Abstract
Natural chemical systems are an excellent object for studying the properties of various elements. The most diverse and informative geological complexes are crystalline rocks of the Precambrian. These rocks are exposed near the northern and southern margins of the Siberian craton. The chemical composition of rocks, the contents of impurity elements, and metals were studied by us using chemical and spectral analysis methods. Microprobe studies were performed. Using regression and multivariate statistical methods of analysis, the regularities of the distribution of chemical elements were found. It is shown that the distribution of precious metals and carbon dioxide in rocks is attributed to their chemical properties and comparable with close in-chemical properties’ rock-forming elements. It is found that the factor analysis reflects the uniform regularities of the distribution of elements in different regions and rocks. These regularities are similar on macro and micro levels. Comparison of the distribution patterns with the results of geochemical and petrological studies of other authors shows the leading role of the redox potential and acidity of the environment in the formation of rocks and minerals. The role of mathematical statistics for solving problems of chemical petrology and chemical systems analysis is underlined.
1. Introduction
The origin and development of the Earth are associated with chemical processes, in which the properties of the atoms of chemical elements play an important role. The various stages of this history are recorded in the rocks and minerals of the Earth’s crust. The age of the studied rocks is associated with the crystallization of a significant part of the Earth’s crust and the formation of ancient stable structures—cratons with a significant part of mineral deposits [1]. The formation of the considered Precambrian rocks corresponds to the conditions of amphibolite and granulite facies of metamorphism with the temperatures 650–950 °C, and pressure 5–7 kb. The Earth’s crust is the site of chemical exchange between the hydrosphere, atmosphere, and the Earth’s interior. Human societies rely on the discovery and mining of ores to produce the raw materials needed to build the edifices of civilization. Understanding the geochemistry of crustal processes is required for the development and maintenance of our society [2]. Geochemical data are commonly composed of thousands of observations and analyzed for 50 or more elements. This abundance of data provides an opportunity to discover a wide range of geochemical processes that may have occurred within a survey area. However, it is often a challenge to examine and interpret the significance of many of the elements, in terms of their exploration potential, and even more difficult to observe or understand the relationships between elements. The application of multivariate data analysis and statistical techniques help make the task of data interpretation and model building easier [3].
2. Materials and Methods
The chemical composition of fragments of Precambrian crystalline rocks from the south and north of the Siberian craton has been studied. In the Department of Physical and Chemical methods of Analysis of the Diamond and Precious Metal Geology Institute, Siberian Branch, Russian Academy of Sciences (DPMGI SB RAS) (Russia, Yakutsk), the contents of chemical elements were identified: (1) rock-forming elements in the form of their oxides by the method of “wet chemistry”; (2) Au and Ag by atomic absorption method; (3) Bi, Mo, Hg, Sb, Zn, Pb, Cu, Sn, B, Co, Ni, Cr, V, Ga, Ge, Nb, La, Y, Li, and Be by semi-quantitative spectral analysis; and (4) Au, Ag, Pd, Bi, Mo, Hg, Sb, Zn, Se, Pb, Sn, Co, Ni, Cr, V, Nb, W, Zr, As, S, Cl, Sr, Rb, Ba, Cs, Sc, Cd, Th, U, Si, Al, Ca, K, Mg, Fe, Mn, P, and Ti by X-ray fluorescence spectral analysis with Niton XL3tGOLDD spectrometer (XRF).
Atomic emission spectral analysis of Au, Ag, Pt, and Pd contents was performed at the A.P. Vinogradov Institute of Geochemistry SB RAS (Russia, Irkutsk). The analysis of the chemical composition of minerals was conducted on polished thin sections using a JSM-6480LV microscope (Jeol Scanning Microscope—Low Vacuum) equipped with Oxford Instruments energy and wavelength-dispersion spectrometers (accelerating voltage 20 kV, probe current 1.09 nA, and measurement time 7 s). The analytical lines were Au—Mα, Ag—α, and other elements—Kα. The standards were gold 850 ‰—Au, Ag; FeS2 (pyrite)—Fe, S; CuFeS2 (chalcopyrite)—Cu; FeAsS (arsenopyrite)—As; ZnS (sphalerite)—Zn; Ca5[PO4]3(F) (fluorapatite)—Ca, P, F; Na [AlSi3O8] (albite)—Na, Al, Si; K[AlSi3O8] (orthoclase)–K; and CeO2 (cerianite)—Ce. Limits of element detection (in wt. %) were: Au 1.81, Ag 1.11, Fe 1.02, S 0.71, Cu 1.22, As 1.07, Zn 1.73, Ca 0.62, F 0.92, P 0.44, Na 0.44, Al 0.36, Si 0.57, K 0.45, and Ce 1.68. The analysis was performed by N. Khristoforova.
To process the results of analysis, we used diagrams that show trends for differentiation of chemical composition of igneous and sedimentary rocks [4] and diagrams reflecting the factors affecting the distribution of elements. The alleged and known properties of the elements, oxides, and factors have been displayed on the diagrams in accordance with the current tasks. To calculate and create trends in the diagrams, the abilities of software Microsoft Excel were used. To calculate factors loadings, Multivariate Exploratory Techniques of information processing in the software Statistica 8 were used. Factor Analysis was performed by the principal components method without axis rotation. Cluster Analysis was performed in the Statistica 8 software by the complete linkage method using Pearson’s Correlation.
Advanced descriptions of the software are available at the sites https://en.wikipedia.org/wiki/Microsoft_Excel and https://en.wikipedia.org/wiki/Statistica (accessed on 24 February).
Descriptions of used Exploration Analysis Technologies are available at the site http://statsoft.ru/products/STATISTICA_Advanced/statistica-multivariate-exploratory-techniques.php (accessed on 24 February).
The data used for calculations and calculation results can be found in the files attached to the article.
