Recent Advances on the Development of Chemosensors for the Detection of Mercury Toxicity: A Review
Abstract
:1. Introduction
2. Types of Probing Agents for Mercury
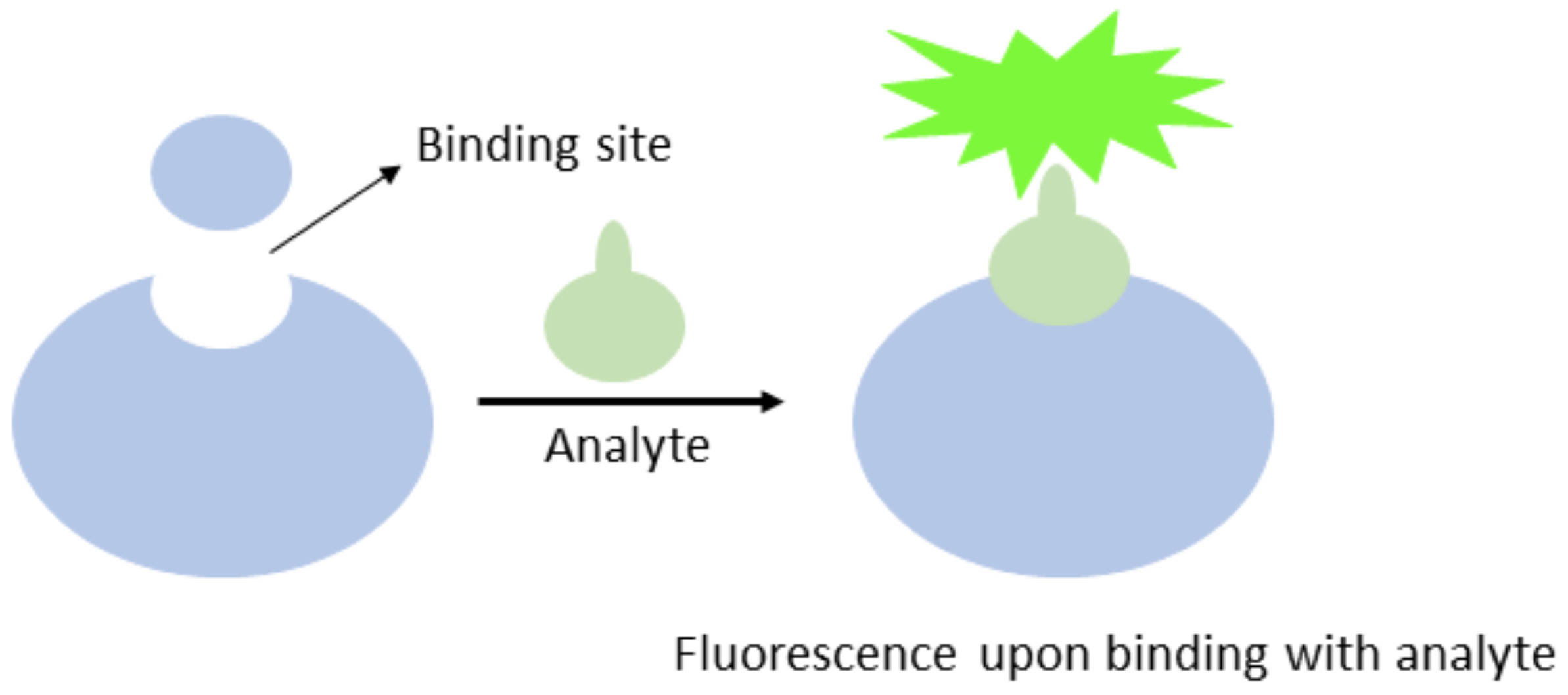
2.1. Chemosensors
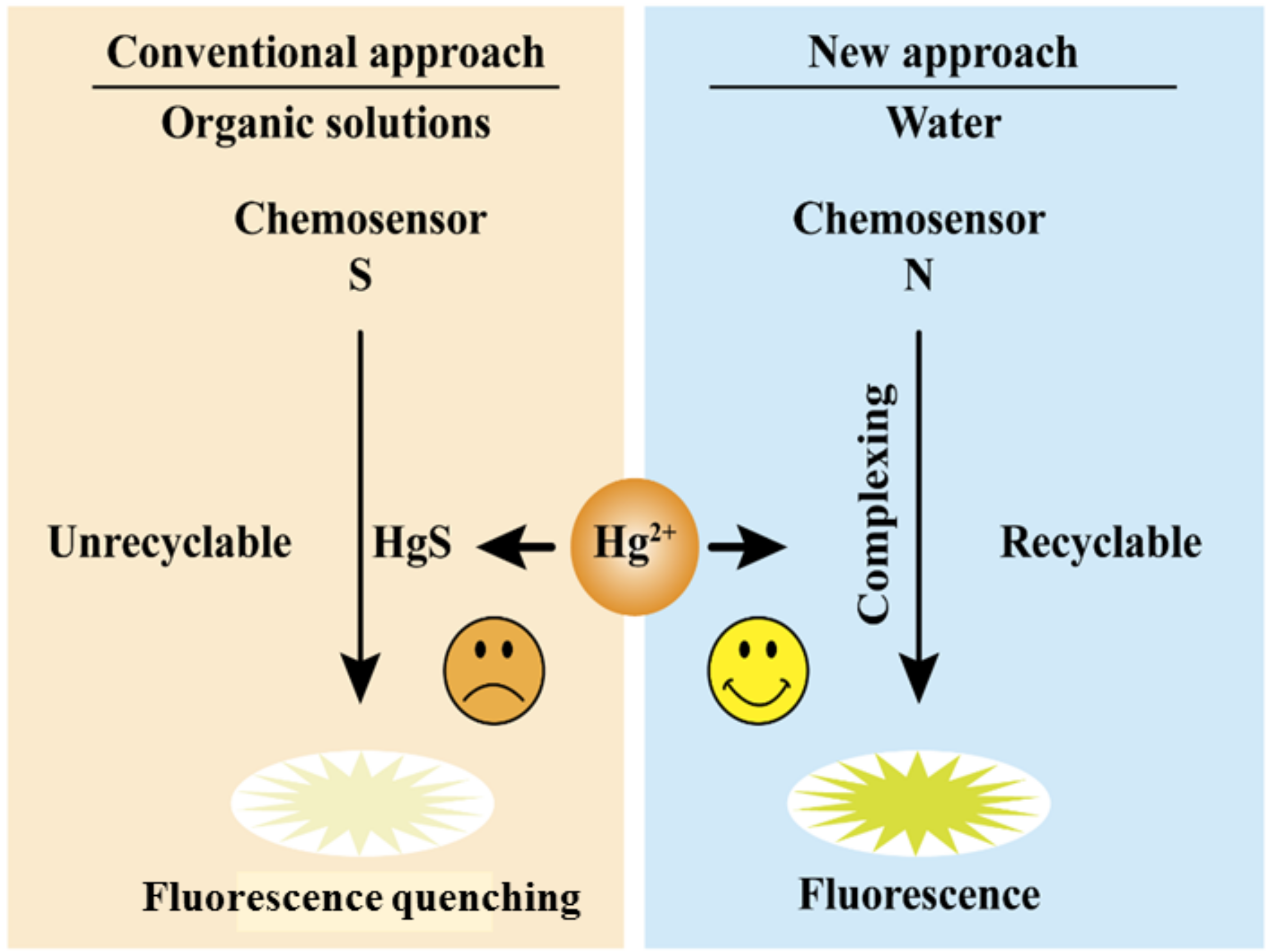
2.2. Mercury Chemosensors
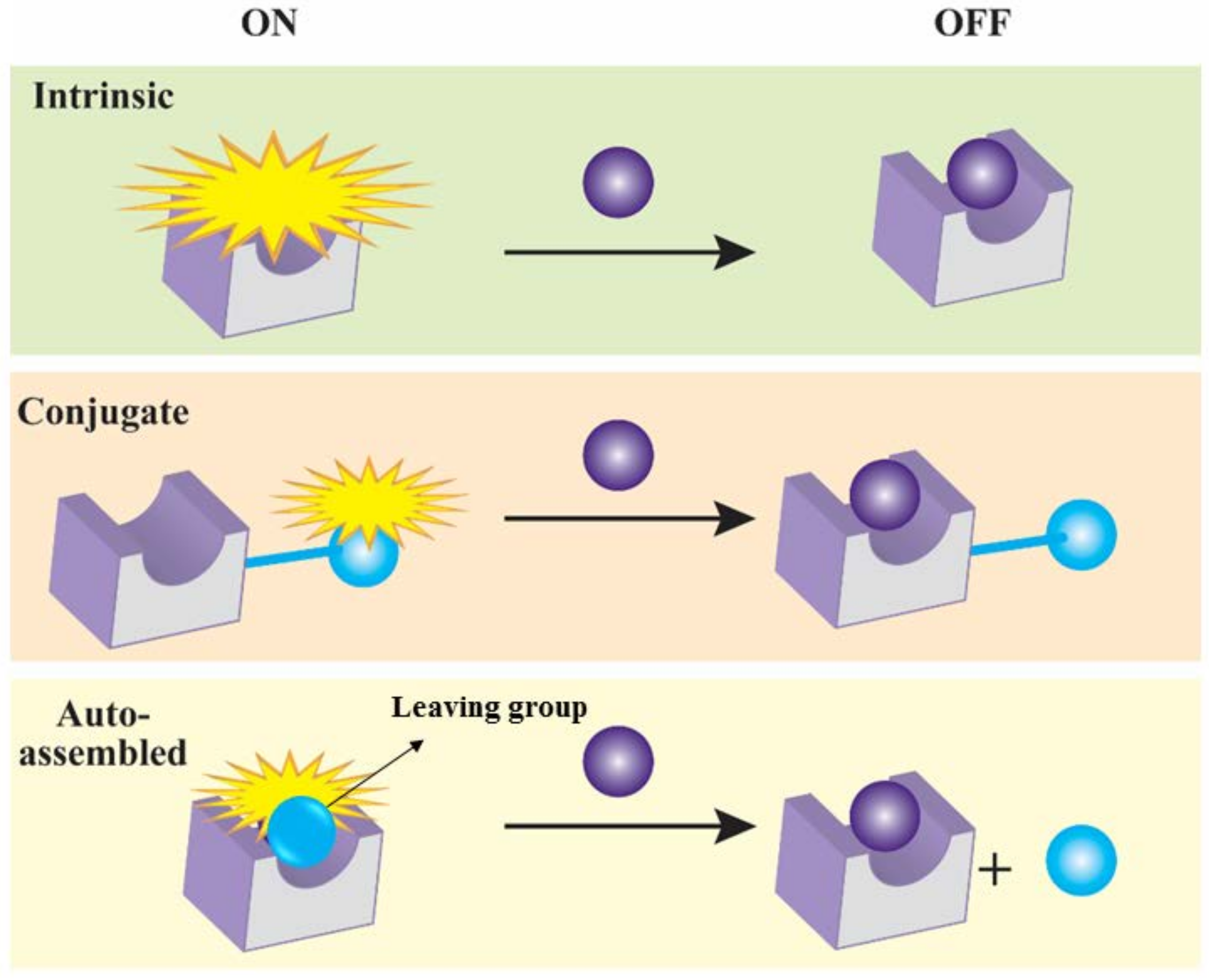
2.3. Types of Mercury Detecting Chemosensors
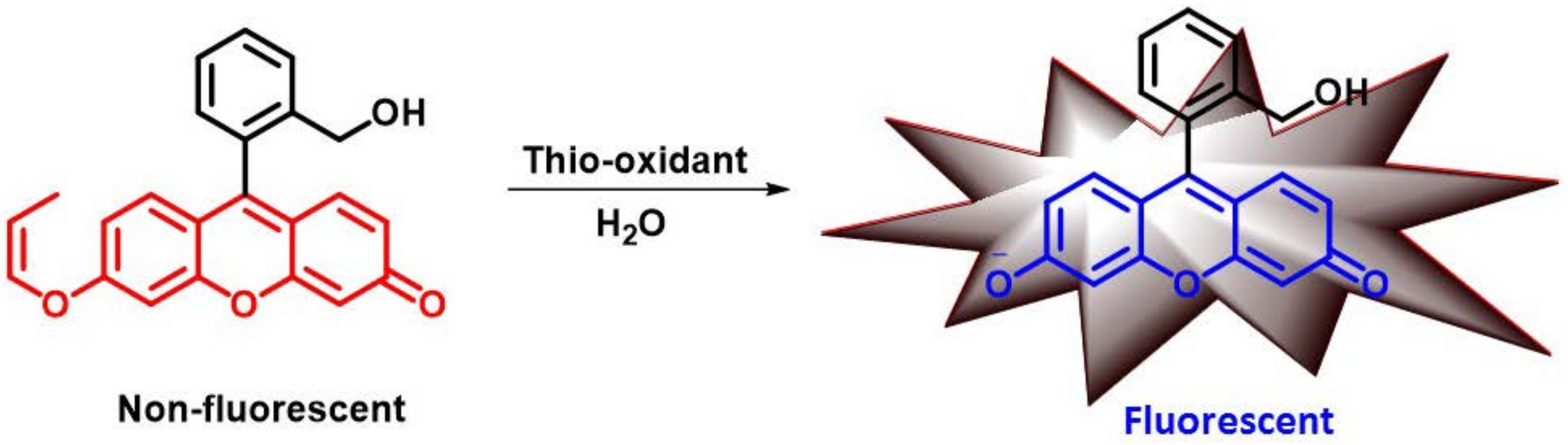
2.3.1. Molecular Sensors
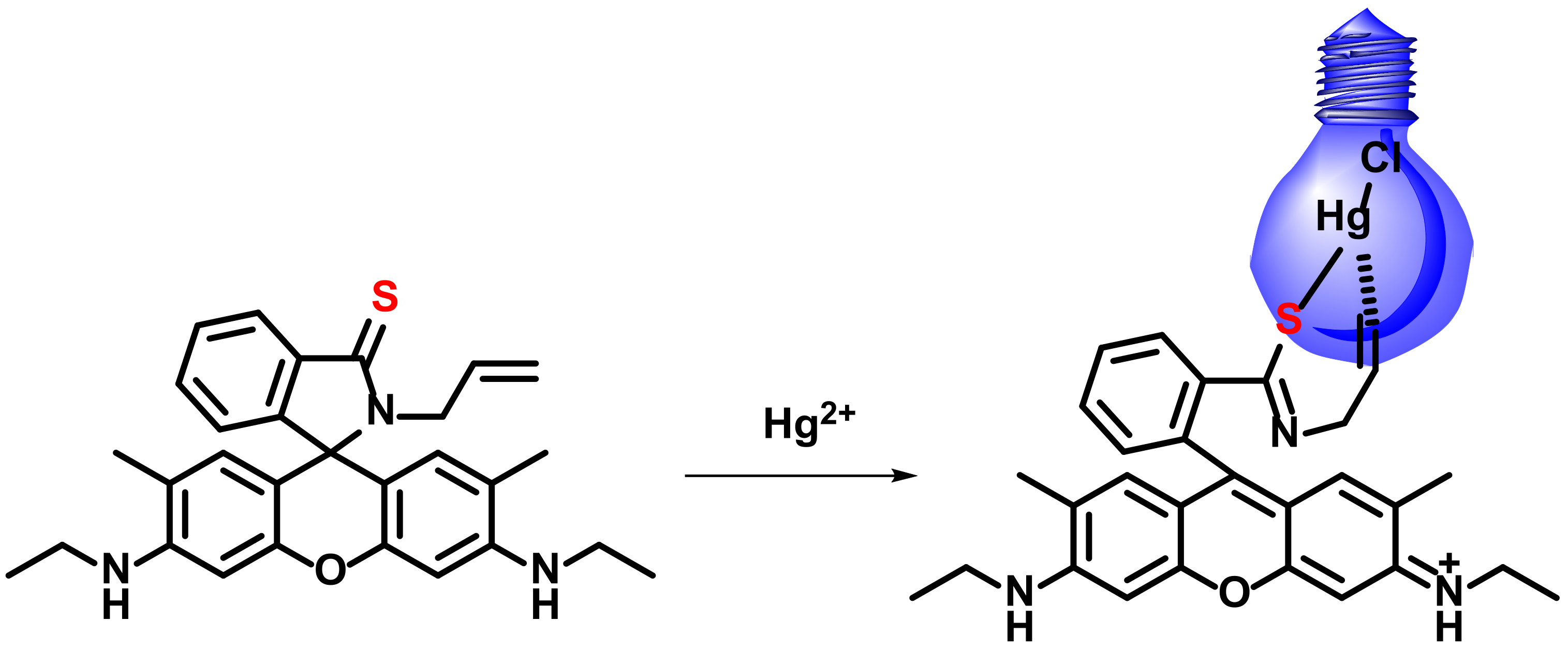
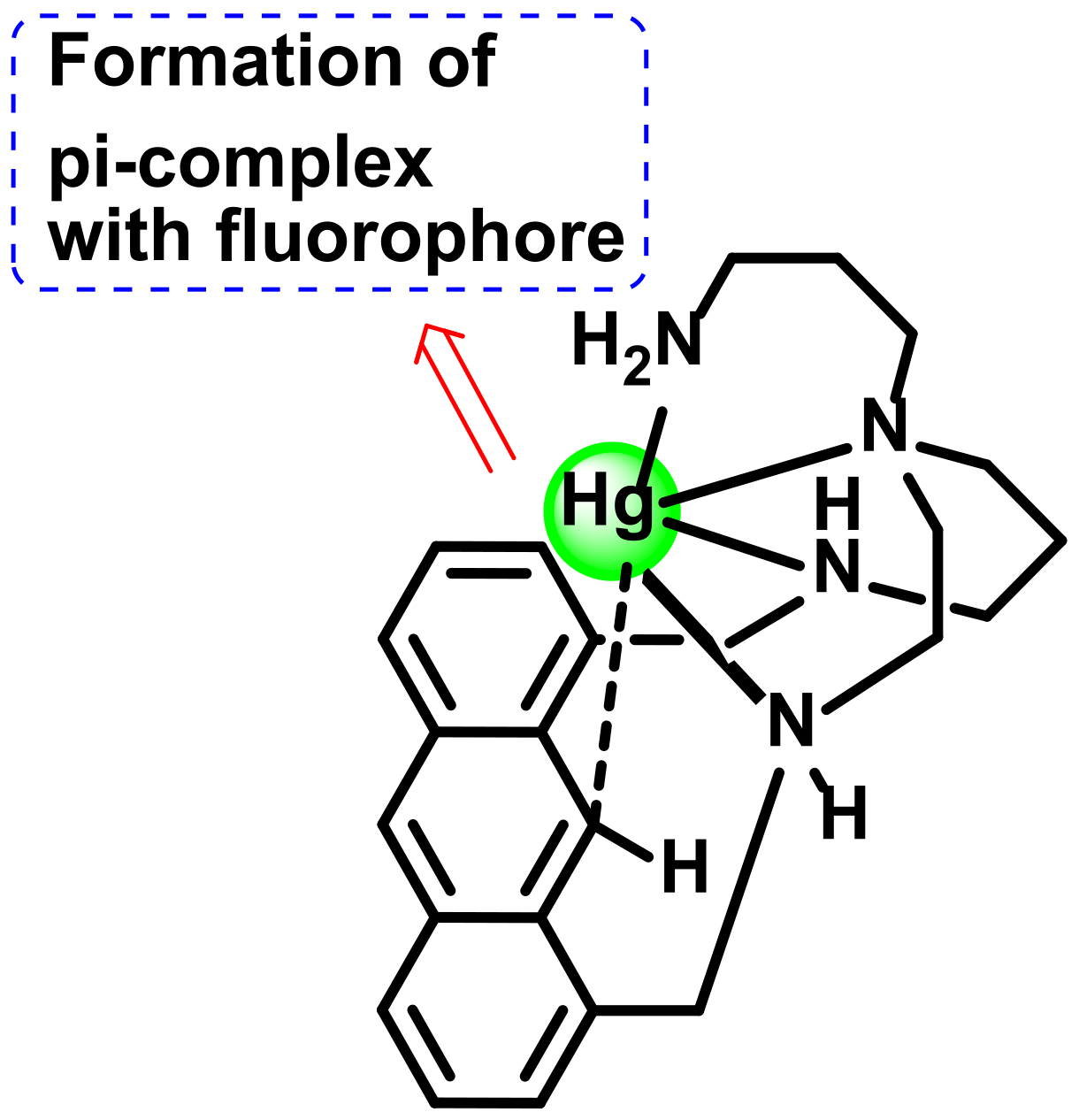
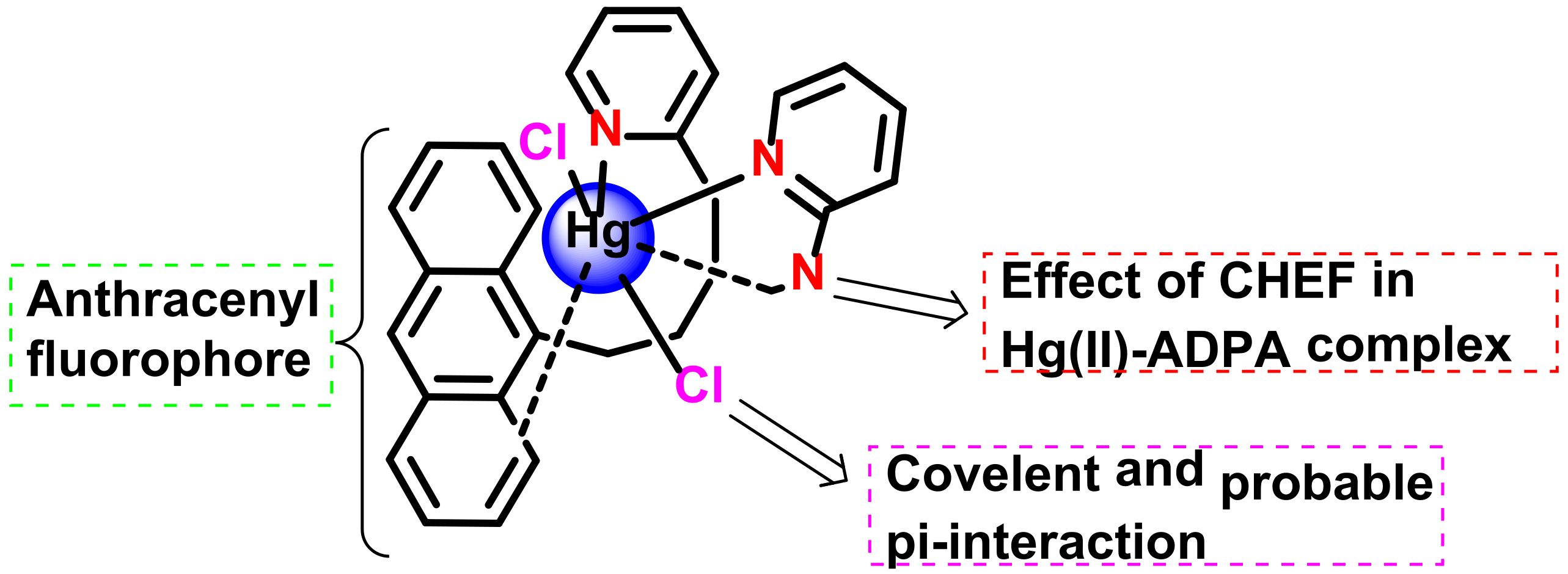
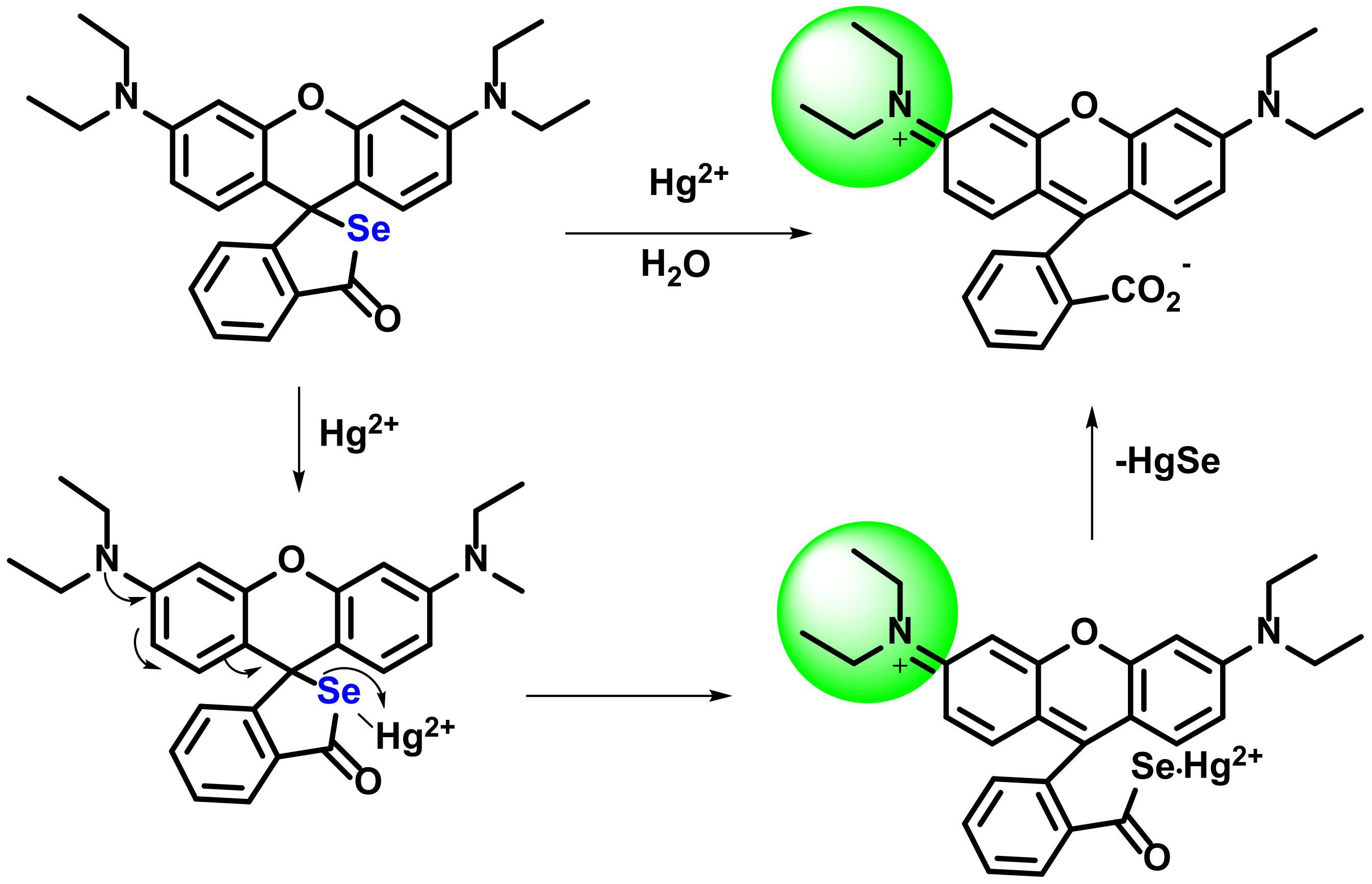
2.3.2. Formation of Hg-C Bond
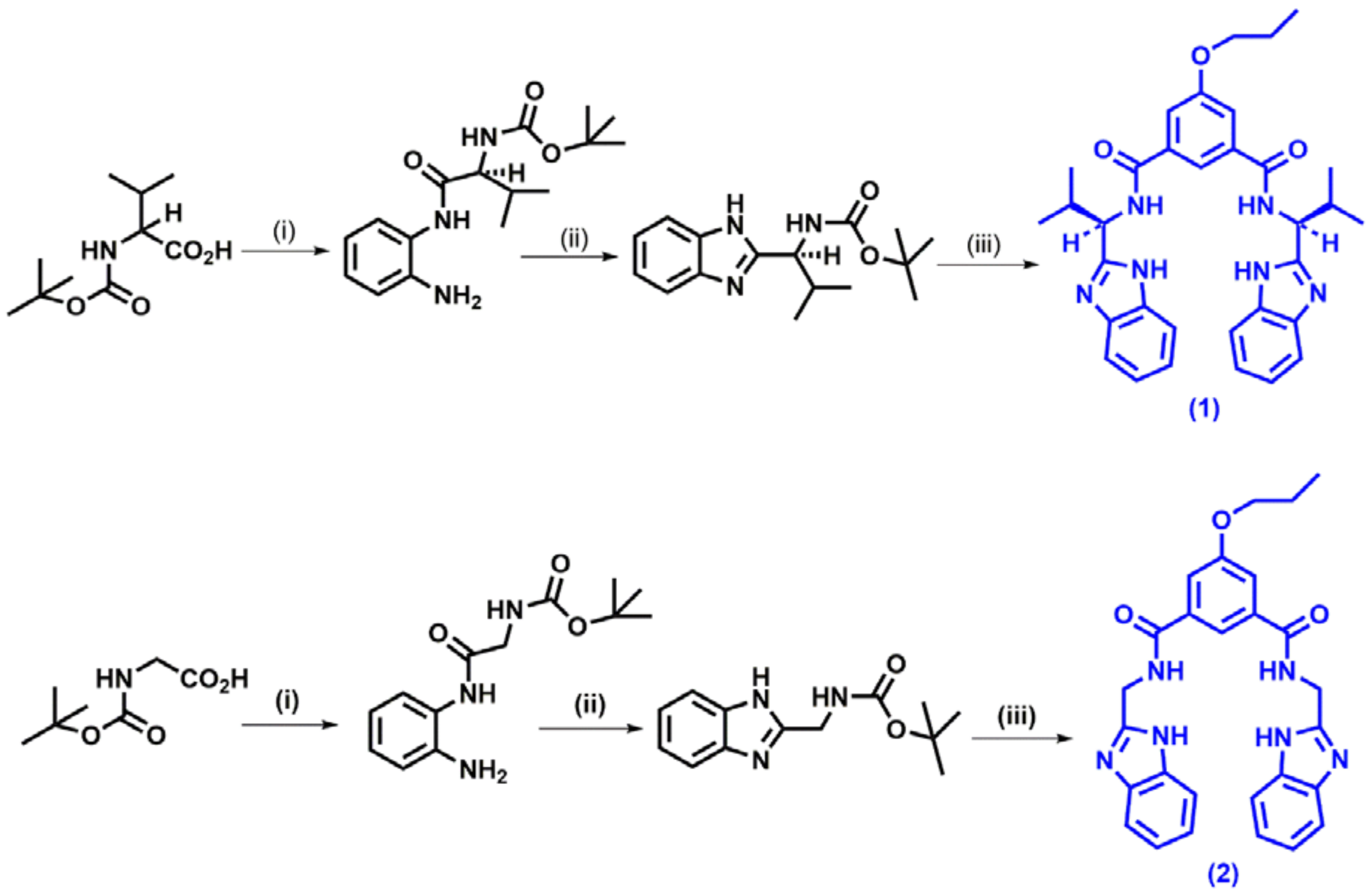
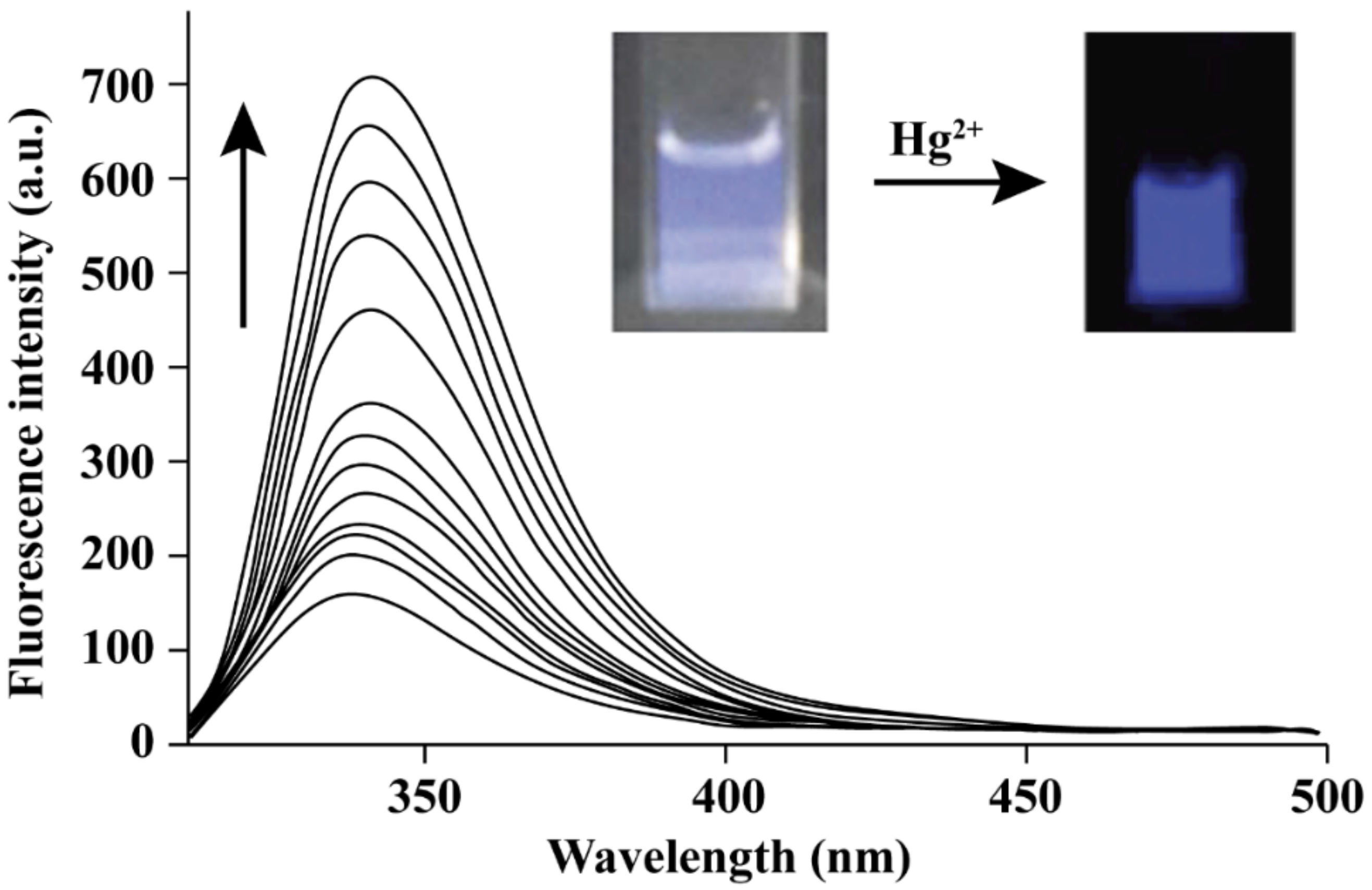
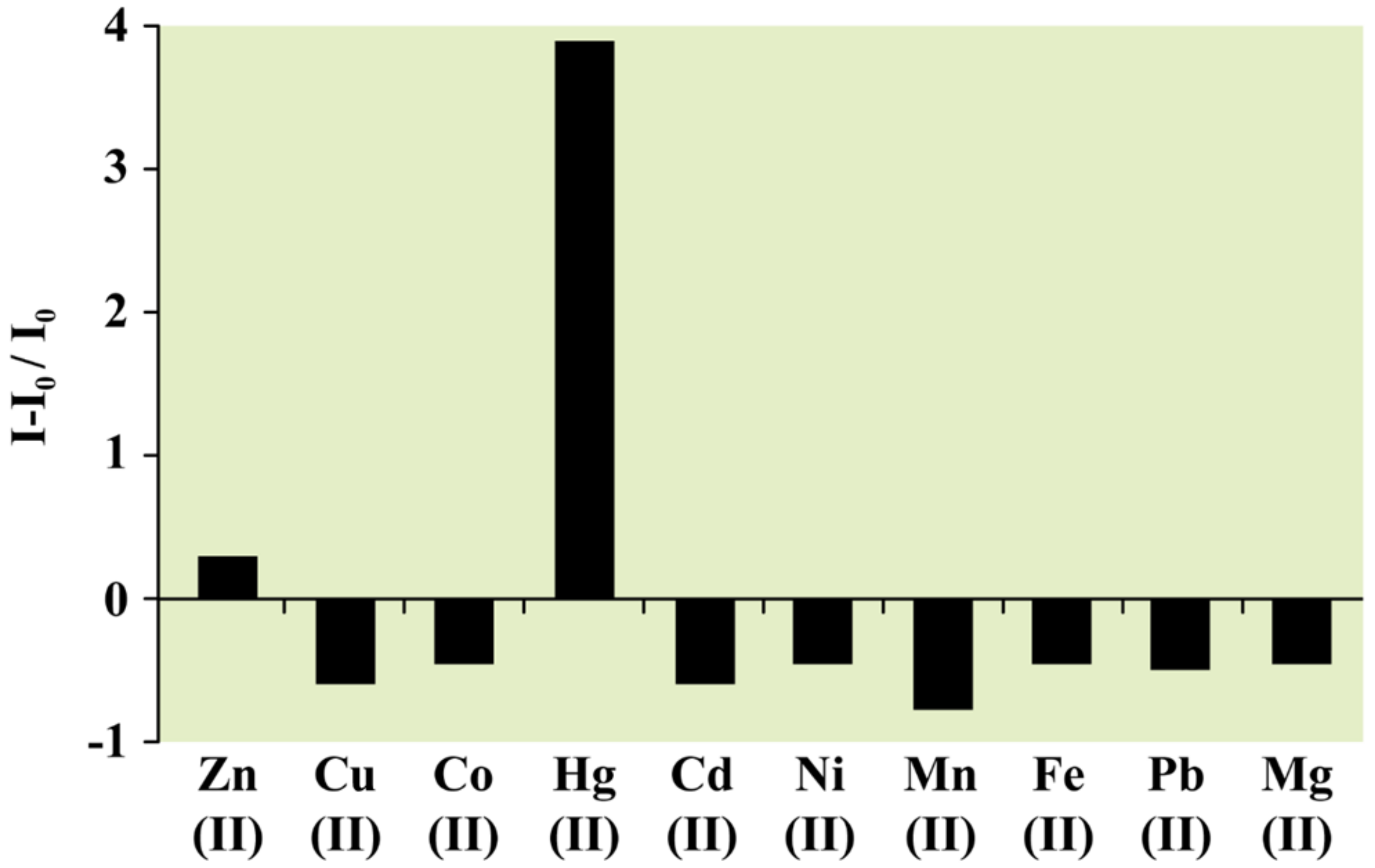
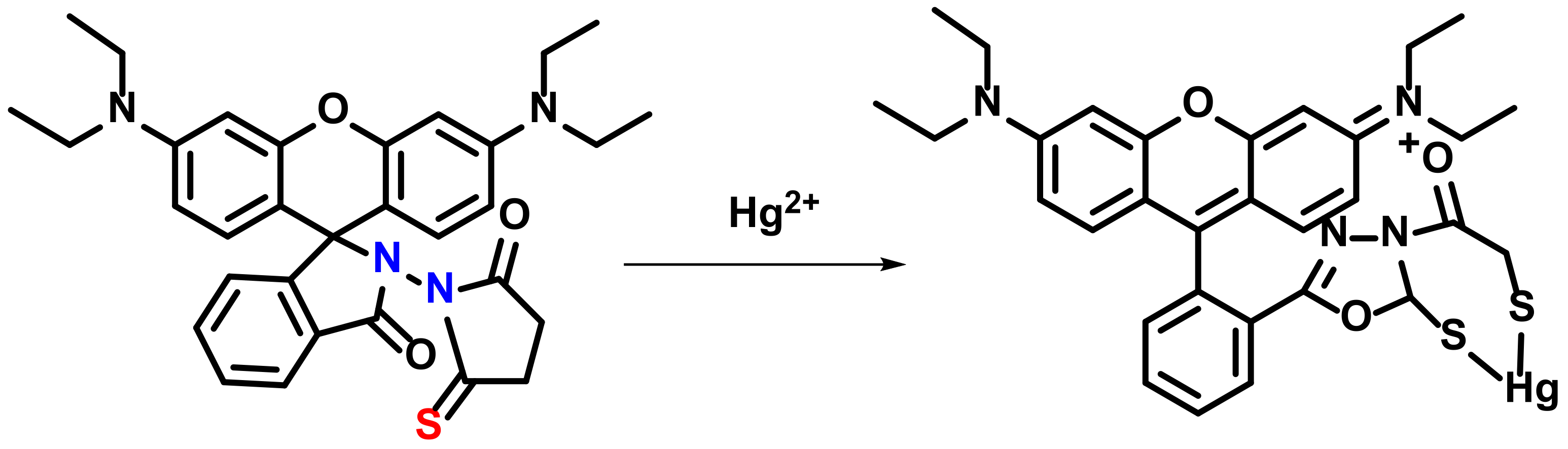
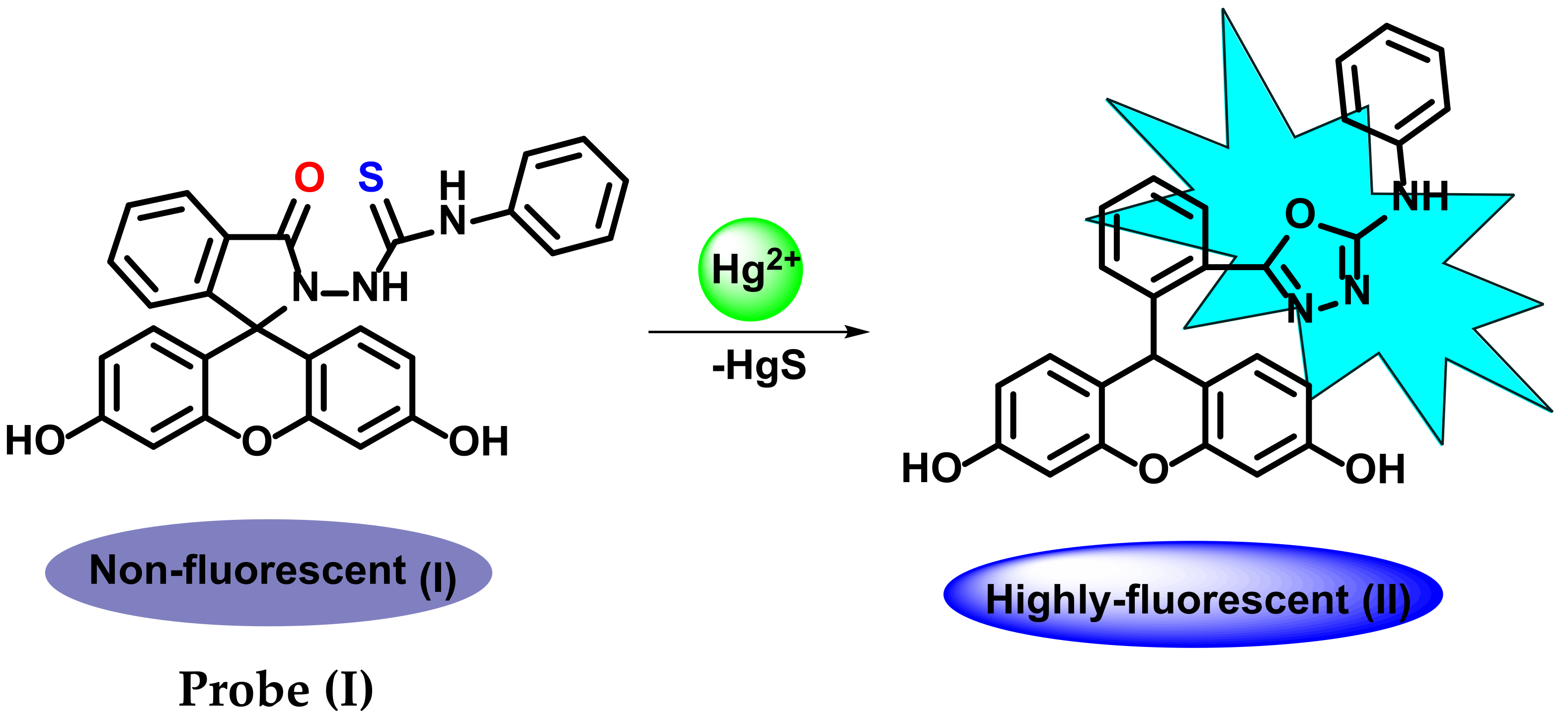
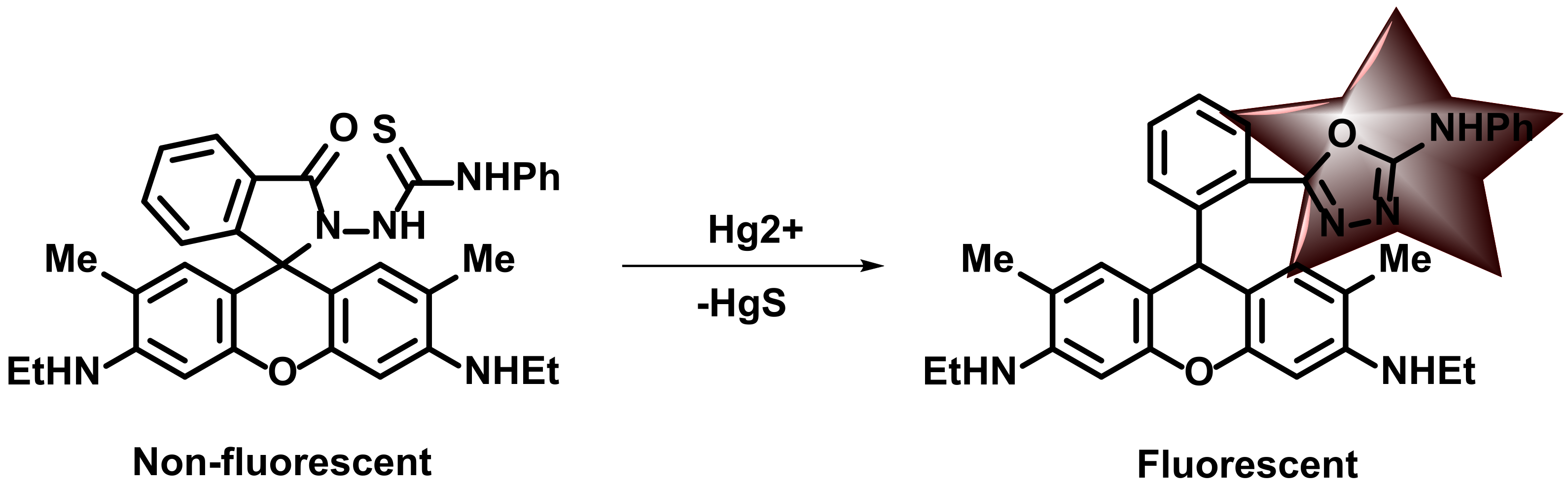
3. Hg2+ Detection via Amide Binding Site
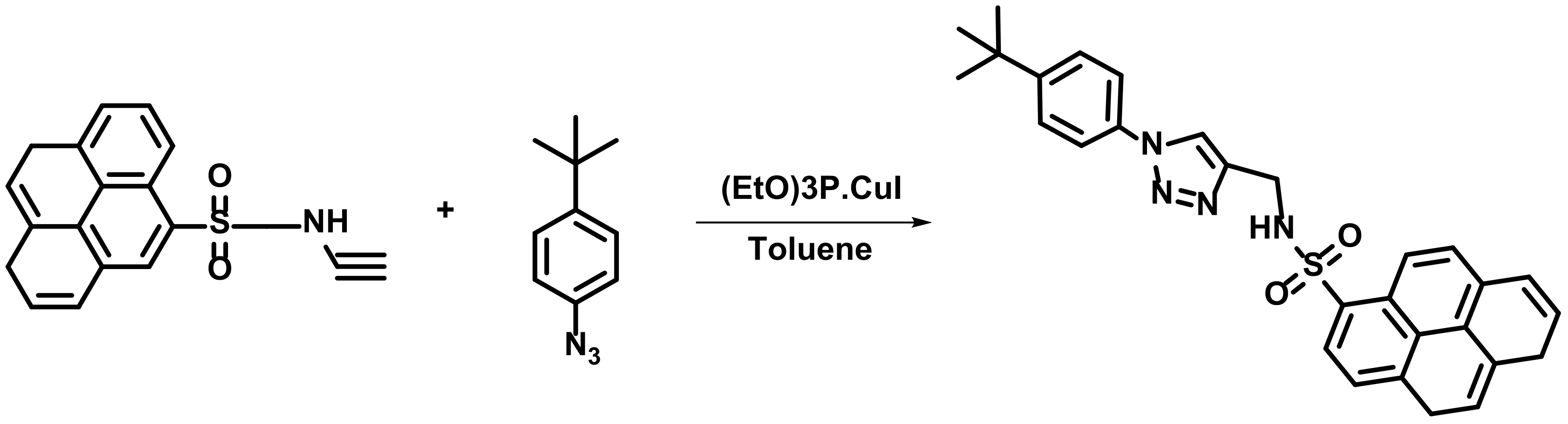
4. Hg2+ Detection through Sulfonamide Binding Site
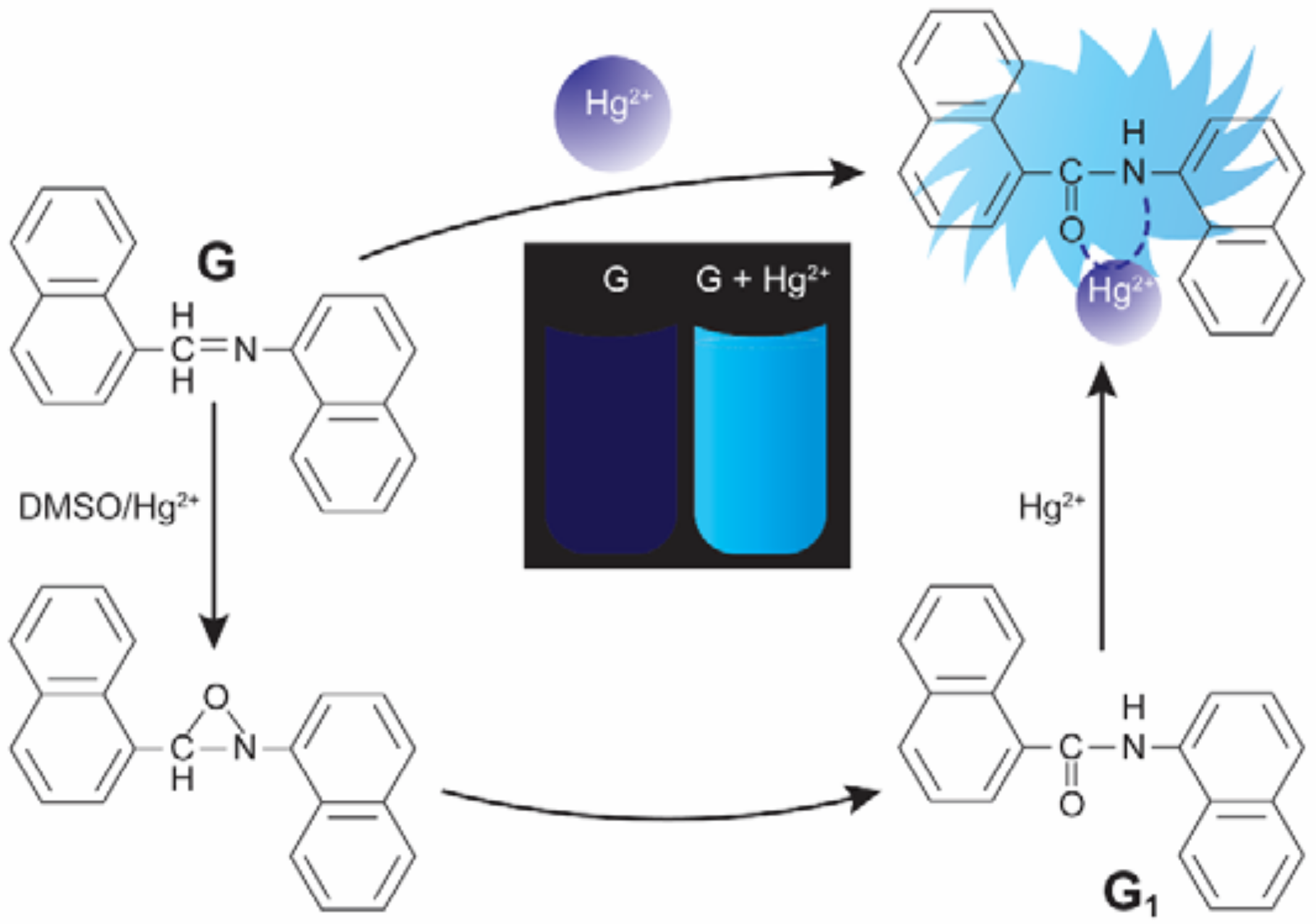
5. Schiff Base Ligands in Hg2+ Ion Detection
6. With Porphyrine Binding Group
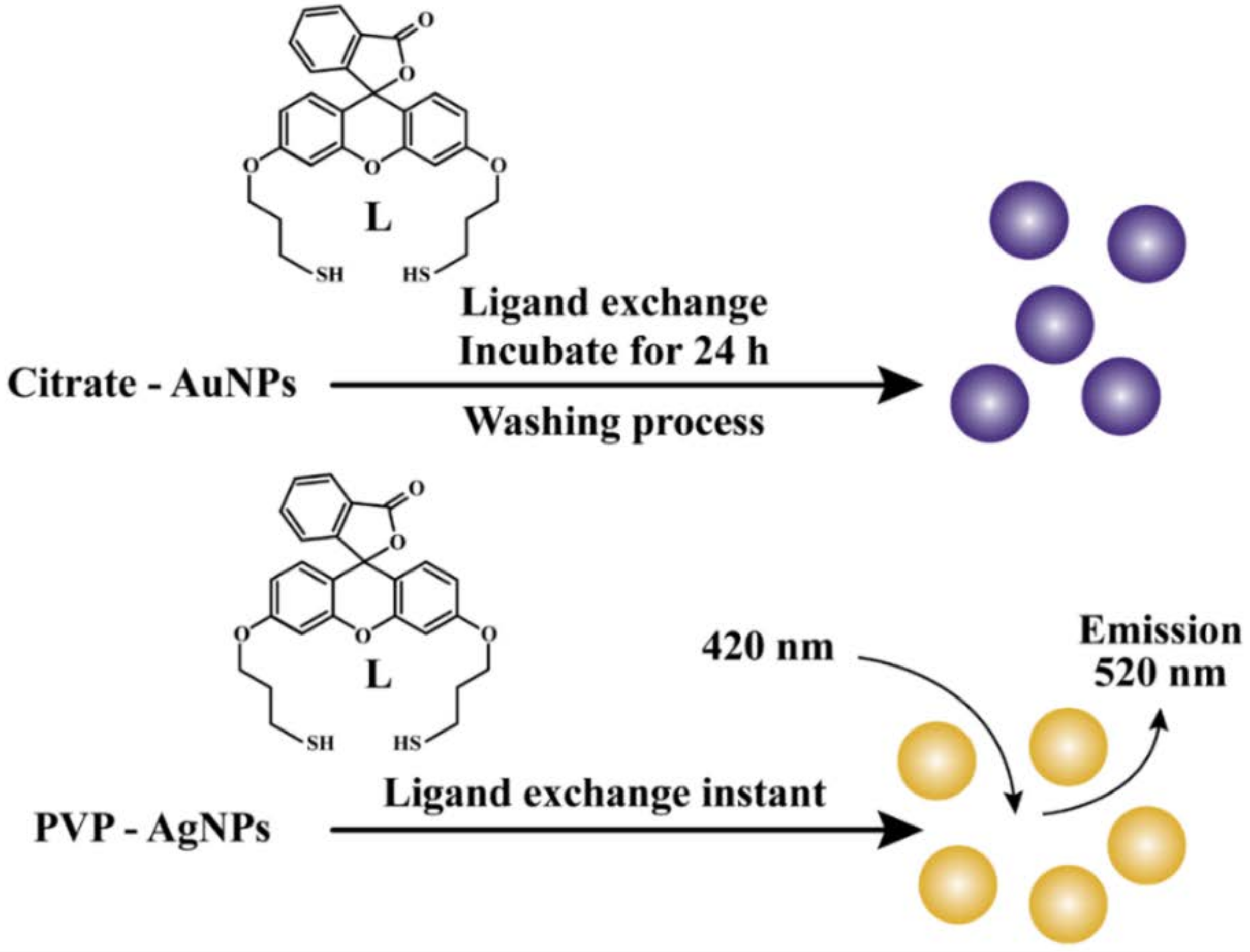
7. Nanostructures as Efficient Hg2+ Probes
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Momidia, B.K.; Tekuria, V.; Trivedi, D.R. Selective detection of mercury ions using benzothiazole based colorimetric chemosensor. Inorgan. Chem. Comm. 2016, 74, 1–5. [Google Scholar] [CrossRef]
- Bernard, V.; Isabelle, L. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar]
- Yu, Y.; Lin, L.R.; Yang, K.B.; Zhong, X.; Huang, R.B.; Zheng, L.S. A fluorescent probe for rapid detection of low concentration mercury ions and its application in biological cells. Talanta 2006, 69, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Amin-Zaki, L.; Elhassani, S.; Majeed, M.A.; Clarkson, T.W.; Doherty, R.A.; Greenwood, M. Intra-uterine methylmercury poisoning in Iraq. Pediatrics 1974, 54, 587–595. [Google Scholar] [PubMed]
