Development of a New LC-MS/MS Screening Method for Detection of 120 NPS and 43 Drugs in Blood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Treatment
2.3. LC-MS/MS
2.4. Validation Parameters
2.4.1. Selectivity and Specificity
2.4.2. Sensitivity
2.4.3. Linearity, Accuracy and Precision
2.4.4. Relative Recovery (RR), Matrix Effect (ME), Stability and Carry over
3. Results and Discussion
3.1. MRM Transitions and Chromatographic Separation
3.2. Method Validation
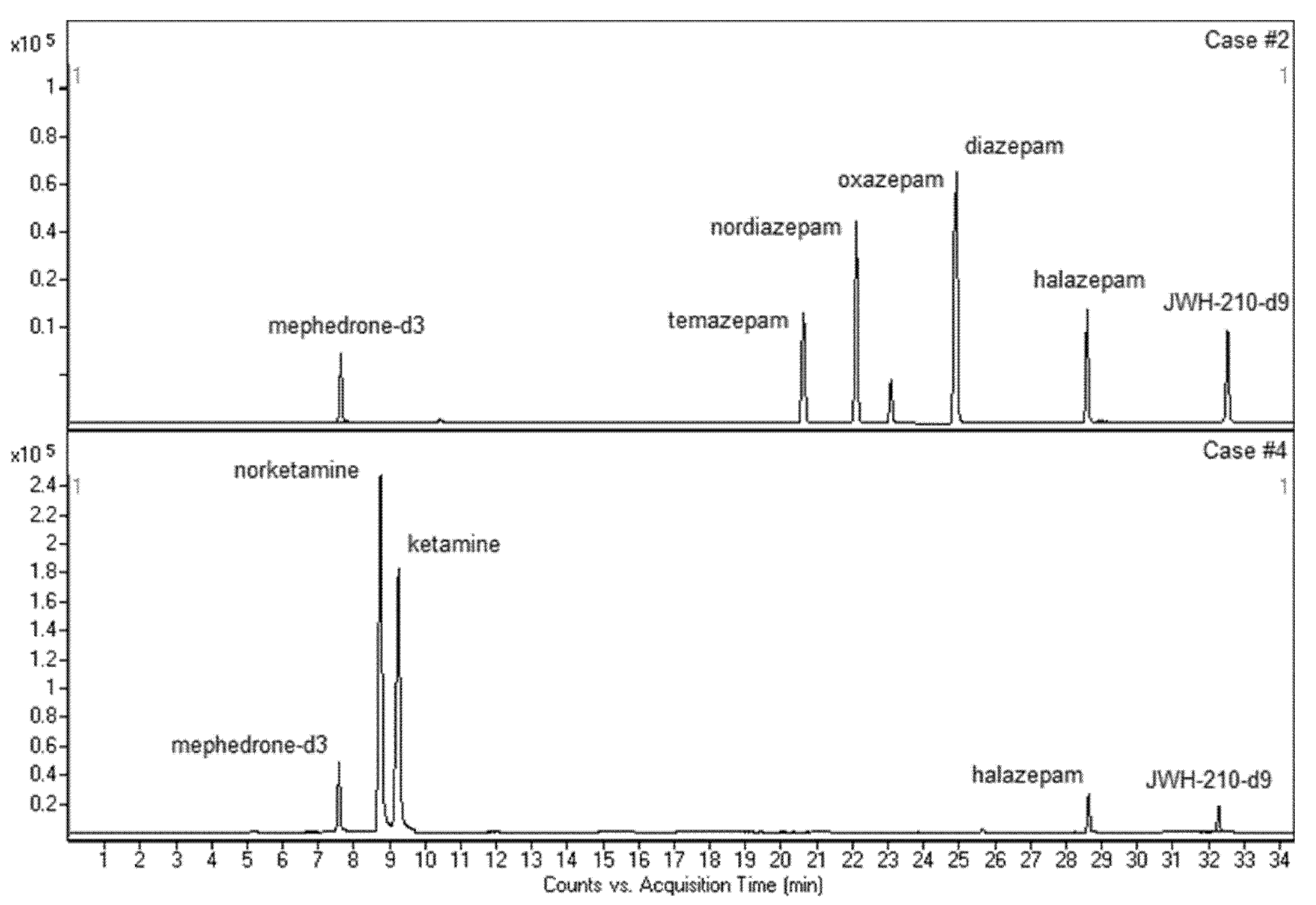
3.3. Application to Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sauvage, F.L.; Picard, N.; Saint-Marcoux, F.; Gaulier, J.M.; Lachâtre, G.; Marquet, P. General unknown screening procedure for the characterization of human drug metabolites in forensic toxicology: Applications and constraints. J. Sep. Sci. 2009, 32, 3074–3083. [Google Scholar] [CrossRef]
- Maurer, H.H. Systematic toxicological analysis of drugs and their metabolites by gas chromatography-mass spectrometry. J. Chromatogr. 1992, 580, 3–41. [Google Scholar] [CrossRef]
- Maurer, H.H. Liquid chromatography-mass spectrometry in forensic and clinical toxicology. J. Chromatogr. B Biomed. Sci. Appl. 1998, 713, 3–25. [Google Scholar] [CrossRef]
- Drummer, O.H. Chromatographic screening techniques in systematic toxicologicalanalysis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 733, 27–45. [Google Scholar] [CrossRef]
- Ambroziak, K.; Adamowicz, P. Simple screening procedure for 72 synthetic cannabinoids in whole blood by liquid chromatography-tandem mass spectrometry. Forensic Toxicol. 2018, 36, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Fagiola, M.; Hahn, T.; Avella, J. Screening of Novel Psychoactive Substances in Postmortem Matrices by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS-MS). J. Anal. Toxicol. 2018, 42, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, M.F.; Papsun, D.M.; Logan, B.K. Analysis of Fentanyl and 18 Novel Fentanyl Analogs and Metabolites by LC-MS-MS, and report of Fatalities Associated with Methoxyacetylfentanyl and Cyclopropylfentanyl. J. Anal. Toxicol. 2018, 42, 592–604. [Google Scholar] [CrossRef]
- Michely, J.A.; Maurer, H.H. A multi-analyte approach to help in assessing the severity of acute poisonings—Development and validation of a fast LC-MS/MS quantification approach for 45 drugs and their relevant metabolites with one-point calibration. Drug Test. Anal. 2018, 10, 164–176. [Google Scholar] [CrossRef]
- Vaiano, F.; Mari, F.; Busardò, F.P.; Bertol, E. Enhancing the sensitivity of the LC-MS/MS detection of propofol in urine and blood by azo-coupling derivatization. Anal. Bioanal. Chem. 2014, 406, 3579–3587. [Google Scholar] [CrossRef]
- Toyo’oka, T. Derivatization-based High-throughput Bioanalysis by LC-MS. Anal. Sci. 2017, 33, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joachico, A.; Sangaraju, D.; Shahidi-Latham, S.K. A rapid derivatization based LC-MS/MS method for quantitation of short chain fatty acids in human plasma and urine. Bioanalysis 2019, 11, 741–753. [Google Scholar] [CrossRef]
- Roemmelt, A.T.; Steurer, A.E.; Poetzsch, M.; Kraemer, T. Liquid chromatography, in combination with a quadrupole time-of-flight instrument (LC QTOF), with sequential window acquisition of all theoretical fragment-ion spectra (SWATH) acquisition: Systematic studies on its use for screenings in clinical and forensic toxicology and comparison with information-dependent acquisition (IDA). Anal. Chem. 2014, 86, 11742–11749. [Google Scholar] [CrossRef]
- Broecker, S.; Herre, S.; Wust, B.; Zweigenbaum, J.; Pragst, F. Development and practical application of a library of CID accurate mass spectra of more than 2500 toxic compounds for systematic toxicological analysis by LC-QTOF-MS with data-dependent acquisition. Anal. Bioanal. Chem. 2011, 400, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Maurer, H.H. Review: LC coupled to low- and high-resolution mass spectrometry for new psychoactive substance screening in biological matrices—Where do we stand today? Anal. Chim. Acta 2016, 927, 13–20. [Google Scholar] [CrossRef]
- Pasin, D.; Cawley, A.; Bidny, S.; Fu, S. Current applications of high-resolution mass spectrometry for the analysis of new psychoactive substances: A critical review. Anal. Bioanal. Chem. 2017, 409, 5821–5836. [Google Scholar] [CrossRef]
- Vaiano, F.; Pascali, J.P.; Bertol, E. New psychoactive substances: An actual problem or an overestimated phenomenon? Forensic Sci. Int. 2019, 304, 109941. [Google Scholar] [CrossRef] [PubMed]
- European Drug Report 2021: Trends and Developments. Available online: https://www.emcdda.europa.eu/publications/edr/trends-developments/2021_en (accessed on 16 October 2021).
- Italian Department of Anti-Drug Policies, P. of the C. of M. Annual Report on Addictions. 2021. Available online: https://www.politicheantidroga.gov.it/media/3076/rap2021pdf.pdf (accessed on 16 November 2021).
