Abstract
In this study, supercritical carbon dioxide (scCO2) extractions of cannabinoids were conducted at four different densities (231, 590, 818, and 911 kg/m3) using ethanol (5% w/v) as a co-solvent. The chemical profiles of these cannabinoids were analysed via reverse-phase high-performance liquid chromatography (RP-HPLC). It was determined that scCO2, at low density (231 kg/m3), produced an extract yield of 6.1% w/v. At high scCO2 density (~818 kg/m3), the yield was 16.1% w/v. More specifically, the amounts of tetrahydrocannabinol (THC) and cannabidiol (CBD) in the scCO2 extract at 818 kg/m3 were 10.8 and 15.6% w/v, respectively. It was also found that the use of 5% w/v ethanol increased scCO2 extract yields at both low and high densities (7.6% w/v and 18.2% w/v, respectively). Additionally, the use of co-solvent increased this yield further under both low- and high-density conditions, to 13.7 and 19.1% w/v, respectively. Interestingly, higher scCO2 density (911 kg/m3) with and without ethanol did not improve the scCO2 extract yield or the amount of cannabinoids. Although this study provides new insights into the correlation between scCO2 density and ethanol co-extraction of CBD and THC, more studies are needed to determine how different scCO2 densities and co-solvents influence the extraction of cannabinoids.
1. Introduction
To obtain bioactive substances from various plant-based matrices, scCO2 has been used widely and is now considered a conventional extraction method. CO2 is considered a useful solvent for the extraction of thermo-sensitive substances because its supercritical fluid state can be reached at mild temperatures (from 31 °C) [1,2]. As a small molecule, CO2 also has high diffusivity that increases with higher temperature. It is also inexpensive, non-flammable, readily available, and inert [3]. Additionally, by controlling the pressure and/or temperature of the process, physical properties such as the density and diffusivity of scCO2 can be adjusted; thus, the solvent’s power, and potentially the extraction’s selectivity, can be refined [4,5].
Based on the theory that like-dissolves-like, the main limitation of CO2 is its non-polar nature. The solvent ability of CO2 is similar to that of hexane; it dissolves mostly non-polar substances and some moderately polar substances. However, using a small amount of co-solvent or modifier such as ethanol and water, one can further improve the affinity and solvating power of scCO2 towards more polar solutes [6,7,8]. Furthermore, the use of organic solvents in scCO2 extraction can improve the extraction yield and the selectivity of specific compounds within the extract. This creates a complex solvent/co-solvent mixture and further studies are required to monitor the solutes’ effects in such extracts. Recently, scCO2 extraction of cannabinoids was compared with pressurised dimethyl-ether, and propane, extractions (at 55 °C and 40 bar). The study claimed that cannabinoid extraction was higher (95 mg/g CBD and 1.20 mg/g THC) in extracts obtained at 100 bar and constant temperature (40 °C) compared to samples operated at 200 and 300 bar. The yield of CO2 extract (12.0%wt) was relatively higher than the yield of propane extract (8.2%wt) [9].
Previously, it was reported that scCO2 extractions of cannabinoids (CBD and THC) at 340 bar, 55 °C (328 K), and ethanol (>2.5% v/v) as a co-solvent, were more selective when compared to extractions using organic solvent alone [10]. However, it is unclear whether lower temperatures and higher density of scCO2 offers an extraction advantage when ethanol is used as a co-solvent. Another study used two different strategies for scCO2/ethanol (6% v/v) extraction from the flower; one with decarboxylated samples at 128 bar to 249 bar and 140 °C; the other with non-decarboxylated samples at 165 bar to 240 bar and 50 °C [11]. The decarboxylated sample extract contained 5- to 10-fold higher CBD and THC content. A similar finding was observed in a study where higher amounts of cannabinoids were extracted, resulting in decarboxylated samples [12]. However, the effect of lower temperatures and the use of a co-solvent on the scCO2 extraction of cannabinoids has not been investigated in detail. Therefore, in this study, ethanol was used at different scCO2 conditions as a function of estimated densities to develop and optimise the scCO2 extraction process, including THC and CBD selectivity.
2. Materials and Methods
2.1. Chemicals and Reagents
Cannabis flower was obtained by the School of Pharmacy, the University of Queensland under Queensland Health Approval license UNIR008335019. Carbon dioxide (99.99% purity) was supplied by BOC (Sydney, NSW, Australia). Ethanol and methanol were purchased from Sigma Aldrich (Sydney, NSW, Australia). Cannabinoids: cannabidiol (CBD), cannabidiol acid (CBDA), tetrahydrocannabinol (THC), and Δ9-tetrahydrocannabinol acid (THCA) were supplied by Novachem Pty Ltd, Victoria, Australia. All other chemicals and reagents used were of analytical or HPLC grade.
2.2. Sample Preparation
The cannabis strain, referred to as ‘balanced strain’ hereafter (having ~55% w/v cannabidiol and ~45% w/v tetrahydrocannabinol), was used for extraction. The cannabis sample with Sativa genotype was planted on 4 May 2017 under best growing conditions (12–18 h light exposure at 23 °C). The flower of the cannabis sample was collected at the fluorescence stage and dried for 5 to 8 days at 20 °C (with total moisture <10%). The flower sample was pulverised in a coffee grinder (Breville, model BCG200, China) for 2 min to reduce the particle size. After that, it was decarboxylated in a vacuum oven at 160 °C for 30 min [13].