- Liu, D.B.; Qu, W.S.; Chen, W.W.; Zhang, W.; Wang, Z.; Jiang, X.Y. Highly sensitive, colorimetric detection of mercury(II) in aqueous media by quaternary ammonium group-capped gold nanoparticles at room temperature. Anal. Chem. 2010, 82, 9606–9610. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, L.; Wang, H.; Ouyang, X.; Zheng, J.; Li, J.; Yang, R. Metal-induced aggregation of mononucleotides-stabilized gold nanoparticles: An efficient approach for simple and rapid colorimetric detection of Hg (II). Chem. Comm. 2011, 47, 6039–6041. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, L.; Zhu, D.; He, P.; Fang, Y.; Cheng, G. Nanomaterial-based optical sensors for mercury ions. Anal. Chim. Acta 2011, 694, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Jung-Duck, P.; Wei, Z. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar]
- Björkman, L.; Lundekvam, B.F.; Laegreid, T.; Bertelsen, B.I.; Morild, I.; Lilleng, P. Mercury in human brain, blood, muscle and toenails in relation to exposure: An autopsy study. Environ. Health 2007, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Cariccio, V.L.; Samà, A.; Bramanti, P.; Mazzon, E. Mercury and Alzheimer’s disease: A look at the links and evidence. Biol. Trace Elem. Res. 2019, 187, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Kevin, M.R.; Ernest, M.W., Jr.; Miaozong, W.; Chris, G.; Eric, R.B. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar]
- Byeong-Jin, Y.; Byoung-Gwon, K.; Man-Joong, J.; Se-Yeong, K.; Hawn-Cheol, K.; Tae-Won, J.; Hong-Jae, C.; Won-Jun, C.; Mi-Na, H.; Young-Seoub, H. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar]
- Ying, G.; Zeming, S.; Zhou, L.; Peng, W.; Chengbin, Z.; Xiandeng, H. Determination and speciation of mercury in environmental and biological samples by analytical atomic spectrometry. Microchem. J. 2012, 103, 1–14. [Google Scholar]
- Lakshmi, N.S.; Young-Kyo, S.; Sung-Ok, B. Speciation and determination of mercury by various analytical techniques. Rev. Anal. Chem. 2013, 32, 225–245. [Google Scholar]
- Qisong, Z.; Jian, Z.; Hujin, Z.; Chengyun, W.; Shen, Y. A novel near-infrared chemosensor for mercury ion detection based on DA structure of triphenylamine and benzothiadiazole. Tetrahedron 2017, 73, 2824–2830. [Google Scholar]
