HILIC-MS/MS Analysis of Adenosine in Patient Blood
Abstract
1. Introduction
2. Results and Discussion
2.1. LC-MS/MS Method
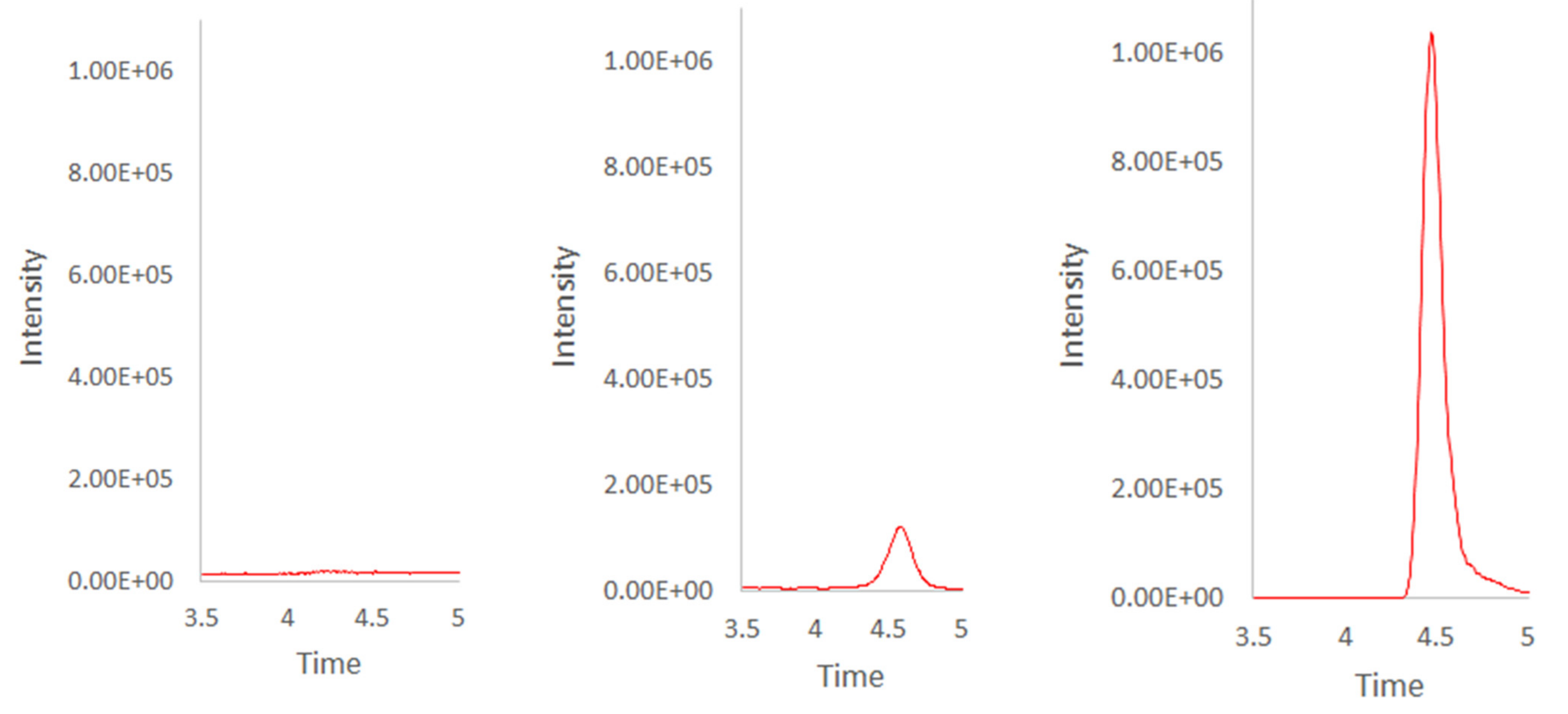
2.2. Method Validation
2.3. Application to the Analysis of Real Samples
3. Materials and Methods
3.1. Standards and Sample Preparation
3.2. Sample Collection and Preparation
3.3. HILIC- MS/MS Targeted Analysis
3.4. Method Validation
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meester, B.J.; Shankley, N.P.; Welsh, N.J.; Meijler, F.L.; Black, J.W. Pharmacological Analysis of the Activity of the Adenosine Uptake Inhibitor, Dipyridamole, on the Sinoatrial and Atrioventricular Nodes of the Guinea-Pig. Br. J. Pharmacol. 1998, 124, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine Receptors: Therapeutic Aspects for Inflammatory and Immune Diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Peragut, J.C.; Roussel, P.; Hassani, H.; Sampieri, F.; Bechis, G.; Gola, R.; Rochat, H. Adenosine and Neuropathic Pain. Pain 1996, 68, 271–274. [Google Scholar] [CrossRef]
- Ruf, J.; Paganelli, F.; Bonello, L.; Kipson, N.; Mottola, G.; Fromonot, J.; Condo, J.; Boussuges, A.; Bruzzese, L.; Kerbaul, F.; et al. Spare Adenosine A2a Receptors Are Associated with Positive Exercise Stress Test in Coronary Artery Disease. Mol. Med. 2016, 22, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Deharo, J.-C.; Brignole, M.; Guieu, R. Adenosine Hypersensitivity and Atrioventricular Block. Herzschrittmacherther. Elektrophysiol. 2018, 29, 166–170. [Google Scholar] [CrossRef]
- Martin, C.; Leone, M.; Viviand, X.; Ayem, M.L.; Guieu, R. High Adenosine Plasma Concentration as a Prognostic Index for Outcome in Patients with Septic Shock. Crit. Care Med. 2000, 28, 3198–3202. [Google Scholar] [CrossRef]
- Sinkovec, M.; Grad, A.; Rakovec, P. Role of Endogenous Adenosine in Vasovagal Syncope. Clin. Auton. Res. 2001, 11, 155–161. [Google Scholar] [CrossRef]
- Möser, G.H.; Schrader, J.; Deussen, A. Turnover of Adenosine in Plasma of Human and Dog Blood. Am. J. Physiol. 1989, 256, C799–C806. [Google Scholar] [CrossRef]
- Löfgren, L.; Pehrsson, S.; Hägglund, G.; Tjellström, H.; Nylander, S. Accurate Measurement of Endogenous Adenosine in Human Blood. PLoS ONE 2018, 13, e0205707. [Google Scholar] [CrossRef]
- Huszár, É.; Barát, E.; Kollai, M. Isocratic High-Performance Liquid Chromatographic Determination of Plasma Adenosine. Chromatographia 1996, 42, 318–322. [Google Scholar] [CrossRef]
- Dolezalová, P.; Krijt, J.; Chládek, J.; Nemcová, D.; Hoza, J. Adenosine and Methotrexate Polyglutamate Concentrations in Patients with Juvenile Arthritis. Rheumatol. Oxf. Engl. 2005, 44, 74–79. [Google Scholar] [CrossRef][Green Version]