- Bertol, E.; Vaiano, F.; Mari, F.; Di Milia, M.G.; Bua, S.; Supuran, C.T.; Carta, F. Advances in new psychoactive substances identification: The U.R.I.To.N. Consortium. J. Enzyme Inhib. Med. Chem. 2017, 32, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Vaiano, F.; Mari, F.; Bertol, E.; Supuran, C.T. Psychoactive substances belonging to the amphetamine class potently activate brain carbonic anhydrase isoforms VA, VB, VII, and XII. J. Enzyme Inhib. Med. Chem. 2017, 32, 1253–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephenson, J.B.; Flater, M.L.; Austin, J.; Bain, L.T.; Holt, L.A.; Mehan, J.M. Comprehensive Drug Screening of Whole Blood by LC-HRMS-MS in a Forensic Laboratory. J. Anal. Toxicol. 2021, 45, 243–251. [Google Scholar] [CrossRef]
- Ong, R.S.; Kappatos, D.C.; Russell, S.G.G.; Poulsen, H.A.; Banister, S.D.; Gerona, R.R.; Glass, M.; Johnson, C.S.; McCarthy, M.J. Simultaneous analysis of 29 synthetic cannabinoids and metabolites, amphetamines, and cannabinoids in human whole blood by liquid chromatography-tandem mass spectrometry—A New Zealand perspective of use in 2018. Drug Test. Anal. 2020, 12, 195–214. [Google Scholar] [CrossRef]
- Strayer, K.E.; Antonides, H.M.; Juhascik, M.P.; Daniulaityte, R.; Sizemore, I.E. LC-MS/MS-based method for the multiplex detection of 24 fentanyl analogues and metabolites in whole blood at Sub ng mL−1 concentrations. ACS Omega 2018, 3, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, P.; Tokarczyk, B. Simple and rapid screening procedure for 143 new psychoactive substances by liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2016, 8, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Di Rago, M.; Pantatan, S.; Hargreaves, M.; Wong, K.; Mantinieks, D.; Kotsos, A.; Glowacki, L.; Drummer, O.H.; Gerostamoulos, D. High Throughput Detection of 327 Drugs in Blood by LC-MS-MS with Automated Data Processing. J. Anal. Toxicol. 2021, 45, 154–183. [Google Scholar] [CrossRef]
- Palmquist, K.B.; Swortwood, M.J. Data-independent screening method for 14 fentanyl analogs in whole blood and oral fluid using LC-QTOF-MS. Forensic Sci. Int. 2019, 297, 189–197. [Google Scholar] [CrossRef]
- Lehmann, S.; Kieliba, T.; Beike, J.; Thevis, M.; Mercer-Chalmers-Bender, K. Determination of 74 new psychoactive substances in serum using automated in-line solid-phase extraction-liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1064, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Lader, M. Benzodiazepine harm: How can it be reduced? Br. J. Clin. Pharmacol. 2014, 77, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Bramness, J.G.; Skurtveit, S.; Mørland, J. Testing for benzodiazepine inebriation—Relationship between benzodiazepine concentration and simple clinical tests for impairment in a sample of drugged drivers. Eur. J. Clin. Pharmacol. 2003, 59, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.L.; Peltekian, S.M.; Helwig, M.; Macdonald, M.; Martin-Misener, R.; Saini, B.; Neyedli, H.; Giacomantonio, C.; Gardner, D.M. Driving performance assessments for benzodiazepine receptor agonist-related impairment: A scoping review protocol. JBI Evid. Synth. 2021, 19, 242–250. [Google Scholar] [CrossRef]
- Van Der Sluiszen, N.N.J.J.M.; Vermeeren, A.; Jongen, S.; Vinckenbosch, F.; Ramaekers, J.G. Influence of Long-Term Benzodiazepine use on Neurocognitive Skills Related to Driving Performance in Patient Populations: A Review. Pharmacopsychiatry 2017, 50, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Ross, J.; Cohen, J. The use of benzodiazepines among regular amphetamine users. Addiction 1994, 89, 1683–1690. [Google Scholar] [CrossRef]
- Altun, B.; Çok, İ. Psychoactive bath salts and neurotoxicity risk. Turkish J. Pharm. Sci. 2020, 17, 235–241. [Google Scholar] [CrossRef]
- Joyce, J.R.; Bal, T.S.; Ardrey, R.E.; Stevens, H.M.; Moffat, A.C. The decomposition of benzodiazepines during analysis by capillary gas chromatography/mass spectrometry. Biomed. Mass Spectrom. 1984, 11, 284–289. [Google Scholar] [CrossRef]
- Perez, E.R.; Knapp, J.A.; Horn, C.K.; Stillman, S.L.; Evans, J.E.; Arfsten, D.P. Comparison of LC–MS-MS and GC–MS Analysis of Benzodiazepine Compounds Included in the Drug Demand Reduction Urinalysis Program. J. Anal. Toxicol. 2016, 40, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Bertol, E.; Vaiano, F.; Furlanetto, S.; Mari, F. Cross-reactivities and structure-reactivity relationships of six benzodiazepines to EMIT(®) immunoassay. J. Pharm. Biomed. Anal. 2013, 84, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, F.; Busardò, F.P.; Palumbo, D.; Kyriakou, C.; Fioravanti, A.; Catalani, V.; Mari, F.; Bertol, E. A novel screening method for 64 new psychoactive substances and 5 amphetamines in blood by LC–MS/MS and application to real cases. J. Pharm. Biomed. Anal. 2016, 129, 441–449. [Google Scholar] [CrossRef] [PubMed]
- SWGTOX. Scientific working group for forensic toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [CrossRef]
- Bertol, E.; Pascali, J.; Palumbo, D.; Catalani, V.; Di Milia, M.G.; Fioravanti, A.; Mari, F.; Vaiano, F. 3-MeO-PCP intoxication in two young men: First in vivo detection in Italy. Forensic Sci. Int. 2017, 274, 7–12. [Google Scholar] [CrossRef]