- Mahnaz, D.G.; Sergei, M.; Prashant, S.; Godwin, A.A.; Emad, L.I. Dual chemosensor for the rapid detection of mercury(II) pollution and biothiols. Analyst 2019, 144, 4908–4916. [Google Scholar]
- Qu, W.J.; Gao, G.Y.; Shi, B.B.; Wei, T.B.; Zhang, Y.M.; Lin, Q.; Yao, H. A highly selective and sensitive fluorescent chemosensor for mercury ions based on the mechanism of supramolecular self-assembly. Sens. Actuators B Chem. 2014, 204, 368–374. [Google Scholar] [CrossRef]
- Jia, H.; Yang, M.; Meng, Q.; He, G.; Wang, Y.; Hu, Z.; Zhang, R.; Zhang, Z. Synthesis and Application of an Aldazine-Based Fluorescence Chemosensor for the Sequential Detection of Cu2+ and Biological Thiols in Aqueous Solution and Living Cells. Sensors 2016, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Kyle, P.C.; Alexandra, M.Y.; Amy, E.P. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar]
- Bruno, B.C.; Manuel, A.; Beatriz, A.; Carmen, M.C.; Joaquim, C.G.; da Silva, E. Mercury(ii) sensing based on the quenching of fluorescence of CdS–dendrimer nanocomposites. RSC Adv. 2009, 134, 2447–2452. [Google Scholar]
- Prasenjit, M.; Sukdeb, S.; Priyadip, D.; Hridesh, A.; Amitava, D. An overview of the recent developments on Hg2+ recognition. RSC Adv. 2014, 4, 36140–36174. [Google Scholar]
- Yang, X.F.; Li, Y.; Bai, Q. A highly selective and sensitive fluorescein-based chemodosimeter for Hg2+ ions in aqueous media. Anal. Chim. Acta 2006, 584, 95–100. [Google Scholar] [CrossRef]
- Guiqiu, C.; Zhi, G.; Guangming, Z.; Lin, T. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400–5443. [Google Scholar]
- Lin, W.Y.; Cao, X.W.; Ding, Y.D.; Yuan, L.; Yu, Q.X. A reversible fluorescent Hg2+ chemosensor based on a receptor composed of a thiol atom and an alkene moiety for living cell fluorescence imaging. Org. Biomol. Chem. 2010, 8, 3618–3620. [Google Scholar] [CrossRef]
- Wei, G.; Bart, J.S.; Joseph, H.R.; Robert, D.H. A fluorescent ligand rationally designed to be selective for zinc(II) over larger metal. Inorg. Chim. Acta 2005, 358, 3958–3966. [Google Scholar]
- Michael, E.H.; Engin, U.A.; Anthony, W.C. Chelation enhanced fluorescence detection of non-metal ions. JACS 1989, 111, 8735–8737. [Google Scholar]