2.3. SFE Equipment and Setup
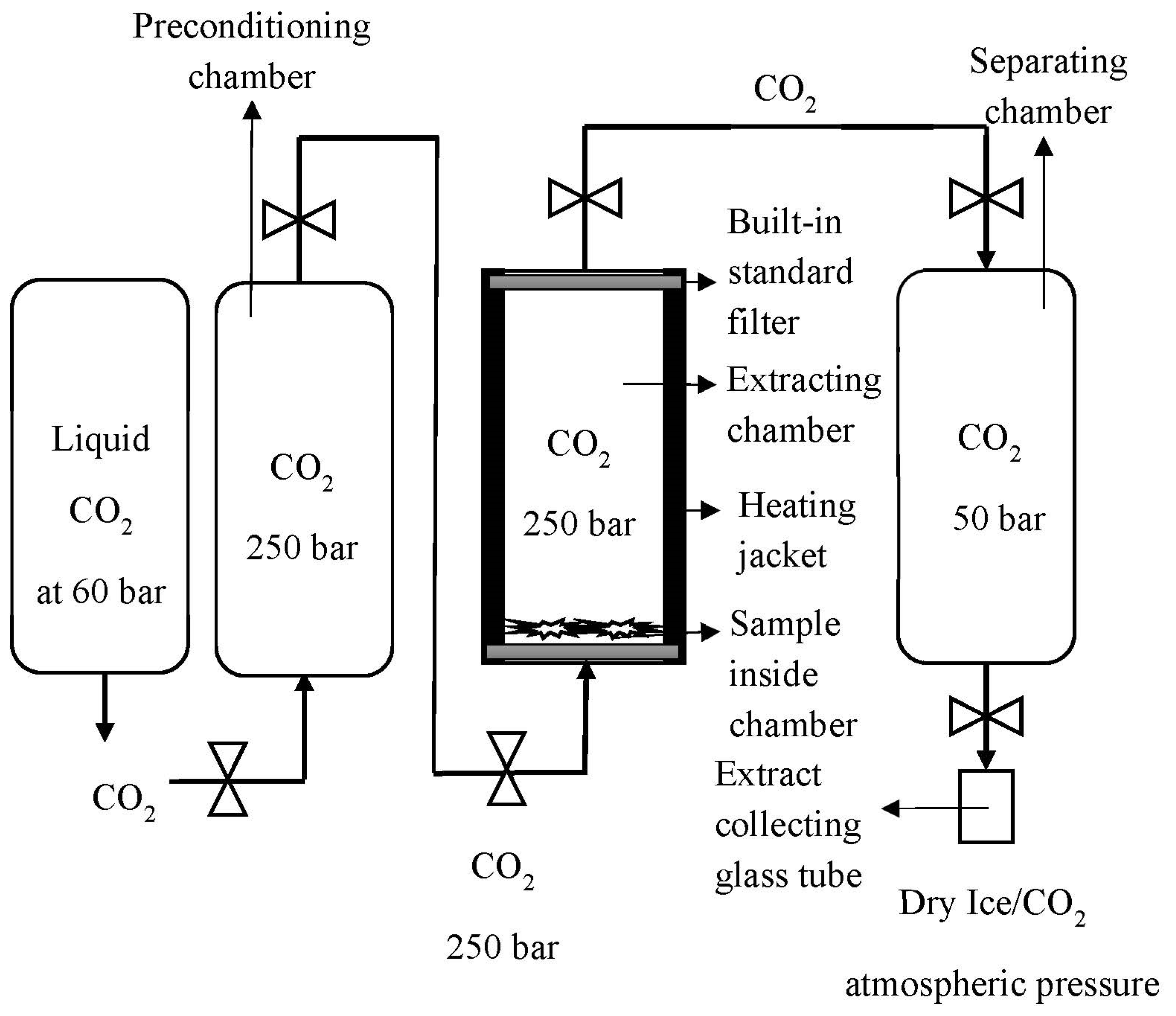
A Helix unit (Applied Separations, Allentown, TX, USA) was used as the core setup for the scCO2 extraction of cannabinoids. The scCO2 unit consisted of four chambers (excluding the stored liquid CO2 cylinder) in series, as shown in Figure 1. This unit was developed and tested in previous experimental studies. Liquid CO2 was converted into the supercritical state in a preconditioning chamber (200 mL). scCO2 was flushed into the sample chamber (100 mL) for the extraction phase. After the extraction, separation of the extract (150 mL sample cylinder, Swagelok, USA) from the raw material was performed via a 10 min cycle procedure of ‘valve open until lower set pressure met’ in the separation chamber, then ‘closed and re-pressurised’. This was completed three times to help maximise the collection of extract. This separation procedure also helped to minimise the throttling effect, which can commonly result in tube blocking. Finally, the extract was collected in a glass sample chamber (10 mL) and the separator chamber was washed with ethanol to collect the total extract.
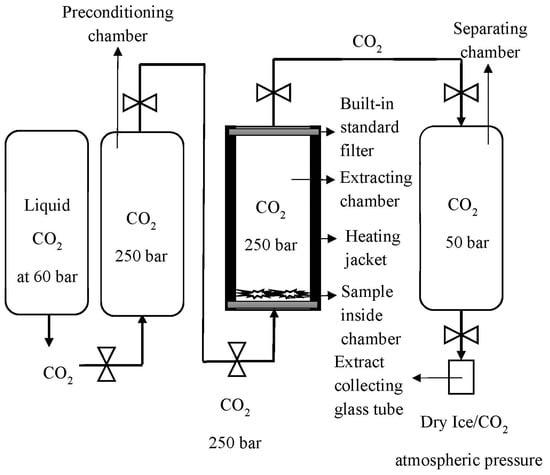
Figure 1.
