Revisiting Chiral Recognition Mechanism on Chicken Alpha 1-Acid Glycoprotein: Location of Chiral Binding Sites and Insight into Chiral Binding Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of W26-Modified cAGP
2.3. Preparation of Native and W26-Modified cAGP Columns
2.4. Generation of Model Structure of cAGP and Docking Simulations
3. Results
3.1. Enantioseparations on Native and W26-Modified cAGP Columns
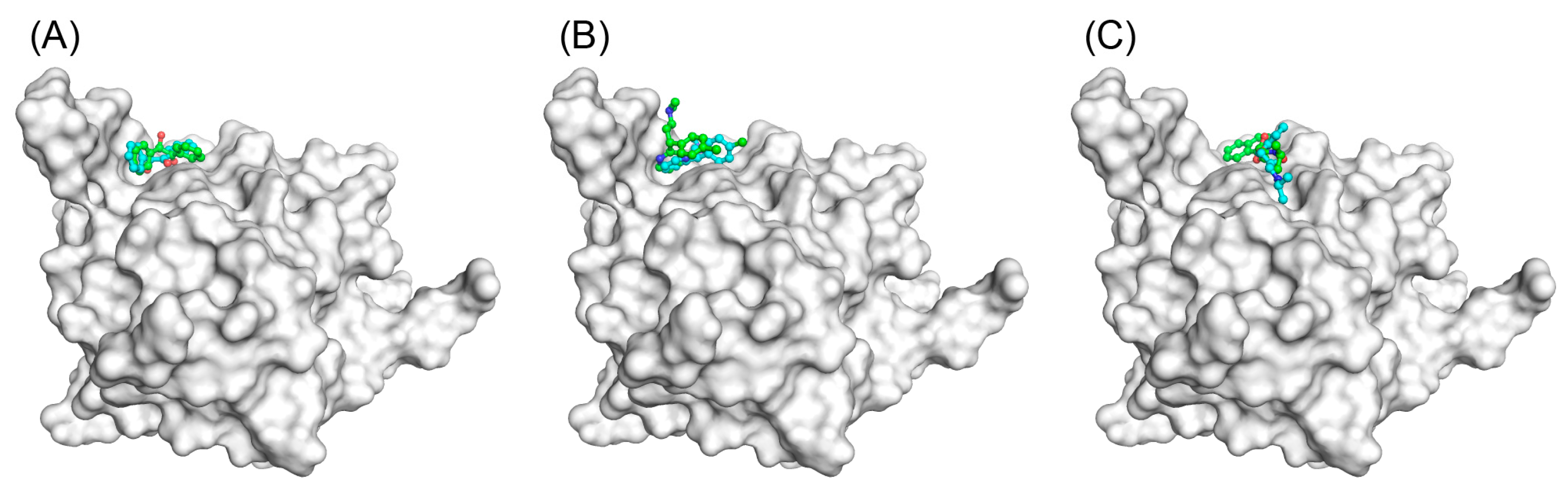
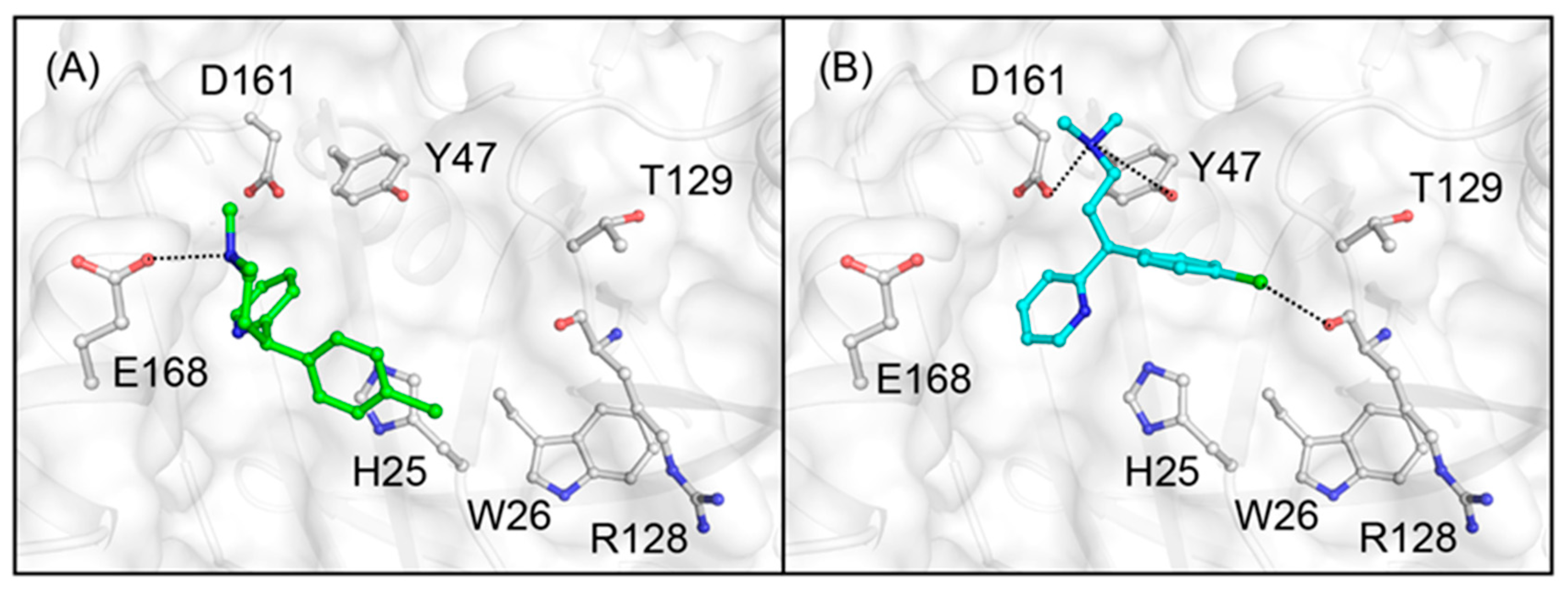
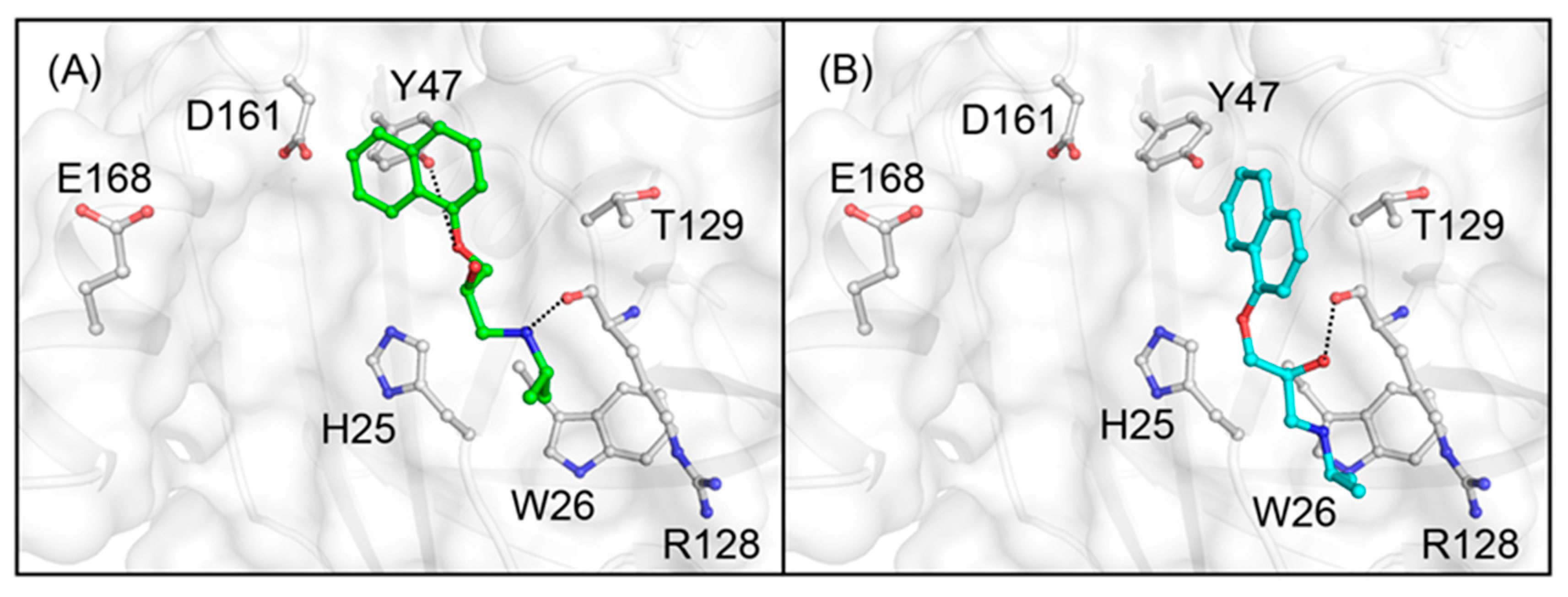
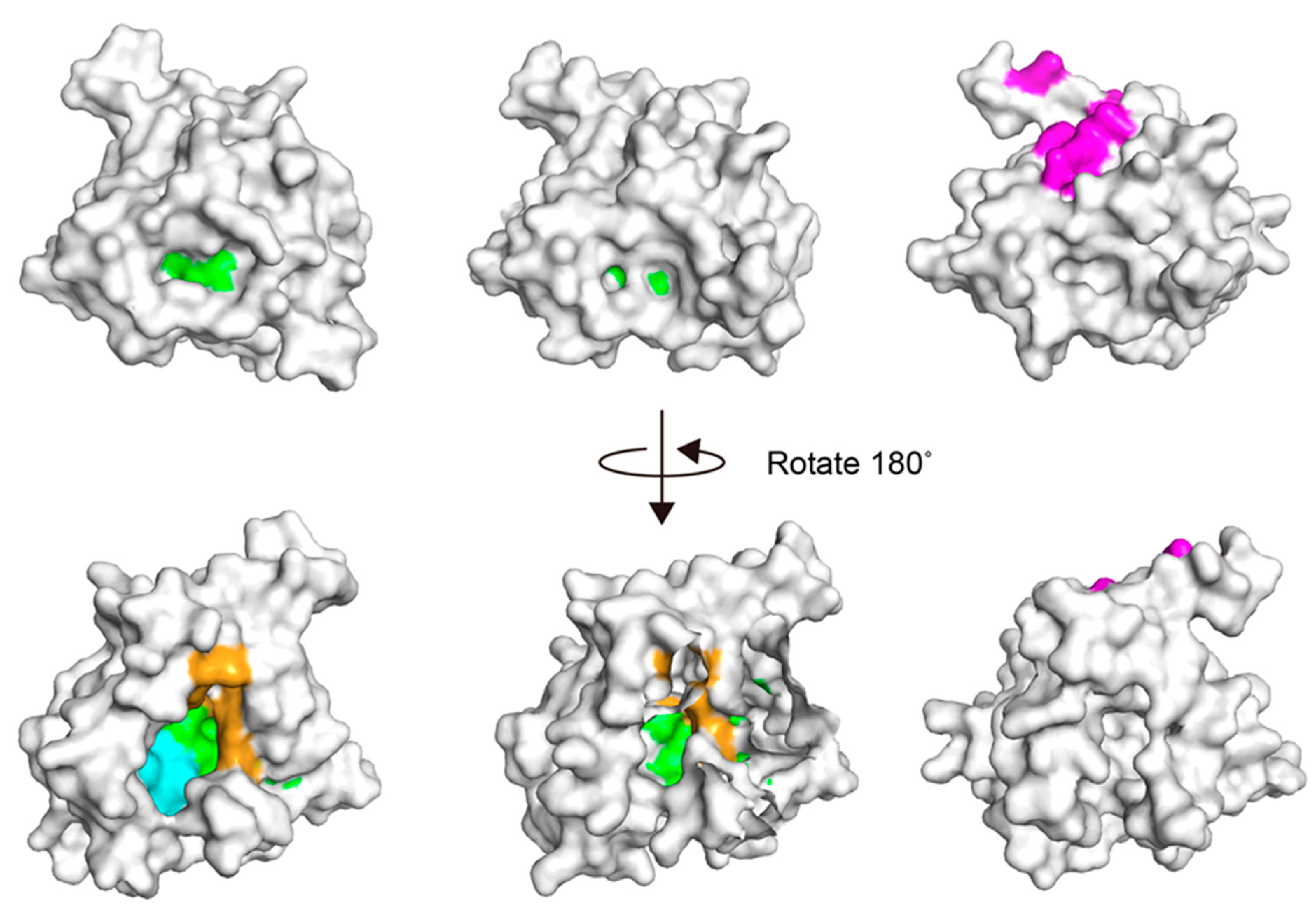
3.2. Docking Simulations of Studied Compounds into Generated Model Structure of cAGP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haginaka, J. Mechanistic Aspects of Chiral Recognition on Protein-Based Stationary Phases. Adv. Chromatogr. 2011, 49, 37–69. [Google Scholar] [CrossRef]
- Bi, C.; Zheng, X.; Azaria, S.; Beeram, S.; Li, Z.; Hage, D.S. Chromatographic Studies of Protein-Based Chiral Separations. Separations 2016, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Allenmark, S.; Bomgren, B.; Borén, H. Direct Liquid Chromatographic Separation of Enantiomers on Immobilized Protein Stationary Phases. IV. Molecular Interaction Forces and Retention Behaviour in Chromatography on Bovine Serum Albumin as a Stationary Phase. J. Chromatogr. 1984, 316, 617–624. [Google Scholar] [CrossRef]
- Domenici, E.; Bertucci, C.; Salvadori, P.; Felix, G.; Cahagne, I.; Motellier, S.; Wainer, I.W. Synthesis and Chromatographic Properties of an HPLC Chiral Stationary Phase Based upon Human Serum Albumin. Chromatographia 1990, 29, 170–176. [Google Scholar] [CrossRef]
- Hermansson, J. Direct Liquid Chromatographic Resolution of Racemic Drugs Using α1-Acid Glycoprotein as the Chiral Stationary Phase. J. Chromatogr. A 1983, 269, 71–80. [Google Scholar] [CrossRef]