- Marin, R.M.; Franchini, K.G.; Rocco, S.A. Analysis of Adenosine by RP-HPLC Method and Its Application to the Study of Adenosine Kinase Kinetics. J. Sep. Sci. 2007, 30, 2473–2479. [Google Scholar] [CrossRef]
- Jacobson, M.K.; Hemingway, L.M.; Farrell, T.A.; Jones, C.E. Sensitive and Selective Assay for Adenosine Using High-Pressure Liquid Chromatography with Fluorometry. Am. J. Physiol. 1983, 245, H887–H890. [Google Scholar] [CrossRef]
- Huang, L.-F.; Guo, F.-Q.; Liang, Y.-Z.; Li, B.-Y.; Cheng, B.-M. Simple and Rapid Determination of Adenosine in Human Synovial Fluid with High Performance Liquid Chromatography-Mass Spectrometry. J. Pharm. Biomed. Anal. 2004, 36, 877–882. [Google Scholar] [CrossRef]
- Carrozzo, M.M.; Troisi, L.; Cannazza, G.; Cazzato, A.S.; Braghiroli, D.; Parenti, C.; Guiducci, S.; Zoli, M. An Improved LC–S/MS Method for the Quantitation of Adenosine Concentration in Mice Brain Microdialysates. J. Pharm. Biomed. Anal. 2012, 70, 563–566. [Google Scholar] [CrossRef]
- Goodwin, K.J.; Gangl, E.; Sarkar, U.; Pop-Damkov, P.; Jones, N.; Borodovsky, A.; Woessner, R.; Fretland, A.J. Development of a Quantification Method for Adenosine in Tumors by LC-MS/MS with Dansyl Chloride Derivatization. Anal. Biochem. 2019, 568, 78–88. [Google Scholar] [CrossRef]
- Jimmerson, L.C.; Bushman, L.R.; Ray, M.L.; Anderson, P.L.; Kiser, J.J. A LC-MS/MS Method for Quantifying Adenosine, Guanosine and Inosine Nucleotides in Human Cells. Pharm. Res. 2017, 34, 73–83. [Google Scholar] [CrossRef]
- Guieu, R.; Kipson, N.; Ruf, J.; Fournier, N.; Laine, M.; Foucher, M.C.; Fromonot, J.; Mottola, G.; Bruzzese, L.; Boussuges, A.; et al. Low Basal Expression of A2A Adenosine Receptors and Increase in Adenosine Plasma Concentration Are Associated with Positive Exercise Stress Testing. Int. J. Cardiol. 2015, 180, 15–17. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, R.; Giri, S.; Rajagopal, S.; Mullangi, R. Highly Sensitive Method for the Determination of Adenosine by LC-MS/MS-ESI: Method Validation and Scope of Application to a Pharmacokinetic/Pharmacodynamic Study. Biomed. Chromatogr. 2012, 26, 81–88. [Google Scholar] [CrossRef]
- Marlinge, M.; Vairo, D.; Marolda, V.; Bruzzese, L.; Adjriou, N.; Guiol, C.; Kipson, N.; Bonnardel, A.; Gastaldi, M.; Kerbaul, F.; et al. Rapid Measurement of Adenosine Concentration in Human Blood Using Fixed Potential Amperometry: Comparison with Mass Spectrometry and High-Performance Liquid Chromatography. J. Anal. Bioanal. Tech. 2017, 8, 371–374. [Google Scholar] [CrossRef]