- Engin, U.A.; Michael, E.H.; Anthony, W.C. Spectral shifts in acid-base chemistry. 1. van der Waals contributions to acceptor numbers. JACS 1990, 112, 3590–3593. [Google Scholar]
- Hyunjung, L.; Hee-Seung, L.; Joseph, H.R.; Robert, D.H. Mechanism of “Turn-on” Fluorescent Sensors for Mercury(II) in Solution and Its Implications for Ligand Design. Inorg. Chem. 2012, 51, 10904–10915. [Google Scholar]
- Andrea, B.; Vito, L. 1,10-Phenanthroline: A versatile building block for the construction of ligands for various purpose. Coord. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar]
- Parthiban, V.; Natesan, T.; Shu-Pao, W. A rhodamine-based chemosensor with diphenylselenium for highly selective fluorescence turn-on detection of Hg2+ in vitro and in vivo. RSC Adv. 2017, 7, 21733–21739. [Google Scholar]
- Cesar, S.H.; Olalla, C.L.; Arnan, M.; Laura, M.L. Advanced Evanescent-Wave Optical Biosensors for the Detection of Nucleic Acids: An Analytic Perspective. Front. Chem. 2019, 7, 724. [Google Scholar]
- Sudibya, H.G.; He, Q.; Zhang, H.; Chen, P. Electrical de-tection of metal ions using field-effect transistors based on micropatterned reduced graphene oxide films. ACS Nano 2011, 5, 1990–1994. [Google Scholar] [CrossRef]
- Marimuthuu, P.; Umamahesh, B.; Baskaran, S.; Sathiyanarayanan, K.I.; Venkatachalapathy, B.; Ravichandran, C.; Karthikeyan, N.S. Development of paper-based chemosensor for the detection of mercury ions using mono- and tetra-sulfur bearing phenanthridines. New J. Chem. 2018, 42, 8530–8536. [Google Scholar]
- Ando, S.; Koide, K. Development and Applications of Fluorogenic Probes for Mercury(II) Based on Vinyl Ether Oxymercuration. JACS 2011, 133, 2556–2566. [Google Scholar] [CrossRef] [Green Version]
- Krishnendu, P.; Priyabrata, S.; Dipankar, B. Semi-quantitative colorimetric and supersensitive electrochemical sensors for mercury using rhodamine b hydrazide thio derivative. J. Mol. Liq. 2019, 276, 141–152. [Google Scholar]
- Sung-Kyun, K.; Young-Keun, Y.; Jinsung, T.; Injae, S. In Vivo Monitoring of Mercury Ions Using a Rhodamine-Based Molecular Probe. JACS 2006, 128, 14150–14155. [Google Scholar]
- Qian-Yong, C.; Yuan-Ming, H.; Hong-Ming, W.; Yu, X. A new pyrenyl-appended triazole for fluorescent recognition of Hg2+ ion in aqueous solution. Dye. Pigment. 2013, 99, 798–802. [Google Scholar]