- Schulz, M.; Schmoldt, A.; Andresen-Streichert, H.; Iwersen-Bergmann, S. Revisited: Therapeutic and toxic blood concentrations of more than 1100 drugs and other xenobiotics. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef]
- Adamowicz, P. Blood concentrations of synthetic cathinones. Clin. Toxicol. 2021, 59, 648–654. [Google Scholar] [CrossRef]
- Adamowicz, P. Blood concentrations of synthetic cannabinoids. Clin. Toxicol. 2021, 59, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Bertol, E.; Di Milia, M.G.; Fioravanti, A.; Mari, F.; Palumbo, D.; Pascali, J.P.; Vaiano, F. Proactive drugs in DFSA cases: Toxicological findings in an eight-years study. Forensic Sci. Int. 2018, 291, 207–215. [Google Scholar] [CrossRef] [PubMed]


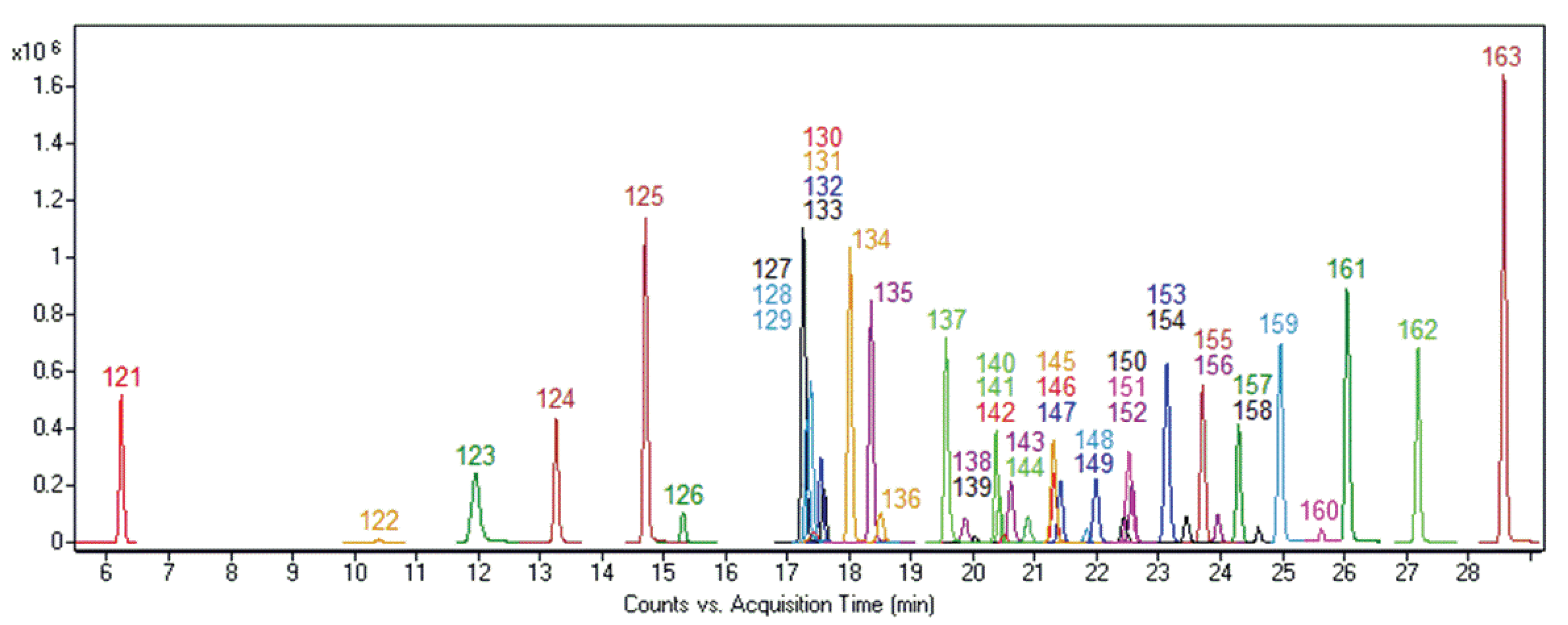
| Compound | MRM Transitions (m/z) | Retention Time (min) | Compound | MRM Transitions (m/z) | Retention Time (min) |
|---|---|---|---|---|---|
| BDZ/Antidepressants | isobutyryl-fentanyl | 351: 188, 105 | 18.9 | ||
| 3-OH-flunitrazepam | 330: 311, 284 | 19.9 | isotonitazene | 411: 100, 72 | 19.8 |
| 7-aminoclonazepam | 286: 222, 121 | 10.3 | MeOAc-fentanyl | 353: 188, 105 | 6.3 |
| 7-aminoflunitrazepam | 284: 135, 227 | 13.2 | MeOAc-norfentanyl | 249: 84, 55 | 15.3 |
| 7-aminonitrazepam | 252: 121, 208 | 6.5 | norfentanyl | 233: 84, 55 | 9.5 |
| alprazolam | 309: 281, 205 | 21.9 | ocfentanil | 371: 188, 105 | 15.7 |
| amitriptyline | 278: 91, 105 | 20.9 | pF-furanyl-fentanyl | 393: 188, 105 | 18.7 |
| bentazepam | 297: 166, 269 | 17.3 | Synthetic Cannabinoids | ||
| bromazepam | 316: 182, 209 | 17.6 | 5Cl-AB-PINACA | 365: 320, 249 | 26.7 |
| brotizolam | 393: 314, 279 | 23.5 | 5Cl-THJ-018 | 377: 249, 145 | 31.9 |
| chlordiazepoxide | 300: 283, 282 | 15.4 | 5F-AKB-48 | 384: 135, 93 | 32.1 |
| cinazepam | 465: 347, 319 | 24.7 | 5F-APINACA | 384: 135, 93 | 31.3 |
| citalopram | 325: 109, 262 | 18.5 | 5F-APP-PICA | 396: 232, 379 | 26.4 |
| clonazepam | 316: 270, 214 | 21.4 | 5F-APP-PINACA | 397: 233, 352 | 26.8 |
| clonazolam | 354: 308, 280 | 20.4 | 5F-Cumyl-P7AICA | 368: 250, 145 | 28.4 |
| delorazepam | 305: 165, 140 | 24 | 5F-Cumyl-PeGACLONE | 391: 273, 185 | 30.5 |
| diazepam | 285: 154, 193 | 25 | 5F-Cumyl-PINACA | 368: 233, 250 | 30.5 |
| diclazepam | 319: 227, 154 | 26.1 | 5F-MDMB-7PAICA | 378: 318, 145 | 27.8 |
| duloxetina | 298: 154, 157 | 20.8 | 5F-MDMB-PICA | 377: 232, 144 | 29.7 |
| etizolam | 343: 314, 289 | 23.8 | 5F-NNEI-2-naphtyl-isomer | 375: 232, 144 | 30.6 |
| flualprazolam | 327: 292, 299 | 21.3 | AB-CHMINACA | 357: 241, 312 | 28.7 |
| flunitrazepam | 314: 268, 239 | 22.6 | AB-FUBINACA | 369: 324, 109 | 26 |
| fluoxetine | 310: 148, 117 | 21.8 | ADB-FUBINACA | 383: 338, 253 | 27.3 |
| flurazepam | 388: 315, 317 | 18.1 | ADB-PINACA | 345: 215, 300 | 28.7 |
| halazepam | 353: 241, 222 | 28.7 | AM-2201 | 360: 155, 127 | 30.7 |
| levomepromazine | 329: 148, 130 | 25.7 | AM-2233 | 459: 98, 112 | 19.9 |
| lorazepam | 321: 275, 303 | 21.5 | AM-694 | 436: 190, 272 | 31.2 |
| lormetazepam | 335: 289, 317 | 24.4 | APP-FUBINACA | 417: 372, 109 | 27.6 |
| midazolam | 326: 291, 223 | 17.5 | CB-13 | 369: 155, 127 | 33.9 |
| mirtazapine | 266: 195, 72 | 12.2 | cumyl-PeGLACONE | 373: 255, 185 | 31.2 |
| nordiazepam | 271: 165, 140 | 22.5 | JWH-007 | 356: 155, 127 | 32 |
| oxazepam | 287: 269, 241 | 20.6 | JWH-016 | 342: 127, 155 | 31.4 |
| oxcarbazepine | 253: 208, 236 | 17.4 | JWH-018 | 342: 155, 127 | 31.7 |
| paroxetine | 330: 70, 192 | 20.1 | JWH-019 | 356: 155, 127 | 32.4 |
| pinazepam | 309: 241, 269 | 27.2 | JWH-073 | 328: 155, 127 | 31.2 |
| prazepam | 325: 271, 140 | 28.6 | JWH-081 | 372: 185, 157 | 32 |
| promazine | 285: 86, 58 | 19.7 | JWH-098 | 386: 185, 157 | 32.2 |
| quetiapine | 384: 253, 221 | 17.6 | JWH-122 | 356: 169, 141 | 32.2 |
| temazepam | 301: 255, 283 | 23.2 | JWH-147 | 382: 155, 127 | 32.9 |
| trazodone | 372: 176, 148 | 17.5 | JWH-200 | 385: 155, 114 | 21.4 |
| triazolam | 343: 308, 315 | 22.6 | JWH-203 | 340: 125, 238 | 31.5 |
| zolpidem | 308: 235, 236 | 14.4 | JWH-210 | 370: 183, 155 | 32.7 |
| zoplicone | 389: 217, 245 | 18.3 | JWH-210-d9 | 379: 183, 155 | 32.6 |
| α-OH-alprazolam | 325: 297, 216 | 20.5 | JWH-250 | 336: 121, 91 | 31.1 |
| α-OH-midazolam | 342: 168, 203 | 17.7 | JWH-251 | 320: 105, 144 | 31.4 |
| Miscellaneous | JWH-302 | 336: 214, 121 | 30.8 | ||
| 2-AI | 134: 117, 115 | 3.7 | JWH-307 | 386: 155, 127 | 32.2 |
| 2F-deschloroketamine | 222: 109, 163 | 7.8 | JWH-398 | 376: 189, 161 | 32.7 |
| 3,4MD-α-PHP | 290: 135, 140 | 15.6 | MDMB-CHMICA | 385: 240, 144 | 31.3 |
| 3-MeO-PCP | 274: 189, 121 | 16.7 | MMB-2201 | 363: 232, 144 | 28.8 |
| 3-MeO-PCE | 234: 189, 121 | 15.8 | pravadoline | 379: 135, 114 | 19 |
| 4-MeO-PCP | 274: 86, 121 | 16.7 | RCS-4 | 322: 135, 77 | 30.8 |
| 4-OH-DiPT | 261: 160, 114 | 10.4 | RCS-8 | 376: 121, 91 | 32.4 |
| 5-IAI | 260: 116, 243 | 11.6 | UR-144 | 312: 125, 55 | 32.5 |
| 5-MeO-DiPT | 275: 114, 174 | 14.1 | WIN 55,212-2 | 427: 155, 127 | 28.4 |
