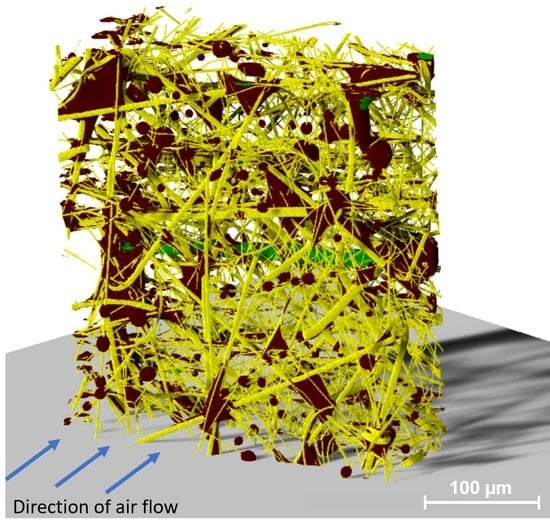
Identification of Deposited Oil Structures on Thin Porous Oil Mist Filter Media Applying µ-CT Imaging Technique
Abstract
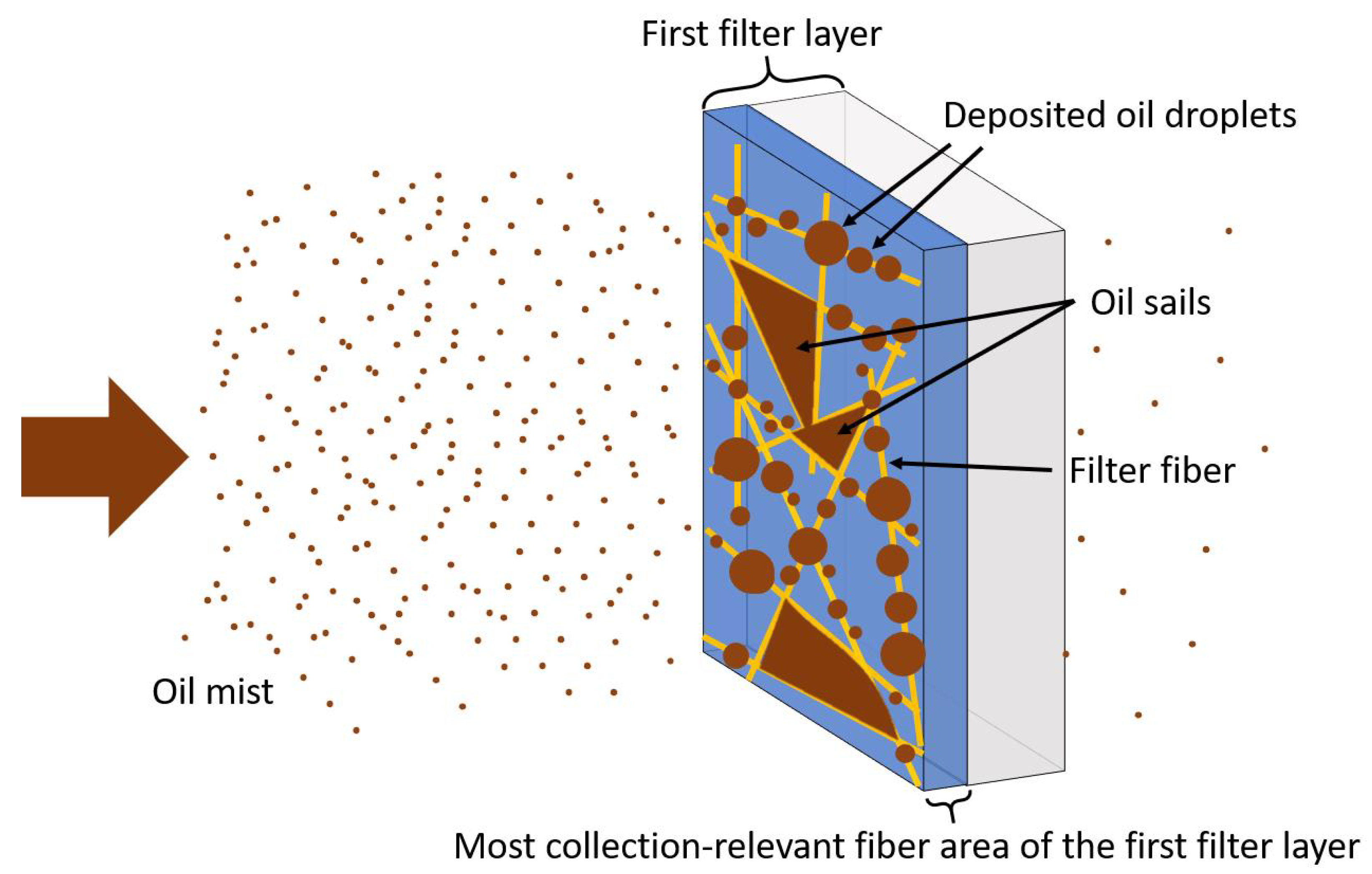
:1. Introduction
2. Material and Methods
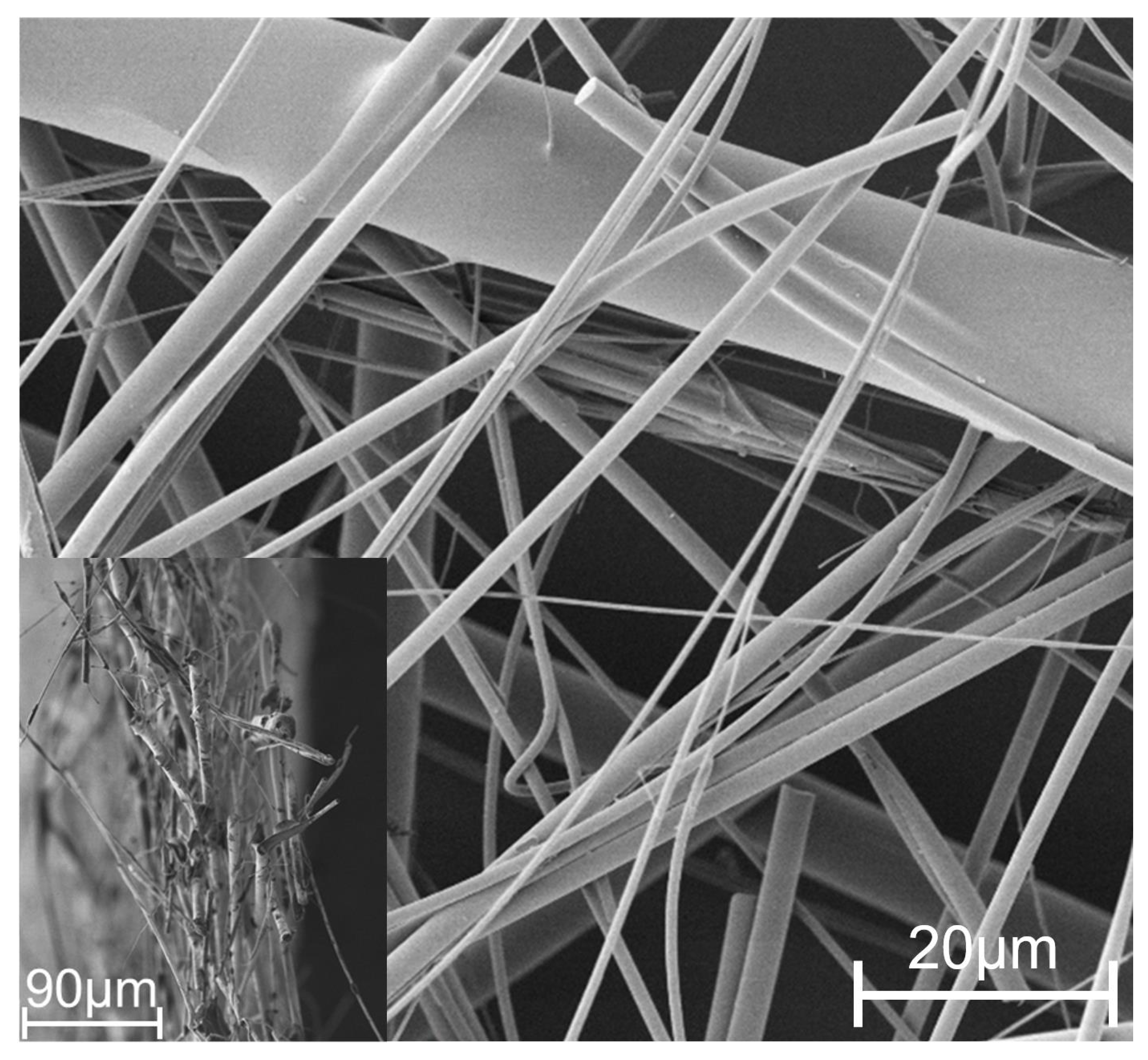