- Murakami, H.; Otani, E.; Iwata, T.; Esaka, Y.; Aoyama, T.; Kawasaki, M.; Tanaka, T.; Minatoguchi, S.; Uno, B. Simple Pretreatment and HILIC Separation for LC-ESI-MS/MS Determination of Adenosine in Human Plasma. Anal. Sci. 2015, 31, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Olesti, E.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Ramaekers, J.G.; de la Torre, R.; Pozo, O.J. Quantification of Endogenous Neurotransmitters and Related Compounds by Liquid Chromatography Coupled to Tandem Mass Spectrometry. Talanta 2019, 192, 93–102. [Google Scholar] [CrossRef]
- Haink, G.; Deussen, A. Liquid Chromatography Method for the Analysis of Adenosine Compounds. J. Chromatogr. B 2003, 784, 189–193. [Google Scholar] [CrossRef]
- Van Dycke, A.; Verstraete, A.; Pil, K.; Raedt, R.; Vonck, K.; Boison, D.; Boon, P. Quantitative Analysis of Adenosine Using Liquid Chromatography/Atmospheric Pressure Chemical Ionization-Tandem Mass Spectrometry (LC/APCI-MS/MS). J. Chromatogr. B 2010, 878, 1493–1498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Virgiliou, C.; Sampsonidis, I.; Gika, H.G.; Raikos, N.; Theodoridis, G.A. Development and Validation of a HILIC-MS/MS Multitargeted Method for Metabolomics Applications. Electrophoresis 2015, 36, 2215–2225. [Google Scholar] [CrossRef]
- Sotiriadou, M.; Antoniadis, A.; Vergopoulos, S.; Lazaridis, C.; Konstantinidis, P.; Bakogiannis, C.; Virgiliou, C.; Gkika, E.; Theodoridis, G.; Mpalaouri, I.; et al. Baseline Adenosine Plasma Levels Indicate Differential Response to Adenosine Test and Head-up Tilt Test in Syncopal Patients. Eur. Heart J. 2020, 41, 704. [Google Scholar] [CrossRef]
- Sotiriadou, M.; Antoniadis, A.; Vergopoulos, S.; Lazaridis, C.; Konstantinidis, P.; Bakogiannis, C.; Virgiliou, C.; Gkika, E.; Theodoridis, G.; Mpalaouri, I.; et al. P6573Adenosine Plasma Levels May Determine Tilt Table Test Outcome in Syncopal Patients with Prodromal Symptoms. Eur. Heart J. 2019, 40, 605. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Mechulan, A.; Giorgi, R.; Franceschi, F.; Prevot, S.; Peyrouse, E.; Condo, J.; By, Y.; Ruf, J.; Brignole, M.; et al. Adenosine Plasma Level and A2A Adenosine Receptor Expression: Correlation with Laboratory Tests in Patients with Neurally Mediated Syncope. Heart 2012, 98, 855. [Google Scholar] [CrossRef]
- Guieu, R.; Deharo, J.-C.; Ruf, J.; Mottola, G.; Kipson, N.; Bruzzese, L.; Gerolami, V.; Franceschi, F.; Ungar, A.; Tomaino, M.; et al. Adenosine and Clinical Forms of Neurally-Mediated Syncope. J. Am. Coll. Cardiol. 2015, 66, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Administration, F. United States Food and Drug Administration. In Guidance for Industry: Bioanalytical Method Validation; US Food and Drug Administration, Department of Health and Human Services: Hampton, VA, USA, 2001; p. 66. [Google Scholar]