| BZP | 177: 91, 65 | 1.9 | Synthetic Cathinones | ||
| deschloro-N-et-ketamine | 218: 91, 173 | 8.8 | 1-naphyrone | 282: 126, 141 | 17.8 |
| ketamine | 238: 125, 179 | 9.4 | 2F-methcathinone | 182: 164, 149 | 4.8 |
| m-CPP | 197: 154, 118 | 10.4 | 3,4-DMMC | 192: 174, 159 | 11.7 |
| MDAI | 178: 161, 103 | 4.9 | 3-MMC | 178: 160, 145 | 7.8 |
| methoxetamine | 248: 203, 121 | 11.8 | 4F-methcathinone | 182: 164, 149 | 4.8 |
| norketamine | 224: 125, 207 | 8.8 | 4-methyl-ethcathinone | 192: 174, 144 | 8.8 |
| ritalinic acid | 220: 84, 56 | 8.6 | buphedrone | 178: 131, 160 | 6.9 |
| α-PHP | 246: 140, 91 | 14.6 | butylone | 222: 204, 174 | 7.7 |
| Opioids | dimethylcathinone | 178: 133, 105 | 4.9 | ||
| (±)-cis-3-met-norfentanyl | 247: 98, 69 | 11.7 | ethcathinone | 178: 160, 132 | 5.6 |
| (±)-trans-3-met-norfentanyl | 247: 98, 69 | 11.9 | ethylone | 222: 204, 174 | 6.7 |
| 2-methyl-AP-237 | 287: 117, 115 | 14.8 | eutylone | 236: 218, 188 | 8.4 |
| acetyl-fentanyl | 323: 188, 105 | 15.6 | MDPV | 276: 126, 135 | 13.1 |
| acetyl-norfentanyl | 219: 84, 55 | 6.5 | mephedrone | 178: 160, 145 | 7.7 |
| alfentanil | 417: 268, 197 | 17.3 | mephedrone-d3 | 181: 148, 163 | 7.6 |
| AP-237 | 273: 117, 115 | 13.8 | methcathinone | 164: 146, 131 | 3.7 |
| butyryl-fentanyl | 351: 188, 105 | 19.2 | methedrone | 194: 176, 161 | 6.8 |
| butyryl-norfentanyl | 247: 84, 55 | 13.3 | methylone | 208: 160, 132 | 5.4 |
| carfentanyl | 395: 335, 113 | 18.9 | naphyrone | 282: 141, 211 | 18.5 |
| cyclopropyl-fentanyl | 349: 188, 105 | 18.3 | N-ethylpentylone | 250: 202, 232 | 12.2 |
| fentanyl | 337: 188, 132 | 17.5 | pentedrone | 192: 174, 132 | 10 |
| furanyl-fentanyl | 375: 188, 105 | 18.2 | pentylone | 236: 218, 188 | 11.2 |
| furanyl-norfentanyl | 271: 84, 55 | 10.9 | |||
| Compound | LOD (ng/mL) | LOQ (ng/mL) | R2 | Accuracy (%) | Intra-Day Precision (%) | Inter-Day Precision (%) | ME (%) | RR (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 * | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | ||||||
| (±)-cis-3-met-norfentanyl | 0.02 | 0.1 | 0.9949 | −20.6 | −14.8 | 13.5 | −2.6 | 18.8 | 15.7 | 14.0 | 10.0 | 19.2 | 11.0 | 12.8 | 5.6 | −0.4 | 78.4 |
| (±)-trans-3-met-norfentanyl | 0.01 | 0.1 | 0.9923 | −19.7 | −18.1 | 5.0 | −7.9 | 11.0 | 17.2 | 11.7 | 9.4 | 10.8 | 16.7 | 8.1 | 3.7 | −20.1 | 94.3 |
| 1-naphyrone | 0.1 | 0.5 | 0.9992 | 20.7 | 19.6 | −14.3 | −7.9 | 19.6 | 14.0 | 12.0 | 1.0 | 11.8 | 11.4 | 9.5 | 6.6 | −19.6 | 98.5 |
| 25D-NBOMe | 0.01 | 0.05 | 0.9997 | −16.5 | 10.7 | 5.5 | −7.3 | 14.2 | 13.2 | 12.1 | 5.1 | 20.5 | 17.0 | 7.2 | 9.9 | 16.8 | 86.6 |
| 25H-NBOMe | 0.03 | 0.1 | 0.9984 | −15.1 | −19.0 | 14.0 | −5.7 | 19.7 | 19.5 | 5.6 | 9.9 | 18.3 | 18.5 | 14.5 | 10.7 | 16.2 | 90.5 |
| 2-AI | 0.05 | 0.1 | 0.9909 | −16.1 | 19.6 | −14.8 | −8.4 | 4.7 | 13.7 | 6.4 | 3.6 | 19.6 | 17.8 | 10.0 | 6.3 | −2.2 | 83.6 |
| 2C-E | 0.3 | 0.5 | 0.9987 | 20.8 | −14.8 | −14.8 | −9.9 | 5.1 | 19.2 | 10.0 | 5.3 | 13.9 | 20.0 | 13.2 | 10.3 | 15.6 | 85.6 |
| 2C-N | 0.2 | 0.5 | 0.9988 | 13.8 | 19.4 | 15.1 | −3.5 | 13.7 | 19.0 | 14.3 | 3.8 | 19.8 | 14.3 | 10.9 | 4.8 | 11.6 | 90.6 |
| 2F-deschloroketamine | 0.5 | 1 | 0.9975 | −19.7 | −17.5 | 4.1 | −7.4 | 19.9 | 13.5 | 9.4 | 9.9 | 10.9 | 17.4 | 6.4 | 7.7 | −5.3 | 98.0 |
| 2F-methcathinone | 0.5 | 1.5 | 0.9961 | 11.0 | 21.2 | −10.9 | −2.3 | 9.7 | 19.2 | 2.4 | 1.3 | 19.0 | 18.8 | 12.1 | 8.1 | −14.7 | 89.5 |
| 2-methyl-AP-237 | 0.1 | 0.5 | 0.9981 | −9.5 | 15.5 | 2.2 | −8.8 | 12.2 | 19.1 | 11.1 | 9.5 | 12.7 | 19.5 | 6.2 | 3.7 | −14.1 | 87.4 |
| 3.4-DMMC | 0.1 | 0.3 | 0.9973 | −15.0 | 0.1 | 15.9 | −7.6 | 19.5 | 20.0 | 9.9 | 3.7 | 19.6 | 15.9 | 15.6 | 10.7 | −14.2 | 79.8 |
| 3.4MD-α-PHP | 0.2 | 0.5 | 0.9997 | 21.4 | 19.0 | −10.0 | −7.5 | 19.3 | 14.7 | 15.0 | 6.0 | 18.3 | 19.1 | 15.6 | 7.5 | −20.4 | 78.8 |
| 3-OH-flunitrazepam | 0.01 | 0.05 | 0.9989 | −11.6 | 11.2 | −0.5 | −1.8 | 3.9 | 5.1 | 14.7 | 8.1 | 18.1 | 15.0 | 10.5 | 4.2 | 18.1 | 98.8 |
| 3−MeO-PCP | 0.03 | 0.1 | 0.9918 | −20.3 | 19.2 | −1.1 | 2.6 | 19.9 | 5.2 | 6.1 | 10.0 | 19.6 | 10.9 | 9.3 | 10.0 | −3.2 | 92.6 |
| 3-MeO-PCE | 0.2 | 0.5 | 0.9993 | 19.0 | −11.7 | 12.5 | 7.6 | 7.6 | 15.2 | 14.6 | 9.5 | 11.5 | 10.3 | 9.5 | 6.8 | −6.8 | 97.8 |
| 3-MMC | 0.5 | 1 | 0.9947 | 17.8 | −20.3 | −8.3 | 8.1 | 11.1 | 19.7 | 14.0 | 10.0 | 17.3 | 11.8 | 15.7 | 5.7 | -1.8 | 83.0 |
| 4F-amphetamine | 0.1 | 0.5 | 0.9922 | 20.6 | 12.3 | −0.6 | −2.4 | 5.7 | 19.6 | 6.1 | 10.0 | 11.0 | 16.9 | 15.6 | 8.3 | 10.3 | 75.3 |
| 4F-methcathinone | 0.1 | 0.3 | 0.9989 | 14.5 | 12.8 | 4.5 | −4.0 | 19.8 | 19.4 | 13.4 | 1.5 | 19.3 | 10.0 | 13.3 | 3.0 | −18.8 | 98.8 |
| 4-MeO-PCP | 0.1 | 0.5 | 0.9950 | 19.6 | −14.5 | 14.8 | 1.7 | 6.2 | 18.2 | 6.0 | 9.3 | 15.3 | 12.0 | 11.4 | 9.1 | 11.3 | 92.7 |
| 4-methyl-ethcathinone | 0.1 | 0.3 | 0.9992 | −11.1 | 14.8 | 15.3 | −5.0 | 19.8 | 4.5 | 14.8 | 9.5 | 13.7 | 12.4 | 7.3 | 9.1 | −6.7 | 80.9 |
| 4-OH-DiPT | 0.02 | 0.2 | 0.9966 | 15.9 | 17.0 | −12.3 | −4.7 | 15.0 | 19.4 | 14.1 | 9.4 | 17.6 | 14.5 | 10.8 | 9.8 | −16.4 | 80.2 |
| 5-APB | 0.2 | 0.5 | 0.9992 | 10.2 | −20.5 | 11.0 | −9.0 | 7.4 | 19.2 | 14.5 | 5.8 | 14.1 | 13.7 | 12.2 | 2.2 | −17.6 | 96.5 |
