On Process Intensification through Membrane Storage Reactors
Abstract
1. Introduction
2. Mathematical Formulation
2.1. Limiting Reactant Conversion
2.2. Desired Product Ratio
2.3. Desired Product Recovery Fraction
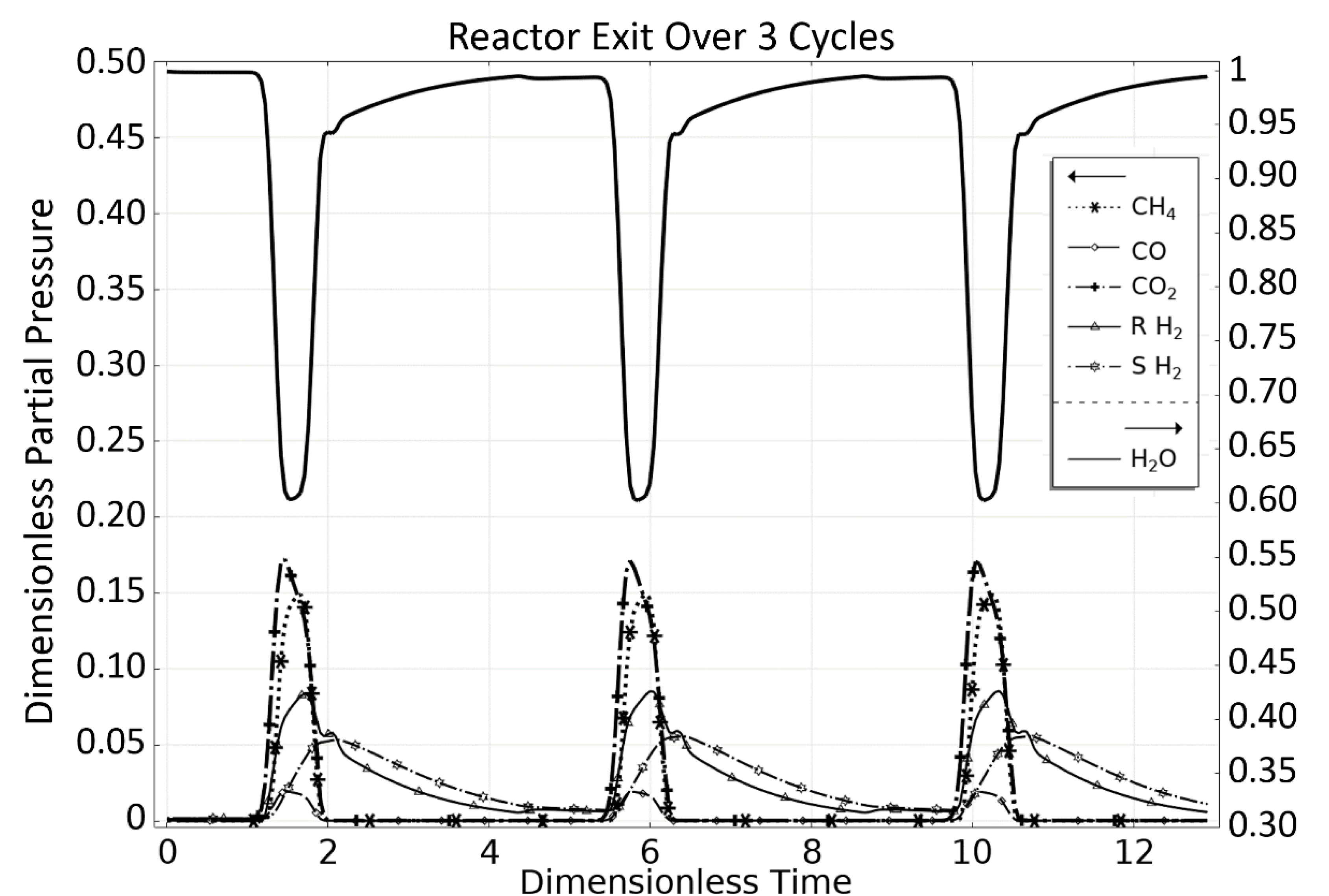
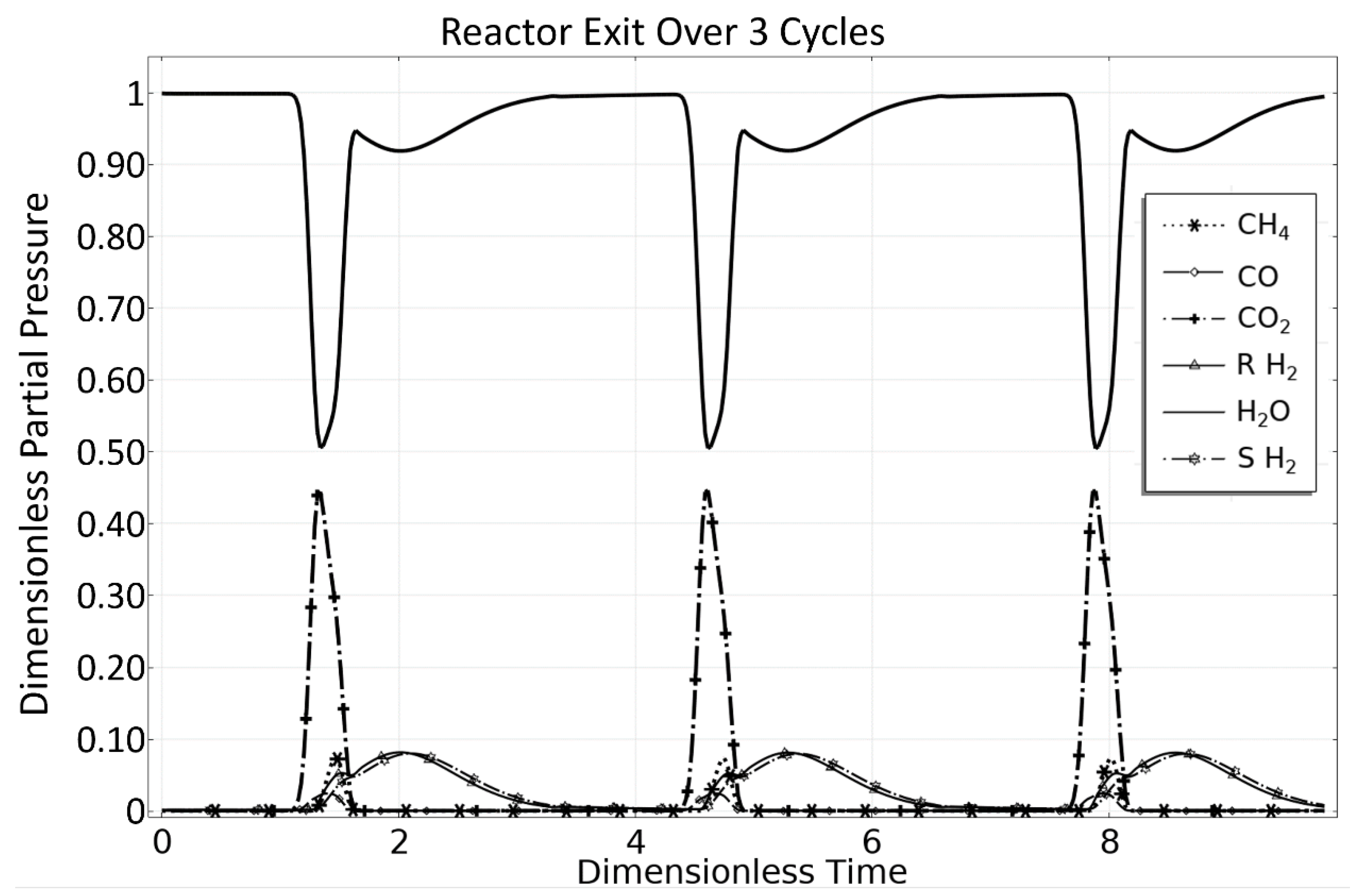
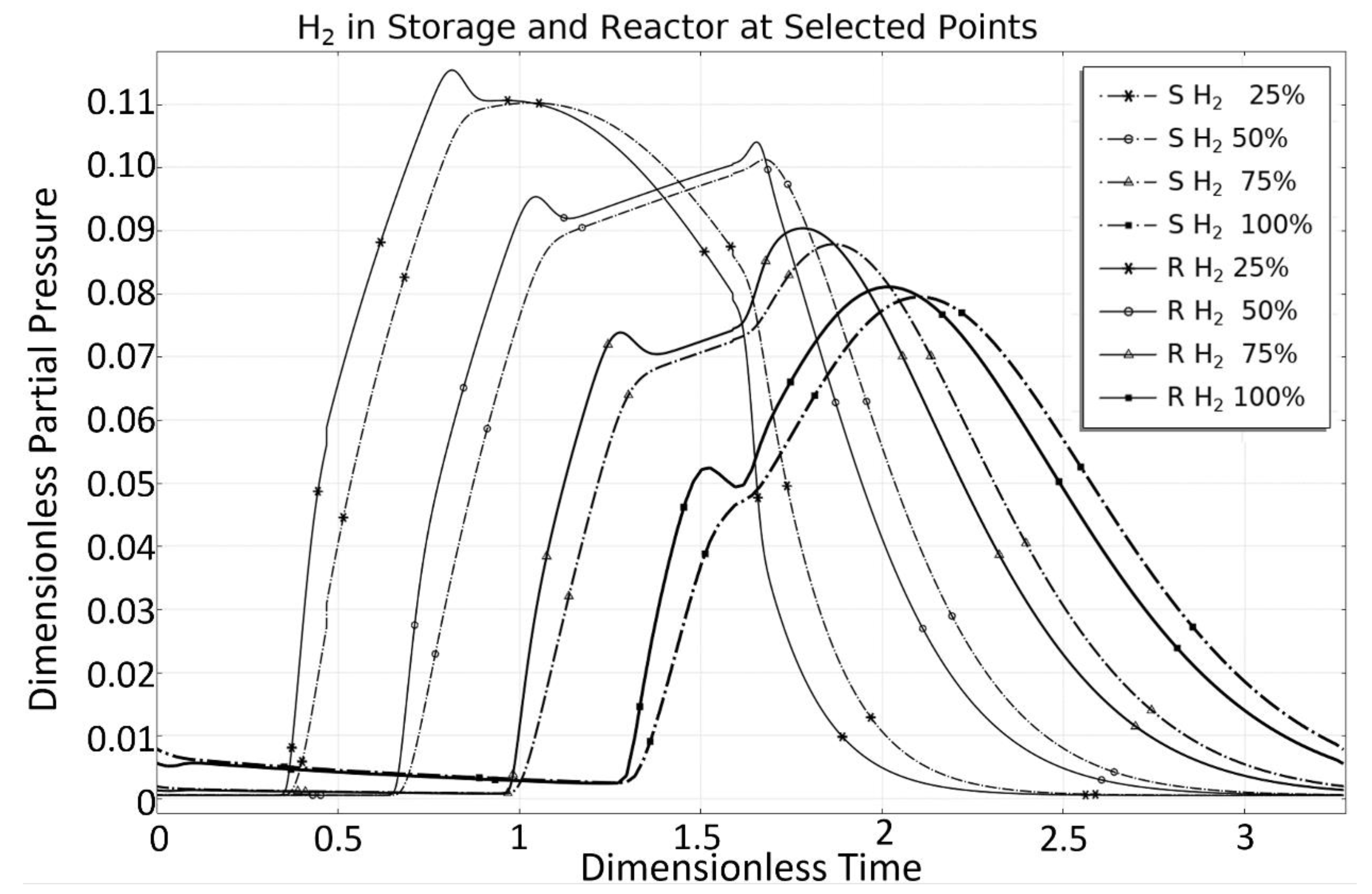
3. Steam Methane Reforming (SMR) Case Study
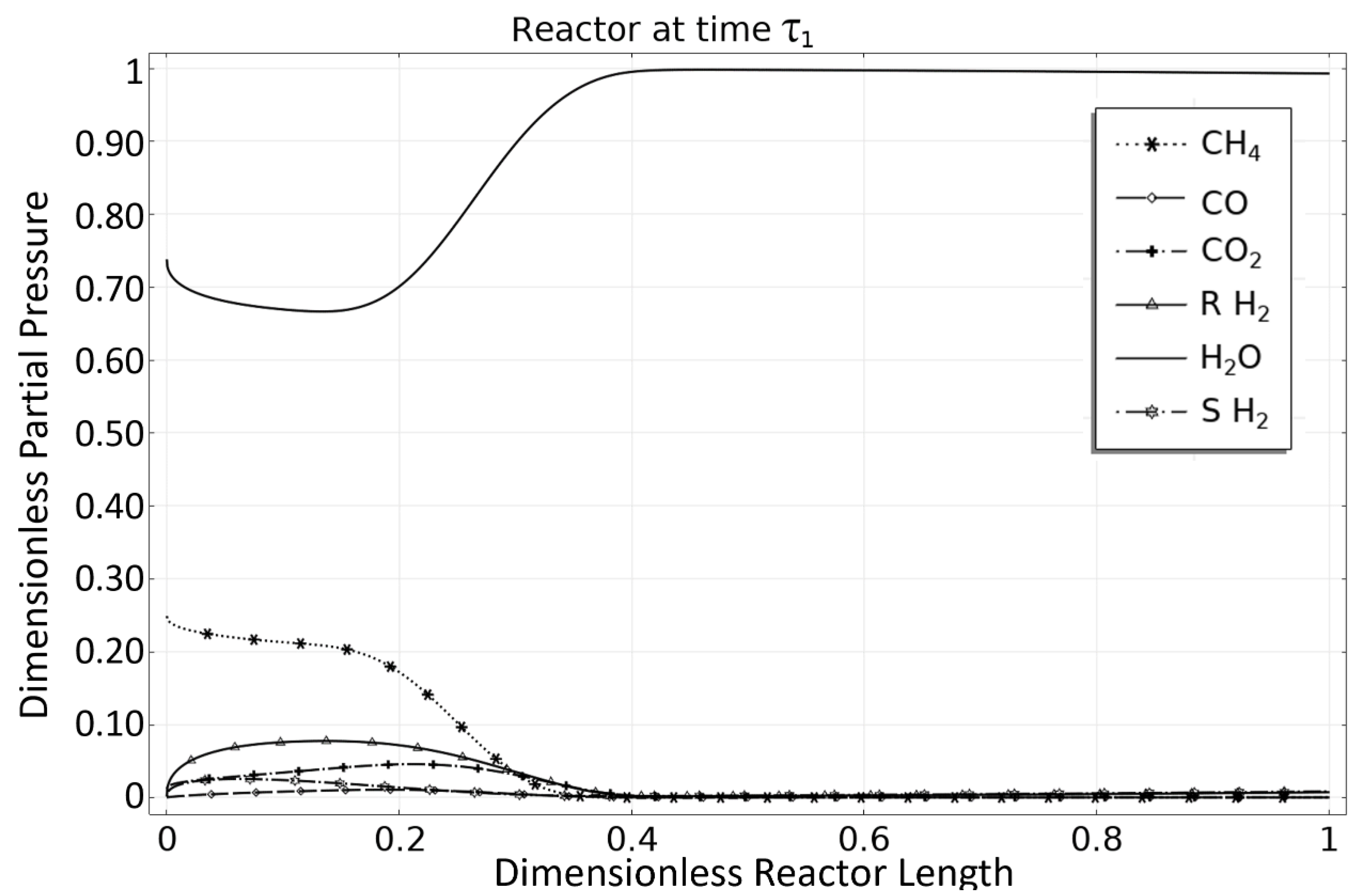
3.1. OM 1: MSR Loading-Reaction/Storage Phase
3.2. OM 2: MSR Decarbonization/Maintenance Phase
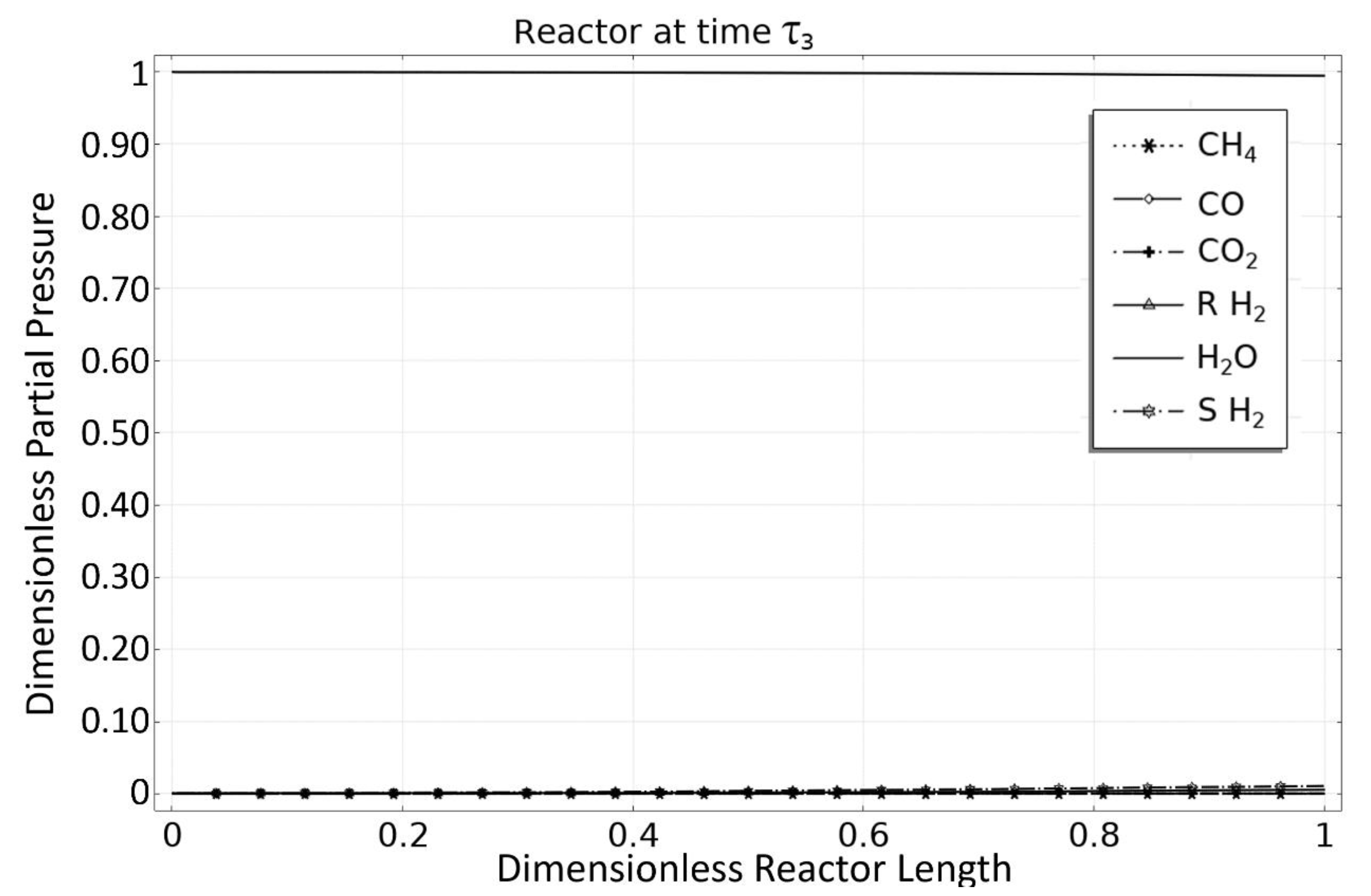
3.3. Phase 3: MSR Unloading-Production/Emptying Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| English Symbols | |
| Verhulst function parameter. | |
| Reactor cross section area | |
| Verhulst function parameter. | |
| species concentration in gas phase of void and storage domains | |
| effective diffusivity | |
| binary diffusion coefficient | |
| Reference Damköhler number | |
| Pellet diameter | |
| species | |
| Reference axial molar flowrate | |
| species | |
| operating mode (OM) specific Heaviside function. | |
| Rate coefficients for SMR reactions | |
| Equilibrium constants for SMR reactions | |
| Species adsorption constants for SMR reactions | |
| Reactor Length | |
| Characteristic length | |
| Number of species | |
| Number of reactor operating modes | |
| Operating mode | |
| species partial pressure in gas phase of void and storage domains | |
| species dimensionless partial pressure in gas phase of void and storage domains | |
| Ratio of inlet partial pressure for species i for operating mode k-1, based on operating mode k = 1 | |
| Ratio of inlet partial pressure for species i for operating mode k, based on operating mode k = 1 | |
| Reference pressure | |