3. Results
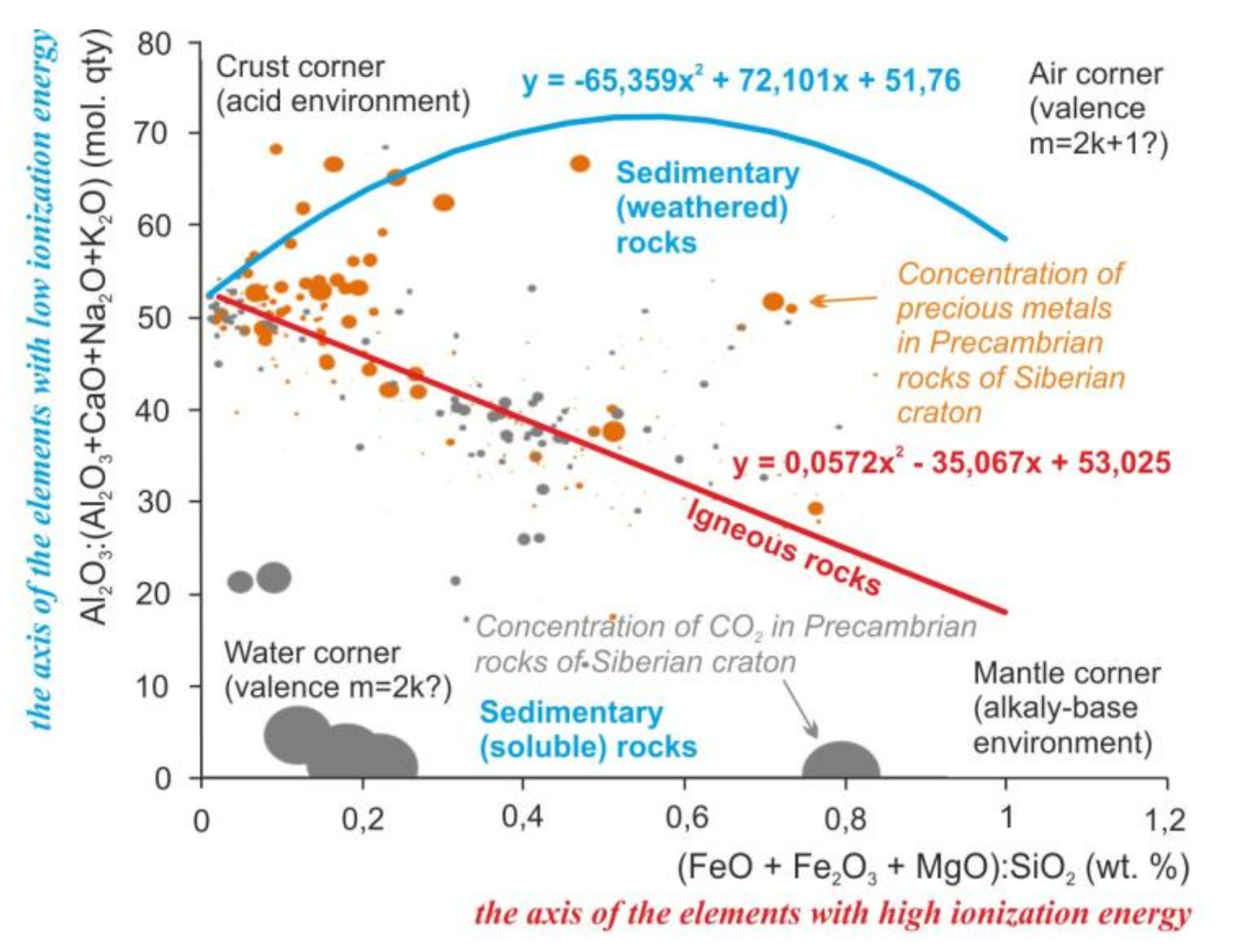
The main elements of rocks by the amount of energy required for ionization can be divided into two groups: Al, Ca, Na, and K with an ionization energy < 600 kJ/mol and Fe, Mg, and Si with ionization energy > 700 kJ/mol [5]. Elements with low ionization energy are involved in the formation of chemical specificity of sedimentary rocks; elements with high ionization energy are involved in magmatic rocks (Figure 1, Supplementary Materials).
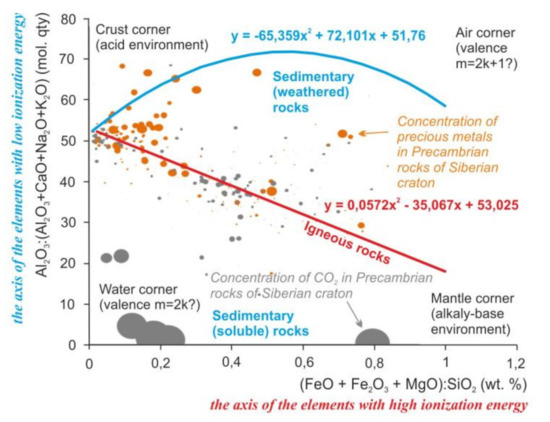
Figure 1.
Regularities of distribution of the rock-forming oxides, precious metals, and carbon dioxide in the Earth’s crust of the Siberian craton on the scattering diagrams. Lines mark the trends of compositions of sedimentary and igneous rocks. Equations of the trends are the mathematical formulas of the variability of chemical composition of the igneous and sedimentary rocks. Dots mark the samples of Precambrian metamorphic rocks with igneous and sedimentary protoliths and concentration of carbon dioxide and precious metals. Size of the dots is the average concentration of the precious metals and carbon dioxide in these rocks. For the diagram on the X-axis, the ratio is a modified parameter F [6] (Femicity); Y-axis is the CIA index (Chemical Index of Alteration) [7] modified (excluding CO2).