- van Amsterdam, P.; Companjen, A.; Brudny-Kloeppel, M.; Golob, M.; Luedtke, S.; Timmerman, P. The European Bioanalysis Forum Community’s Evaluation, Interpretation and Implementation of the European Medicines Agency Guideline on Bioanalytical Method Validation. Bioanalysis 2013, 5, 645–659. [Google Scholar] [CrossRef] [PubMed]
| Level | Concentration | Matrix Effect | Recovery | |
|---|---|---|---|---|
| μg/mL | μM | (%) | (%) | |
| LLOQ | 0.005 | 0.019 | 104 | 104 |
| LQC | 0.02 | 0.075 | 94 | 96 |
| MQC | 0.5 | 1.873 | 95 | 98 |
| HQC | 1.8 | 6.742 | 85 | 114 |
| QC Level | Concentration | Intraday (n = 5) | Inter-Day (n = 15) | |||||
|---|---|---|---|---|---|---|---|---|
| μg/mL | μM | Mean (μg/mL) ± SD | Accuracy %R | Precision %RSD | Mean (μg/mL) ± SD | Accuracy %R | Precision %RSD | |
| LLOQ | 0.005 | 0.019 | 0.006 ± 0.0001 | 120 | 2 | 0.006 ± 0.0002 | 119 | 3 |
| LQC | 0.02 | 0.075 | 0.020 ± 0.001 | 100 | 4 | 0.023 ± 0.004 | 115 | 20 |
| MQC | 0.5 | 1.873 | 0.445 ± 0.05 | 89 | 5 | 0.485 ± 0.05 | 97 | 11 |
| HQC | 1.8 | 6.742 | 1.566 ± 0.08 | 87 | 1 | 1.233 ± 0.10 | 85 | 7 |
| Concentration | Stability %R | ||||
|---|---|---|---|---|---|
| μg/mL | μM | 24 h (4 °C) | %RSD | 3 Days | %RSD |
| (−22 °C) | |||||
| 0.005 | 0.019 | 104 | 0.4 | 101 | 4 |
| 0.02 | 0.075 | 106 | 4.2 | 109 | 4.3 |
| 0.5 | 1.873 | 100 | 1.4 | 115 | 13 |
| 1.8 | 6.742 | 113 | 0.9 | 92 | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgiliou, C.; Fragakis, N.; Sotiriadou, M.; Vassilikos, V.; Gerou, S.; Theodoridis, G.; Gika, H. HILIC-MS/MS Analysis of Adenosine in Patient Blood. Separations 2021, 8, 222. https://doi.org/10.3390/separations8110222
Virgiliou C, Fragakis N, Sotiriadou M, Vassilikos V, Gerou S, Theodoridis G, Gika H. HILIC-MS/MS Analysis of Adenosine in Patient Blood. Separations. 2021; 8(11):222. https://doi.org/10.3390/separations8110222
Chicago/Turabian StyleVirgiliou, Christina, Nikolaos Fragakis, Melani Sotiriadou, Vassilios Vassilikos, Spiros Gerou, Georgios Theodoridis, and Helen Gika. 2021. "HILIC-MS/MS Analysis of Adenosine in Patient Blood" Separations 8, no. 11: 222. https://doi.org/10.3390/separations8110222
APA StyleVirgiliou, C., Fragakis, N., Sotiriadou, M., Vassilikos, V., Gerou, S., Theodoridis, G., & Gika, H. (2021). HILIC-MS/MS Analysis of Adenosine in Patient Blood. Separations, 8(11), 222. https://doi.org/10.3390/separations8110222