| 5Cl-AB-PINACA | 0.2 | 0.5 | 0.9932 | −20.4 | −17.1 | −11.3 | −7.3 | 19.0 | 19.6 | 5.7 | 9.8 | 19.0 | 14.9 | 5.9 | 7.7 | −9.7 | 76.3 |
| 5Cl-THJ-018 | 0.2 | 0.5 | 0.9969 | −14.0 | 16.4 | −14.2 | −9.7 | 6.2 | 5.7 | 14.7 | 1.7 | 14.2 | 13.7 | 6.7 | 2.1 | 10.0 | 77.2 |
| 5F-AKB-48 | 0.05 | 0.2 | 0.9998 | 21.3 | −11.1 | 14.5 | −8.0 | 13.4 | 19.0 | 5.4 | 10.0 | 12.9 | 19.9 | 15.2 | 2.0 | −3.6 | 78.5 |
| 5F-APINACA | 0.05 | 0.1 | 0.9918 | 21.0 | 20.2 | 10.0 | 6.5 | 12.1 | 17.8 | 13.2 | 9.9 | 14.3 | 14.5 | 15.4 | 7.9 | −17.5 | 90.9 |
| 5F-APP-PICA | 0.2 | 0.5 | 0.9983 | 18.6 | 21.0 | −14.0 | 9.7 | 19.4 | 2.4 | 9.7 | 10.0 | 18.9 | 17.0 | 5.3 | 2.0 | 20.4 | 87.4 |
| 5F-APP-PINACA | 0.1 | 0.5 | 0.9918 | 22.2 | −13.0 | 14.6 | −5.1 | 2.2 | 11.5 | 14.3 | 1.6 | 16.0 | 17.9 | 8.6 | 5.9 | −2.4 | 99.0 |
| 5F-Cumyl-P7AICA | 0.2 | 0.5 | 0.9999 | 0.2 | 13.6 | −3.5 | −7.1 | 13.1 | 5.9 | 14.1 | 9.7 | 18.1 | 12.9 | 6.8 | 9.7 | 19.4 | 94.3 |
| 5F-Cumyl-PeGACLONE | 0.05 | 0.2 | 0.9991 | 13.8 | −17.5 | −14.1 | 5.7 | 16.8 | 11.1 | 12.1 | 9.9 | 12.2 | 15.9 | 8.1 | 2.1 | 4.9 | 77.9 |
| 5F-Cumyl-PINACA | 0.05 | 0.2 | 0.9979 | 21.8 | 16.6 | −12.8 | −8.5 | 14.0 | 19.7 | 14.4 | 9.5 | 16.2 | 19.9 | 10.2 | 5.8 | −20.1 | 90.3 |
| 5F-MDMB-7PAICA | 0.2 | 0.5 | 0.9991 | 19.5 | −15.1 | −10.2 | −3.1 | 3.4 | 19.0 | 14.3 | 7.0 | 11.0 | 15.1 | 14.1 | 8.3 | 8.4 | 77.5 |
| 5F-MDMB-PICA | 0.05 | 0.2 | 0.9985 | 17.3 | 12.6 | −11.9 | −9.1 | 13.4 | 10.0 | 14.9 | 7.3 | 15.5 | 15.8 | 13.8 | 3.3 | 3.9 | 93.9 |
| 5F-NNEI-2′-naphtyl-isomer | 0.1 | 0.5 | 0.9998 | −20.2 | 18.3 | −11.7 | −1.7 | 19.2 | 19.0 | 14.9 | 5.5 | 10.5 | 14.8 | 8.3 | 8.0 | 13.1 | 82.1 |
| 5-IAI | 0.1 | 1 | 0.9945 | 12.3 | −11.5 | −11.1 | 2.1 | 1.9 | 17.0 | 14.8 | 3.1 | 19.2 | 11.2 | 14.5 | 4.0 | 7.0 | 81.1 |
| 5-MAPB | 0.5 | 1 | 0.9990 | 12.1 | −9.6 | −10.5 | 7.2 | 9.7 | 6.7 | 15.0 | 4.0 | 20.6 | 11.2 | 6.7 | 8.3 | 21.0 | 94.1 |
| 5-MeO-DiPT | 0.1 | 0.3 | 0.9939 | −20.1 | −12.2 | −4.1 | −8.8 | 17.7 | 8.5 | 8.4 | 1.4 | 13.8 | 19.9 | 5.2 | 2.2 | −31.1 | 80.2 |
| 6-APB | 0.2 | 1 | 0.9989 | 21.8 | 15.0 | −14.2 | −5.8 | 13.2 | 4.6 | 1.6 | 10.0 | 12.0 | 16.7 | 10.6 | 4.2 | −2.5 | 88.0 |
| 6-MAPB | 0.5 | 1 | 0.9999 | 21.1 | 14.3 | 14.7 | 9.5 | 19.0 | 14.6 | 3.8 | 10.0 | 14.5 | 20.2 | 14.0 | 7.0 | −4.9 | 95.8 |
| 7-aminoclonazepam | 0.5 | 1 | 0.9960 | 15.5 | 18.1 | 7.6 | −4.4 | 7.0 | 19.2 | 8.7 | 9.0 | 11.5 | 13.1 | 6.1 | 4.3 | 3.5 | 77.2 |
| 7-aminoflunitrazepam | 0.01 | 0.05 | 0.9950 | 17.0 | 0.5 | 3.8 | 9.4 | 17.9 | 9.2 | 6.5 | 9.9 | 16.2 | 14.3 | 14.9 | 9.5 | −15.8 | 96.9 |
| 7-aminonitrazepam | 0.05 | 0.2 | 0.9967 | −12.1 | 19.3 | 14.6 | −3.2 | 5.6 | 19.5 | 6.5 | 1.7 | 20.8 | 17.9 | 15.8 | 5.4 | 7.6 | 78.8 |
| AB-CHMINACA | 0.02 | 0.1 | 0.9985 | 20.0 | 19.2 | 5.9 | −8.3 | 4.2 | 2.9 | 3.4 | 10.0 | 19.3 | 15.6 | 14.0 | 6.6 | 14.3 | 88.5 |
| AB-FUBINACA | 0.1 | 0.5 | 0.9941 | −15.3 | 19.1 | −2.0 | −9.9 | 7.8 | 1.7 | 14.1 | 6.1 | 18.7 | 18.5 | 14.5 | 3.5 | 6.0 | 85.2 |
| acetyl-fentanyl | 0.1 | 0.5 | 0.9990 | 21.1 | 19.2 | −11.8 | −8.0 | 19.1 | 14.2 | 14.1 | 9.6 | 15.3 | 19.2 | 9.5 | 9.2 | −0.7 | 76.0 |
| acetyl-norfentanyl | 0.5 | 1 | 0.9969 | −10.7 | −16.5 | 14.8 | −7.9 | 15.0 | 19.4 | 9.8 | 9.0 | 15.0 | 17.1 | 10.0 | 9.7 | 12.2 | 78.2 |
| ADB-FUBINACA | 0.5 | 1 | 0.9927 | −18.0 | −20.4 | 10.7 | −9.0 | 17.1 | 20.0 | 9.6 | 8.8 | 14.7 | 11.1 | 9.6 | 8.2 | −8.3 | 92.7 |
| ADB-PINACA | 0.1 | 0.5 | 0.9991 | 14.5 | 19.0 | −11.4 | 0.9 | 13.5 | 17.2 | 3.3 | 2.7 | 19.4 | 12.7 | 15.9 | 3.7 | 1.5 | 87.6 |
| alfentanil | 0.2 | 1 | 0.9966 | −14.2 | −12.2 | 8.0 | −6.4 | 18.1 | 5.2 | 13.0 | 9.1 | 12.5 | 10.3 | 5.2 | 9.5 | −0.9 | 93.9 |
| alprazolam | 0.05 | 0.1 | 0.9996 | 12.1 | 21.4 | −13.9 | −9.8 | 14.9 | 18.2 | 3.6 | 9.9 | 20.9 | 20.3 | 6.8 | 3.6 | −20.1 | 76.4 |
| AM-2201 | 0.05 | 0.1 | 0.9979 | 18.3 | 13.2 | −1.3 | −6.2 | 19.2 | 19.7 | 12.5 | 8.0 | 19.7 | 20.4 | 14.9 | 7.5 | 15.8 | 93.9 |
| AM-2233 | 0.05 | 0.1 | 0.9997 | 20.0 | −16.7 | 4.9 | 5.0 | 19.2 | 15.2 | 14.4 | 5.1 | 19.6 | 20.9 | 12.0 | 6.9 | 19.3 | 82.4 |
| AM-694 | 0.1 | 0.5 | 0.9986 | −14.8 | 0.3 | 2.7 | −7.5 | 12.9 | 4.7 | 5.8 | 9.9 | 19.0 | 15.4 | 7.1 | 2.6 | 15.1 | 91.1 |
| amitriptyline | 0.5 | 1 | 0.9933 | −20.6 | 19.8 | 0.7 | −5.0 | 19.0 | 19.2 | 1.4 | 9.0 | 13.8 | 20.2 | 5.9 | 4.2 | −2.8 | 80.5 |
| amphetamine | 0.1 | 0.5 | 0.9941 | −18.0 | −20.9 | −13.9 | −6.3 | 3.6 | 11.2 | 9.9 | 4.3 | 16.0 | 10.5 | 8.4 | 5.2 | 15.4 | 77.9 |
| AP-237 | 0.05 | 0.1 | 0.9955 | −21.4 | 15.3 | 12.2 | −3.0 | 3.5 | 12.5 | 6.0 | 9.9 | 12.8 | 19.7 | 13.8 | 10.5 | −2.5 | 95.4 |
| APP-FUBINACA | 0.5 | 1 | 0.9978 | −13.6 | 19.5 | 3.5 | −2.0 | 7.7 | 11.8 | 2.8 | 2.6 | 19.3 | 11.2 | 7.4 | 3.6 | −19.8 | 77.0 |
| bentazepam | 0.2 | 0.5 | 0.9989 | 18.2 | −16.8 | −5.1 | 9.5 | 19.0 | 8.6 | 3.9 | 9.8 | 14.1 | 17.2 | 6.6 | 8.3 | 11.1 | 90.6 |
| bromazepam | 0.2 | 0.5 | 0.9959 | 20.3 | −17.2 | −14.6 | −7.9 | 1.8 | 18.2 | 14.9 | 9.0 | 19.1 | 19.8 | 13.8 | 8.0 | 20.4 | 91.9 |
| brotizolam | 0.03 | 0.1 | 0.9982 | 1.0 | −15.6 | −8.6 | −8.0 | 11.7 | 6.4 | 3.8 | 9.1 | 19.5 | 20.9 | 5.3 | 2.2 | 20.1 | 77.1 |
| buphedrone | 0.1 | 0.5 | 0.9959 | 15.9 | 19.0 | −13.5 | 0.1 | 6.4 | 19.8 | 14.4 | 10.0 | 20.2 | 13.7 | 14.7 | 7.9 | 12.3 | 84.4 |