| Peclet number for convective to diffusive mass transport | |
| Peclet number for membrane to convective transport | |
| species reaction-based generation rate | |
| species dimensionless reaction-based generation rate | |
| Reference reaction generation rate | |
| species produced during all OM’s | |
| SMR reaction | |
| SMR reaction | |
| Universal Gas Constant | |
| Limiting reactant used in performance metric calculations | |
| species into the gas phase of the voids domain due to transport from the gas phase in the storage domain | |
| species into the gas phase of the storage domain due to transport from the gas phase in the voids domain | |
| Time | |
| Dimensionless time | |
| Reference time, chosen as the residence time | |
| Temperature in all reactor domains | |
| Total reactor volume | |
| effective velocity | |
| gas velocity in reactor void domain | |
| Reference velocity, chosen as gas inlet velocity during OM 1 | |
| Dimensionless gas velocity in reactor void domain | |
| over all OM’s | |
| Verhulst function for switching between inlet boundary conditions during OM change. | |
| Reactor axial coordinate | |
| Reactor dimensionless axial coordinate | |
| Geek Symbols | |
| Storage-void domain interfacial area per unit volume of reactor system | |
| species permeance through storage medium permselective layer | |
| Volume fractions of voids, catalyst, storage, gas phase in storage domain, and solid phase in storage domain | |
| Catalyst effectiveness factor | |
| Dimensionless number quantifying membrane permeation to convection (inverse Peclet) | |
| Catalyst pellet density | |
| over limiting reactant fed throughout all OM’s | |
| species produced during all OMs over limiting reactant fed throughout all OM’s | |
