.32% of ABTS•+ inhibition; FRAP 21.33 mg GA/g | [57] | ||
| MetOH: H2O | Percolation overnight | NR | Sexually potent and sexually sluggish/impotent male rats were treated orally with different amounts. | [60] | ||
| Reflux | EtOH | 2 g/80 °C/3 h | 590 ± 16.4 mg/g of plant | 236.27 ± 0.36 mg GAE/dw of TPC and 377.21 ± 0.08 mg Trolox/dw | [39] | |
| EtOH 70% | 1:4 w/v ratio, 60 °C/2 h | 96.4 ± 31.1 mg/g of plant | 9.64 ± 3.11 mg GAE/g; 76.03% to 91.96% DPPH inhibition; 65% in the LOI and 50% in ABTS•+. Quercetin was identified. | [61] | ||
| Soxhlet | EtOH 50% | 1.66.6 w/v | 409 ± 12.1 mg/g of plant | 161.62 ± 0.12 mg GAE/dw of TPC and 186.62 ± 0.007 mg Trolox/dw | [39] | |
| Lippia turbinata | ||||||
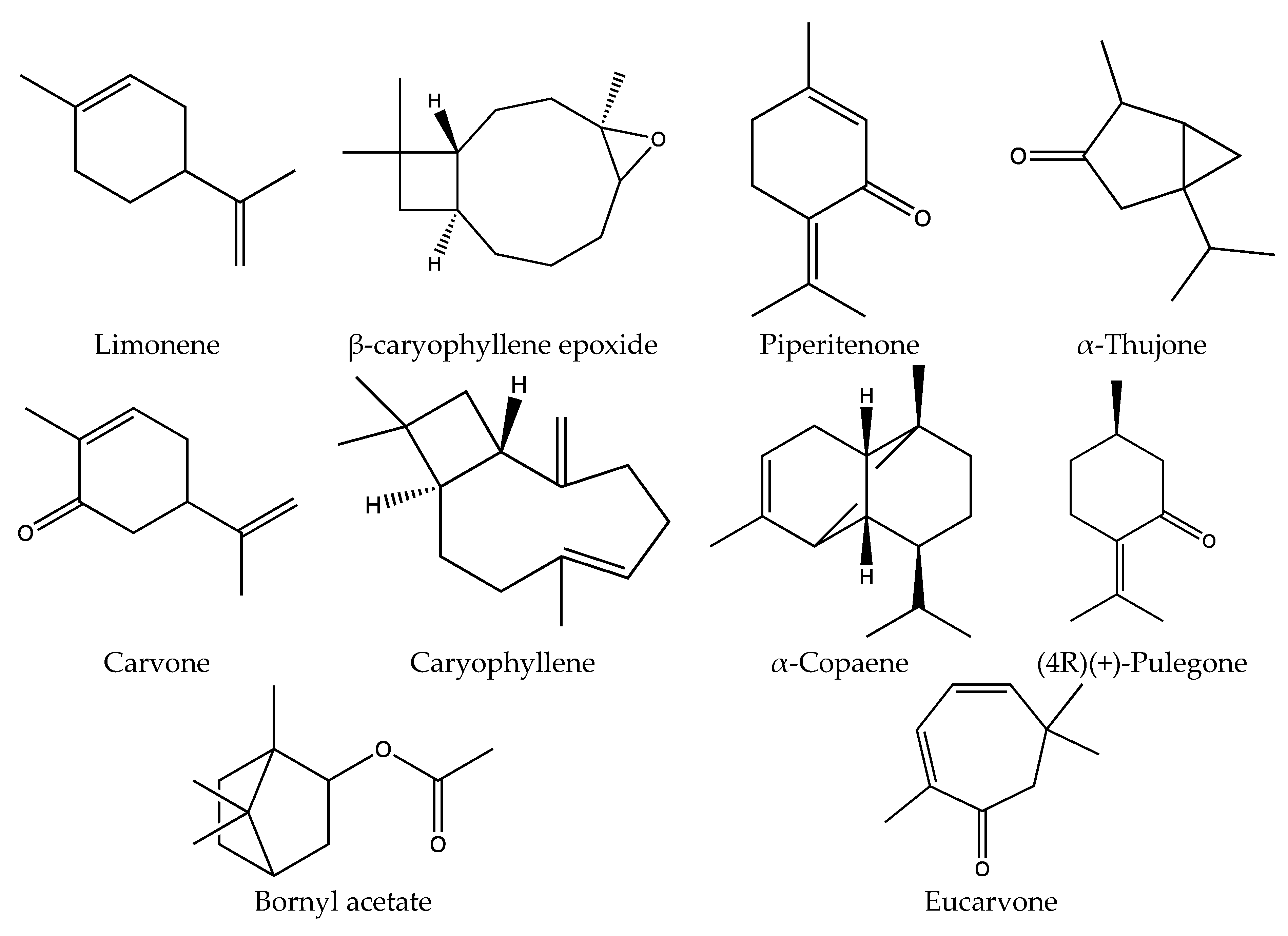
| Oil | Distillation | H2O | 100 g/3 h in a CTA | 10.2 ± 1.1 μL/g of plant | Limonene was the main component. TPC 14.03 ± 0.12 mg GAE/100 g fresh vegetal material | [11] |
| Distillation | H2O | 100 g/3 h in a CTA | 10.2 μL/g of plant | Limonene (48.83%), β-caryophyllene epoxide (18.06%), and piperitenone (7.67%) | [14] | |
| Distillation | H2O | 2 h in a CTA | NR | α-Thujone (48.3%), Carvone (17.4%), β-Caryophyllene (10.0%), Limonene (3.5%), α-Copaene (3.1%) | [16] | |
| Distillation | H2O | 2 h in a CTA | NR | (4R)(+)-Pulegone (3.56%) | [62] | |
| Distillation | H2O | Using a CTA | NR | Limonene (60.8%), Bornyl acetate (8.2%) and Eucarvone (5.8%) | [63] | |
| NR | NR | MeOH-CH2Cl2 (1:1) | NR | NR | Four novel triterpenoids 3β,25-epoxy-3α,21α-dihydroxy-22β-(3-methylbut-2-en-1- oyloxy)olean-12-ene-28-oic acid (1); 3β,25-epoxy-3α,21α-dihydroxy-22β-angeloyloxyolean-12-ene-28-oic acid (2); 3β,25-epoxy-3α,21α-dihydroxy-22β-tigloyloxyolean-12-ene-28-oic acid (3); and 3α,25-epoxy-3α-hydroxy- 22β-(2-methylbutan-1-oyloxy)olean-12-ene-28-oic acid (4) | [64] |
| TPP | Maceration | EtOH 50% | 1:5 w/v, 24 h Rotavapor 70 °C | NR | Gastroprotective and antispasmodic activity was evaluated. | [55] |
2.6. The Most Important Compounds Identified and Their Properties
3. Ecofriendly Extraction Methods Used for Lippia turbinata and Turnera diffusa
3.1. Microwave Assisted Extraction
3.2. Ultrasound-Assisted Extraction
4. Other Ecofriendly Extraction Methods Used to Extract Essential Oils and Antioxidants
5. Preconcentration and Purification of Essential Oils
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tousson, E.; Hafez, E.; Zaki, S.; Gad, A.; Elgharabawy, R.M. Evaluation of the testicular protection conferred by damiana (Turnera diffusa Willd.) against amitriptyline-induced testicular toxicity, DNA damage and apoptosis in rats. Biomed. Pharmacother. 2020, 132, 110819. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.S.B.; Thomson, M.; Infield, D.G. Renewable energy powered desalination in Baja California Sur, Mexico. Desalination 2008, 220, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Garza-Juárez, A.; de la Luz Salazar-Cavazos, M.; Salazar-Aranda, R.; Pérez-Meseguer, J.; de Torres, N.W. Correlation between chromatographic fingerprint and antioxidant activity of Turnera diffusa (Damiana). Planta Med. 2011, 77, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, J.; Malheiro, I.; Videira, R.A.; Valentão, P.; Santos, A.C.; Veiga, F.; Andrade, P.B. Trichilia catigua and Turnera diffusa extracts: In vitro inhibition of tyrosinase, antiglycation activity and effects on enzymes and pathways engaged in the neuroinflammatory process. J. Ethnopharmacol. 2021, 271, 113865. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Carro-Juárez, M.; Mota, L.A.M. Pro-sexual effects of Turnera diffusa Wild (Turneraceae) in male rats involves the nitric oxide pathway. J. Ethnopharmacol. 2013, 146, 164–172. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Ortiz-López, P.; Gutiérrez-Ortíz, J.; Mota, L.A.M. Turnera diffusa Wild (Turneraceae) recovers sexual behavior in sexually exhausted males. J. Ethnopharmacol. 2009, 123, 423–429. [Google Scholar] [CrossRef]
- Zhao, J.; Dasmahapatra, A.K.; Khan, S.I.; Khan, I.A. Anti-aromatase activity of the constituents from damiana (Turnera diffusa). J. Ethnopharmacol. 2008, 120, 387–393. [Google Scholar] [CrossRef]
- Taha, M.M.E.; Salga, M.S.; Ali, H.M.; Abdulla, M.A.; Abdelwahab, S.; Hadi, A.H.A. Gastroprotective activities of Turnera diffusa Willd. ex Schult. revisited: Role of arbutin. J. Ethnopharmacol. 2012, 141, 273–281. [Google Scholar] [CrossRef]
