Quality Assessment of Camellia oleifera Oil Cultivated in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Oil Content Assay
2.3. Oil Extraction Assay
2.4. Determination of Acid, Peroxide and Iodine Values
2.5. Determination of Fatty Acid Composition
2.6. Determination of Tocopherols
2.7. Determination of Polyphenols
2.8. Determination of Squalene
2.9. Determination of Trace Elements
2.10. Statistical Analysis
3. Results and Discussion
3.1. Oil Content
3.2. Acid, Peroxide and Iodine Values
3.3. Fatty Acid Composition and Content
3.4. Tocopherols Content
3.5. Polyphenol Content
3.6. Squalene Content
3.7. Trace Elements Concentration
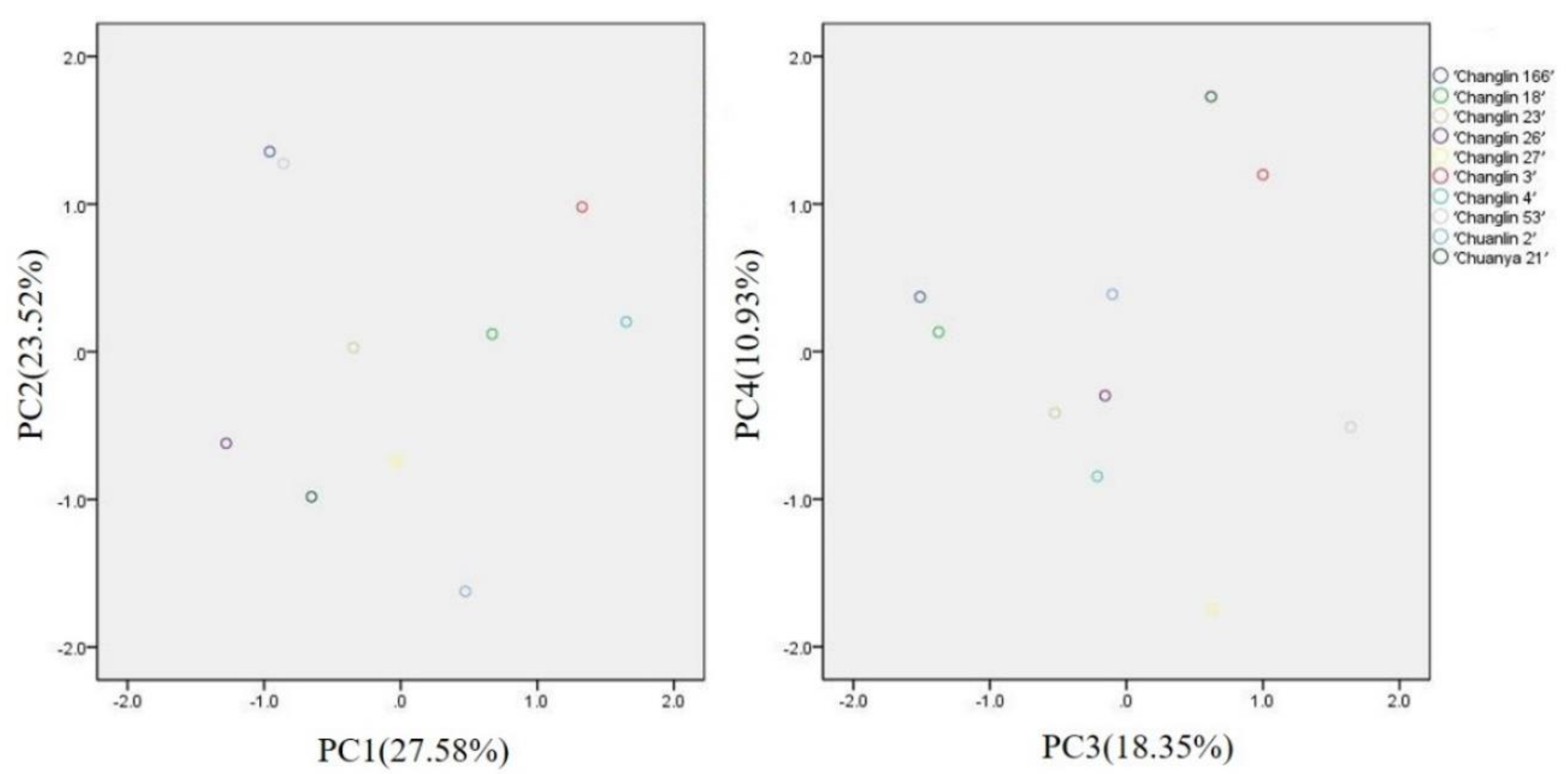
3.8. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ma, J.L.; Ye, H.; Rui, Y.K.; Chen, G.C.; Zhang, N.Y. Fatty acid composition of Camellia oleifera oil. J. Verbrauch. Lebensm. 2010, 6, 9–12. [Google Scholar] [CrossRef]
- Zhu, M.T.; Shi, T.; Chen, Y.; Luo, S.H.; Leng, T.; Wang, Y.; Guo, C.; Xie, M.Y. Prediction of fatty acid composition in camellia oil by 1H NMR combined with PLS regression. Food Chem. 2018, 279, 339–346. [Google Scholar] [CrossRef]
- Yang, J.Y.; Li, M.; Zou, X.G.; Peng, B.; Yin, Y.L.; Deng, Z.Y. A novel aqueous extraction for camellia oil by emulsified oil-frozen/thawed method. Eur. J. Lipid Sci. Tech. 2019, 121, 1800431. [Google Scholar] [CrossRef]
- Feas, X.; Estevinho, L.M.; Salinero, C.; Vela, P.; Sainz, M.J.; Vazquez-Tato, M.P.; Seijas, J.A. Triacylglyceride, antioxidant and antimicrobial features of virgin Camellia oleifera, C. reticulata and C. sasanqua Oils. Molecules 2013, 18, 4573–4587. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, C.; Chen, H.; Zhou, H.; Ye, J. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Triacylglycerols composition, oxidation and oxidation compounds in camellia oil using liquid chromatography-mass spectrometry. Chem. Phys. Lipids 2012, 165, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.N.; Song, D.H.; Zhang, F.Q.; Xiao, X.J.; Wang, Q. Comparison of nutritional value of camellia seed oil and olive oil. China Oil Fat 2008, 33, 39–41. [Google Scholar]