- Haginaka, J.; Seyama, C.; Kanasugi, N. The Absence of Chiral Recognition Ability in Ovomucoid: Ovoglycoprotein-bonded HPLC Stationary Phases for Chiral Recognition. Anal. Chem. 1995, 67, 2539–2547. [Google Scholar] [CrossRef]
- Sadakane, Y.; Matsunaga, H.; Nakagomi, K.; Hatanaka, Y.; Haginaka, J. Protein Domain of Chicken α1-Acid Glycoprotein is Responsible for Chiral Recognition Ability. Biochem. Biophys. Res. Commun. 2002, 295, 587–590. [Google Scholar] [CrossRef]
- Thelohan, S.; Jadaud, P.; Wainer, I.W. Immobilized Enzymes as Chromatographic Phases for HPLC: The Chromatography of Free and Derivatized Amino Acids on Immobilized Trypsin. Chromatographia 1989, 28, 551–555. [Google Scholar] [CrossRef]
- Erlandsson, P.; Marle, I.; Hansson, L.; Isaksson, R.; Petterson, C.; Petterson, G. Immobilized Cellulase (CBH I) as a Chiral Stationary Phase for Direct Resolution of Enantiomers. J. Am. Chem. Soc. 1990, 112, 4573–4574. [Google Scholar] [CrossRef]
- Haginaka, J.; Miyano, Y.; Saizen, Y.; Seyama, C.; Murashima, T. Separation of Enantiomers on a Pepsin-bonded Column. J. Chromatogr. A 1995, 708, 161–168. [Google Scholar] [CrossRef]
- Nystrom, A.; Strandberg, A.; Aspergren, A.; Behr, S.; Karlsson, A. Use of Immobilized Amyloglucosidase as Chiral Selector in Chromatography. Immobilization and Performance in Liquid Chromatography. Chromatographia 1999, 50, 209–214. [Google Scholar] [CrossRef]
- Hofstetter, H.; Hofstetter, O. Antibodies as Tailor-made Chiral Selectors for Detection and Separation of Stereoisomers. Trends Anal. Chem. 2005, 24, 869–879. [Google Scholar] [CrossRef]
- Massolini, G.; de Lorenzi, E.; Calleri, E.; Bertucci, C.; Monaco, H.L.; Perduca, M.; Caccialanza, G.; Wainer, I.W. Properties of a Stationary Phase Based on Immobilized Chicken Liver Basic Fatty Acid-binding Protein. J. Chromatogr. B 2001, 751, 117–130. [Google Scholar] [CrossRef]
- Schmid, K.; Nimerg, R.B.; Kimura, A.; Yamaguchi, H.; Binette, J.P. The Carbohydrate Units of Human Plasma Alpha1-acid Glycoprotein. Biochim. Biophys. Acta 1977, 492, 291–302. [Google Scholar] [CrossRef]