- Tai-bao, W.; Guo-ying, G.; Wen-juan, Q.; Bing-bing, S.; Qi, L.; Hong, Y.; You-ming, Z. Selective fluorescent sensor for mercury(II) ion based on an easy to prepare double naphthalene Schiff base. Sens. Actuators B Chem. 2014, 199, 142–147. [Google Scholar]
- Chun-Yan, L.; Xiao-Bing, Z.; Li, Q.; Yan, Z.; Chun-Mei, H.; Shuang-Yan, H.; Li-Min, L.; Li-Xin, J.; Guo-Li, S.; Ru-Qin, Y. Naphthalimide−Porphyrin Hybrid Based Ratiometric Bioimaging Probe for Hg2+: Well-Resolved Emission Spectra and Unique Specificity. Anal. Chem. 2009, 81, 9993–10001. [Google Scholar]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotech. 2004, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Lei, D.; Chong, W.; Lan, M.; Sai-Feng, X.; Zhu, T. Highly sensitive chemosensor for Cu(II) and Hg(II) based on the tripodal rhodamine receptor. Sens. Actuators B Chem. 2009, 141, 506–510. [Google Scholar]
- Da-Hye, K.; Junho, S.; Hyunsook, L.; Keun-Hyeung, L. Ratiometric fluorescence detection of Hg(II) in aqueous solutions at physiological pH and live cells with a chemosensor based on tyrosine. Sens. Actuators B Chem. 2014, 196, 421–428. [Google Scholar]
- Nuriman, B.K.; Willem, V. Selective chemosensor for Hg(II) ions based on tris[2 -(4-phenyldiazenyl)phenylaminoethoxy]cyclotriveratrylene in aqueous samples. Anal. Chim. Acta 2009, 655, 75–79. [Google Scholar] [CrossRef]
- Zhuo, W.; Deqing, Z.; Daoben, Z. A sensitive and selective “turn on” fluorescent chemosensor for Hg(II) ion based on a new pyrene–thymine dyad. Anal. Chim. Acta 2005, 549, 10–13. [Google Scholar]
- Hossein, A.Z.; Elaheh, R. A novel chemosensor based on graphitic carbon nitride quantum dots and potassium ferricyanide chemiluminescence system for Hg(II) ion detection. Sens. Actuators B Chem. 2016, 225, 258–266. [Google Scholar]
- Xiaohong, P.; Yujiao, W.; Xiaoliang, T.; Weisheng, L. Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles as selectivity-enhanced chemosensor for Hg(II). Dye. Pigment. 2011, 91, 26–32. [Google Scholar]