| butylone | 0.1 | 0.3 | 0.9973 | 19.8 | 13.5 | 12.2 | 9.5 | 19.6 | 8.2 | 14.9 | 2.3 | 19.1 | 13.7 | 10.3 | 2.8 | −3.0 | 86.4 |
| butyryl-fentanyl | 0.1 | 0.5 | 0.9967 | 11.5 | −16.5 | 7.0 | −7.0 | 2.1 | 18.5 | 8.8 | 9.9 | 15.1 | 11.2 | 14.7 | 2.8 | 9.9 | 98.5 |
| butyryl-norfentanyl | 0.1 | 0.5 | 0.9970 | 21.6 | −14.0 | −1.6 | −5.3 | 19.0 | 2.5 | 2.8 | 10.0 | 10.2 | 20.2 | 14.9 | 8.8 | 12.7 | 92.2 |
| BZP | 0.05 | 0.5 | 0.9989 | −21.9 | −17.2 | −14.6 | −5.4 | 9.9 | 12.6 | 2.6 | 6.0 | 16.5 | 20.3 | 13.3 | 8.9 | 15.5 | 90.5 |
| carfentanyl | 0.1 | 0.5 | 0.9952 | 11.4 | 12.2 | −11.5 | −9.8 | 19.8 | 5.8 | 14.7 | 9.9 | 16.3 | 11.4 | 11.9 | 10.4 | −9.5 | 87.0 |
| CB-13 | 0.1 | 0.5 | 0.9983 | −10.1 | −11.5 | 13.0 | −8.4 | 7.4 | 9.7 | 14.4 | 9.8 | 14.9 | 14.5 | 9.0 | 4.5 | 10.9 | 94.0 |
| chlordiazepoxide | 0.2 | 0.5 | 0.9996 | 12.7 | −17.9 | 14.9 | 9.7 | 12.9 | 15.7 | 14.5 | 2.3 | 19.7 | 19.4 | 14.6 | 5.2 | −0.7 | 91.6 |
| cinazepam | 0.5 | 1 | 0.9984 | −20.4 | −11.8 | −0.6 | −3.4 | 19.0 | 19.7 | 4.1 | 9.6 | 19.6 | 13.9 | 9.6 | 5.8 | 14.2 | 88.9 |
| citalopram | 0.03 | 0.1 | 0.9969 | 19.6 | 21.7 | −13.3 | −7.4 | 19.0 | 19.5 | 14.0 | 9.8 | 13.6 | 14.3 | 6.4 | 10.4 | −6.7 | 94.5 |
| clonazepam | 0.1 | 0.5 | 0.9981 | 19.6 | 19.3 | −9.1 | −6.6 | 19.7 | 1.8 | 13.5 | 5.2 | 14.1 | 18.6 | 15.0 | 3.6 | 8.0 | 86.4 |
| clonazolam | 0.2 | 0.5 | 0.9987 | −9.4 | 17.1 | −15.0 | −6.4 | 19.0 | 4.1 | 14.1 | 2.7 | 16.4 | 15.5 | 14.5 | 6.2 | 5.9 | 81.3 |
| cumyl-PeGLACONE | 0.2 | 0.5 | 0.9927 | 22.7 | −18.2 | 3.6 | 8.0 | 1.7 | 19.2 | 14.4 | 9.5 | 16.6 | 16.4 | 14.9 | 6.6 | 16.2 | 95.4 |
| cyclopropyl-fentanyl | 0.1 | 0.5 | 0.9988 | −17.2 | −16.0 | −14.2 | 6.5 | 17.2 | 19.8 | 14.5 | 9.3 | 19.6 | 20.1 | 8.0 | 3.2 | 6.9 | 80.9 |
| delorazepam | 0.1 | 0.5 | 0.9996 | 19.9 | 11.1 | 16.9 | −4.7 | 1.2 | 19.2 | 4.4 | 9.6 | 10.1 | 12.0 | 9.3 | 9.3 | −18.8 | 81.6 |
| deschloro-N-et-ketamine | 0.2 | 0.5 | 0.9958 | −19.3 | −11.5 | 11.1 | −3.5 | 19.0 | 19.7 | 9.3 | 9.9 | 20.1 | 13.5 | 6.0 | 5.5 | −7.3 | 96.0 |
| diazepam | 0.01 | 0.05 | 0.9980 | 15.9 | 16.8 | 12.3 | −7.9 | 7.1 | 12.4 | 4.1 | 9.7 | 12.2 | 20.7 | 14.4 | 2.6 | 20.5 | 78.3 |
| diclazepam | 0.2 | 0.5 | 0.9908 | 15.1 | −20.7 | 5.4 | −2.0 | 19.6 | 18.6 | 14.1 | 7.6 | 10.8 | 19.8 | 9.9 | 8.7 | 20.2 | 98.9 |
| dimethylcathinone | 0.1 | 0.3 | 0.9987 | −18.5 | −20.4 | −9.9 | 5.5 | 8.2 | 13.0 | 9.5 | 9.8 | 19.1 | 20.9 | 9.1 | 9.9 | −14.4 | 93.9 |
| duloxetina | 0.1 | 0.5 | 0.9976 | 11.3 | −13.8 | 7.2 | 7.4 | 19.8 | 2.9 | 5.5 | 2.4 | 10.0 | 13.2 | 7.7 | 3.0 | −15.6 | 89.6 |
| ethcathinone | 0.1 | 0.3 | 0.9911 | −20.9 | 16.6 | 14.2 | −4.7 | 19.9 | 19.2 | 14.3 | 1.6 | 11.6 | 16.3 | 8.5 | 4.3 | −2.2 | 98.8 |
| ethylone | 0.1 | 0.3 | 0.9997 | −12.3 | 15.0 | 14.5 | −9.0 | 14.6 | 19.7 | 3.7 | 8.6 | 18.2 | 10.9 | 7.2 | 3.2 | 21.5 | 89.5 |
| etizolam | 0.1 | 0.5 | 0.9928 | 15.0 | −16.4 | 12.5 | 8.2 | 13.5 | 17.2 | 14.0 | 8.9 | 16.6 | 17.1 | 6.2 | 9.8 | −6.8 | 93.8 |
| eutylone | 0.5 | 1 | 0.9997 | 21.6 | −15.6 | −11.0 | −4.8 | 1.7 | 14.1 | 12.5 | 9.7 | 19.1 | 20.4 | 9.1 | 9.9 | 6.4 | 83.5 |
| fentanyl | 0.05 | 0.1 | 0.9977 | 14.1 | −20.2 | −2.6 | −1.4 | 18.5 | 19.2 | 6.3 | 6.8 | 19.4 | 10.7 | 11.2 | 9.0 | −1.1 | 90.3 |
| flualprazolam | 0.2 | 0.5 | 0.9905 | −9.9 | 18.0 | −9.9 | 0.6 | 13.8 | 11.8 | 11.9 | 9.9 | 18.3 | 18.5 | 5.8 | 7.2 | −13.0 | 80.2 |
| flunitrazepam | 0.1 | 0.5 | 0.9988 | 11.3 | −11.1 | 11.0 | 7.4 | 14.7 | 12.5 | 14.5 | 9.9 | 20.1 | 11.9 | 7.7 | 9.2 | 21.4 | 77.7 |
| fluoxetine | 0.5 | 1 | 0.9918 | −20.8 | 19.8 | 13.6 | −9.4 | 19.0 | 12.2 | 2.9 | 9.0 | 16.8 | 16.0 | 11.5 | 9.7 | 4.0 | 80.5 |
| flurazepam | 0.01 | 0.05 | 0.9954 | −18.2 | −12.2 | 15.1 | 9.5 | 17.1 | 13.7 | 14.7 | 9.7 | 15.7 | 13.8 | 7.3 | 10.4 | −7.5 | 76.0 |
| furanyl-fentanyl | 0.1 | 0.5 | 0.9979 | −20.8 | 18.5 | 14.3 | 0.3 | 19.8 | 19.0 | 2.4 | 10.0 | 15.9 | 11.2 | 14.8 | 9.4 | 10.6 | 76.0 |
| furanyl-norfentanyl | 0.01 | 0.2 | 0.9955 | 12.6 | −18.0 | −12.1 | −6.3 | 18.0 | 19.0 | 14.1 | 9.8 | 11.9 | 13.4 | 9.6 | 9.7 | 1.3 | 86.6 |
| isobutyryl-fentanyl | 0.1 | 0.5 | 0.9912 | 14.1 | −17.2 | 13.1 | −8.4 | 3.8 | 15.7 | 15.0 | 6.9 | 15.5 | 19.8 | 12.5 | 7.2 | 4.2 | 87.2 |
| isotonitazene | 0.05 | 0.2 | 0.9985 | 20.6 | −17.3 | 14.9 | −3.9 | 4.9 | 3.7 | 5.3 | 1.5 | 12.3 | 18.0 | 9.1 | 7.7 | 10.6 | 90.6 |
| JWH-007 | 0.5 | 1 | 0.9991 | −13.0 | 21.4 | −11.9 | −4.9 | 5.2 | 6.7 | 14.6 | 2.7 | 13.7 | 15.5 | 10.1 | 7.6 | 27.4 | 83.7 |
| JWH-016 | 0.3 | 0.1 | 0.9999 | −15.0 | 13.3 | −11.8 | 0.7 | 19.4 | 19.9 | 1.7 | 6.1 | 16.3 | 13.2 | 15.2 | 2.0 | −4.8 | 80.1 |
| JWH-018 | 0.3 | 0.1 | 0.9997 | 14.6 | 20.2 | 14.2 | 3.0 | 3.8 | 19.4 | 14.1 | 10.0 | 14.1 | 13.9 | 10.8 | 4.7 | 1.1 | 78.1 |
| JWH-019 | 0.1 | 0.5 | 0.9913 | 12.7 | 19.8 | 16.4 | −1.4 | 14.9 | 1.2 | 12.6 | 10.0 | 12.3 | 19.6 | 11.9 | 10.7 | −19.8 | 76.4 |
| JWH-073 | 0.1 | 0.5 | 0.9944 | −16.5 | 15.9 | 14.5 | −0.6 | 1.7 | 3.6 | 14.8 | 10.0 | 16.5 | 12.2 | 12.7 | 2.4 | −14.6 | 94.7 |
| JWH-081 | 0.1 | 0.5 | 0.9987 | −19.5 | −18.2 | 12.6 | −0.9 | 19.1 | 7.2 | 3.1 | 9.7 | 11.4 | 14.1 | 12.0 | 7.1 | 21.4 | 93.0 |