- Barbieri, N.; Costamagna, M.; Gilabert, M.; Perotti, M.; Schuff, C.; Isla, M.I.; Benavente, A. Antioxidant activity and chemical composition of essential oils of three aromatic plants from La Rioja province. Pharm. Biol. 2015, 54, 168–173. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Grosso, N.R.; Lante, A.; Lomolino, G.; Zygadlo, J.A.; Nepote, V. Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and Lippia turbinata essential oils. Int. J. Food Sci. Technol. 2012, 48, 642–649. [Google Scholar] [CrossRef]
- Girardi, N.S.; García, D.; Passone, M.A.; Nesci, A.; Etcheverry, M. Microencapsulation of Lippia turbinata essential oil and its impact on peanut seed quality preservation. Int. Biodeterior. Biodegrad. 2017, 116, 227–233. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Passone, M.A.; Etcheverry, M. Antifungal impact of volatile fractions of Peumus boldus and Lippia turbinata on Aspergillus section Flavi and residual levels of these oils in irradiated peanut. Int. J. Food Microbiol. 2014, 168-169, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.; Talarico, L.; Almeida, N.; Colombres, S.; Duschatzky, C.; Damonte, E.B. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother. Res. 2003, 17, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Gleiser, R.M.; Zygadlo, J.A. Insecticidal properties of essential oils from Lippia turbinata and Lippia polystachya (Verbenaceae) against Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2007, 101, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Corzo, F.L.; Traverso, L.; Sterkel, M.; Benavente, A.; Ajmat, M.T.; Ons, S. Plodia interpunctella (Lepidoptera: Pyralidae): Intoxication with essential oils isolated from Lippia turbinata (Griseb.) and analysis of neuropeptides and neuropeptide receptors, putative targets for pest control. Arch. Insect Biochem. Physiol. 2020, 104, e21684. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crop. Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2020, 119, 57–74. [Google Scholar] [CrossRef]
- Mehany, T.; Khalifa, I.; Barakat, H.; Althwab, S.A.; Alharbi, Y.M.; El-Sohaimy, S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: A review with research evidence and underlying mechanisms. Food Biosci. 2021, 40, 100891. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H. Ohmic accelerated steam distillation of essential oil from lavender in comparison with conventional steam distillation. Innov. Food Sci. Emerg. Technol. 2018, 50, 34–41. [Google Scholar] [CrossRef]
- Damyeh, M.S.; Niakousari, M. Ohmic hydrodistillation, an accelerated energy-saver green process in the extraction of Pulicaria undulata essential oil. Ind. Crop. Prod. 2017, 98, 100–107. [Google Scholar] [CrossRef]
- Damyeh, M.S.; Niakousari, M. Impact of ohmic-assisted hydrodistillation on kinetics data, physicochemical and biological properties of Prangos ferulacea Lindle. essential oil: Comparison with conventional hydrodistillation. Innov. Food Sci. Emerg. Technol. 2016, 33, 387–396. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Farhoosh, R.; Javidnia, K.; Shahidi, F. Extraction of essential oils from Mentha piperita using advanced techniques: Microwave versus ohmic assisted hydrodistillation. Food Bioprod. Process. 2015, 94, 50–58. [Google Scholar] [CrossRef]
- Peng, X.; Feng, C.; Wang, X.; Gu, H.; Li, J.; Zhang, X.; Zhang, X.; Yang, L. Chemical composition and antioxidant activity of essential oils from barks of Pinus pumila using microwave-assisted hydrodistillation after screw extrusion treatment. Ind. Crop. Prod. 2021, 166, 113489. [Google Scholar] [CrossRef]
- Angoy, A.; Ginies, C.; Goupy, P.; Bornard, I.; Ginisty, P.; Sommier, A.; Valat, M.; Chemat, F. Development of a green innovative semi-industrial scale pilot combined microwave heating and centrifugal force to extract essential oils and phenolic compounds from orange peels. Innov. Food Sci. Emerg. Technol. 2020, 61, 102338. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind. Crop. Prod. 2019, 143, 111908. [Google Scholar] [CrossRef]
- Khalili, G.; Mazloomifar, A.; Larijani, K.; Tehrani, M.S.; Azar, P.A. Solvent-free microwave extraction of essential oils from Thymus vulgaris L. and Melissa officinalis L. Ind. Crop. Prod. 2018, 119, 214–217. [Google Scholar] [CrossRef]
- Guzmán-Albores, J.M.; Bojórquez-Velázquez, E.; De León-Rodríguez, A.; Calva-Cruz, O.D.J.; de la Rosa, A.P.B.; Ruíz-Valdiviezo, V.M. Comparison of Moringa oleifera oils extracted with supercritical fluids and hexane and characterization of seed storage proteins in defatted flour. Food Biosci. 2020, 40, 100830. [Google Scholar] [CrossRef]
- Ferrentino, G.; Giampiccolo, S.; Morozova, K.; Haman, N.; Spilimbergo, S.; Scampicchio, M. Supercritical fluid extraction of oils from apple seeds: Process optimization, chemical characterization and comparison with a conventional solvent extraction. Innov. Food Sci. Emerg. Technol. 2020, 64, 102428. [Google Scholar] [CrossRef]