- Luo, Z.; Lei, H.; Sun, Y.; Liu, X.; Su, D.F. Orosomucoid, an Acute Response Protein with Multiple Modulating Activities. J. Physiol. Biochem. 2015, 71, 329–340. [Google Scholar] [CrossRef]
- Kremer, J.M.; Wilting, J.; Janssen, L.H. Drug Binding to Human Alpha-1-acid Glycoprotein in Health and Disease. Pharmacol. Rev. 1988, 40, 1–47. [Google Scholar]
- Israili, Z.H.; Dayton, P.G. Human Alpha-1-Glycoprotein and Its Interactions with Drugs. Drug Metab. Rev. 2001, 33, 161–235. [Google Scholar] [CrossRef]
- Dente, L.; Pizza, M.G.; Metspalu, A.; Cortese, R. Structure and Expression of the Genes Coding for Human Alpha 1-acid Glycoprotein. EMBO J. 1987, 6, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Eap, C.B.; Baumann, P. The Genetic Polymorphism of Human Alpha 1-acid Glycoprotein. Prog. Clin. Biol. Res. 1989, 300, 111–125. [Google Scholar]
- Yuasa, I.; Weidinger, S.; Umetsu, K.; Suenaga, K.; Ishimoto, G.; Eap, B.C.; Duche, J.C.; Baumann, P. Orosomucoid System: 17 Additional Orosomucoid Variants and Proposal for a New Nomenclature. Vox Sang. 1993, 64, 47–55. [Google Scholar] [CrossRef]
- Schönfeld, D.L.; Ravelli, R.B.; Mueller, U.; Skerra, A. The 1.8-Å Crystal Structure of Alpha1-acid Glycoprotein (Orosomucoid) Solved by UV RIP Reveals the Broad Drug-binding Activity of This Human Plasma Lipocalin. J. Mol. Biol. 2008, 384, 393–405. [Google Scholar] [CrossRef]
- Nishi, K.; Ono, T.; Nakamura, T.; Fukunaga, N.; Izumi, M.; Watanabe, H.; Suenaga, A.; Maruyama, T.; Yamagata, Y.; Curry, S.; et al. Structural Insights into Differences in Drug-binding Selectivity between Two Forms of Human Alpha1-acid Glycoprotein Genetic Variants, the A and F1*S Forms. J. Biol. Chem. 2011, 286, 14427–14434. [Google Scholar] [CrossRef]
- Zsila, F.; Bikádi, Z.; Simonyi, M. Induced Circular Dichroism Spectra Reveal Binding of the Antiinflammatory Curcumin to Human Alpha1-acid Glycoprotein. Bioorg. Med. Chem. 2004, 12, 3239–3245. [Google Scholar] [CrossRef]