- Siger, A.; Nogala-kalucka, M.; Lampart-szczapa, E. The content and antixoidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipid 2007, 15, 137–149. [Google Scholar] [CrossRef]
- Cayuela, J.A.; García, J.F. Nondestructive measurement of squalene in olive oil by near infrared spectroscopy. LWT 2018, 88, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.S.; Barbosa, J.T.; Fernandes, A.P.; Lemos, V.A.; Santos, W.N.; Korn, M.G.; Teixeira, L.S. Multi-element determination of Cu, Fe, Ni and Zn content in vegetable oils samples by high-resolution continuum source atomic absorption spectrometry and microemulsion sample preparation. Food Chem. 2011, 127, 780–783. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Zeng, Q.M.; Verardo, V.; Contreras, M.D.M. Fatty acid and sterol composition of tea seed oils: Their comparison by the ‘‘FancyTiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Wu, S.L.; Ho, C.Y.; Huang, S.M.; Cheng, C.L.; Yen, G.C. Beneficial effects of camellia oil (Camellia oleifera Abel.) on ketoprofen-induced gastrointestinal mucosal damage through upregulation of HO-1 and VEGF. J. Agric. Food Chem. 2014, 62, 642–650. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pavadhgul, P.; Kalpravidh, R.W. Camellia oil-enriched diet attenuates oxidative stress and inflammatory markers in hypercholesterolemic subjects. J. Med. Food 2016, 19, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Yeh, W.J.; Huang, W.C.; Yang, H.Y. Camellia Oleifera Seed Extract Mildly Ameliorates Carbon Tetrachloride-Induced Hepatotoxicity in Rats by Suppressing Inflammation. J. Food Sci. 2019, 84, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Nasir, S. Taxonomic perspective of plant species yielding vegetable oils used in cosmetics and skin care products. Afr. J. Biotechnol. 2005, 4, 36–44. [Google Scholar]
- Lin, P.; Yao, X.H.; Wang, K.L.; Zheng, T.T.; Teng, J.H. Identification and genetic analysis of camellia oleifera changlin series superior clones by SRAP molecular marker. J. Agr. Biotech. 2010, 18, 272–279. [Google Scholar]
- Du, Y.W.; Deng, X.Z.; Zhou, W.G.; Yao, X.H.; Cheng, J.Y. Introduction and comprehensive evaluation of Camellia oleifera ‘Changlin’ cultivars. Develop. Tech. 2014, 28, 96–100. [Google Scholar]