- Bendif, H.; Adouni, K.; Miara, M.D.; Baranauskienė, R.; Kraujalis, P.; Venskutonis, P.R.; Nabavi, S.M.; Maggi, F. Essential oils (EOs), pressurized liquid extracts (PLE) and carbon dioxide supercritical fluid extracts (SFE-CO2) from Algerian Thymus munbyanus as valuable sources of antioxidants to be used on an industrial level. Food Chem. 2018, 260, 289–298. [Google Scholar] [CrossRef]
- Khajeh, M.; Moghaddam, M.G.; Shakeri, M. Application of artificial neural network in predicting the extraction yield of essential oils of Diplotaenia cachrydifolia by supercritical fluid extraction. J. Supercrit. Fluids 2012, 69, 91–96. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Ascacio-Valdés, J.A.; Cruz, K.; Reyes-Luna, C.; Rodríguez, R.; Aguilar, C.N. Chapter 5—Extraction of Bioactive Phenolic Compounds by Alternative Technologies. In Ingredients Extraction by Physicochemical Methods in Food; Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 229–252. [Google Scholar] [CrossRef]
- Jan, S.; Khan, A.L.; Bashir, K.; Jan, K. Ohmic Processing of Plant-Related Food Products. Innov. Food Process. Technol. 2020, 699–705. [Google Scholar] [CrossRef]
- Bertolini, M.; Romagnoli, G. An Italian case study for the Process-Target-Cost evaluation of the ohmic treatment and aseptic packaging of a vegetable soup (minestrone). J. Food Eng. 2012, 110, 214–219. [Google Scholar] [CrossRef]
- Perović, A.; Stanković, M.Z.; Veljković, V.B.; Kostić, M.D.; Stamenković, O.S. A further study of the kinetics and optimization of the essential oil hydrodistillation from lavender flowers. Chin. J. Chem. Eng. 2020, 29, 126–130. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Márquez, D.; Rodriguez, R.; Balagurusamy, N.; Carrillo, M.; Belmares, R.; Contreras, J.; Nevárez, G.; Aguilar, C. Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L. and Amaranthus hybridus L. CyTA J. Food 2013, 12, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Tsaltaki, C.; Katsouli, M.; Kekes, T.; Chanioti, S.; Tzia, C. Comparison study for the recovery of bioactive compounds from Tribulus terrestris, Panax ginseng, Gingko biloba, Lepidium meyenii, Turnera diffusa and Withania somnifera by using microwave-assisted, ultrasound-assisted and conventional extraction methods. Ind. Crop. Prod. 2019, 142, 111875. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Martínez-Ávila, G.C.G.; Rojas-Molina, R.; Sánchez-Alejo, E.J. Natural Beverages and Sensory Quality Based on Phenolic Contents. In Antioxidants in Foods and Its Applications; Shalaby, E., Azzam, G.M., Eds.; Books on Demand: Norderstedt, Germany, 2018; Volume 1, pp. 69–85. [Google Scholar]
- Olalere, O.A.; Abdurahman, N.H.; Yunus, R.B.M.; Alara, O.R. Multi-response optimization and neural network modeling for parameter precision in heat reflux extraction of spice oleoresins from two pepper cultivars (Piper nigrum). J. King Saud Univ. Sci. 2019, 31, 789–797. [Google Scholar] [CrossRef]
- Martins, S.; Aguilar, C.N.; de la Garza-Rodriguez, I.; Mussatto, S.I.; Teixeira, J.A. Kinetic study of nordihydroguaiaretic acid recovery from Larrea tridentata by microwave-assisted extraction. J. Chem. Technol. Biotechnol. 2010, 85, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Tatke, P.; Rajan, M. Comparison of Conventional and Novel Extraction Techniques for the Extraction of Scopoletin from Convolvulus pluricaulis. Indian J. Pharm. Educ. Res. 2014, 48, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.M.; Pietrantonio, E.; Romanelli, G.; Di Muccio, A. Comparison of Soxhlet, Shaking, and Microwave Assisted Extraction Techniques for Determination of PCB Congeners in a Marine Sediment. Bull. Environ. Contam. Toxicol. 2000, 65, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Diz, P.; Prieto, M.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Porto, C.; Decorti, D.; Natolino, A. Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: A comparison and a proposal. J. Supercrit. Fluids 2014, 87, 1–8. [Google Scholar] [CrossRef]
- García-Becerra, L.; Mitjans, M.; Rivas-Morales, C.; Verde-Star, J.; Oranday-Cárdenas, A.; María, P.V. Antioxidant comparative effects of two grape pomace Mexican extracts from vineyards on erythrocytes. Food Chem. 2016, 194, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crop. Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Manna, L.; Bugnone, C.A.; Banchero, M. Valorization of hazelnut, coffee and grape wastes through supercritical fluid extraction of triglycerides and polyphenols. J. Supercrit. Fluids 2015, 104, 204–211. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem. Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Dutta, R.; Sarkar, U.; Mukherjee, A. Extraction of oil from Crotalaria Juncea seeds in a modified Soxhlet apparatus: Physical and chemical characterization of a prospective bio-fuel. Fuel 2013, 116, 794–802. [Google Scholar] [CrossRef]
- Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Niño-Medina, G.; Martínez-Ávila, G.C.; Morata, A.; Loira, I. Phenolic Compound Recovery from Grape Fruit and By- Products: An Overview of Extraction Methods. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen Book Series; IntechOpen: London, UK, 2016; pp. 103–123. [Google Scholar]
- Wojdyło, A.; Samoticha, J.; Chmielewska, J. Effect of different pre-treatment maceration techniques on the content of phenolic compounds and color of Dornfelder wines elaborated in cold climate. Food Chem. 2020, 339, 127888. [Google Scholar] [CrossRef] [PubMed]
- Toso, R.E.; Toribio, M.S.; Mengelle, P.; Boeris, M.A. Plantas de la provincia de La Pampa, Argentina, con actividad gastroprotectora y antiespasmódica. InVet 2007, 9, 145–151. [Google Scholar]
- Alcaraz-Meléndez, L.; Real-Cosío, S.; Suchy, V.; Švajdlenka, E. Differences in essential oil production and leaf structure in pheno-types of damiana (Turnera diffusa Willd.). J. Plant Biol. 2007, 50, 378–382. [Google Scholar] [CrossRef]
- Urbizu-González, A.L.; Castillo-Ruiz, O.; Martínez-Ávila, G.C.G.; Torres-Castillo, J.A. Natural variability of essential oil and antioxidants in the medicinal plant Turnera diffusa. Asian Pac. J. Trop. Med. 2017, 10, 121–125. [Google Scholar] [CrossRef]
- Godoi, A.F.L.; Vilegas, W.; Godoi, R.H.M.; Van Vaeck, L.; Van Grieken, R. Application of low-pressure gas chromatography–ion-trap mass spectrometry to the analysis of the essential oil of Turnera diffusa (Ward.) Urb. J. Chromatogr. A 2003, 1027, 127–130. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Ginera, P.; Silva-Jara, J.; Macias, A.; Velazquez-Carriles, C.; Alcaraz-Meléndez, L.; Angulo, C. Assessment of chemical, biological and immunological properties of “Damiana de California” Turnera diffusa Willd extracts in Longfin yellowtail (Seriola rivoliana) leukocytes. Fish Shellfish. Immunol. 2020, 100, 418–426. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Cavazzuti, E.; Scarpetta, G.; Bertolini, A. Stimulating property of Turnera diffusa and Pfaffia paniculata extracts on the sexual-behavior of male rats. Psychopharmacology 1999, 143, 15–19. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.I.L.; Moorillón, G.V.N.; Aguilar, C.N. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac. J. Trop. Med. 2015, 8, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Palacios, S.M.; Bertoni, A.; Rossi, Y.; Santander, R.; Urzúa, A. Insecticidal activity of essential oils from native medicinal plants of Central Argentina against the house fly, Musca domestica (L.). Parasitol. Res. 2009, 106, 207–212. [Google Scholar] [CrossRef]
- Garcia, F.; Brunetti, M.A.; Lucini, E.I.; Scorcione Turcato, M.C.; Moreno, M.V.; Frossasco, G.P.; Colombatto, D.; Martínez, M.J.; Martínez Ferrer, J. Essential oils from Argentinean native species reduce in vitro methane production. RIA 2018, 44, 76–83. [Google Scholar]
- Wächter, G.A.; Valcic, S.; Franzblau, S.G.; Suarez, E.; Timmermann, B.N. Antitubercular Activity of Triterpenoids from Lippia turbinata. J. Nat. Prod. 2000, 64, 37–41. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, M.A.; Crespo, R.; Galle, M.; de Bravo, M.G. Anti-cancer mechanisms of linalool and 1,8-cineole in non-small cell lung cancer A549 cells. Heliyon 2020, 6, e05639. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, A.; Demirci, B.; Çaşkurlu, A.; Okur, M.; Orak, D.; Sipahi, H.; Başer, K. In vitro antibacterial, antioxidant, anti-inflammatory and analgesic evaluation of Rosmarinus officinalis L. flower extract fractions. S. Afr. J. Bot. 2019, 125, 214–220. [Google Scholar] [CrossRef]
- Rossi, Y.E.; Palacios, S.M. Insecticidal toxicity of Eucalyptus cinerea essential oil and 1,8-cineole against Musca domestica and possible uses according to the metabolic response of flies. Ind. Crop. Prod. 2015, 63, 133–137. [Google Scholar] [CrossRef]