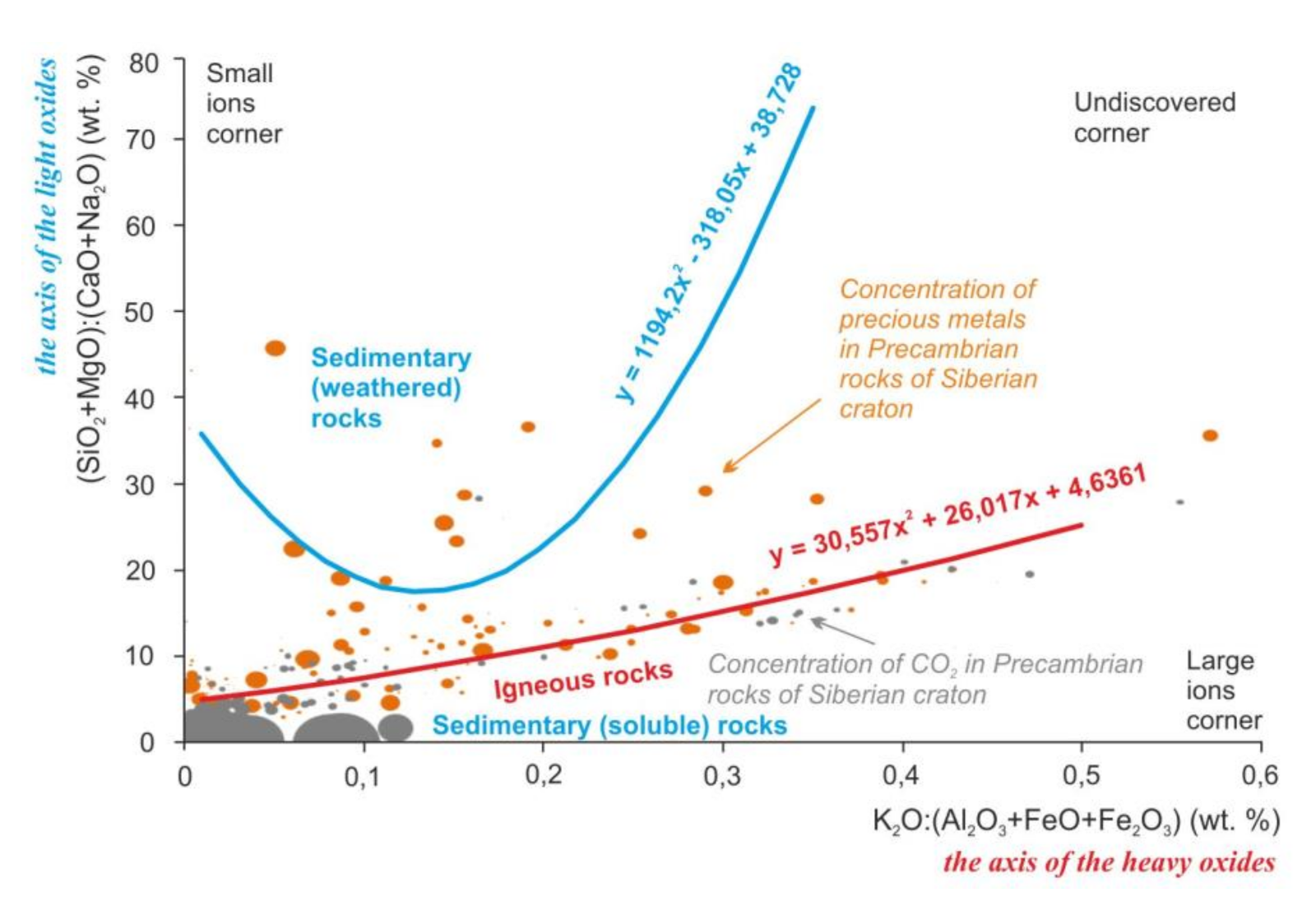
The main oxides of rocks form two groups according to the values of molecular weight: oxides with a mass < 65 and oxides with a mass > 90. Light oxides are involved in the formation of the chemical specificity of sedimentary rocks, and heavy oxides are involved in magmatic rocks (Figure 2).
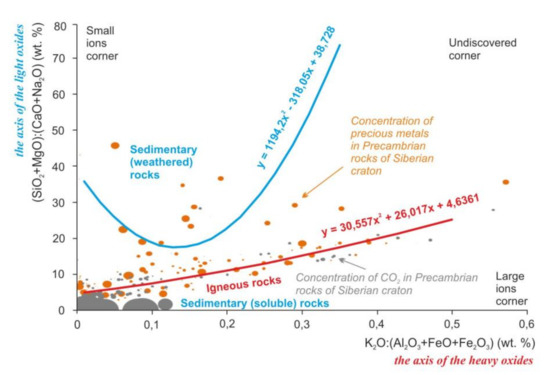
Figure 2.
Regularities of distribution of the rock-forming oxides, precious metals, and carbon dioxide in the Earth’s crust of the Siberian craton on the scattering diagrams. Lines mark the trends of compositions of sedimentary and igneous rocks. Equations of the trends are the mathematical formulas of the variability of chemical composition of the igneous and sedimentary rocks. Dots mark the samples of Precambrian metamorphic rocks with igneous and sedimentary protoliths and concentration of carbon dioxide and precious metals. Size of the dots is the average concentration of the precious metals and carbon dioxide in the rocks. For this diagram, ratios (SiO2+MgO): (CaO+Na2O) and K2O:(Al2O3+FeO+Fe2O3) were proposed by the authors [4].
The behavior of elements probably is controlled by acidity and redox. The influence of acidity is noticeable in the change of chemical elements in the igneous rocks from Fe and Mg to Si and Al during differentiation (Figure 1, Crust and Mantle corners). The influence of redox is noticeable in the distribution of low and high valence elements in sedimentary rocks (Figure 1, Air and Water corners).
Other two factors can be the physical factors such as the gravity and the chemical or mechanical strength. That is seen by the ratios of the oxides masses (axes of Figure 2) and by the interatomic distances for Si and Mg (“small ions” corner, insoluble and hard quartz sands, and spinel) against K, Na, and Ca (“large ions” corner, soluble and soft calcite, halite, and sylvine).
These regularities of the elements’ distribution persist after metamorphic recrystallization of the rocks. Similar regularities are visible on the example of precious metals in Precambrian metamorphic and magmatic rocks of the Siberian craton. Precious metals prefer to form high concentrations closer to the “air” corner (Figure 1) and to the “small ions” corner (Figure 2). For those oxides like the Al2O3 and Fe2O3, as for precious metals, low reactivity or inertness is characteristic. A non-metal such as carbon forms high concentrations located in the “water” and in the areas of distribution of chemogenic sedimentary rocks. That is probably caused by the solubility of the carbon dioxide in water.