- Yang, C.Y.; Liu, X.M.; Chen, Z.Y.; Lin, Y.S.; Wang, S.Y. Comparison of oil content and fatty acid profile of ten new Camellia oleifera cultivars. J. Lipid. 2016, 10, 3982486. [Google Scholar]
- International Organization for Standardization. Oilseeds-Determination of Oil Content; ISO 659-2009; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Guo, Y.B.; Tang, B.; Qiu, A.Y.; Ji, C.G.; Liu, T.S. Technology for aqueous extraction of camellia seed oil. Tran. CSAE 2008, 24, 249–252. [Google Scholar]
- International Organization for Standardization. Animal and Vegetable Fats and Oils-Determination of Acid Value and Acidity; ISO 660-2009; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. Animal and Vegetable Fats and Oils-Determination of Peroxide Value; ISO 3960-2007; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- International Organization for Standardization. Animal and Vegetable Fats and Oils-Determination of Iodine Value; ISO 3961-2018; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Ren, C.Y.; Zhang, Y.P.; Tang, F.B.; Shen, D.Y.; Mo, R.H. Analysis of main chemical components in camellia oil, olive oil, walnut oil and torreya seeds oil. J. Food Safe. Qua. Test. 2015, 6, 5011–5016. [Google Scholar]
- Su, M.H.; Shih, M.C.; Lin, K.H. Chemical composition of seed oils in native Taiwanese Camellia species. Food Chem. 2014, 156, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, H.Y.; Xia, X.; Zou, X.G.; Li, J.; Zhu, X.M.; Deng, Z.Y. Effect of fatty acid and tocopherol on oxidative stability of vegetable oils with limited air. Int. J. Food Prop. 2015, 18, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.X. The Study of Polyphenol and Oxidation Stability of Oil from Camellia; Central South University of Forestry and Technology: Hunan, China, 2013; pp. 9–11. [Google Scholar]
- Liu, L.Y. Determination of squalene in camellia oil by High Performance Liquid Chromatography. Mod. Food 2017, 11, 84–85. [Google Scholar]
- Ni, Z.L.; Tang, F.B.; Liu, Y.H.; Shen, D.Y.; Mo, R.H. Multielemental analysis of camellia oil by microwave dry ashing and inductively coupled plasma mass spectrometry. Anal. Lett. 2015, 48, 1777–1786. [Google Scholar] [CrossRef]