- Aydın, B.; Barbas, L.A. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Juergens, L.J.; Tuleta, I.; Stoeber, M.; Racké, K.; Juergens, U.R. Regulation of monocyte redox balance by 1,8-cineole (eucalyptol) controls oxidative stress and pro-inflammatory responses in vitro: A new option to increase the antioxidant effects of combined respiratory therapy with budesonide and formoterol? Synergy 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; De Oliveira, M.R.C.; Tintino, C.D.M.; Castro, F.F.E.; Alcântara, I.S.; Fernandes, M.N.M.; De Albuquerque, T.R.; Da Silva, M.S.A.; et al. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1,8-cineole (eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef]
- Juergens, L.J.; Racké, K.; Tuleta, I.; Stoeber, M.; Juergens, U.R. Anti-inflammatory effects of 1,8-cineole (eucalyptol) improve glucocorticoid effects in vitro: A novel approach of steroid-sparing add-on therapy for COPD and asthma? Synergy 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Dammak, I.; Hamdi, Z.; El Euch, S.K.; Zemni, H.; Mliki, A.; Hassouna, M.; Lasram, S. Evaluation of antifungal and anti-ochratoxigenic activities of Salvia officinalis, Lavandula dentata and Laurus nobilis essential oils and a major monoterpene constituent 1,8-cineole against Aspergillus carbonarius. Ind. Crop. Prod. 2018, 128, 85–93. [Google Scholar] [CrossRef]
- Upadhyay, S.; Bisht, K.; Bahukhandi, A.; Bisht, M.; Mehta, P.; Bisht, A. Chapter 3.2.6—Rosmarinus officinalis L. In Naturally Occurring Chemicals against Alzheimer’s Disease; Belwal, T., Nabavi, S.M., Nabavi, S.F., Dehpour, A.R., Shirooie, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 271–281. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Noacco, N.; de Berti, I.P.; Stewart, S.; Cabrera, A.; Alvarez, V.; de Bravo, M.G.; Durán, N.; Castro, G.; Islan, G. Design of magnetic hybrid nanostructured lipid carriers containing 1,8-cineole as delivery systems for anticancer drugs: Physicochemical and cytotoxic studies. Colloids Surf. B Biointerfaces 2021, 202, 111710. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Mo, H.; Zhao, Y.; Li, H.; Zhang, H.; Hu, L.; Zhou, X. Thymol Mediates Bactericidal Activity against Staphylococcus aureus by Targeting an Aldo–Keto Reductase and Consequent Depletion of NADPH. J. Agric. Food Chem. 2019, 67, 8382–8392. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control. 2021, 126, 108063. [Google Scholar] [CrossRef]
- Yuan, H.; Pan, Y.; Young, C.Y. Overexpression of c-Jun induced by quercetin and resverol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2004, 213, 155–163. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; David, A.V.A.; Arulmoli, R. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-C.; Zhang, F.; Pakrasi, H.B. Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Metab. Eng. Commun. 2021, 12, e00164. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Petrillo, F.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Calabrò, V. A non-conventional method to extract D-limonene from waste lemon peels and comparison with traditional Soxhlet extraction. Sep. Purif. Technol. 2014, 137, 13–20. [Google Scholar] [CrossRef]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Baysal, T.; Starmans, D. Supercritical carbon dioxide extraction of carvone and limonene from caraway seed. J. Supercrit. Fluids 1999, 14, 225–234. [Google Scholar] [CrossRef]
- Estrella, G.-R.A.; Eva, G.-T.M.; Alberto, H.-L.; Guadalupe, V.-D.M.; Azucena, C.-V.; Sandra, O.-S.; Noé, A.-V.; Javier, L.-M.F. Limonene from Agastache mexicana essential oil produces antinociceptive effects, gastrointestinal protection and improves experimental ulcerative colitis. J. Ethnopharmacol. 2021, 280, 114462. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, Y.; Li, S.; Zhao, Y.; Li, J.; Hu, Z.; Zhang, C.; Xiao, D.; Yu, A. Engineering the oleaginous yeast Yarrowia lipolytica to produce limonene from waste cooking oil. Biotechnol. Biofuels 2019, 12, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.-Q.; Wei, L.-J.; Lv, Y.-B.; Chen, J.; Hua, Q. Elevating Limonene Production in Oleaginous Yeast Yarrowia lipolytica via Genetic Engineering of Limonene Biosynthesis Pathway and Optimization of Medium Composition. Biotechnol. Bioprocess Eng. 2019, 24, 500–506. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, L.; Li, H.; Li, P.; Weng, Y.; Zhang, C.; Yu, A.; Xiao, D. Engineering Saccharomyces cerevisiae for production of the valuable monoterpene d-limonene during Chinese Baijiu fermentation. J. Ind. Microbiol. Biotechnol. 2020, 47, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Meng, Y.; Zhang, L.; Qiao, J.; Zhao, G.-R. Combinatorial engineering of Saccharomyces cerevisiae for improving limonene production. Biochem. Eng. J. 2021, 176, 108155. [Google Scholar] [CrossRef]