| operating mode | |
| operating mode | |
References
- Mannan, M.S.; Reyes-Valdes, O.; Jain, P.; Tamim, N.; Ahammad, M. The Evolution of Process Safety: Current Status and Future Direction. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 135–162. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Moulijn, J.A. Re-Engineering the Chemical Processing Plant: Process Intensification; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Baldea, M. From process integration to process intensification. Comput. Chem. Eng. 2015, 81, 104–114. [Google Scholar] [CrossRef]
- Basile, A.; Campanari, S.; Manzolini, G.; Iulianelli, A.; Longo, T.; Liguori, S.; De Falco, M.; Piemonte, V. Methane steam reforming in a Pd–Ag membrane reformer: An experimental study on reaction pressure influence at middle temperature. Int. J. Hydrogen Energy 2011, 36, 1531–1539. [Google Scholar] [CrossRef]
- Asprion, N.; Kaibel, G. Dividing wall columns: Fundamentals and recent advances. Chem. Eng. Process. Process Intensif. 2010, 49, 139–146. [Google Scholar] [CrossRef]
- LeViness, S.; Deshmukh, S.R.; Richard, L.A.; Robota, H.J. Velocys Fischer–Tropsch Synthesis Technology—New Advances on State-of-the-Art. Top. Catal. 2014, 57, 518–525. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, L.; Xu, D.; Ciora, R.; Liu, P.K.; Manousiouthakis, V.I.; Tsotsis, T.T. A carbon molecular sieve membrane-based reactive separation process for pre-combustion CO2 capture. J. Membr. Sci. 2020, 605, 118028. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, L.; Xu, D.; Parsley, D.; Ciora, R.; Liu, P.K.; Manousiouthakis, V.I.; Tsotsis, T.T. A reactive separation process for pre-combustion CO2 capture employing oxygen-blown coal gasifier off-gas. Chem. Eng. J. 2021, 420 Pt 2, 127694. [Google Scholar] [CrossRef]