Schematic representation of the Helix unit used for extraction and separation of cannabis flower.
2.4. SFE Conditions
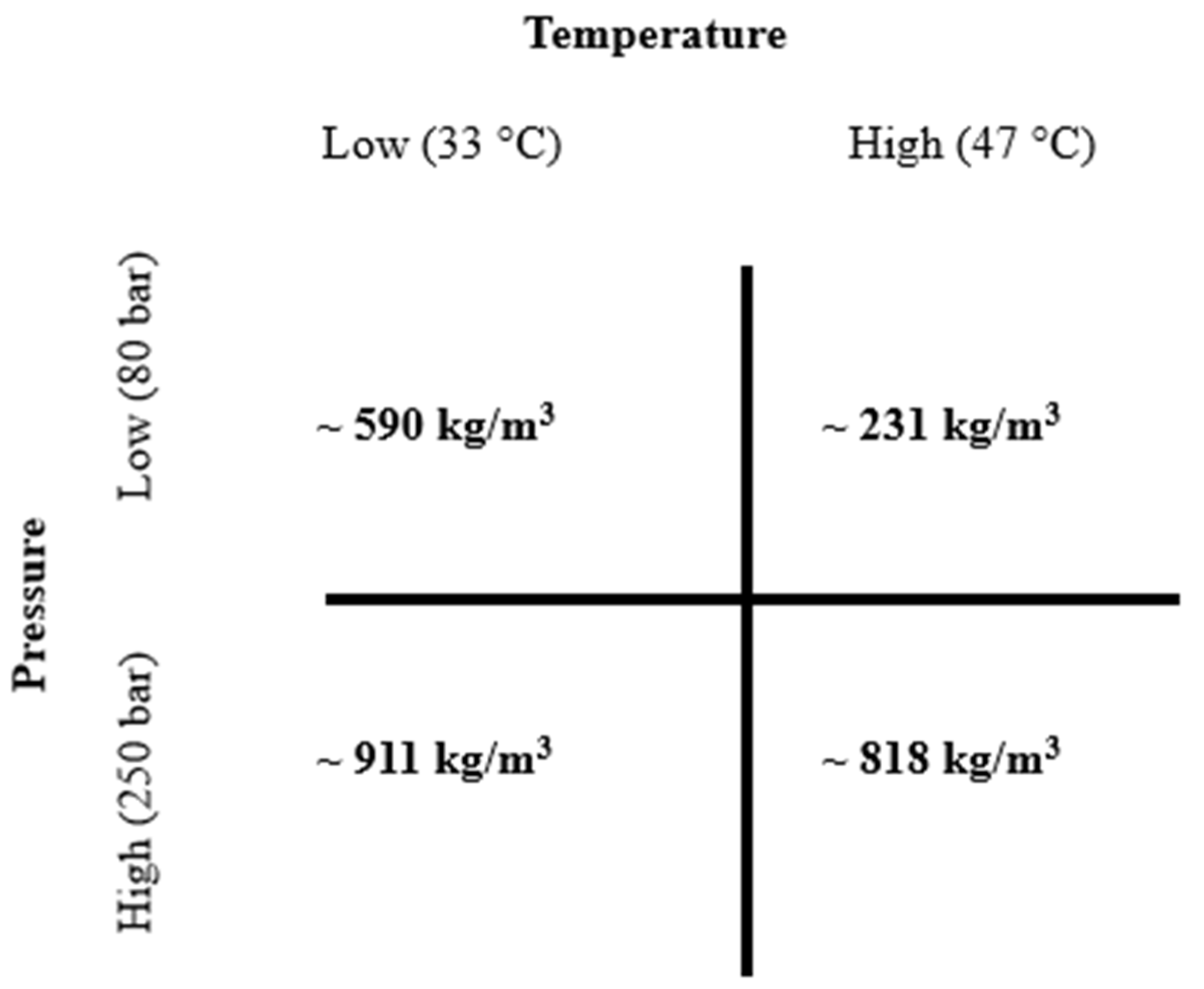
Supercritical CO2 (scCO2) operating conditions were selected according to estimated density tables previously detailed by Span and Wagner [14]. In this study, four different scCO2 density conditions were chosen according to low (231 kg/m3), medium (590 kg/m3), and high (818 kg/m3 and 911 kg/m3) densities as functions of temperature and pressure (as shown in Figure 2). The sample amount used was 1 g and the extraction time was fixed at 3 h.
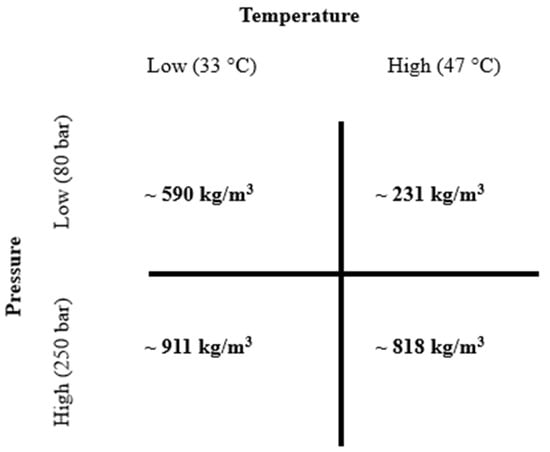
Figure 2.
Supercritical carbon dioxide (scCO2) and estimated operating densities (in bold) according to Span and Wagner’s density tables. (~means approximately).