- de Medeiros, T.D.M.; Alexandrino, T.D.; Pastore, G.M.; Bicas, J.L. Extraction and purification of limonene-1,2-diol obtained from the fungal biotransformation of limonene. Sep. Purif. Technol. 2020, 254, 117683. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2020, 10, 32. [Google Scholar] [CrossRef]
- Friedman, M.I.; Preti, G.; Deems, R.O.; Friedman, L.S.; Munoz, S.J.; Maddrey, W.C. Limonene in expired lung air of patients with liver disease. Dig. Dis. Sci. 1994, 39, 1672–1676. [Google Scholar] [CrossRef]
- O’Hara, M.E.; Del Río, R.F.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; A Mayhew, C. Limonene in exhaled breath is elevated in hepatic encephalopathy. J. Breath Res. 2016, 10, 046010. [Google Scholar] [CrossRef]
- Morisco, F.; Aprea, E.; Lembo, V.; Fogliano, V.; Vitaglione, P.; Mazzone, G.; Cappellin, L.; Gasperi, F.; Masone, S.; De Palma, G.D.; et al. Rapid “Breath-Print” of Liver Cirrhosis by Proton Transfer Reaction Time-of-Flight Mass Spectrometry. A Pilot Study. PLoS ONE 2013, 8, e59658. [Google Scholar] [CrossRef] [Green Version]
- Guneser, O.; Demirkol, A.; Yuceer, Y.K.; Togay, S.O.; Hosoglu, M.I.; Elibol, M. Production of flavor compounds from olive mill waste by Rhizopus oryzae and Candida tropicalis. Braz. J. Microbiol. 2016, 48, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Felipe, L.D.O.; de Oliveira, A.M.; Bicas, J.L. Bioaromas—Perspectives for sustainable development. Trends Food Sci. Technol. 2017, 62, 141–153. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, S.; Jin, G.; Yang, X.; Zhou, Y.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 107628. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.H.; Rosenfjeld, M. A rapid GC-FID method for determination of sabinene, β-pinene, α-thujone and β-thujone in the essential oil of Kitchen Sage (Salvia officinalis L.). J. Chromatogr. B 2020, 1149, 122159. [Google Scholar] [CrossRef]
- Dybowski, M.P.; Dawidowicz, A. The determination of α- and β-thujone in human serum—Simple analysis of absinthe congener substance. Forensic Sci. Int. 2016, 259, 188–192. [Google Scholar] [CrossRef]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2012, 65, 100–107. [Google Scholar] [CrossRef]
- Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.C.; Diop, M.M.; Harman, C.L.; Hayashi, S.-M.; Krammer, G.; et al. Dietary administration of β-caryophyllene and its epoxide to Sprague-Dawley rats for 90 days. Food Chem. Toxicol. 2019, 135, 110876. [Google Scholar] [CrossRef]
- Kumar, R.C.; Benal, M.; Prasad, B.D.; Krupashankara, M.; Kulkarni, R.; Siddaligaswamy, N. Microwave assisted extraction of oil from pongamia pinnata seeds. Mater. Today: Proc. 2018, 5, 2960–2964. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Niakousari, M. Sweet bay (Laurus nobilis L.) essential oil and its chemical composition, antioxidant activity and leaf micromorphology under different extraction methods. Sustain. Chem. Pharm. 2018, 9, 12–18. [Google Scholar] [CrossRef]
- Ríos, N.; Stashenko, E.; Duque, J.E. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae). Rev. Bras. De Entomol. 2017, 61, 307–311. [Google Scholar] [CrossRef]
- Stevanato, N.; da Silva, C. Radish seed oil: Ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind. Crop. Prod. 2019, 132, 283–291. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Muthumanickam, S.; Kamaladevi, A.; Karthika, C.; Jothi, R.; Boomi, P.; Maniazhagu, D.; Pandian, S.K. Promising phytochemicals of traditional Indian herbal steam inhalation therapy to combat COVID-19—An in silico study. Food Chem. Toxicol. 2021, 148, 111966. [Google Scholar] [CrossRef]
- de Melo, C.N.; Meireles, A.M.; da Silva, V.S.; Robles-Azocar, P.; DeFreitas-Silva, G. Manganese complex catalyst for valencene oxidation: The first use of metalloporphyrins for the selective production of nootkatone. Inorg. Chim. Acta 2020, 515, 120031. [Google Scholar] [CrossRef]
- Matsudaira, A.; Hoshino, Y.; Uesaka, K.; Takatani, N.; Omata, T.; Usuda, Y. Production of glutamate and stereospecific flavors, (S)-linalool and (+)-valencene, by Synechocystis sp. PCC6803. J. Biosci. Bioeng. 2020, 130, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Khanam, S. Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J. Clean. Prod. 2018, 188, 816–824. [Google Scholar] [CrossRef]
- Gomes, C.F.; Sarkis, J.R.; Marczak, L.D.F. Ohmic blanching of Tetsukabuto pumpkin: Effects on peroxidase inactivation kinetics and color changes. J. Food Eng. 2018, 233, 74–80. [Google Scholar] [CrossRef]
- Guida, V.; Ferrari, G.; Pataro, G.; Chambery, A.; Di Maro, A.; Parente, A. The effects of ohmic and conventional blanching on the nutritional, bioactive compounds and quality parameters of artichoke heads. LWT 2013, 53, 569–579. [Google Scholar] [CrossRef]