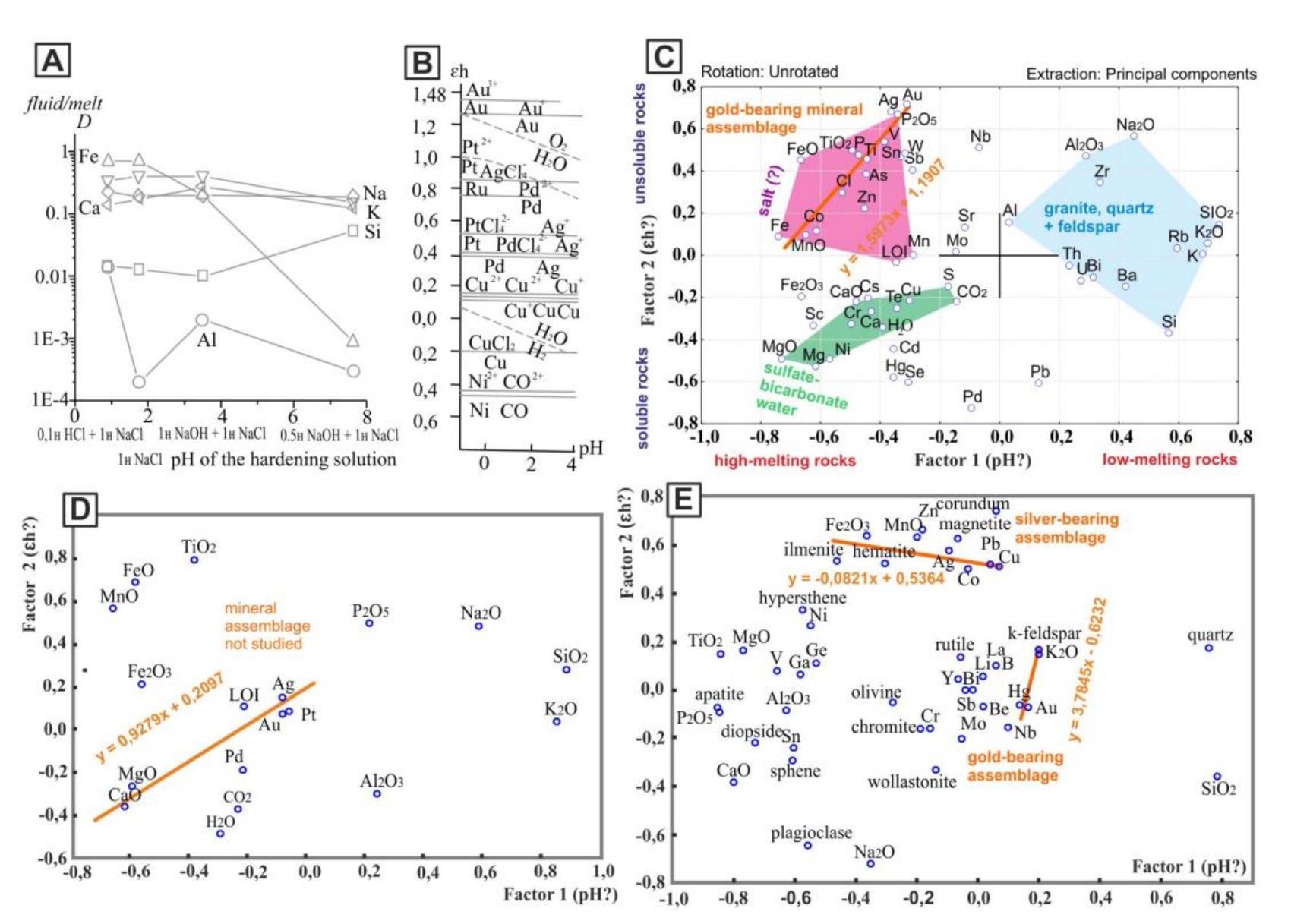
The results of factor analysis of the chemical compositions of rocks (Figure 3) reflect the regularities similar to Figure 1. The distribution of the main elements in the fluid/melt system and the opposition of iron and silicon (Figure 1A) are controlled by the pH factor [8] (Figure 3A). A factor influencing the distribution of elements along the X-axis in the Figure 3 probably is a measure of acidity that is well noticeable if compared to the behavior of rock-forming elements in the fluid/melt system in Figure 3A with the distribution of elements along the X-axis in Figure 3C. During the crystallization of rocks from the melt, silicon, unlike iron, tends to accumulate in the fluid phase. With the accumulation of silicon, the acidity of the fluid phase increases [8]. The factor affecting the distribution of elements along the Y-axis is probably the redox potential. This is clearly visible if we compare the distribution of elements along the Y-axis in Figure 3B with one in Figure 3C.
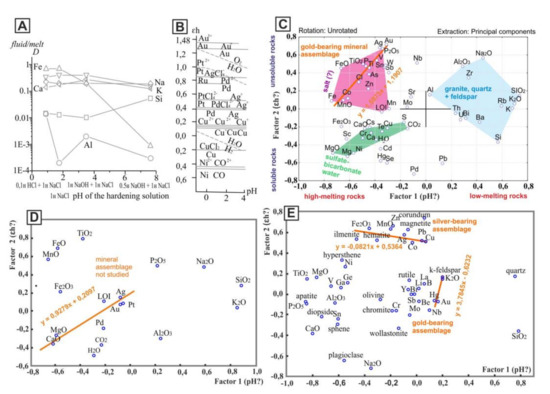
Figure 3.
Regularities of distribution of the elements and oxides in the scattering diagrams. (A)—the distribution of rock-forming components between the fluid and the granite melt depending on the acidity–alkalinity of the fluid of complex composition 1n NaCl + HCl(NaOH) at T = 750°C, P = 100 Mpa [8]. (B)—the stability fields of ions and hydroxides of noble and other metals of the VIII and 1b Mendeleev groups at T = 25 °C, p = 100 Pa, and ion concentrations of 10–7 mol/L [9]. (C–E)—results of factor analysis: (C,D)—rocks of the Aldan granulite–gneiss region in the south of the craton; (E)—rocks of the Anabar shield in the north of the craton. For the C diagram, the contents of elements were determined by XRF, rock-forming oxides by wet chemistry methods; for the (D) diagram, the contents of rock-forming oxides were determined by wet chemistry, and precious metals by atomic emission spectral analysis; for the E diagram, the contents of rock-forming oxides were determined by wet chemistry, elements by semi-quantitative spectral analysis, and the contents of normative minerals by the CIPW method [10].