3. CO2 Extraction with Co-Solvent
In this study, ethanol was used as a modifier or co-solvent with scCO2 extraction of cannabinoids. For this purpose, 0.5 mL of ethanol was added into the 1 g of cannabis sample (5% w/v), and then CO2 extraction was performed for three hours.
3.1. Conventional Extraction
To compare the results and effectiveness of scCO2 extraction, an organic solvent extraction process was also performed. Here, the alcoholic extract was prepared by mixing the grinded cannabis material with ethanol (1:10 w/v). Next, sonication was performed for 15 min and the obtained mixture was stirred for 24 h in the dark at cold temperature (2–8 °C). This mixture was finally filtered and dried using a nitrogen evaporator. The obtained extract was dissolved in methanol (2 mL) and centrifuged at 10,000 rpm for 15 min. Further dilutions were performed to quantify cannabinoids in the extract using RP-HPLC [15].
3.2. Statistical Analysis of the Experimental Design
The factorial design runs and data analysis were performed using Minitab 17.1, with 90% (p < 0.01) and 95% (p < 0.05) confidence levels considered statistically significant.
4. HPLC Quantification
4.1. Mobile Phase Elution Program
Separations and quantifications of selected cannabinoids in a single run (45 min) were performed using reverse-phase HPLC. A C18 chromatographic column with 2.2 µm particle size (Shimadzu Scientific) and the Cannabis Analyser or Lab Solutions software (Shimadzu Scientific Instruments, Sydney, NSW, Australia) were used to standardise the method.
The mobile phase A (Milli-Q water) and B (methanol) were mixed with phosphoric acid (99.93/0.07% v/v) and sonicated for 15 min. The pH of the mobile phase A (2.22 to 2.26) and B (2.43 to 2.48) were also monitored to avoid peak shifting. The flow rate was 1.0 mL/min and the column oven temperature was maintained at 50 °C to obtain a constant column pressure (5400–5600 psi). The volume of injection was 10 µL and detection was performed at 220 nm. The mobile phase B ratio was gradually increased from 65% (v/v) to 72% (v/v) over 25 min. After that, it inclined to 95% over 5 min. Following this, the column was re-equilibrated for 12 min.
4.2. Standard Solution Preparation
The standard solution of each cannabinoid (at 1000 µg/mL concentrations) was diluted in methanol to make 250 µg/mL as a primary stock solution. Calibration curves for 11 cannabinoid solution mixtures were prepared by using methanol at various concentrations (ranging from 1.0 to 25.0 µg/mL). All stock, and standard, solutions were stored at −80 °C until ready for testing.
4.3. Sample Preparation for Cannabinoid Quantification
The solvent-free scCO2 extract was added to methanol (2 mL) and mixed via sonication for 15 min. Following this, the primary extract was centrifuged at 10,000 rpm for 10 min. The 20-times-diluted secondary extract was prepared and vortexed. The secondary extract was either directly quantified or further diluted to fit the area under the curve. All samples and stock solutions were stored at −80 °C until they were ready for testing.
5. Results and Discussion
In this study, the solubility of two main neutral cannabinoids (CBD and THC) and their carboxylic acids (CBDA and THCA) in organic solvent and scCO2 extracts were examined. To increase the amount of neutral cannabinoids in our extracts, the decarboxylation of the cannabis sample was performed; results are presented in Table 1. It was observed that the decarboxylation process increases the amount of neutral cannabinoids in the organic solvent extract. Therefore, decarboxylated samples were used for the scCO2 extraction of cannabinoids. Ethanol was used as an organic solvent for the extraction because, according to FDA guidelines, it can be considered a safe solvent. Additionally, it can extract both polar and slightly non-polar compounds [16].

Table 1.
Organic solvent extraction of cannabinoids from cannabis plant material.
5.1. Effect of scCO2 Density on the Extraction of Cannabinoids
In this study, scCO2 extractions were performed at four different densities. CO2 density can be changed by varying temperature and pressure. The solubility of any compound in scCO2 is mainly dependent on the operating temperature and pressure. High pressure increases the solvent power and density of scCO2, which forms the basis of this study, where the influence of scCO2 density on the selective extraction of CBD and THC was evaluated [17]. From results represented in Table 2 and Table 3, high operating densities (at 818 kg/m3 and 911 kg/m3) yielded greater scCO2 extract (16.10% and 16.13%, respectively) than low (231 kg/m3) and medium (590 kg/m3) densities.

Table 2.
Supercritical carbon dioxide extraction at low pressure (80 bar).

Table 3.
Supercritical carbon dioxide extraction at high pressure (250 bar).