- Icier, F.; Yildiz, H.; Sabanci, S.; Cevik, M.; Cokgezme, O.F. Ohmic heating assisted vacuum evaporation of pomegranate juice: Electrical conductivity changes. Innov. Food Sci. Emerg. Technol. 2017, 39, 241–246. [Google Scholar] [CrossRef]
- Cokgezme, O.F.; Sabanci, S.; Cevik, M.; Yildiz, H.; Icier, F. Performance analyses for evaporation of pomegranate juice in ohmic heating assisted vacuum system. J. Food Eng. 2017, 207, 1–9. [Google Scholar] [CrossRef]
- Moreno, J.; Gonzales, M.; Zúñiga, P.; Petzold, G.; Mella, K.; Muñoz, O. Ohmic heating and pulsed vacuum effect on dehydration processes and polyphenol component retention of osmodehydrated blueberries (cv. Tifblue). Innov. Food Sci. Emerg. Technol. 2016, 36, 112–119. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.; Genisheva, Z.; Oliveira, H.; Freitas, V.; Teixeira, J.; Vicente, A. Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT 2016, 74, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Termrittikul, P.; Jittanit, W.; Sirisansaneeyakul, S. The application of ohmic heating for inulin extraction from the wet-milled and dry-milled powders of Jerusalem artichoke (Helianthus tuberosus L.) tuber. Innov. Food Sci. Emerg. Technol. 2018, 48, 99–110. [Google Scholar] [CrossRef]
- Mesías, M.; Wagner, M.; George, S.; Morales, F.J. Impact of conventional sterilization and ohmic heating on the amino acid profile in vegetable baby foods. Innov. Food Sci. Emerg. Technol. 2016, 34, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Jha, S.N.; Narsaiah, K.; Basediya, A.L.; Sharma, R.; Jaiswal, P.; Kumar, R.; Bhardwaj, R. Measurement techniques and application of electrical properties for nondestructive quality evaluation of foods—A review. J. Food Sci. Technol. 2011, 48, 387–411. [Google Scholar] [CrossRef] [Green Version]
- Aamir, M.; Jittanit, W. Ohmic heating treatment for Gac aril oil extraction: Effects on extraction efficiency, physical properties and some bioactive compounds. Innov. Food Sci. Emerg. Technol. 2017, 41, 224–234. [Google Scholar] [CrossRef]
- Grémy, C.; Lanoisellé, J.; Vorobiev, E. Electrotechnologies for Extraction from Food Plants and Biomaterials, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef] [Green Version]
- Imbimbo, P.; Romanucci, V.; Pollio, A.; Fontanarosa, C.; Amoresano, A.; Zarrelli, A.; Olivieri, G.; Monti, D.M. A cascade extraction of active phycocyanin and fatty acids from Galdieria phlegrea. Appl. Microbiol. Biotechnol. 2019, 103, 9455–9464. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Song, L.; Sommerfeld, M.; Hu, Q. Automated accelerated solvent extraction method for total lipid analysis of microalgae. Algal Res. 2020, 51, 102080. [Google Scholar] [CrossRef]
- Schäfer, K. Accelerated solvent extraction of lipids for determining the fatty acid composition of biological material. Anal. Chim. Acta 1998, 358, 69–77. [Google Scholar] [CrossRef]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenéz, D.; Muñoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Mezquita, P.C. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161, 104824. [Google Scholar] [CrossRef]
- Khanam, S. Selection of suitable model for the supercritical fluid extraction of carrot seed oil: A parametric study. LWT 2019, 119, 108815. [Google Scholar] [CrossRef]
- Ocaña-Fuentes, A.; Arranz-Gutiérrez, E.; Señorans, F.; Reglero, G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: Anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 2010, 48, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Fornari, T.; Vicente, G.; Vázquez, E.; Garcia-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loypimai, P.; Moongngarm, A.; Chottanom, P.; Moontree, T. Ohmic heating-assisted extraction of anthocyanins from black rice bran to prepare a natural food colourant. Innov. Food Sci. Emerg. Technol. 2015, 27, 102–110. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Al-Anaki, W.S.; Ismail, F.A.; Al-Jishi, F. Solvent and temperature effect of accelerated solvent extraction (ASE) coupled with ultra-high-pressure liquid chromatography (UHPLC-PDA) for the determination of methyl xanthines in commercial tea and coffee. Food Chem. 2019, 311, 126021. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, N.; Alkhars, S.; Alkhars, A.; Alyousif, M.; Bukhamseen, A.; Abuthayn, S.; Aqeel, M.; Aljamea, A. Green accelerated solvent extraction (ASE) with solvent and temperature effect and green UHPLC-DAD analysis of phenolics in pepper fruit (Capsicum annum L.). J. Food Compos. Anal. 2020, 97, 103766. [Google Scholar] [CrossRef]