- Hazai, E.; Visy, J.; Fitos, I.; Bikádi, Z.; Simonyi, M. Selective Binding of Coumarin Enantiomers to Human Apha1-acid Glycoprotein Genetic Variants. Bioorg. Med. Chem. 2006, 14, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Huang, J.X.; Cooper, M.A.; Roberts, K.D.; Thompson, P.E.; Nation, R.L.; Li, J.; Velkov, T. Structure-activity Relationships for the Binding of Polymyxins with Human α-1-Acid Glycoprotein. Biochem Pharmacol. 2012, 84, 278–291. [Google Scholar] [CrossRef]
- Huang, J.X.; Cooper, M.A.; Baker, M.A.; Azad, M.A.; Nation, R.L.; Li, J.; Velkov, T. Drug-binding Energetics of Human α-1-Acid Glycoprotein Assessed by Isothermal Titration Calorimetry and Molecular Docking Simulations. J. Mol. Recognit. 2012, 25, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.R.; Abdelhameed, A.S.; Alam, P.; Khan, R.H. Interaction of New Kinase Inhibitors Cabozantinib and Tofacitinib with Human Serum Alpha-1 Acid Glycoprotein. A Comprehensive Spectroscopic and Molecular Docking Approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 159, 199–208. [Google Scholar] [CrossRef]
- Ajmal, M.R.; Almutairi, F.; Zaidi, N.; Alam, P.; Siddiqi, M.K.; Khan, M.V.; Zaman, M.; Ishtikhar, M.; Khan, R.H. Biophysical Insights into the Interaction of Clofazimine with Human Alpha 1-acid Glycoprotein: A Multitechnique Approach. J. Biomol. Struct. Dyn. 2019, 37, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Sakurama, K.; Kobashigawa, Y.; Morioka, H.; Udo, N.; Hashimoto, M.; Imoto, S.; Yamasaki, K.; Otagiri, M. Interaction of Aripiprazole With Human α1-Acid Glycoprotein. J. Pharm. Sci. 2019, 108, 3911–3916. [Google Scholar] [CrossRef]