However, the values of the redox potential K+ ↔ K = −2.92 E°, V; Ca2+ ↔ Ca = −2.87; Na+ ↔ Na = −2.71; Mg2+ ↔ Mg = −2.34; Al3+ ↔ Al = −1.66; Mn2+ ↔ Mn = −1.19; Fe2+ ↔ Fe = −0.44; Fe3+ ↔ Fe = −0.04 [11] correspond with the order of these elements in the non-orthogonal projection on the Y-axis (Figure 3C).
In the diagrams with the results of factor analysis, it is possible to distinguish the corner in which the elements that make up mineralized hydrosulfate–carbonate waters are located. It is possible to distinguish the corner in which chlorine and elements that make up losses of ignition (LOI) of rocks are localized, and the corner with elements of the quartz, feldspar, and granites. Oxides located in the diagram close to volatile elements are characteristic of basalts and rocks of carbonate composition. Thus, the role of volatile elements is probably greater in rocks of basaltic and carbonate composition (rocks of the oceanic crust and mantle). The melts of these rocks are more mobile than melts of granites (rocks of the continental crust) [12]. The carbon and precious metals are confined to rocks with volatile elements. The distribution of these elements is associated with an alkaline (basic) environment and is probably controlled by redox reactions (Figure 3C–E).
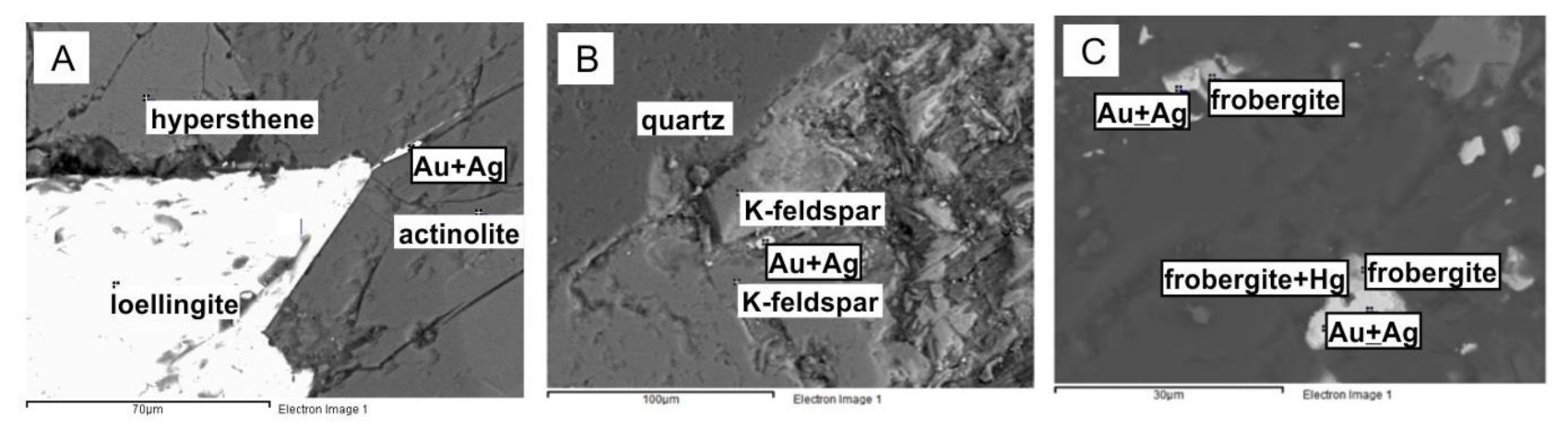
Along the diagonals of the factor diagrams, sets of elements composing certain minerals and mineral assemblages are notable. Along diagonals with a positive correlation, mineral assemblages are more obvious. It was found that gold in rocks (crystalline schists) corresponding in chemical composition to basalts on the southern margin of the craton is closely related to iron arsenide (mineral loellingite) and forms high concentrations in that mineral or independent secretions nearby [13] (Figure 4A). In the factor diagram, the elements characteristic of this mineral assemblage can be connected by a diagonal line Fe-Co-As-Ag-Au (Figure 3C). Therefore, based on the results of factor analysis, the mineral assemblages prospective for searching the metals can be defined.

According to the report of Yevgeny Kardash (Yakutskgeologiya company), one of the signs of this type of mineralization is the presence of carbonate rocks at the contact with mafic crystalline schists. In some gold-bearing zones, high concentrations of platinum and palladium are found. For such objects, we have studied only the chemical composition of rocks and then not fully. The mineral composition was not analyzed. However, the regularities in the distribution of the elements of the studied rocks in the diagram (Figure 3D) reflect the relationship of noble metals with the carbonate component and apatite, similar to the mineralization in the carbonatites of the Tomtor massif [14].
Gold in Precambrian rocks of the northern margin of the craton is associated with granitoids and potassium feldspar [15]. The line drawn through K2O, Au, and Hg (Figure 3E) reflects the features of this type mineralization (Figure 4B,C). The addition in the factor analysis of the contents of normative minerals calculated by the CIPW method [10] gives more objective results (Figure 3E).
Despite the fact that the factor analysis was carried out for rocks of different location and composition, the regularities of the distribution of elements in the factor diagrams is similar (Figure 3C–E). This suggests that factor analysis objectively reflects geochemical factors that affect the distribution of elements, regardless of the type of crystalline rocks and their location.