- Ponce-Ortega, J.M.; Al-Thubaiti, M.M.; El-Halwagi, M.M. Process intensification: New understanding and systematic approach. Chem. Eng. Process. Process Intensif. 2012, 53, 63–75. [Google Scholar] [CrossRef]
- Sharma, S.; Rangaiah, G.P. An improved multi-objective differential evolution with a termination criterion for optimizing chemical processes. Comput. Chem. Eng. 2013, 56, 155–173. [Google Scholar] [CrossRef]
- Carvalho, A.; Matos, H.A.; Gani, R. SustainPro—A tool for systematic process analysis, generation and evaluation of sustainable design alternatives. Comput. Chem. Eng. 2013, 50, 8–27. [Google Scholar] [CrossRef]
- Li, M. Thermodynamic analysis of adsorption enhanced reforming of ethanol. Int. J. Hydrogen Energy 2009, 34, 9362–9372. [Google Scholar] [CrossRef]
- Karagöz, S.; Tsotsis, T.T.; Manousiouthakis, V.I. Multi-scale model based design of membrane reactor/separator processes for intensified hydrogen production through the water gas shift reaction. Int. J. Hydrogen Energy 2020, 45, 7339–7353. [Google Scholar] [CrossRef]
- Da Cruz, F.E.; Karagöz, S.; Manousiouthakis, V.I. Parametric Studies of Steam Methane Reforming Using a Multiscale Reactor Model. Ind. Eng. Chem. Res. 2017, 56, 14123–14139. [Google Scholar] [CrossRef]
- Da Cruz, F.E.; Manousiouthakis, V.I. Process Intensification of Multipressure Reactive Distillation Networks Using Infinite Dimensional State-Space (IDEAS). Ind. Eng. Chem. Res. 2019, 58, 5968–5983. [Google Scholar] [CrossRef]
- Pichardo, P.A.; Manousiouthakis, V.I. Intensified energetically enhanced steam methane reforming through the use of membrane reactors. AIChE J. 2020, 66, e16827. [Google Scholar] [CrossRef]