- State Forestry Administration. Regulations of Selection and Breeding for Plus Tree and Superior Clone; LY/T 1730.1-2008; State Forestry Administration: Beijing, China, 2008. [Google Scholar]
- China National Standardization Committee. Oil-Tea Camellia Seed Oil; GB/T 11765-2018; China National Standardization Committee: Beijing, China, 2018. [Google Scholar]
- Zhu, J.L.; Chai, Z.L.; Wu, C.R.; Huang, Y.T.; Wu, X.Y. Study on the comprehensive characters of Toona sinensis and its oil in Zhejiang Province. J. Chin. Cer. Oil Asso. 2019, 34, 67–73. [Google Scholar]
- Chen, Y.H. Effects of five extraction methods on the quality of camellia oil. Grain Fats 2019, 32, 33–37. [Google Scholar]
- Jiang, H. Study on the Process and Quality of Extracting Camellia Seed Oil by Aqueous Enzymatic Method; Shanghai Normal University: Shanghai, China, 2013; pp. 15–30. [Google Scholar]
- Yang, X.; Yin, S.S.; Bi, H.S. Standard Identification of Camellia Oil and Other Cooking Oils. J. Hefei Norm. Univ. 2017, 35, 31–35, 53. [Google Scholar]
- Nie, M.; Yang, X.H.; Yao, X.H.; Fang, X.Z.; Wang, Y.P. Effects of processing methods on physicochemical property and nutritient component of tea oil. Fore. Res. 2010, 23, 165–169. [Google Scholar]
- Ni, X.R.; Qin, Y.C.; Liu, B.T.; Wang, Y.B.; Ding, M.; Qian, H. Anlysis of oil content and fatty acid composition of Changlin series Camellia olefera seeds. Jiangxi Fore. Sci. Tech. 2014, 42, 18–20. [Google Scholar]
- Zhu, G.L.; Zhou, W.G.; Li, S.G.; Wang, K.L.; Yu, Q.F.; Hu, Z.Z. Research on oil content and fatty acid composition of in different Changlin varieties Camellia oleifera seeds. Hubei Fore. Sci. Tech. 2013, 42, 21–23. [Google Scholar]
- He, Y.C.; Wu, S.J.; Wang, Y.J.; Zhu, P.L. Active components of twenty different kinds of geographical provenances in Jiangxi Province on the base of Camellia oleifera oil by grey relational grade analysis. South. China Fore. Sci. 2017, 45, 26–32. [Google Scholar]
- Zou, Y.J. Geographical Patterns of Variations in Main Chemical Components of Oil-Tea Camellia Seeds; Nanchang University: Nanchang, China, 2019; pp. 30–37. [Google Scholar]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef]