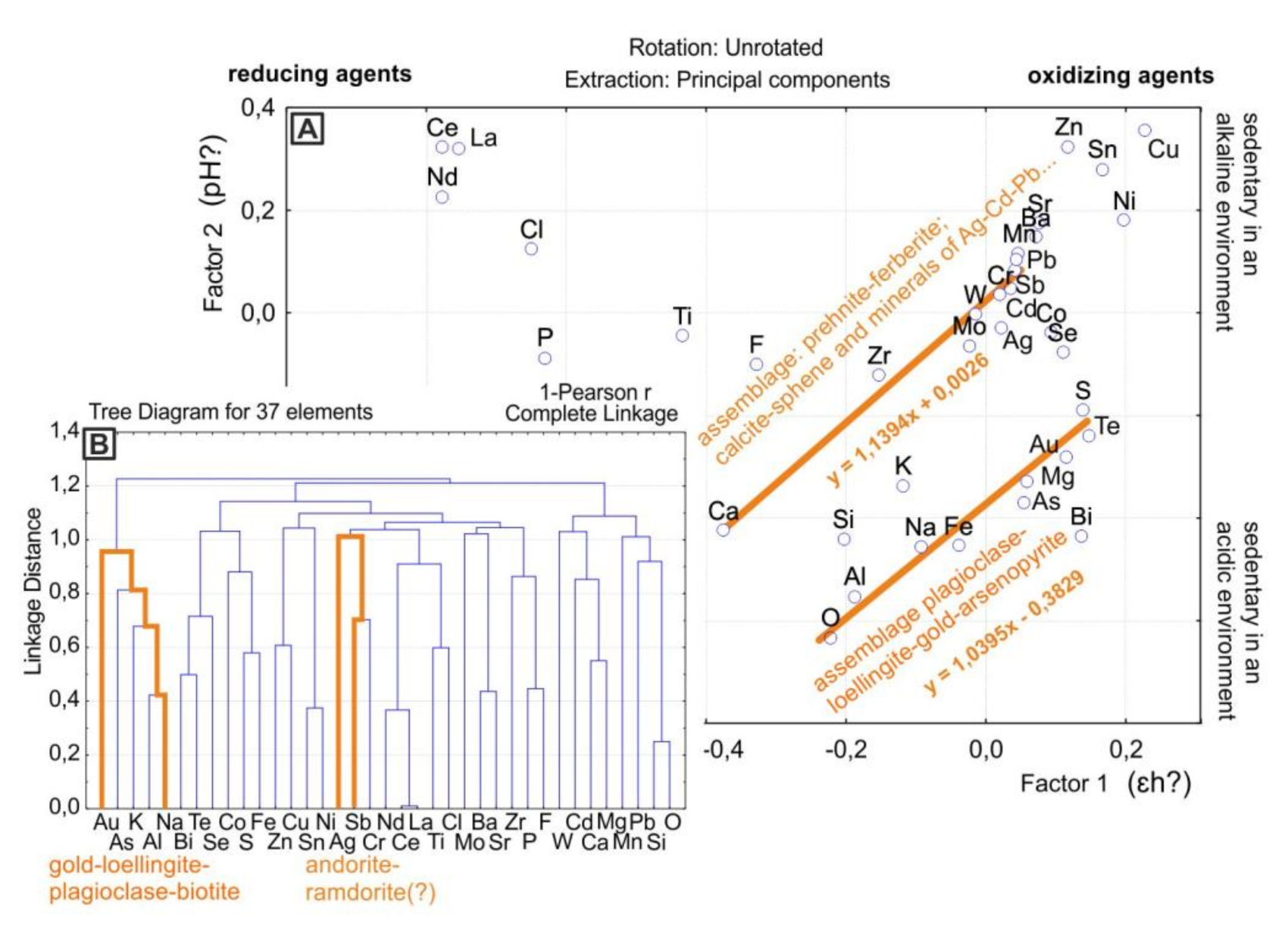
To verify this assumption, a calculation was made based on the results of microprobe analysis. For the calculation from a single observation point, the minimum and maximum values of the content of elements in the mineral assemblages were taken. A total of 111 points were analyzed in seven samples from the Precambrian P. Pinigin gold deposit (south of the craton) [13]. The result of factor analysis of these data show close to those discussed above distribution of chemical elements. Rare earth elements, good reducing agents [16], are concentrated in the left side of the diagram. Good oxidizing agents, such as copper, are located on the right side (Figure 5A). The elements that ions precipitate in an alkaline environment are located at the top of the diagram. Elements that ions precipitate in an acidic environment are located in the lower part of the diagram (Figure 5A). Along the diagonal with a direct dependence of factors, mineral assemblages can be deciphered (Figure 5A).
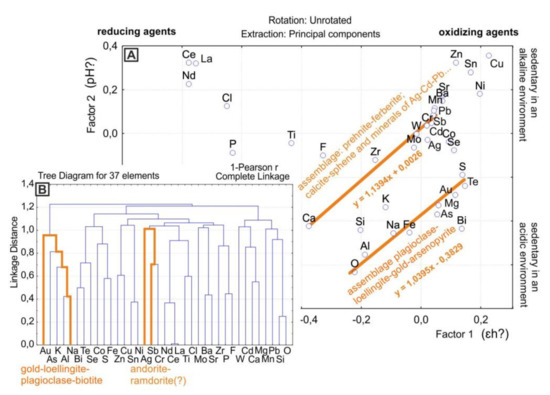
Figure 5.
Results of statistical analysis of the chemical composition of mineral assemblages in rocks of P. Pinigin gold deposit. (A)—Results of factor analysis. (B)—Tree diagram of cluster analysis.