- Zderic, A.; Zondervan, E. Polyphenol extraction from fresh tea leaves by pulsed electric field: A study of mechanisms. Chem. Eng. Res. Des. 2016, 109, 586–592. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Wallace, E.D.; Graf, T.N.; Oberlies, N.H.; Cech, N.B. Conventional and accelerated-solvent extractions of green tea (Camellia sinensis) for metabolomics-based chemometrics. J. Pharm. Biomed. Anal. 2017, 145, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Sun, B.; Wang, S.; Ito, Y. Isolation and purification of nootkatone from the essential oil of fruits of Alpinia oxyphylla Miquel by high-speed counter-current chromatography. Food Chem. 2009, 117, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.M.; Fingolo, C.E.; Kaplan, M.A.C. HSCCC separation and enantiomeric distribution of key volatile constituents of Piper claussenianum (Miq.) C. DC. (Piperaceae). Food Chem. Toxicol. 2017, 109, 1111–1117. [Google Scholar] [CrossRef]
- Memarzadeh, S.M.; Gholami, A.; Pirbalouti, A.G.; Masoum, S. Bakhtiari savory (Satureja bachtiarica Bunge.) essential oil and its chemical profile, antioxidant activities, and leaf micromorphology under green and conventional extraction techniques. Ind. Crop. Prod. 2020, 154, 112719. [Google Scholar] [CrossRef]
- Chen, G.; Sun, F.; Wang, S.; Wang, W.; Dong, J.; Gao, F. Enhanced extraction of essential oil from Cinnamomum cassia bark by ultrasound assisted hydrodistillation. Chin. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Drinić, Z.; Pljevljakušić, D.; Živković, J.; Bigović, D.; Šavikin, K. Microwave-assisted extraction of O. vulgare L. spp. hirtum essential oil: Comparison with conventional hydro-distillation. Food Bioprod. Process. 2020, 120, 158–165. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, X.; Li, J.; Liu, P.; Yang, Y.; Ni, Y. Preparative isolation and purification of cuminaldehyde and p-menta-1,4-dien-7-al from the essential oil of Cuminum cyminum L. by high-speed counter-current chromatography. Anal. Chim. Acta 2011, 689, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, G.; Tong, S.; Feng, Y.; Sheng, L.; Lou, J. Preparative isolation and purification of germacrone and curdione from the essential oil of the rhizomes of Curcuma wenyujin by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1070, 207–210. [Google Scholar] [CrossRef]
- Dai, X.; Huang, Q.; Zhou, B.; Gong, Z.; Liu, Z.; Shi, S. Preparative isolation and purification of seven main antioxidants from Eucommia ulmoides Oliv. (Du-zhong) leaves using HSCCC guided by DPPH-HPLC experiment. Food Chem. 2013, 139, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lin, Y.; Lv, R.; Yan, H.; Yu, J.; Zhao, H.; Wang, X.; Wang, D. An Efficient Method for the Preparative Isolation and Purification of Flavonoids from Leaves of Crataegus pinnatifida by HSCCC and Pre-HPLC. Molecules 2017, 22, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Extract | Method | Solvent | Conditions | Yield | Main Results | Ref. |
|---|---|---|---|---|---|---|
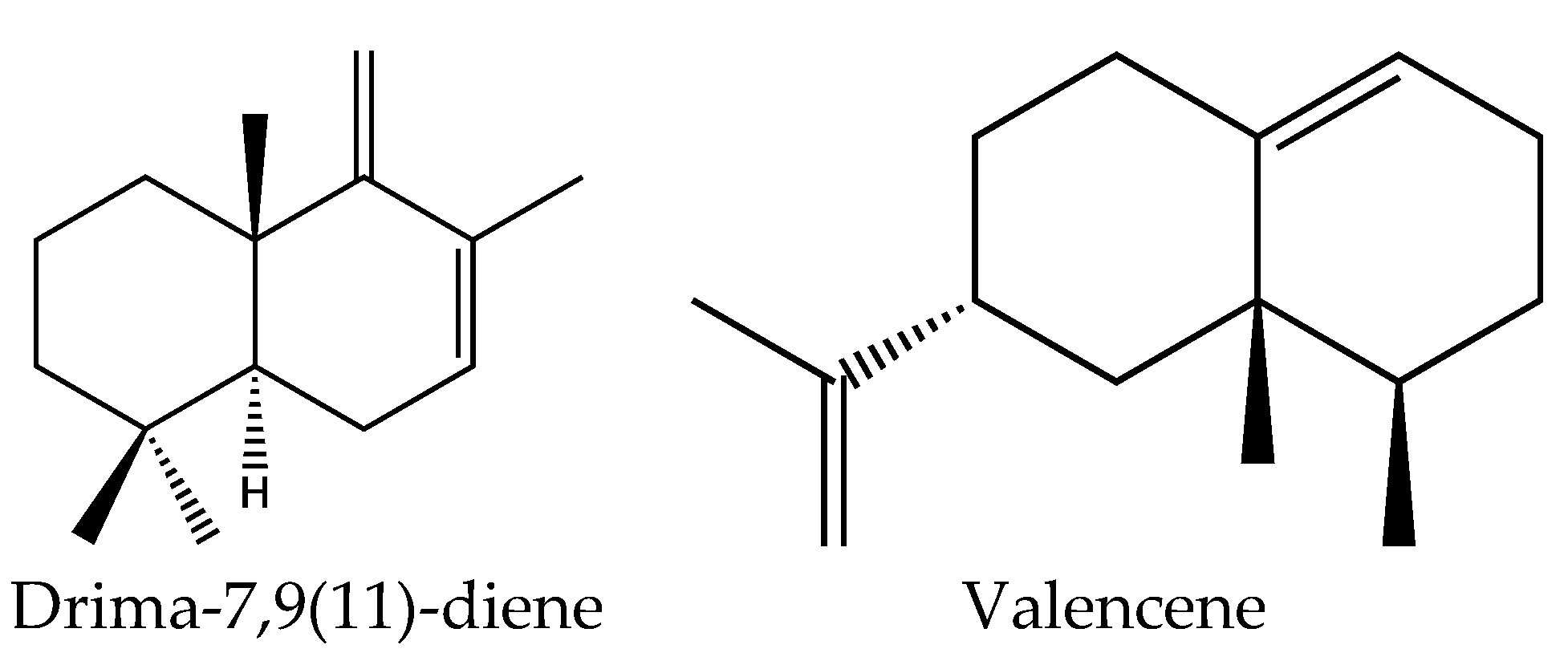
| Oil | MAE | H2O | 2.45 GHz, 800 W/30 min | 7 μL/g of plant | Drima-7,9(11)-diene (22.9%), β-viridiflorene (6.6%), α-silinene (5.9%), valencene (5.5%) | [105] |
| TPP | MAE | H2O | 2.75 g/300 W/220 rpm/50 °C/15 min | 606 ± 15.8 mg/g of plant | 239.52 ± 0.31 mg GAE/dw of TPC and 116.24 ± 0.08 mg Trolox/dw | [39] |
| UAE | EtOH 50% | 2 g/40 °C | 516 ± 16.7 mg/g of plant | 203.96 ± 0.35 mg GAE/dw of TPC and 201.94 ± 0.07 mg Trolox/dw | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ávila, G.C.G.; Aguilar-Zarate, P.; Rojas, R. Currently Applied Extraction Processes for Secondary Metabolites from Lippia turbinata and Turnera diffusa and Future Perspectives. Separations 2021, 8, 158. https://doi.org/10.3390/separations8090158
Martínez-Ávila GCG, Aguilar-Zarate P, Rojas R. Currently Applied Extraction Processes for Secondary Metabolites from Lippia turbinata and Turnera diffusa and Future Perspectives. Separations. 2021; 8(9):158. https://doi.org/10.3390/separations8090158
Chicago/Turabian StyleMartínez-Ávila, Guillermo C. G., Pedro Aguilar-Zarate, and Romeo Rojas. 2021. "Currently Applied Extraction Processes for Secondary Metabolites from Lippia turbinata and Turnera diffusa and Future Perspectives" Separations 8, no. 9: 158. https://doi.org/10.3390/separations8090158
APA StyleMartínez-Ávila, G. C. G., Aguilar-Zarate, P., & Rojas, R. (2021). Currently Applied Extraction Processes for Secondary Metabolites from Lippia turbinata and Turnera diffusa and Future Perspectives. Separations, 8(9), 158. https://doi.org/10.3390/separations8090158