- Lowd, J.; Tsotsis, T.T.; Manousiouthakis, V.I. On process intensification through storage reactors: A case study on methane steam reforming. Comput. Chem. Eng. 2020, 133, 106601. [Google Scholar] [CrossRef]
- Dabir, S.; Deng, W.; Sahimi, M.; Tsotsis, T. Fabrication of silicon carbide membranes on highly permeable supports. J. Membr. Sci. 2017, 537, 239–247. [Google Scholar] [CrossRef]
- Huysmans, M.; Dassargues, A. Review of the use of Péclet numbers to determine the relative importance of advection and diffusion in low permeability environments. Hydrogeol. J. 2005, 13, 895–904. [Google Scholar] [CrossRef]
- Horseman, S.T.; Higgo, J.J.W.; Alexander, J.; Harrington, J.F. Water, Gas and Solute Movement through Argillaceous Media; Nuclear Energy Agency of the OECD (NEA), Organization for Economic Co-Operation and Development: Paris, France, 1996. [Google Scholar]
- Carapellucci, R.; Milazzo, A. Membrane systems for CO2 capture and their integration with gas turbine plants. Proc. Inst. Mech. Eng. Part A J. Power Energy 2003, 217, 505–517. [Google Scholar] [CrossRef]
- Caravella, A.; Hara, S.; Drioli, E.; Barbieri, G. Sieverts law pressure exponent for hydrogen permeation through Pd-based membranes: Coupled influence of non-ideal diffusion and multicomponent external mass transfer. Int. J. Hydrogen Energy 2013, 38, 16229–16244. [Google Scholar] [CrossRef]
- Elyassi, B.; Sahimi, M.; Tsotsis, T.T. A novel sacrificial interlayer-based method for the preparation of silicon carbide membranes. J. Membr. Sci. 2008, 316, 73–79. [Google Scholar] [CrossRef]
- Alavi, M.; Eslamloueyan, R.; Rahimpour, M.R. Multi Objective Optimization of a Methane Steam Reforming Reaction in a Membrane Reactor: Considering the Potential Catalyst Deactivation due to the Hydrogen Removal. Int. J. Chem. React. Eng. 2018, 16, 20170066. [Google Scholar] [CrossRef]