- Nai-bo, Z.; Jian-jun, X.; Chen-guang, X. Core–shell structured mesoporous silica nanoparticles equipped with pyrene-based chemosensor: Synthesis, characterization, and sensing activity towards Hg(II). J. Lumin. 2011, 131, 2021–2025. [Google Scholar]
- Venkatesan, M.; Sathiyanarayanan, K. Twisted pyrene with perfect hetero atomic cavity optical sensor for Hg22+ and Pb2+. Inorgan. Chem. Comm. 2020, 121, 108187. [Google Scholar]
- Nguyen, K.H.; Nguyen, C.B.; Nguyen, T.A.N.; Nguyen, T.T. Pham Cam Nam, Tran Duong, Jong Seung Kim, Duong Tuan Quang, A highly sensitive fluorescent chemosensor for simultaneous determination of Ag(I), Hg(II), and Cu(II) ions: Design, synthesis, characterization and application. Dye. Pigment. 2015, 116, 89–96. [Google Scholar]
- Juyoung, Y.; Norman, E.O.; David, H.V.; Wade, D.A.; Anthony, W.C. A fluorescent chemosensor signalling only Hg(II) and Cu(II) in water. Tetrahedron. Lett. 1997, 38, 3845–3848. [Google Scholar]
- Ali, C.; Deniz, Y.M.; Engin, U.A. Bis(2-pyridyl)-Substituted Boratriazaindacene as an NIR-Emitting Chemosensor for Hg(II). Org. Lett. 2007, 9, 607–609. [Google Scholar]
- Zhi-Xiang, H.; Hong-Yuan, L.; Xiao-Bing, Z.; Rong-Mei, K.; Guo-Li, S.; Ru-Qin, Y. A ratiometric chemosensor for fluorescent determination of Hg2+ based on a new porphyrin-quinoline dyad. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 1084–1088. [Google Scholar]
- Reham, A.; Ibrahim, A.I.A.; Sabri, M.; Fahad, M.A.; Sayed, M.S. An effective optical chemosensor film for selective detection of mercury ions. J. Mol. Liq. 2021, 336, 116122. [Google Scholar]
- Buddhadeb, S.; Manjira, M.; Siddhartha, P.; Koushik, D.; Sushil Kumar, M.; Anisur, R.K.; Pabitra, C. A water soluble FRET-based ratiometric chemosensor for Hg(II) and S2− applicable in living cell staining. RSC Adv. 2014, 4, 14919–14927. [Google Scholar]
- Xu, D.; Yu, S.; Yin, Y.; Wang, S.; Lin, Q.; Yuan, Z. Sensitive Colorimetric Hg2+ Detection via Amalgamation-Mediated Shape Transition of Gold Nanostars. Front. Chem. 2018, 6, 566. [Google Scholar] [CrossRef] [PubMed]
- Zihao, D.; Wenying, J.; Qiaobo, Y.; Jinkun, H.; Ziheng, H.; Haiyan, F.; Yali, Y.; Jianmei, Z.; Jinfang, N.; Yun, Z. Ultrasensitive visual detection of Hg2+ ions via the Tyndall effect of gold nanoparticles. Chem. Comm. 2021, 57, 2613–2616. [Google Scholar]