- Wang, B.L.; Kou, S.B.; Lin, Z.Y.; Shi, J.H.; Liu, Y.X. Insights on the Interaction Mechanism of Brigatinib to Human α-1-Acid Glycoprotein: Experimental and Computational Approaches. Int. J. Biolog. Macromolec. 2020, 157, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Sukimoto, K.; Otagiri, M. Investigation of the Interaction Mode of Phenothiazine Neuroleptics with Apha 1-acid Glycoprotein. J. Pharm. Pharmacol. 1992, 44, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Iwao, Y. The Drug Binding Site of Human Alpha1-acid Glycoprotein: Insight from Induced Circular Dichroism and Electronic Absorption Spectra. Biochim. Biophys. Acta. 2007, 1770, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Chuang, V.T.; Nishi, K.; Kawahara, K.; Nakayama, H.; Yamaotsu, N.; Hirono, S.; Otagiri, M. Use of Photoaffinity Labeling and Site-directed Mutagenesis for Identification of the Key residue Responsible for Extraordinarily High Affinity Binding of UCN-01 in Human Alpha1-acid Glycoprotein. J. Biol. Chem. 2005, 280, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.A.; Larive, C.K. Probing the Binding of Propranolol Enantiomers to α1-Acid Glycoprotein with Ligand-Detected NMR Experiments. J. Phys. Chem. B 2008, 112, 13581–13587. [Google Scholar] [CrossRef]
- Miwa, T.; Ichikawa, M.; Tsuno, M.; Hattori, T.; Miyakawa, T.; Kayano, M.; Miyake, Y. Direct Liquid Chromatographic Resolution of Racemic Compounds. Use of Ovomucoid as a Column Ligand. Chem. Pharm. Bull. 1987, 35, 682–686. [Google Scholar] [CrossRef]
- Miwa, T.; Miyakawa, T.; Kayano, M.; Miyake, Y. Application of an Ovomucoid-conjugated Column for the Optical Resolution of Some Pharmaceutically Important Compounds. J. Chromatogr. A 1987, 408, 316–322. [Google Scholar] [CrossRef]
- Matsunaga, H.; Sadakane, Y.; Haginaka, J. Identification of Disulfide Bonds and Site-specific Glycosylation in Chicken Alpha1-acid Glycoprotein by Matrix-assisted Laser Desorption Ionization Time-of-flight Mass Spectrometry. Anal. Biochem. 2004, 331, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, H.; Haginaka, J. Investigation of Chiral Recognition Mechanism on Chicken Alpha1-acid Glycoprotein Using Separation System. J. Chromatogr. A 2006, 1106, 124–130. [Google Scholar] [CrossRef]