| JWH-098 | 0.1 | 0.5 | 0.9951 | −13.0 | 17.0 | −7.9 | 4.6 | 4.7 | 19.4 | 9.1 | 2.9 | 10.7 | 18.3 | 9.1 | 9.1 | 4.7 | 80.0 |
| JWH-122 | 0.1 | 0.5 | 0.9971 | −20.6 | 19.8 | −10.4 | −7.0 | 8.2 | 11.2 | 8.4 | 9.1 | 12.9 | 19.3 | 10.3 | 5.6 | 15.4 | 98.9 |
| JWH-147 | 0.02 | 0.05 | 0.9961 | −11.7 | −12.0 | −4.6 | −1.9 | 19.9 | 19.6 | 14.7 | 9.7 | 10.3 | 19.7 | 12.5 | 10.4 | 13.2 | 84.2 |
| JWH-200 | 0.1 | 0.5 | 0.9945 | −19.9 | 12.9 | −14.6 | 9.1 | 19.6 | 19.5 | 14.6 | 9.9 | 11.4 | 18.7 | 11.8 | 10.4 | −8.2 | 89.6 |
| JWH-203 | 0.1 | 0.5 | 0.9908 | −12.7 | 18.8 | −13.9 | −4.1 | 5.1 | 14.1 | 14.4 | 5.4 | 20.0 | 19.6 | 11.7 | 10.9 | −4.1 | 98.1 |
| JWH-210 | 0.1 | 0.5 | 0.9985 | 18.3 | −16.2 | −3.6 | −6.0 | 19.4 | 11.8 | 5.0 | 10.0 | 17.3 | 17.8 | 11.9 | 9.5 | 10.7 | 95.3 |
| JWH-250 | 0.1 | 0.5 | 0.9965 | 16.8 | 15.0 | 1.6 | −4.0 | 5.2 | 19.1 | 19.0 | 10.0 | 20.9 | 18.6 | 15.0 | 9.6 | −12.0 | 93.1 |
| JWH-251 | 0.1 | 0.5 | 0.9944 | −13.0 | 12.2 | -12.4 | 8.8 | 3.2 | 1.4 | 14.7 | 9.6 | 19.2 | 19.8 | 7.7 | 2.3 | 7.7 | 76.2 |
| JWH-302 | 0.1 | 0.5 | 0.9926 | 11.0 | −18.8 | 2.2 | 3.6 | 18.0 | 3.0 | 12.6 | 10.0 | 14.0 | 10.0 | 8.8 | 9.5 | −11.0 | 84.5 |
| JWH-307 | 0.05 | 0.1 | 0.9934 | −13.7 | −18.9 | −15.0 | 3.6 | 6.2 | 16.2 | 14.3 | 8.6 | 19.1 | 16.6 | 8.2 | 6.5 | −1.0 | 97.1 |
| JWH-398 | 0.1 | 0.5 | 0.9991 | 21.7 | 13.8 | 14.5 | −7.1 | 19.9 | 2.0 | 10.9 | 8.0 | 12.6 | 11.1 | 11.3 | 9.9 | 6.3 | 82.9 |
| ketamina | 0.05 | 0.2 | 0.9926 | 12.6 | −13.1 | −13.8 | −5.6 | 3.4 | 2.2 | 8.7 | 9.5 | 19.1 | 19.7 | 6.1 | 5.5 | −1.1 | 84.1 |
| levomepromazine | 0.01 | 0.1 | 0.9914 | −11.8 | 15.8 | 10.0 | −3.1 | 19.4 | 9.3 | 9.1 | 5.7 | 17.6 | 13.5 | 10.9 | 5.8 | 14.6 | 89.1 |
| lorazepam | 0.1 | 0.5 | 0.9997 | 21.8 | −11.0 | −8.9 | −8.4 | 2.2 | 19.2 | 8.1 | 2.5 | 12.9 | 15.9 | 7.3 | 2.7 | −11.0 | 89.3 |
| lormetazepam | 0.05 | 0.1 | 0.9999 | 10.9 | −18.4 | −12.0 | 4.5 | 4.2 | 19.1 | 7.0 | 9.5 | 19.4 | 14.8 | 14.8 | 9.6 | −9.3 | 92.2 |
| m-CPP | 0.1 | 0.5 | 0.9971 | −12.3 | −9.6 | −1.0 | −9.0 | 19.5 | 19.4 | 6.0 | 10.0 | 19.8 | 13.2 | 7.9 | 9.5 | 10.1 | 81.6 |
| MDA | 0.1 | 0.3 | 0.9979 | 19.9 | 19.0 | −11.0 | −1.0 | 13.2 | 4.0 | 14.9 | 9.7 | 14.9 | 16.6 | 10.2 | 5.6 | 20.7 | 75.6 |
| MDAI | 0.1 | 0.5 | 0.9946 | 12.4 | -18.6 | 14.8 | 2.9 | 19.5 | 5.2 | 2.4 | 4.8 | 19.0 | 12.1 | 11.0 | 10.4 | −14.5 | 88.9 |
| MDEA | 0.03 | 0.1 | 0.9919 | 12.7 | 0.6 | −7.4 | 0.9 | 13.7 | 19.0 | 14.8 | 10.0 | 12.0 | 15.6 | 14.2 | 3.2 | −16.9 | 98.4 |
| MDMA | 0.1 | 0.5 | 0.9967 | −20.1 | −18.6 | −6.9 | −1.1 | 19.2 | 15.9 | 14.1 | 10.0 | 19.4 | 13.4 | 6.6 | 6.2 | −6.4 | 94.9 |
| MDMB-CHMICA | 0.2 | 0.5 | 0.9972 | −17.8 | 15.6 | −13.5 | 9.1 | 13.2 | 7.6 | 14.6 | 2.0 | 17.8 | 15.2 | 15.4 | 7.5 | −3.7 | 80.5 |
| MDPV | 0.3 | 0.5 | 0.9935 | 18.5 | 19.5 | −12.9 | −6.4 | 14.5 | 12.0 | 11.2 | 9.7 | 11.1 | 17.0 | 13.6 | 9.5 | −6.0 | 86.6 |
| MeOAc-fentanyl | 0.1 | 0.5 | 0.9942 | 19.8 | −13.2 | 2.2 | 5.9 | 12.7 | 14.1 | 4.7 | 9.0 | 19.0 | 18.8 | 11.3 | 5.5 | −18.3 | 96.7 |
| MeOAc-norfentanyl | 0.1 | 0.5 | 0.9998 | 19.7 | −11.5 | −14.5 | 1.0 | 1.8 | 13.7 | 14.1 | 9.4 | 20.9 | 20.0 | 9.3 | 6.3 | −12.7 | 79.2 |
| mephedrone | 0.1 | 0.5 | 0.9996 | 15.0 | 13.3 | 5.6 | 5.4 | 19.9 | 12.7 | 14.0 | 10.0 | 20.7 | 17.1 | 6.3 | 9.3 | 14.7 | 85.8 |
| methamphetamine | 0.1 | 0.5 | 0.9997 | −18.9 | 19.5 | 0.9 | −7.2 | 15.5 | 19.8 | 9.7 | 9.8 | 12.2 | 15.4 | 8.1 | 8.5 | 7.9 | 96.4 |
| methcathinone | 0.1 | 0.5 | 0.9979 | 19.6 | 20.0 | 13.8 | 9.1 | 19.0 | 16.0 | 6.0 | 2.0 | 19.8 | 17.3 | 11.3 | 5.4 | 27.7 | 90.5 |
| methedrone | 0.1 | 0.5 | 0.9992 | −14.5 | 14.4 | −14.0 | −3.5 | 19.0 | 4.0 | 14.6 | 4.5 | 17.6 | 19.1 | 12.2 | 10.2 | −10.1 | 92.1 |
| methoxetamine | 0.1 | 0.5 | 0.9964 | 19.6 | −18.8 | 14.3 | 0.3 | 18.9 | 2.6 | 1.8 | 9.4 | 15.8 | 11.6 | 7.0 | 5.4 | −5.3 | 94.6 |
| methylone | 0.1 | 0.5 | 0.9941 | 15.0 | -18.4 | 9.5 | 8.7 | 14.0 | 19.1 | 4.7 | 9.5 | 18.9 | 20.7 | 6.2 | 5.7 | -13.7 | 89.0 |
| midazolam | 0.05 | 0.1 | 0.9955 | 19.8 | 14.6 | −10.6 | −2.8 | 11.8 | 14.2 | 14.1 | 10.0 | 16.8 | 13.0 | 7.2 | 2.4 | 8.5 | 79.2 |
| mirtazapine | 0.1 | 0.5 | 0.9974 | −18.9 | −18.1 | 14.9 | −3.0 | 18.9 | 19.0 | 14.9 | 9.5 | 12.3 | 13.0 | 12.5 | 6.7 | 18.1 | 79.4 |
| MMB-2201 | 0.1 | 0.5 | 0.9964 | 22.0 | 15.3 | −7.1 | −1.2 | 4.5 | 19.9 | 9.0 | 9.9 | 12.2 | 19.7 | 7.2 | 8.1 | −8.9 | 91.4 |
| naphyrone | 0.1 | 0.5 | 0.9976 | 18.1 | −10.2 | 6.2 | 9.6 | 14.4 | 16.8 | 5.1 | 9.6 | 17.3 | 19.8 | 14.0 | 9.4 | 2.8 | 79.6 |
| N-ethylpentylone | 0.3 | 0.5 | 0.9906 | 19.1 | 20.9 | −13.8 | −1.0 | 1.2 | 1.8 | 12.7 | 10.0 | 13.6 | 16.5 | 14.4 | 6.3 | −5.8 | 98.5 |
| nordiazepam | 0.1 | 0.3 | 0.9952 | 14.7 | −13.1 | −14.7 | −4.6 | 9.4 | 7.0 | 1.7 | 5.9 | 15.4 | 16.7 | 6.3 | 7.3 | 21.1 | 91.1 |
| norfentanyl | 0.5 | 1 | 0.9973 | 18.8 | 13.5 | 0.3 | −8.1 | 1.2 | 19.0 | 14.7 | 9.8 | 10.7 | 10.0 | 12.0 | 7.2 | 10.3 | 78.2 |
| norketamina | 0.1 | 0.5 | 0.9939 | 12.7 | −16.4 | −13.1 | −2.6 | 1.9 | 13.2 | 15.0 | 3.6 | 10.5 | 19.0 | 15.1 | 9.9 | 16.0 | 87.4 |
| ocfentanil | 0.2 | 0.1 | 0.9994 | 18.2 | 14.0 | 6.1 | 9.7 | 19.6 | 19.2 | 4.5 | 9.9 | 20.0 | 16.2 | 5.9 | 4.5 | −6.4 | 87.9 |
| oxazepam | 0.5 | 1 | 0.9966 | −20.4 | 15.3 | 10.1 | 9.4 | 18.9 | 19.0 | 13.7 | 7.5 | 19.2 | 11.8 | 9.9 | 9.3 | 3.8 | 92.1 |