- Tao, Y.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Poly(acrylic acid)-templated silver nanoclusters as a platform for dual fluorometric turn-on and colorimetric detection of mercury (II) ions. Talanta 2012, 88, 290–294. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Wang, Y.; Zhang, D.; Shi, X.; Dong, F.; Yu, K.; Lin, Q.; Yang, B. Fluorescent silver nanoclusters as effective probes for highly selective detection of mercury(II) at parts-per-billion levels. Chem—Asian J. 2012, 7, 1652–1656. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.; Zhang, L.; Huang, H.; Liu, F.; Liu, X. Synthesis of biocompatible AuAgS/Ag2S nanoclusters and their applications in photocatalysis and mercury detection. J. Nanopar Res. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Zhang, N.; Si, Y.; Sun, Z.; Chen, L.; Li, R.; Qiao, Y.; Wang, H. Rapid, selective, and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold–silver nanoclusters with “silver effect”-enhanced red fluorescence. Anal. Chem. 2014, 86, 11714–11721. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-N.; Guo, Y.-X. One-pot synthesis of dual-emitting BSA-Pt-Au bimetallic nanoclusters for fluorescence ratiometric detection of mercury ions and cysteine. Anal. Meth. 2015, 7, 5787–5793. [Google Scholar] [CrossRef]
- Koneswaran, R. Narayanaswamy, Mercaptoacetic acid capped CdS quantum dots as fluorescence single shot probe for mercury(II). Sens. Actuators B Chem. 2009, 139, 91–96. [Google Scholar] [CrossRef]
- Xie, W.Y.; Huang, W.T.; Luo, H.Q.; Li, N.B. CTAB-capped Mn-doped ZnS quantum dots and label-free aptamer for room-temperature phosphorescence detection of mercury ions. Analyst 2012, 137, 4651–4653. [Google Scholar] [CrossRef]
| Nanosensor | Probing Mechanism | Linear Range | Limit of Detection | Analytical Application | Reference |
|---|---|---|---|---|---|
| AgNCs | Hg2+-induced the changes of poly(acrylic acid)-templated the Ag NCs states. | 0–20 μM | 2 nM | River water and tap water | [58] |
| Au NCs | The addition of Hg2+ caused the aggregation-induced fluorescence quenching of glutathione-capped Ag NCs. | 0.1 nM–10 μM | 0.1 nM | Drinking water | [59] |