- Giakoustidis, D.; Papageorgiou, G.; Iliadis, S.; Kontos, N.; Kostopoulou, E.; Papachrestou, A.; Tsantilas, D.; Spyridis, C.; Takoudas, D.; Botsoglou, N.; et al. Intramuscular administration of very high dose of alpha-tocopherol protects liver from severe ischemia/reperfusion injury. World J. Surg. 2002, 26, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Determination of phenols in six kinds of camellia oil. Anhui Agri. Sci. Bull 2018, 24, 30–31, 34. [Google Scholar]
- Wang, X.; Zeng, Q.; Del Mar Contreras, M.; Wang, L. Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Z.H. Study on polar antioxidant in tea seed oil. J. Chin. Cer. Oil Asso. 2002, 3, 4–9. [Google Scholar]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Von Tungeln, L.S.; Mitkus, R.J.; Anderson, S.A.; Vanlandingham, M.M.; Forshee, R.A.; Beland, F.A. Pharmacokinetics and biodistribution of squalene-containing emulsion adjuvant following intramuscular injection of H5N1 influenza vaccine in mice. Regul. Toxicol. Pharmacol. 2016, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Deng, Z.Y.; Hu, J.N.; Li, J.; Fan, Y.W. Physical-chemical properties and nutrients of oil-tea camellia seed oil in different refining stages. Food Sci. 2015, 36, 111–115. [Google Scholar]
- Cao, Y.Q.; Yao, X.H.; Ren, H.D.; Wang, K.L. Determination of fatty acid composition and metallic element content of four Camellia species used for edible oil extraction in China. J. Consum. Prot. Food Saf. 2017, 12, 165–169. [Google Scholar] [CrossRef]
- Ni, Z.L.; Tang, F.B.; Qu, M.H.; Liu, Y.H.; Shen, D.Y. Determination of five heavy metals in Camellia Seed Oil by microwave digestion-ICP-MS. Food Sci. 2013, 34, 165–167. [Google Scholar]
- Kara, D.; Fisher, A.; Hill, S. Extraction of trace elements by ultrasound-assisted emulsification from edible oils producing detergentless microemulsions. Food Chem. 2015, 188, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Bakircioglu, D.; Kurtulus, Y.B.; Yurtsever, S. Comparison of extraction induced by emulsion breaking, ultrasonic extraction and wet digestion procedures for determination of metals in edible oil samples in Turkey using ICP-OES. Food Chem. 2013, 138, 770–775. [Google Scholar] [CrossRef] [PubMed]