- Tsuru, T.; Yamaguchi, K.; Yoshioka, T.; Asaeda, M. Methane steam reforming by microporous catalytic membrane reactors. AIChE J. 2004, 50, 2794–2805. [Google Scholar] [CrossRef]
- Motamedhashemi, M.M.Y.; Egolfopoulos, F.; Tsotsis, T. Application of a flow-through catalytic membrane reactor (FTCMR) for the destruction of a chemical warfare simulant. J. Membr. Sci. 2011, 376, 119–131. [Google Scholar] [CrossRef]
- Israni, S.H.; Nair, B.K.R.; Harold, M.P. Hydrogen generation and purification in a composite Pd hollow fiber membrane reactor: Experiments and modeling. Catal. Today 2009, 139, 299–311. [Google Scholar] [CrossRef]
- Gokhale, Y.V.; Noble, R.D.; Falconer, J.L. Effects of reactant loss and membrane selectivity on a dehydrogenation reaction in a membrane-enclosed catalytic reactor. J. Membr. Sci. 1995, 103, 235–242. [Google Scholar] [CrossRef]
- Mohan, K.; Govind, R. Analysis of a cocurrent membrane reactor. AIChE J. 1986, 32, 2083–2086. [Google Scholar] [CrossRef]
- Wei, C.-L.; Chen, Y.-C.; Cheng, C.-C.; Kao, K.-S.; Cheng, D.-L.; Chung, C.-J. Highly Sensitive UV Sensors Based on SMR Oscillators. Procedia Eng. 2012, 36, 468–475. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.F.; Conesa, M.; Alhama, I.; Alhama, F.; Cánovas, M. Searching fundamental information in ordinary differential equations. Nondimensionalization technique. PLoS ONE 2017, 12, e0185477. [Google Scholar] [CrossRef]