| AuAgS/Ag2S NCs | Hg2+ closed to the AuAgS/Ag2S NCs could form HgS resulting to the quenching of fluorescent nanoclusters. | - | 10−13 M | Fish samples | [60] |
| Au-Ag NCs | The fluorescence quenching of Au-AgNCs was resulted from the interactions between Hg2+ ions and Au of Au-AgNCs by metallophilic bonding of 5d10 centers. | 0.20–2500 nM | 0.10 nM | Blood samples | [61] |
| Pt-Au NCs | The d10-d10 metallophilic interaction between Hg2+ and Au+ induced the quenching of fluorescent BSA-Pt-Au NCs. | 0.5 nM–22 μM | 0.3 nM | Urine and serum | [62] |
| CdS QDs | Hg2+ could bind carboxyl and carbonyl groups on the surfaces of mercaptoacetic acid capped CdS QDs. | 5–400 nM | 4.2 nM | - | [63] |
| Mn-doped ZnS QDs | The bind between Hg2+ and thymine bases induced electron transfer quenching of the QDs. | 50–800 nM | 1.5 nM | Tap water | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kollur, S.P.; Shivamallu, C.; Prasad, S.K.; Veerapur, R.; Patil, S.S.; Cull, C.A.; Coetzee, J.F.; Amachawadi, R.G. Recent Advances on the Development of Chemosensors for the Detection of Mercury Toxicity: A Review. Separations 2021, 8, 192. https://doi.org/10.3390/separations8100192
Kollur SP, Shivamallu C, Prasad SK, Veerapur R, Patil SS, Cull CA, Coetzee JF, Amachawadi RG. Recent Advances on the Development of Chemosensors for the Detection of Mercury Toxicity: A Review. Separations. 2021; 8(10):192. https://doi.org/10.3390/separations8100192
Chicago/Turabian StyleKollur, Shiva Prasad, Chandan Shivamallu, Shashanka K. Prasad, Ravindra Veerapur, Sharanagouda S. Patil, Charley A. Cull, Johann F. Coetzee, and Raghavendra G. Amachawadi. 2021. "Recent Advances on the Development of Chemosensors for the Detection of Mercury Toxicity: A Review" Separations 8, no. 10: 192. https://doi.org/10.3390/separations8100192
APA StyleKollur, S. P., Shivamallu, C., Prasad, S. K., Veerapur, R., Patil, S. S., Cull, C. A., Coetzee, J. F., & Amachawadi, R. G. (2021). Recent Advances on the Development of Chemosensors for the Detection of Mercury Toxicity: A Review. Separations, 8(10), 192. https://doi.org/10.3390/separations8100192