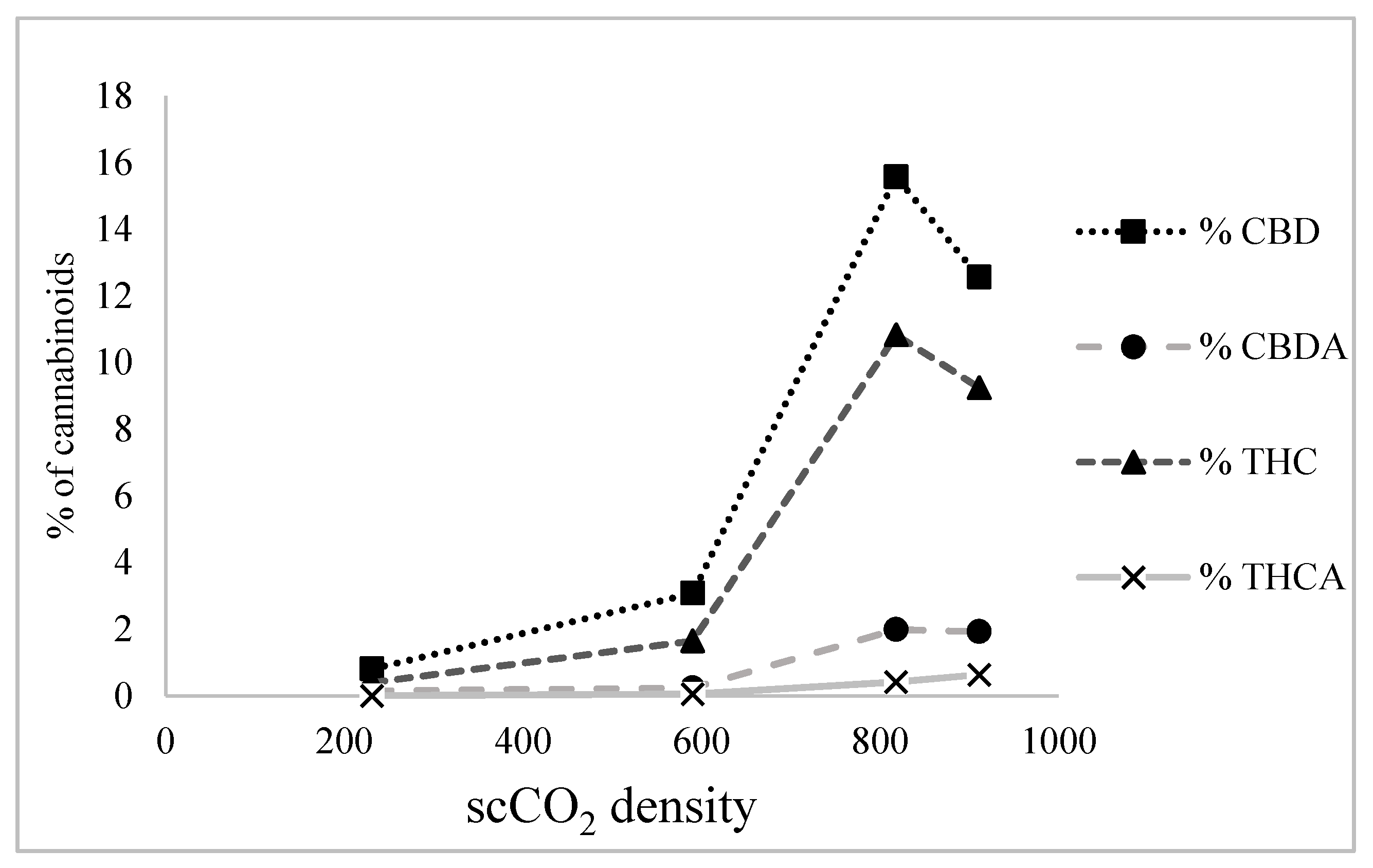
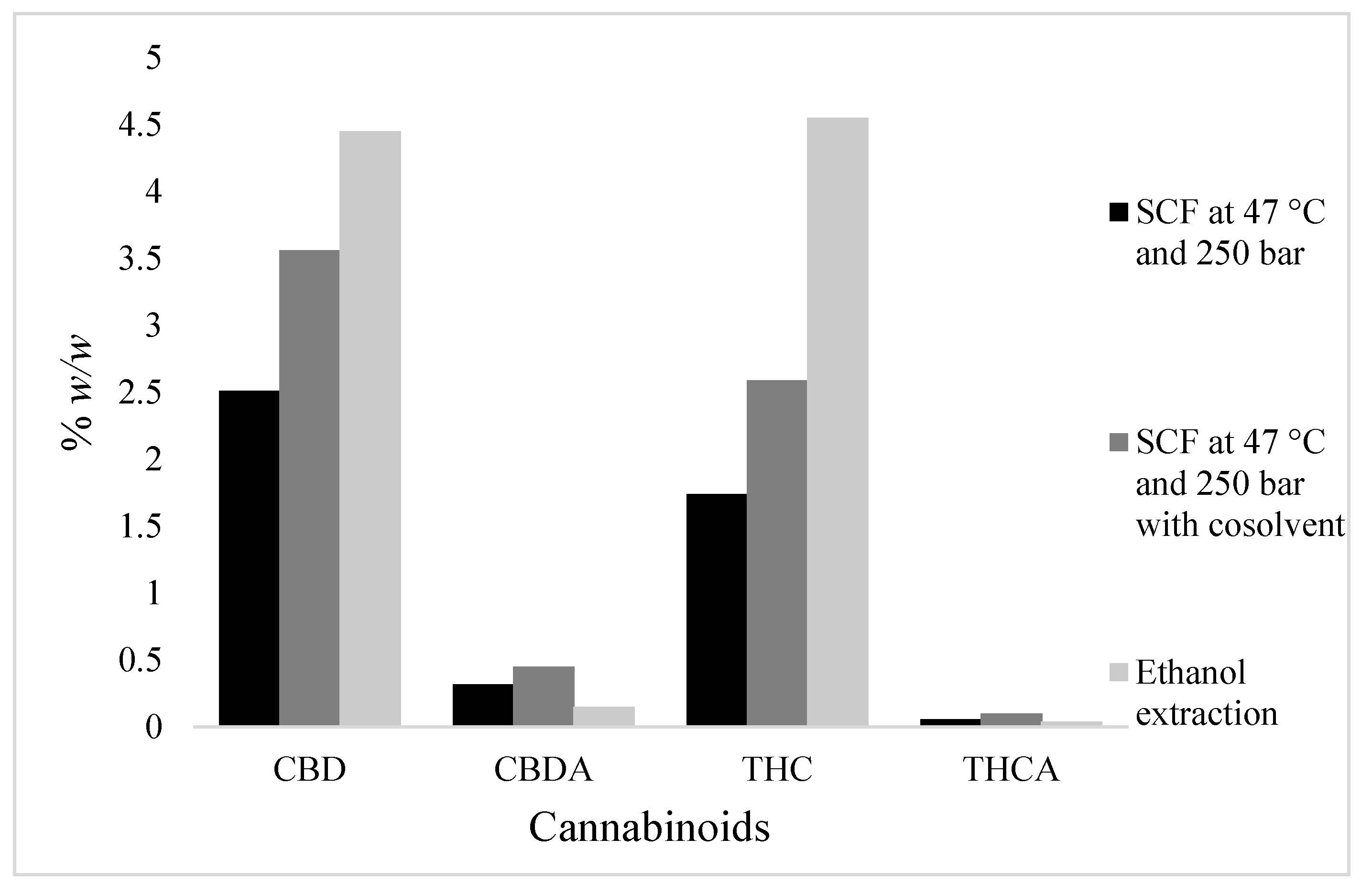
It was observed that, as scCO2 density increased, the amounts of CBD and THC in scCO2 extracts also increased. For example, at low density (231 kg/m3) the percentages of CBD and THC in the scCO2 extract were 0.81% and 0.40%, respectively. At medium density (590 kg/m3) this increased to 3.08% and 1.65%, respectively, whereas at high density (818 kg/m3), percentages sharply increased to 15.57% and 10.83%, respectively. This can be attributed to the fact that, at low density, scCO2 behaves like a highly non-polar solvent (c.f. hexane), while at high density, it acts like a moderately polar solvent (c.f. chloroform) [18].
However, at very high density (911 kg/m3), CBD and THC solubility in scCO2 starts to decline, as shown in Figure 3. This may be due to the low rate of solute mass transfer at a very high density of scCO2 given that the diffusivity of scCO2 decreases with increasing density. This limits the ability of scCO2 to diffuse through the sample and dissolve more of the solute [19].
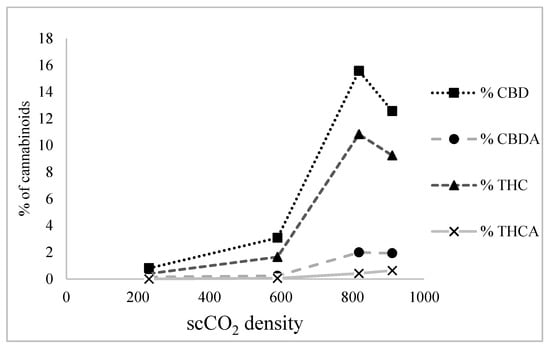
Figure 3.
Effect of supercritical carbon dioxide (scCO2) density on cannabidiol (CBD), cannabidiolic acid (CBDA), Δ9-tetrahydrocannabinol (THC), and Δ9-tetrahydrocannabinol acid (THCA) extraction.
5.2. Effects of Pressure and Temperature
The effects of pressure on the supercritical CO2 extraction of cannabinoids were studied at high (47 °C) and low (33 °C) relative temperatures. It was observed that at low pressure (80 bar), the yield of scCO2 extract and the concentration of cannabinoids was very low compared to high operating pressure. From Table 2, it is also apparent that low pressure, in combination with low temperature, can extract a marginally higher amount of cannabinoids compared to combinations of low pressure and high temperature.
At high pressure (250 bar), the scCO2 extraction yield was somewhat similar at both low and high temperatures. Despite this, the amount of cannabinoids in the resulting scCO2 extract was slightly higher at high temperatures, as shown in Table 3. Previously, it has been reported that changing pressure, while leaving temperature unaltered, can change the strength and density of the scCO2 solvent, directly impacting cannabinoid solubility [10,20]. Additionally, the vapor pressure of the solute also increases above 200 bar, which may explain why, at high pressures, high temperature has a greater influence on solubility than density [21].