- Abdollahi, M.; Yu, J.; Liu, P.K.T.; Ciora, R.; Sahimi, M.; Tsotsis, T.T. Ultra-pure hydrogen production from reformate mixtures using a palladium membrane reactor system. J. Membr. Sci. 2012, 390, 32–42. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Sosio, F.; Manzolini, G.; Campanari, S. Fixed bed membrane reactor for hydrogen production from steam methane reforming: Experimental and modeling approach. Int. J. Chem. React. Eng. 2015, 40, 7559–7567. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 5, pp. 1–117. [Google Scholar] [CrossRef]
- Said, S.A.M.; Simakov, D.S.A.; Waseeuddin, M.; Román-Leshkov, Y. Solar molten salt heated membrane reformer for natural gas upgrading and hydrogen generation: A CFD model. Solar Energy 2016, 124, 163–176. [Google Scholar] [CrossRef]








| Rate Coefficient or Adsorption Constant | Pre-Exponential Factor | Unit Pre-Exponential Factor | Activation Energy or Adsorption Enthalpy |
|---|---|---|---|
| Equilibrium Constant | Units |
|---|---|
| Trial | Da | Θ | |||
|---|---|---|---|---|---|
| 1 | 1 | 1 | 0.717543 | 1.00 | 0.674081 |
| 2 | 1 | 10 | 0.755321 | 1.00 | 2.261037 |
| 3 | 1 | 30 | 0.752953 | 1.00 | 2.049624 |
| 4 | 1 | 40 | 0.762492 | 1.00 | 1.941365 |
| 5 | 1 | 50 | 0.742631 | 1.00 | 1.814203 |
| 6 | 2 | 1 | 0.545116 | 1.00 | 0.367633 |
| 7 | 2 | 10 | 0.565994 | 1.00 | 2.509012 |
| 8 | 2 | 30 | 0.587789 | 1.00 | 1.998005 |
| 9 | 2 | 40 | 0.588387 | 1.00 | 1.8797 |
| 10 | 2 | 50 | 0.597422 | 1.00 | 1.754175 |
| 11 | 4 | 1 | 0.405848 | 1.00 | 0.24924 |
| 12 | 4 | 10 | 0.428648 | 1.00 | 2.455872 |
| 13 | 4 | 50 | 0.466553 | 1.00 | 1.713176 |
| 14 | 6 | 1 | 0.363228 | 1.00 | 0.222919 |
| 15 | 6 | 10 | 0.389041 | 1.00 | 2.457342 |
| 16 | 6 | 50 | 0.388379 | 1.00 | 1.76623 |
| Trial | Da | Θ | |||||
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0.268297 | 0.2205 | 0.925244 | 0.8505 | 0.133693 |
| 2 | 1 | 10 | 0.486804 | 0.2205 | 2.06218 | 0.8505 | 0.851397 |