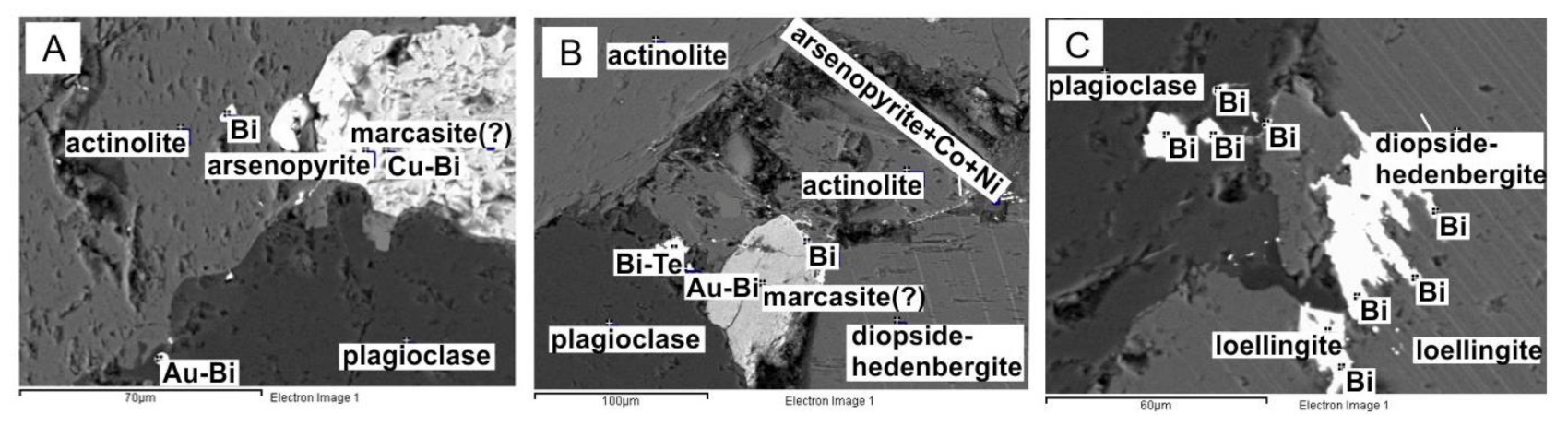
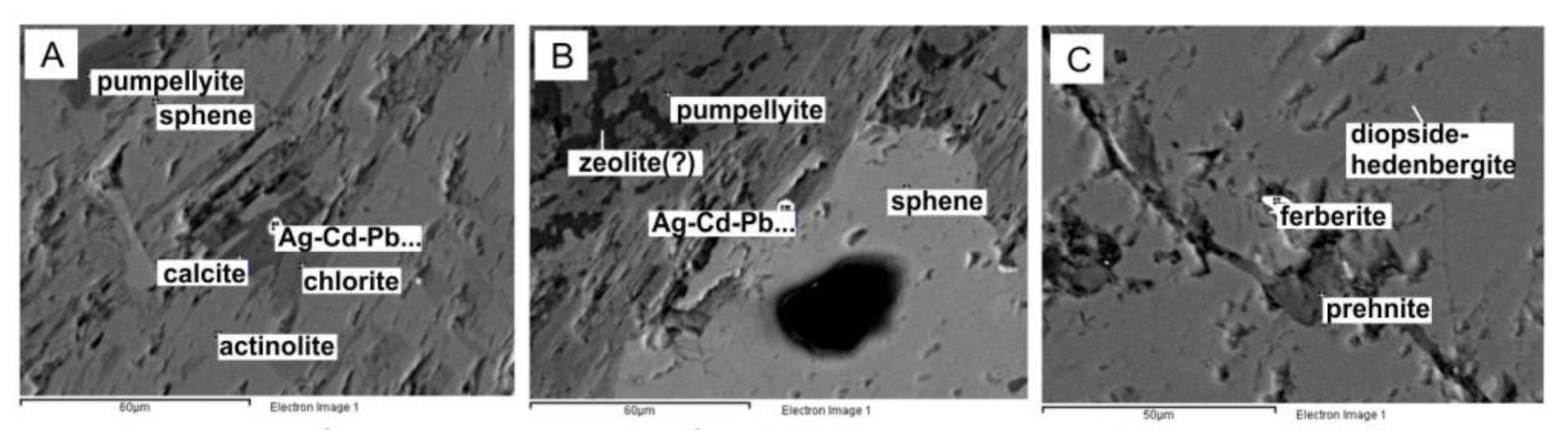
The geochemical associations of elements highlighted diagonally in the factor analysis diagram are confirmed by the results of cluster analysis (Figure 5B). For gold and silver, different assemblages of minerals are distinguished by statistical analysis. The presence of these minerals can serve as forecasting criteria of precious metals for this object. For gold, such minerals are loellingite, arsenopyrite, and plagioclase. For silver, such minerals are sphene and calcite (Figure 5, Figure 6 and Figure 7).

Figure 6.
Results of microprobe analysis of rocks in the P. Pinigin gold deposit illustrating the mineral assemblage of plagioclase–loellingite–gold–arsenopyrite (for Figure 5A).

Figure 7.
Results of microprobe analysis of rocks in the P. Pinigin gold deposit show the mineral assemblage sphen–pumpellyite–calcite–chlorite–silver minerals (for Figure 5A).
4. Discussion
According to this research, redox reactions play one of the leading roles in the formation of deposits of the precious metals. These reactions, as one of the most important factors in the formation of deposits, are widely considered for the surface of the Earth’s crust. Redox processes have been shown to control the behavior of a number of elements in lakes and in the pore waters of sediments where pe–pH diagrams are classically used to calculate the domains of stability of the solids that precipitate (e.g., MnO2, Fe(OH)3, FeS2) [2]. The processes of deep mineral formation due to these reactions are less often considered [17,18,19]. It is known that inorganic compounds (Fe3+, Au3+, Pd2+, Pt2+, Pt4+, Ag+, Hg2+, Cr2O72−, and MnO4−) are reduced, and (Fe2+, Sn2+, and Ti3+) are oxidized upon contact with the carbon surface [20]. In our research, the distribution of all these elements occurs along the axis that characterizes the redox potential as a factor in the formation of rocks and minerals. This distribution is probably due to their interaction. Further modeling of the chemical processes leading to the formation of rocks and minerals should take this probability into account. Mathematical modeling has always been an important activity in science and engineering [21], also as the basis for organizing and planning any geological survey [22]. Modeling involves the use of abstraction and idealization procedures [21]. This is especially important when the subject of modeling is complex geological systems, the behavior of which depends on a large number of interrelated factors of different nature. In the course of cognition, such systems are tested in different models that complement each other [23]. The basis for research of geochemical systems and forecasting the minerals should be statistical models that are most suitable for research of empirical data and/or unstructured systems. Based on algorithms for the distribution of elements detected by statistical methods, it is possible to determine the type of rocks and mineralization, and it is possible to use the discovered algorithms in technology.
5. Conclusions
The building of models of complex geochemical systems by statistical methods has good prospects. The constructed models of the element distribution and rock-forming factors have the potential to predict the minerals–indicators of precious metals for different areas. Comparison of the discovered regularities with the results of studies in the field of chemical petrology by other authors shows the leading role of the redox potential and acidity of the environment in the formation of rocks and minerals. The distribution of carbon and precious metals in the rocks is probably controlled by fluids and redox reactions. Due to the properties of chemical elements, the regularities of their distribution are similar in the different objects and for the macro and micro levels.
Supplementary Materials
The following are available online at https://www.mdpi.com/2297-8739/8/3/23/s1, Word S1: data explanation, Excel S1: data Figures S1 and S2, Excel S2: data Figure S3c, Excel S3: data Figure S3d, Excel S4: data Figure S3e, Excel S5: data Figure S5.