5.3. Effects of Co-Solvent
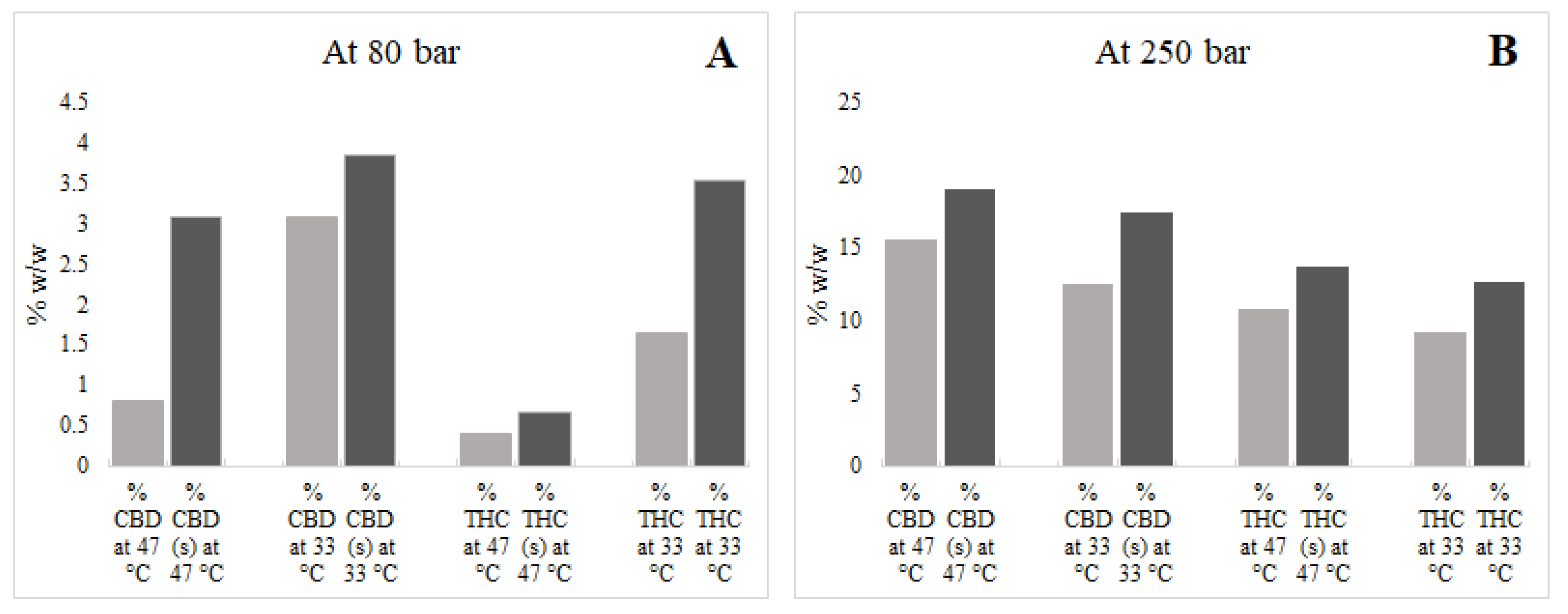
The solubility of cannabinoids in scCO2 using ethanol were also determined. Results are presented in Table 2 and Table 3 and comparisons are shown in Figure 4. It was observed that the use of ethanol as a co-solvent marginally increased the extraction yield, an outcome driven by improved scCO2 solvation power in the presence of the co-solvent that, in turn, increased yield values [22]. In this study, the highest extraction yield obtained using scCO2 and the co-solvent was 18.17%, which was obtained at 47 °C and 250 bar. Previously, Rovetto and Aieta [10] studied the co-solvent effect on scCO2 cannabis extraction yield and obtained an 18.5% yield at high temperature and pressure (55 °C and 340 bar). In another study, [23] obtained a 21.5% extraction yield at 40 °C and 300 bar. The authors of [24] obtained a 26.36% extraction yield at very high temperature (80 °C) and pressure (330 bar) using large amounts of co-solvent (ethanol) and continuous flow during scCO2 extraction. However, in this study, relatively small volumes of co-solvent were used to avoid pigment (e.g., chlorophyll) co-solubilisation along with the target cannabinoids.
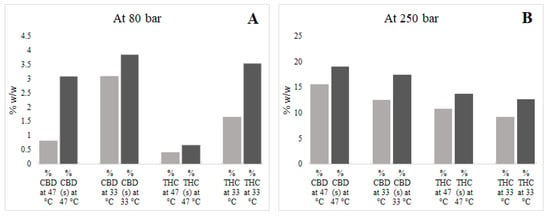
Figure 4.
Effects of co-solvent (s) on supercritical carbon dioxide (scCO2) extraction of cannabidiol (CBD), cannabidiolic acid (CBDA), Δ9-tetrahydrocannabinol (THC), and Δ9-tetrahydrocannabinol acid (THCA). (A); at pressure 80 bar, (B); at pressure 250 bar.
The addition of co-solvent at high temperature (47 °C) and pressure (250 bar) resulted in the extraction of relatively high amounts of CBD and THC in the scCO2 extract. The amounts of CBD + CBDA and THC + THCA were 21.52% w/v and 14.27% w/v, respectively. Reverting to an extraction process with co-solvent at low pressure (80 bar), however, was unfavourable and did not result in the anticipated improvements in cannabinoid extraction, as shown in Figure 4.
In [19], researchers studied the effects of 5% co-solvent on the extraction of cannabinoids (mainly CBDA, CBG, and THC) at 50 °C and 100 to 300 bar. This study showed that the use of ethanol improved the extraction yield and that higher amounts of cannabinoids were extracted at 100 bar with co-solvent. However, previous studies only focused on either the temperature or pressure, not density, of scCO2.
5.4. SFE vs. Organic Solvent Extraction
A relative comparison study between organic solvent (ethanol) extraction and scCO2 extraction was completed and the results are shown in Figure 5. This study showed that our organic solvent-driven method extracted more cannabinoids than the scCO2 method. The addition of the co-solvent in scCO2 increased the amount of cannabinoids extracted. However, the selectivity of scCO2 for cannabinoids is higher than organic solvent extraction, as shown in Figure 6. Additionally, the highest amounts of CBD and THC in the scCO2 extract (at 250 bar and 47 °C with co-solvent) were around 19.0% and 13.73% w/v, respectively. The highest amounts of CBD and THC in decarboxylated samples obtained using conventional solvent extraction through ethanol was around 12.65% and 13.10% w/v, respectively.
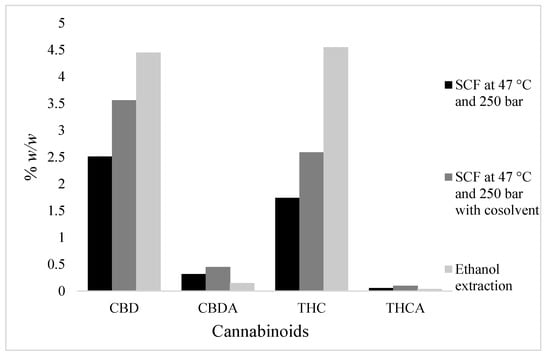
Figure 5.