- China National Food and Drug Supervision and Administration Commission. National Food Safety Standard-Limits of Contaminants in Food; GB 2762-2017; China National Food and Drug Supervision and Administration Commission: Beijing, China, 2017. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
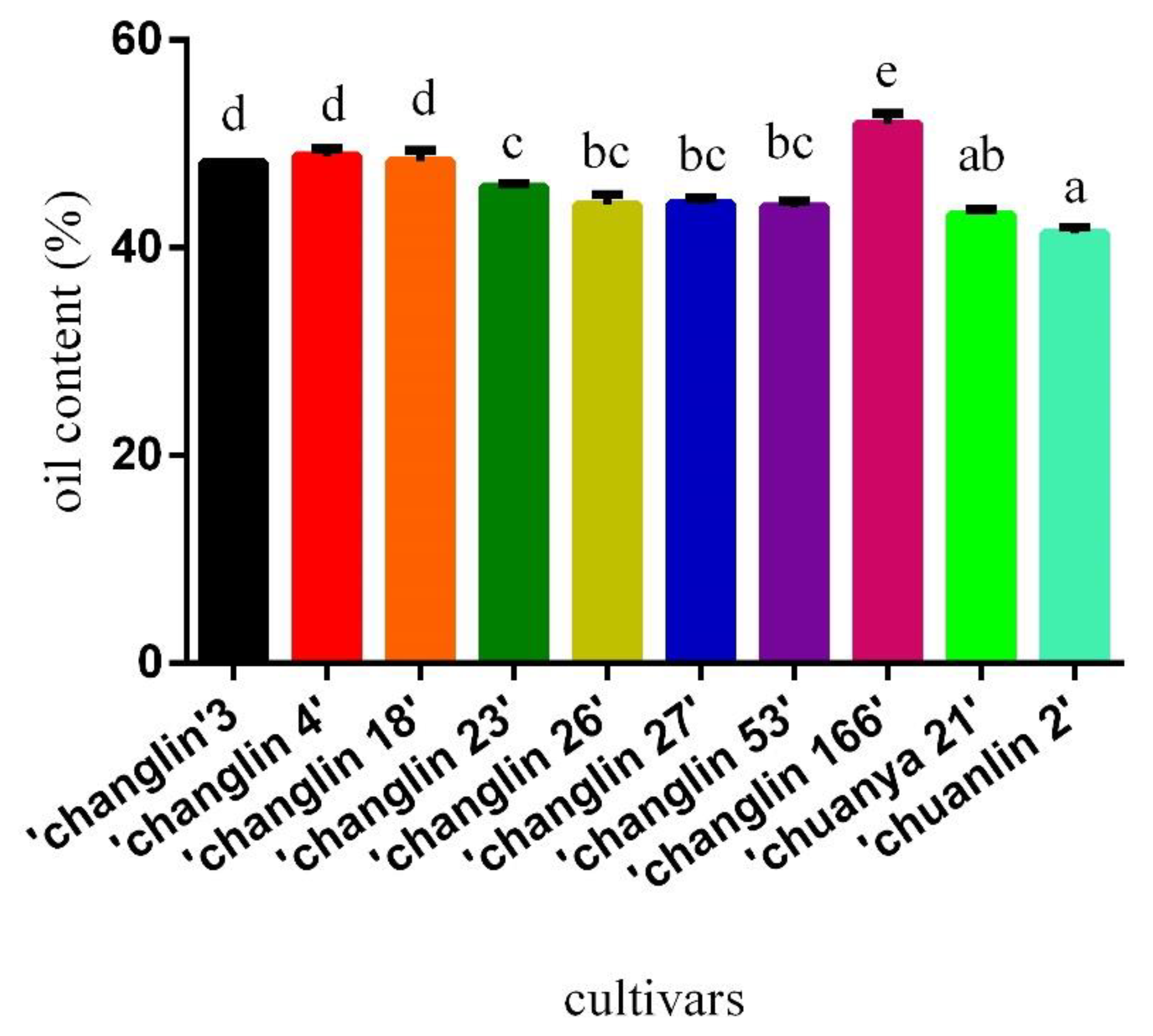

| Acid Value (mg/g) | Peroxide Value (mmol/kg) | Iodine Value (g/100g) | |
|---|---|---|---|
| ‘Changlin 3’ | 1.58 ± 0.11 a | 0.79 ± 0.04 b | 88.72 ± 1.69 bc |
| ‘Changlin 4’ | 1.61 ± 0.17 b | 0.52 ± 0.03 a | 88.92 ± 0.70 bc |
| ‘Changlin 18’ | 1.23 ± 0.05 a | 1.06 ± 0.05 c | 89.89 ± 0.87 bc |
| ‘Changlin 23’ | 1.92 ± 0.19 b | 0.95 ± 0.03 b | 85.33 ± 1.01 a |
| ‘Changlin 26’ | 1.83 ± 0.10 b | 1.22 ± 0.07 d | 88.01 ± 0.54 b |
| ‘Changlin 27’ | 1.74 ± 0.22 b | 1.73 ± 0.03 f | 88.90 ± 1.01 bc |
| ‘Changlin 53’ | 1.60 ± 0.12 b | 0.72 ± 0.01 b | 94.69 ± 0.60 d |
| ‘Changlin 166’ | 1.24 ± 0.06 a | 0.55 ± 0.06 a | 91.17 ± 0.34 c |
| ‘Chuanya 21’ | 1.83 ± 0.02 b | 1.50 ± 0.06 e | 83.80 ± 0.36 a |
| ‘Chuanlin 2’ | 1.89 ± 0.08 b | 1.61 ± 0.09 ef | 84.64 ± 0.61 a |
| C12:0 | C14:1 | C16:0 | C18:0 | C18:1 | C18:2 | C20:1 | |
|---|---|---|---|---|---|---|---|
| ‘Changlin 3’ | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 9.19 ± 0.13 c | 1.16 ± 0.05 ab | 80.53 ± 0.34 a | 8.95 ± 0.18 g | 0.16 ± 0.02 abc |
| ‘Changlin 4’ | - | 0.02 ± 0.01 a | 9.26 ± 0.12 c | 1.43 ± 0.04 b | 81.10 ± 0.32 a | 8.24 ± 0.71 fg | 0.26 ± 0.03 c |
| ‘Changlin 18’ | 0.01 ± 0.01 a | 0.03 ± 0.03 a | 8.06 ± 0.32 b | 1.61 ± 0.13 c | 82.35 ± 1.00 ab | 7.75 ± 0.53 efg | 0.20 ± 0.04 bc |
| ‘Changlin 23’ | 0.01 ± 0.00 a | - | 7.66 ± 0.19 b | 1.25 ± 0.11 b | 85.44 ± 0.62 cd | 5.50 ± 0.35 ab | 0.13 ± 0.02 ab |
| ‘Changlin 26’ | 0.01 ± 0.00 a | - | 7.55 ± 0.16 b | 1.25 ± 0.05 b | 85.67 ± 0.55 d | 5.40 ± 0.31 ab | 0.13 ± 0.02 ab |
| ‘Changlin 27’ | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 8.12 ± 0.08 b | 1.45 ± 0.03 bc | 83.36 ± 0.17 bc | 6.88 ± 0.08 cde | 0.16 ± 0.01 abc |
| ‘Changlin 53’ | 0.02 ± 0.00 a | - | 7.61 ± 0.25 b | 0.84 ± 0.27 a | 85.38 ± 1.28 cd | 5.97 ± 0.82 bc | 0.09 ± 0.01 a |
| ‘Changlin 166’ | 0.02 ± 0.02 a | 0.01 ± 0.00 a | 6.72 ± 0.36 a | 1.22 ± 0.18 b | 86.18 ± 1.23 d | 4.79 ± 0.36 ab | 0.10 ± 0.01 ab |