- Zsila, F.; Matsunaga, H.; Bikádi, Z.; Haginaka, J. Multiple Ligand-binding Properties of the Lipocalin Member Chicken Alpha1-acid Glycoprotein Studied by Circular Dichroism and Electronic Absorption Spectroscopy: The Essential Role of the Conserved Tryptophan Residue. Biochim. Biophys. Acta 2006, 1760, 1248–1273. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Tian, T.; Kortum, L.; Hage, D.S. Development of Tryptophan-modified Human Serum Albumin Columns for Site-specific Studies of Drug-protein Interactions by High-performance Affinity Chromatography. J. Chromatogr. B 1998, 715, 183–190. [Google Scholar] [CrossRef]
- Haginaka, J.; Takehira, H. Separation of Enantiomers on a Chiral Stationary Phase Based on Ovoglycoprotein. I. Influences of the Pore Size of Base Silica Materials and Bound Protein Amounts on Chiral Resolution. J. Chromatogr. A 1977, 773, 85–91. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Studer, G.; Tauriello, G.; Bienert, S.; Biasini, M.; Johner, N.; Schwede, T. ProMod3-A Versatile Homology Modelling Toolbox. PLoS Comput. Biol. 2021, 17, e1008667. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER Server: New Development for Protein Structure and Function Predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed]
- Bharatham, N.; Bharatham, K.; Shelat, A.A.; Bashford, D. Ligand Binding Mode Prediction by Docking: Mdm2/Mdmx Inhibitors as a Case Study. J. Chem. Inf. Model. 2014, 54, 648–659. [Google Scholar] [CrossRef]
- Kumar, G.K.; Prasanna, G.; Marimuthu, T.; Saraswathi, N.T. Structural Basis for Complementary and Alternative Medicine: Phytochemical Interaction with Non-structural Protein 2 Protease-a Reverse Engineering Strategy. Chin. J. Integr. Med. 2015, 21, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Pavan Kumar, T.; Saraswathy, G.R.; Sujatha, S. In Silico Evaluation of Drugs Used in Treatment of Oral Lichen Planus. J. Oral Pathol. Med. 2020, 49, 926–932. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Haginaka, J.; Wakai, J.; Takahashi, K.; Yasuda, H.; Katagi, T. Chiral Separation of Propranolol and Its Ester Derivatives on an Ovomucoid-bonded Silica: Influence of pH, Ionic Strength and Organic Modifier on Retention, Enantioselectivity and Enantiomeric Elution Order. Chromatographia 1990, 29, 587–592. [Google Scholar] [CrossRef]



| Entry | Native cAGP Column | W26-modified cAGP Column | ||||
|---|---|---|---|---|---|---|
| kS 2 | kR 2 | α 3 | kS 2 | kR 2 | α 3 | |
| Benzoin | 5.97 | 19.5 | 3.27 | 4.82 | 8.00 | 1.66 |
| Chlorpheniramine | 8.40 | 4.49 | 1.87 | 4.03 | 2.76 | 1.46 |
| Propranolol | 27.0 | 30.5 | 1.13 | 2.06 | 2.06 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haginaka, J.; Yamashita, T.; Tsujino, H.; Arisawa, M. Revisiting Chiral Recognition Mechanism on Chicken Alpha 1-Acid Glycoprotein: Location of Chiral Binding Sites and Insight into Chiral Binding Mechanism. Separations 2021, 8, 73. https://doi.org/10.3390/separations8060073
Haginaka J, Yamashita T, Tsujino H, Arisawa M. Revisiting Chiral Recognition Mechanism on Chicken Alpha 1-Acid Glycoprotein: Location of Chiral Binding Sites and Insight into Chiral Binding Mechanism. Separations. 2021; 8(6):73. https://doi.org/10.3390/separations8060073
Chicago/Turabian StyleHaginaka, Jun, Taku Yamashita, Hirofumi Tsujino, and Mitsuhiro Arisawa. 2021. "Revisiting Chiral Recognition Mechanism on Chicken Alpha 1-Acid Glycoprotein: Location of Chiral Binding Sites and Insight into Chiral Binding Mechanism" Separations 8, no. 6: 73. https://doi.org/10.3390/separations8060073
APA StyleHaginaka, J., Yamashita, T., Tsujino, H., & Arisawa, M. (2021). Revisiting Chiral Recognition Mechanism on Chicken Alpha 1-Acid Glycoprotein: Location of Chiral Binding Sites and Insight into Chiral Binding Mechanism. Separations, 8(6), 73. https://doi.org/10.3390/separations8060073