Author Contributions
conceptualization: A.K.; methodology: A.K. and B.G.; validation: A.K., B.G., E.L., and A.O.; investigation: A.K., B.G., E.L., A.O., L.G., and V.B.; funding acquisition: B.G., A.K., and E.L. All authors have read and agreed to the published version of the manuscript.
Funding
Statistical models were built under the state task of DPMGI SB RAS No. 0381-2019-0003 and No. 0381-2019-0004. The rocks and analysis were collected under the RFBR project No. 18-45-140018 and contracts with the “Diamonds of Anabar”, “Seligdar Gold”, and Yakutskgeologiya companies.
Acknowledgments
The authors are grateful to Alexander Smelov and Valery Alpatov for starting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Voitkevich, G.V.; Bessonov, O.A. Chemical evolution of the Earth; Nedra: Moscow, Russia, 1986; 212p. (In Russian) [Google Scholar]
- Turekian, K.K.; Holland, H.D. Treatise on Geochemistry, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Grunsky, E.C. Statistical Analysis in the Geosciences; Atkinson, P.M., Ed.; Encyclopedia of Life Support Systems (EOLSS): Oxford, UK, 2002. [Google Scholar]
- Kravchenko, A.A.; Beryozkin, V.I. Identification of the primary nature of granulite complexes with the use of geochemical tendencies of sedimentary and igneous differentiation. In Proceedings of the 6th International Siberian Early Career GeoScientists Conference, Novosibirsk, Russia, 9–23 June 2012; IGM, IPPG SB RAS & NSU: Novosibirsk, Russia, 2012; pp. 101–102. [Google Scholar]
- Gurvich, L.V.; Karachavtsev, G.V.; Kondrat’ev, V.N. The Energies of Breaking Chemical Bonds, Ionization Potentials and Electron Affinity; Handbook; Nauka: Moscow, Russia, 1974. (In Russian) [Google Scholar]
- Predovsky, A.A. Geochemical Reconstruction of the Primary Composition of Metamorphosed Volcanogenic-Sedimentary Formations of the Precambrian; Book Publishing House: Apatity, Russia, 1970; 115p. (In Russian) [Google Scholar]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Chevychelov, V.Y. Partitioning of Volatile Components (Cl, F and CO2) in Water-Saturated Fluid-Magma Systems of Various Composition. Petrology 2019, 27, 585–605. [Google Scholar] [CrossRef]
- Latimer, V. The Oxidation State of the Elements and Their Potentials in Aqueous Solutions; Publishing House of Foreign Literature: Moscow, Russia, 1954; 396p. (In Russian) [Google Scholar]
- Cross, W.; Iddings, P.J.; Pirsson, L.V.; Washington, H.S. A quantitative chemico-mineralogical classification and nomenclature of igneous rocks. J. Geol. 1902, 10, 555–690. [Google Scholar] [CrossRef]
- Pilipenko, A.T. (Ed.) Handbook of Elementary Chemistry, 2nd ed.; Naukova Dumka: Kiev, Ukraine, 1977; 544p. (In Russian) [Google Scholar]
- Mueller, R.F.; Saxena, S.K. Chemical Petrology with Applications to the Terrestrial Planets and Meteorites; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1977; 394p. [Google Scholar]
- Kravchenko, A.A.; Smelov, A.P.; Beryozkin, V.I.; Popov, N.V. Geology and Genesis of Gold-Bearing Precambrian Metabasites of the Central Part of the Aldan-Stanovoy Shield (on the Example of the P. Pinigin Deposit): Monograph; Mazurov, M.P., Ed.; Publishing house of LLC RIC “Offset”: Yakutsk, Russia, 2010; 148p. (In Russian) [Google Scholar]
- Baranov, L.; Tolstov, A.; Okrugin, A. Precious Metals in Alkaline Rocks and Carbonatites; Geology and Mineral Resources of the North-East of Russia; Publishing house NEFU: Yakutsk, Russia, 2020; pp. 188–192. (In Russian) [Google Scholar]
- Gerasimov, B.; Beryozkin, V.; Kravchenko, A. Typomorphic Features of Placer Gold from the Billyakh Tectonic Melange Zone of the Anabar Shield and Its Potential Ore Sources (Northeastern Siberian Platform). Minerals 2020, 10, 281. [Google Scholar] [CrossRef]
- Chertko, N.K. Chertko EN Geochemistry and Ecology of Chemical Elements; Publishing Center of BSU: Minsk, Belarus, 2008; 140p. [Google Scholar]
- Zhimulev, E.I.; Chepurov, A.I.; Sobolev, N.V. Genesis of Diamond in Metal-Carbon and Metal-Sulfur-Carbon Melts: Evidence from Experimental Data. Doklady Earth Sci. 2018, 1473–1474. [Google Scholar] [CrossRef]
- Haggerty, S.E. Diamond genesis in multiply-constrained model. Nature 1986, 320, 34–38. [Google Scholar] [CrossRef]
- Malitch, K.N.; Kadik, A.A.; Badanina, I.Y.; Zharkova, E.V. Redox Conditions of Formation of Osmium-Rich Minerals from the Guli Massif, Russia. Geochem. Int. 2011, 49, 726–730. [Google Scholar] [CrossRef]
- Romanenko, A.V.; Simonov, P.A. Carbon Materials and Their Physicochemical Properties; Industrial Catalysis in Lectures; No. 7; Kalvis: Moscow, Russia, 2007; 128p. (In Russian) [Google Scholar]
- Rasmuson, A.; Andersson, B.; Olsson, L.; Andersson, R. Mathematical Modelling in Chemical Engineering; University Press: Cambridge, UK, 2014; 183p. [Google Scholar]
- Krumbein, W.C.; Graybill, F.A. An Introduction to Statistical Models in Geology; Mir: Moscow, Russia, 1969; 324p. (In Russian) [Google Scholar]
- Korobeynikov, A.F. Theoretical Bases of Modeling of Mineral Deposits, 2nd ed.; Textbook for Universities; Tomsk Polytechnic University Press: Tomsk, Russia, 2009; 183p. (In Russian) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).