| ‘Chuanya 21’ | 0.01 ± 0.01 a | 0.02 ± 0.02 a | 8.99 ± 0.08 c | 1.31 ± 0.05 bc | 85.38 ± 0.49 cd | 4.19 ± 0.42 a | 0.10 ± 0.02 ab |
| ‘Chuanlin 2’ | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 9.01 ± 0.10 c | 1.65 ± 0.04 c | 80.34 ± 0.51 a | 7.35 ± 0.68 def | 0.14 ± 0.09 ab |
| α-Tocopherol | Polyphenols | Squalene | |
|---|---|---|---|
| ‘Changlin 3’ | 280.58 ± 5.62 f | 19.47 ± 2.94 ab | 52.49 ± 5.11 d |
| ‘Changlin 4’ | 268.62 ± 8.33 ef | 14.22 ± 0.19 a | 26.62 ± 3.78 b |
| ‘Changlin 18’ | 195.22 ± 6.79 c | 28.24 ± 3.76 c | 37.93 ± 0.30 c |
| ‘Changlin 23’ | 271.31 ± 4.34 ef | 21.27 ± 0.61 b | 34.33 ± 2.30 bc |
| ‘Changlin 26’ | 257.98 ± 4.61 e | 53.63 ± 2.50 e | 32.49 ± 3.80 bc |
| ‘Changlin 27’ | 410.46 ± 4.20 h | 49.51 ± 1.48 de | 14.80 ± 1.54 a |
| ‘Changlin 53’ | 351.73 ± 5.82 g | 47.25 ± 1.87 d | 33.02 ± 3.66 bc |
| ‘Changlin 166’ | 218.28 ± 2.98 d | 29.04 ± 2.35 c | 30.95 ± 1.05 bc |
| ‘Chuanya 21’ | 140.84 ± 3.24 b | 45.10 ± 1.77 d | 33.06 ± 2.37 bc |
| ‘Chuanlin 2’ | 112.36 ± 3.81 a | 30.16 ± 0.91 c | 16.37 ± 0.66 a |
| Mg | Ca | Mn | Fe | Zn | Cu | Cr | As | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Changlin 3’ | 37767.62 ± 141.24 i | 14781.81 ± 369.93 g | 4223.08 ± 165.36 c | 3039.27 ± 13.13 f | 213.84 ± 10.81 d | - | 105.37 ± 1.82 b | - | 0.01 ± 0.00 | - |
| ‘Changlin 4’ | 34529.46 ± 171.04 f | 10793.01 ± 325.66 f | 3416.64 ± 30.60 b | 880.91 ± 20.89 c | 375.67 ± 0.00 e | - | - | - | - | - |
| ‘Changlin 18’ | 30224.19 ± 123.92 d | 6016.77 ± 14.39 b | 4327.17 ± 65.38 c | 1380.45 ± 78.16 d | 18.32 ± 0.66 a | - | - | - | - | 0.01 ± 0.00 a |
| ‘Changlin 23’ | 26930.09 ± 180.88 c | 8194.09 ± 63.57 c | 3057.80 ± 43.03 b | 606.46 ± 26.99 a | 15.51 ± 0.50 a | - | - | - | - | 0.01 ± 0.00 a |
| ‘Changlin 26’ | 24624.36 ± 145.97 b | 5667.37 ± 157.82 ab | 17247.78 ± 258.31 e | 916.49 ± 66.65 c | 51.82 ± 2.74 b | 0.04 ± 0.00 b | - | - | - | 0.02 ± 0.00 a |
| ‘Changlin 27’ | 36872.35 ± 521.63 h | 8864.91 ± 319.01 de | 17592.39 ± 398.03 e | 922.38 ± 51.66 c | 12.75 ± 1.39 a | - | - | - | - | |
| ‘Changlin 53’ | 37339.69 ± 387.01 hi | 9290.46 ± 49.84 e | 12767.51 ± 103.47 d | 820.84 ± 24.68 bc | 471.67 ± 13.44 f | - | 173.15 ± 8.57 d | - | - | 0.01 ± 0.00 a |