Comparison study between organic solvent (ethanol) extraction and supercritical carbon dioxide (scCO2) extraction.
Figure 6.
(A) Supercritical carbon dioxide (scCO2) extraction. (B) Organic solvent (ethanol) extraction.
6. Conclusions
From considering the parameters explored, scCO2 density appears to have an appreciable impact on both the extraction yield and amount of cannabinoids extracted from the cannabis flower. The optimum yield was obtained at a high operating scCO2 density (818 kg/m3) at 250 bar and 47 °C; however, the highest density investigated (911 kg/m3) did not further improve extraction yield. The use of ethanol as a solvent modifier further increased cannabinoid yields in the scCO2 extract, with improvements in both the CBD (19.05% w/v) and THC (13.73% w/v) yields obtained. Overall, this body of work revealed that optimal SFE parameters for cannabinoid extractions employing ethanol (5% v/w) as a co-solvent are achieved at 250 bar and 47 °C.
Author Contributions
Conceptualization, S.Q. and J.R.F.; Data curation, S.Q. and Y.J.M.T.; Formal analysis, S.Q. and J.R.F.; Funding acquisition, S.Q. and H.S.P.; Investigation, Y.J.M.T. and H.S.P.; Methodology, S.Q.; Project administration, Y.J.M.T. and H.S.P.; Software, S.Q.; Supervision, J.R.F.; Validation, J.R.F.; Visualization, H.S.P.; Writing—original draft, S.Q.; Writing—review & editing, S.Q., Y.J.M.T., H.S.P. and J.R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
S.Q. is a recipient of the Australian Government Research Training Program Scholarship from the University of Queensland, Brisbane, Australia. The authors also thank Andrew K. Whittaker of the Australian Institute for Bioengineering and Nanotechnology (AJBN) and Kristofer Thurecht at the Centre for Advanced Imaging, the University of Queensland (Brisbane, QLD, 4072, Australia), for their support and access to specialised equipment including the CO2 unit used in this search. Authors also acknowledge support from the School of Pharmacy, the University of Queensland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Campone, L.; Celano, R.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response surface methodology to optimize supercritical carbon dioxide/co-solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Abbas, K.A.; Mohamed, A.; Abdulamir, A.; Abas, H. A review on supercritical fluid extraction as new analytical method, American. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar]
- Espinosa, S.; Diaz, M.; Brignole, E. Process optimization for supercritical concentration of orange peel oil. Lat. Am. Appl. Res. 2005, 35, 321–326. [Google Scholar]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, P.N. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Robert Falconer, J. Extraction of medicinal cannabinoids through supercritical carbon dioxide technologies: A review. J. Chromatogr. B 2021, 1167, 122581. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, T.M.; Paulaitis, M.E.; Johnson, D.M.; McNally, D.M. Modifier effects in the supercritical fluid extraction of solutes from clay, soil, and plant materials. Anal. Chem. 1993, 65, 1462–1469. [Google Scholar] [CrossRef]
- King, M.; Bott, T.R. Extraction of Natural Products Using Near-Critical Solvents; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Moreno, T.; Montanes, F.; Tallon, S.J.; Fenton, T.; King, J.W. Extraction of cannabinoids from hemp (Cannabis sativa L.) using high pressure solvents: An overview of different processing options. J. Supercrit. Fluids 2020, 161, 104850. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Grijó, D.R.; Osorio, I.A.V.; Cardozo-Filho, L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J. CO2 Util. 2018, 28, 174–180. [Google Scholar] [CrossRef]
- Grijo, D.R.; Bidoia, D.L.; Nakamura, C.V.; Osorio, I.V.; Cardozo-Filho, L. Analysis of the antitumor activity of bioactive compounds of cannabis flowers extracted by green solvents. J. Supercrit. Fluids 2019, 149, 20–25. [Google Scholar] [CrossRef]
- Hospodor, A.D. Controlled Cannabis Decarboxylation. Google Patents. U.S. Patent No. 8,980,941, 17 March 2015. [Google Scholar]
- Span, R.; Wagner, W.A. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-assisted extraction of cannabinoids from cannabis sativa L. optimized by response surface methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar]
- Konen, B. Why ethanol works so well for cannabis extraction. Leafy, 31 August 2016. [Google Scholar]
- Cornelio-Santiago, H.P.; Gonçalves, C.B.; de Oliveira, N.A.; de Oliveira, A.L. Supercritical CO2 extraction of oil from green coffee beans: Solubility, triacylglycerol composition, thermophysical properties and thermodynamic modelling. J. Supercrit. Fluids 2017, 128, 386–394. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes, 1st ed.; Springer Science & Business Media: Hamburg-Harburg, Germany, 2013. [Google Scholar]
- Rozzi, N.L.; Singh, R.K.; Vierling, R.A.; Watkins, B.A. Supercritical fluid extraction of lycopene from tomato processing byproducts. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Shi, J.; Khatri, M.; Xue, S.J.; Mittal, G.S.; Ma, Y.; Li, D. Solubility of lycopene in supercritical CO2 fluid as affected by temperature and pressure. Sep. Purif. Technol. 2009, 66, 322–328. [Google Scholar] [CrossRef]
- Romano, L.L.; Hazekamp, A. Cannabis oil: Chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids 2013, 1, 1–11. [Google Scholar]
- Da Porto, C.; Voinovich, D.; Decorti, D.; Natolino, A. Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 68, 45–51. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Ramírez, J.A.M.; Monroy, Z.J.R.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).