| 3 | 1 | 30 | 0.51877 | 0.2205 | 2.172158 | 0.8505 | 0.874449 |
| 4 | 1 | 40 | 0.527766 | 0.2205 | 2.211969 | 0.8505 | 0.883636 |
| 5 | 1 | 50 | 0.557738 | 0.2205 | 2.317619 | 0.8505 | 0.895902 |
| 6 | 2 | 1 | 0.332334 | 0.2564 | 1.027561 | 0.9858 | 0.114506 |
| 7 | 2 | 10 | 0.555291 | 0.2564 | 2.311048 | 0.9858 | 0.828753 |
| 8 | 2 | 30 | 0.657883 | 0.2564 | 2.710915 | 0.9858 | 0.90206 |
| 9 | 2 | 40 | 0.683793 | 0.2564 | 2.797332 | 0.9858 | 0.917312 |
| 10 | 2 | 50 | 0.696967 | 0.2564 | 2.856135 | 0.9858 | 0.923089 |
| 11 | 4 | 1 | 0.406878 | 0. 2866 | 1.140335 | 1.102 | 0.122357 |
| 12 | 4 | 10 | 0.719437 | 0. 2866 | 2.918608 | 1.102 | 0.868964 |
| 13 | 4 | 50 | 0.900393 | 0. 2866 | 3.638564 | 1.102 | 0.958944 |
| 14 | 6 | 1 | 0.430822 | 0.2913 | 1.165753 | 1.131 | 0.122353 |
| 15 | 6 | 10 | 0.795136 | 0.2913 | 3.205293 | 1.131 | 0.884476 |
| 16 | 6 | 50 | 0.974497 | 0.2913 | 3.931581 | 1.131 | 0.972301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowd, J., III; Tsotsis, T.; Manousiouthakis, V.I. On Process Intensification through Membrane Storage Reactors. Separations 2021, 8, 195. https://doi.org/10.3390/separations8110195
Lowd J III, Tsotsis T, Manousiouthakis VI. On Process Intensification through Membrane Storage Reactors. Separations. 2021; 8(11):195. https://doi.org/10.3390/separations8110195
Chicago/Turabian StyleLowd, John, III, Theodore Tsotsis, and Vasilios I. Manousiouthakis. 2021. "On Process Intensification through Membrane Storage Reactors" Separations 8, no. 11: 195. https://doi.org/10.3390/separations8110195
APA StyleLowd, J., III, Tsotsis, T., & Manousiouthakis, V. I. (2021). On Process Intensification through Membrane Storage Reactors. Separations, 8(11), 195. https://doi.org/10.3390/separations8110195