| ‘Changlin 166’ | 20549.60 ± 257.41 a | 5939.70 ± 78.35 b | 2298.96 ± 47.66 a | 1345.96 ± 62.36 d | 47.88 ± 1.17 b | - | 77.82 ± 4.64 a | - | - | 0.02 ± 0.01 a |
| ‘Chuanya 21’ | 35398.45 ± 261.88 g | 8347.00 ± 90.15 cd | 17408.37 ± 153.80 e | 1992.82 ± 130.67 e | 103.72 ± 4.61 c | 0.02 ± 0.00 a | 143.96 ± 8.31 c | - | - | 0.02 ± 0.00 a |
| ‘Chuanlin 2’ | 33643.24 ± 162.45 e | 5329.25 ± 69.43 a | 2356.90 ± 12.81 a | 689.45 ± 19.46 ab | 106.53 ± 6.99 c | 0.02 ± 0.00 a | 120.02 ± 6.77 b | - | - | - |
| Cultivars | Component 1 | Component 2 | Component 3 | Component 4 | Synthesis Score |
|---|---|---|---|---|---|
| ‘Changlin 3’ | 1.37 | 4.24 | 1.56 | 0.95 | 1.79 |
| ‘Changlin 4’ | 1.76 | 2.70 | −0.42 | −1.11 | 0.89 |
| ‘Changlin 18’ | 1.30 | 1.23 | −2.71 | −0.26 | 0.11 |
| ‘Changlin 23’ | −0.63 | −0.74 | −1.03 | −0.27 | −0.57 |
| ‘Changlin 26’ | −1.27 | −3.35 | −0.18 | −0.01 | −1.17 |
| ‘Changlin 27’ | 1.18 | −1.42 | 1.12 | −2.97 | −0.21 |
| ‘Changlin 53’ | −3.70 | 0.74 | 3.15 | −0.66 | −0.36 |
| ‘Changlin 166’ | −3.83 | 0.59 | −2.89 | 0.65 | −1.36 |
| ‘Chuanya 21’ | 0.53 | −0.74 | −1.03 | −0.27 | −0.25 |
| ‘Chuanlin 2’ | 1.16 | −1.57 | −0.12 | 0.82 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Feng, S.; Chen, T.; Zhou, L.; Yuan, M.; Liao, J.; Huang, Y.; Yang, H.; Yang, R.; Ding, C. Quality Assessment of Camellia oleifera Oil Cultivated in Southwest China. Separations 2021, 8, 144. https://doi.org/10.3390/separations8090144
Liu L, Feng S, Chen T, Zhou L, Yuan M, Liao J, Huang Y, Yang H, Yang R, Ding C. Quality Assessment of Camellia oleifera Oil Cultivated in Southwest China. Separations. 2021; 8(9):144. https://doi.org/10.3390/separations8090144
Chicago/Turabian StyleLiu, Li, Shiling Feng, Tao Chen, Lijun Zhou, Ming Yuan, Jinqiu Liao, Yan Huang, Hongyu Yang, Ruiwu Yang, and Chunbang Ding. 2021. "Quality Assessment of Camellia oleifera Oil Cultivated in Southwest China" Separations 8, no. 9: 144. https://doi.org/10.3390/separations8090144
APA StyleLiu, L., Feng, S., Chen, T., Zhou, L., Yuan, M., Liao, J., Huang, Y., Yang, H., Yang, R., & Ding, C. (2021). Quality Assessment of Camellia oleifera Oil Cultivated in Southwest China. Separations, 8(9), 144. https://doi.org/10.3390/separations8090144