| oxcarbazepine | 0.5 | 1 | 0.9988 | −11.5 | 19.9 | 12.8 | 9.8 | 2.4 | 6.2 | 5.4 | 9.4 | 19.7 | 14.6 | 5.6 | 6.8 | 18.4 | 89.3 |
| paroxetine | 1 | 2 | 0.9986 | 22.4 | 13.8 | 12.8 | −4.1 | 19.1 | 11.2 | 14.1 | 9.6 | 18.2 | 20.7 | 7.1 | 2.9 | −0.7 | 80.8 |
| pentedrone | 0.1 | 0.3 | 0.9934 | −10.4 | −18.6 | −13.6 | 7.1 | 13.6 | 19.7 | 3.6 | 9.4 | 19.6 | 19.7 | 13.4 | 8.8 | −5.1 | 79.9 |
| pentylone | 0.1 | 0.3 | 0.9966 | 17.2 | −18.6 | 14.0 | −2.8 | 19.0 | 4.5 | 12.5 | 9.8 | 10.3 | 10.5 | 14.8 | 2.1 | −12.7 | 85.0 |
| pF-furanyl-fentanyl | 0.01 | 0.2 | 0.9981 | 19.6 | −9.7 | −3.6 | −9.1 | 4.7 | 20.0 | 1.5 | 9.5 | 16.0 | 11.9 | 7.5 | 8.1 | 2.4 | 90.0 |
| pinazepam | 0.01 | 0.05 | 0.9983 | 18.0 | −9.1 | −6.0 | −7.6 | 12.6 | 15.7 | 1.5 | 4.5 | 10.2 | 10.9 | 7.9 | 7.6 | 20.6 | 79.7 |
| pravadoline | 0.02 | 0.1 | 0.9946 | 0.4 | 16.1 | 5.5 | 9.6 | 18.4 | 2.0 | 1.1 | 3.5 | 20.7 | 20.1 | 13.9 | 10.6 | −16.3 | 82.6 |
| prazepam | 0.01 | 0.02 | 0.9981 | −10.5 | 19.5 | 1.0 | 9.2 | 5.4 | 19.0 | 14.1 | 2.9 | 11.3 | 17.8 | 12.4 | 9.9 | 8.3 | 92.0 |
| promazine | 0.1 | 0.5 | 0.9944 | −20.2 | −17.0 | −6.6 | −0.1 | 4.2 | 19.8 | 14.6 | 1.3 | 19.7 | 16.1 | 13.9 | 7.4 | 21.8 | 91.7 |
| quetiapine | 0.1 | 0.5 | 0.9987 | 18.0 | -20.4 | 6.9 | 6.4 | 19.0 | 4.2 | 9.7 | 2.0 | 19.8 | 12.0 | 11.2 | 10.3 | 20.1 | 87.2 |
| RCS-4 | 0.05 | 0.1 | 0.9994 | 16.8 | 11.4 | 16.8 | 6.0 | 8.8 | 19.6 | 2.4 | 9.0 | 16.8 | 12.1 | 11.7 | 3.5 | 8.5 | 80.2 |
| RCS-8 | 0.02 | 0.05 | 0.9922 | 19.4 | −18.3 | 5.4 | 1.0 | 1.2 | 10.0 | 6.1 | 9.1 | 11.3 | 17.3 | 11.2 | 2.1 | 17.0 | 79.0 |
| Ritalinic acid | 0.2 | 1 | 0.9965 | −14.2 | −11.8 | 8.2 | 0.5 | 14.9 | 18.2 | 12.1 | 6.7 | 14.3 | 17.4 | 8.1 | 4.8 | 15.0 | 76.9 |
| temazepam | 0.1 | 0.5 | 0.9978 | 22.0 | −19.0 | −11.6 | −8.9 | 19.0 | 19.9 | 14.0 | 9.6 | 16.3 | 13.1 | 9.9 | 9.5 | 21.3 | 85.6 |
| trazodone | 0.1 | 0.5 | 0.9986 | 18.8 | 19.3 | 10.9 | 9.7 | 13.9 | 12.4 | 9.4 | 4.8 | 19.6 | 19.5 | 6.8 | 8.0 | 1.2 | 93.6 |
| triazolam | 0.03 | 0.1 | 0.9984 | 21.5 | −15.8 | −14.0 | 1.9 | 2.4 | 14.2 | 14.3 | 10.0 | 15.5 | 11.8 | 15.5 | 4.7 | 7.1 | 86.6 |
| UR-144 | 0.2 | 0.5 | 0.9993 | 19.2 | 14.7 | 13.2 | −6.0 | 6.8 | 14.1 | 7.9 | 9.8 | 13.4 | 19.7 | 12.1 | 3.4 | −8.8 | 84.1 |
| WIN 55.212-2 | 0.05 | 0.1 | 0.9985 | −18.8 | 19.6 | −13.9 | −9.3 | 18.3 | 19.2 | 2.5 | 9.9 | 11.9 | 11.5 | 12.0 | 8.7 | −17.2 | 97.9 |
| zolpidem | 0.05 | 0.1 | 0.9932 | −17.0 | −14.8 | 13.9 | −9.0 | 15.2 | 19.6 | 11.7 | 9.9 | 18.5 | 19.9 | 12.3 | 5.5 | −3.2 | 91.8 |
| zoplicone | 0.05 | 0.1 | 0.9991 | 22.0 | −18.7 | 14.8 | −5.4 | 4.5 | 19.1 | 14.5 | 2.6 | 12.5 | 13.2 | 9.5 | 7.5 | 16.7 | 94.4 |
| α-OH-alprazolam | 0.05 | 1 | 0.9964 | −17.1 | −12.5 | 11.6 | 5.6 | 19.0 | 12.4 | 14.1 | 9.9 | 11.8 | 14.6 | 8.9 | 4.5 | −3.8 | 80.9 |
| α-OH-midazolam | 0.05 | 0.1 | 0.9977 | −18.7 | 14.5 | −12.0 | −7.2 | 9.4 | 19.7 | 14.8 | 9.7 | 10.3 | 11.2 | 9.4 | 10.1 | −7.6 | 89.9 |
| α-PHP | 0.5 | 0.1 | 0.9967 | 11.6 | 18.0 | 8.1 | 9.8 | 19.4 | 9.8 | 12.1 | 9.8 | 18.5 | 14.7 | 14.8 | 7.1 | 10.6 | 93.6 |
| Case | Forensic Casework | Compound | Concentration (ng/mL) |
|---|---|---|---|
| #1 | DUID | alprazolam α-OH-alprazolam | 50.39 3.32 |
| #2 | DUID | diazepam nordiazepam temazepam oxazepam | 346.07 70.82 35.56 15.77 |
| #3 | DUID | lorazepam | 85.30 |
| #4 | DUID | ketamina nor-ketamina | 246.37 177.43 |
| #5 | DUID | diazepam nordiazepam temazepam oxazepam | 1046.36 638.16 18.68 80.93 |
| #6 | DUID | diazepam nordiazepam temazepam oxazepam | 28.89 74.53 10.58 <LOQ |
| #7 | DUID | alprazolam α-OH-alprazolam | 31.30 <LOQ |
| #8 | DUID | ketamina nor-ketamina | 305.01 198.77 |
| #9 | DUID | alprazolam α-OH-alprazolam | 7.27 <LOQ |
| #10 | DUID | diazepam nordiazepam temazepam oxazepam | 38.25 30.78 29.11 26.89 |
| #11 | DUID | diazepam nordiazepam temazepam oxazepam | 1830.17 44.00 19.02 <LOQ |
| #12 | DUID | lorazepam | 178.12 |
| #13 | Acute intoxication | MDPV | 42.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaiano, F.; Bertol, E.; Mineo, M.; Pietrosemoli, L.; Rubicondo, J.; Supuran, C.T.; Carta, F. Development of a New LC-MS/MS Screening Method for Detection of 120 NPS and 43 Drugs in Blood. Separations 2021, 8, 221. https://doi.org/10.3390/separations8110221
Vaiano F, Bertol E, Mineo M, Pietrosemoli L, Rubicondo J, Supuran CT, Carta F. Development of a New LC-MS/MS Screening Method for Detection of 120 NPS and 43 Drugs in Blood. Separations. 2021; 8(11):221. https://doi.org/10.3390/separations8110221
Chicago/Turabian StyleVaiano, Fabio, Elisabetta Bertol, Maria Mineo, Laura Pietrosemoli, Jolanda Rubicondo, Claudiu T. Supuran, and Fabrizio Carta. 2021. "Development of a New LC-MS/MS Screening Method for Detection of 120 NPS and 43 Drugs in Blood" Separations 8, no. 11: 221. https://doi.org/10.3390/separations8110221
APA StyleVaiano, F., Bertol, E., Mineo, M., Pietrosemoli, L., Rubicondo, J., Supuran, C. T., & Carta, F. (2021). Development of a New LC-MS/MS Screening Method for Detection of 120 NPS and 43 Drugs in Blood. Separations, 8(11), 221. https://doi.org/10.3390/separations8110221