2.1. Thin Porous Filter Media
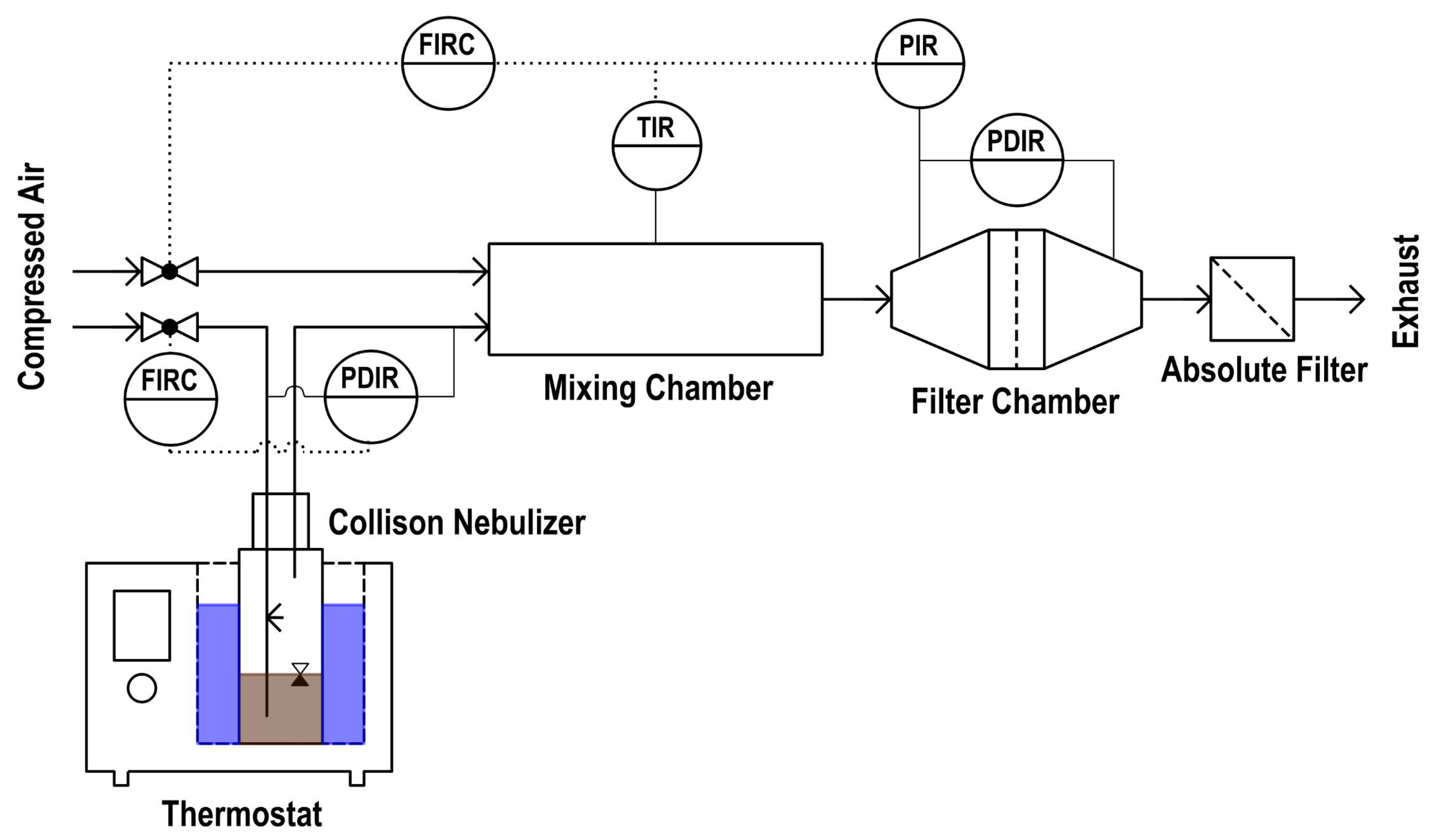
2.2. Oil Deposition on the Thin Porous Filter Medium
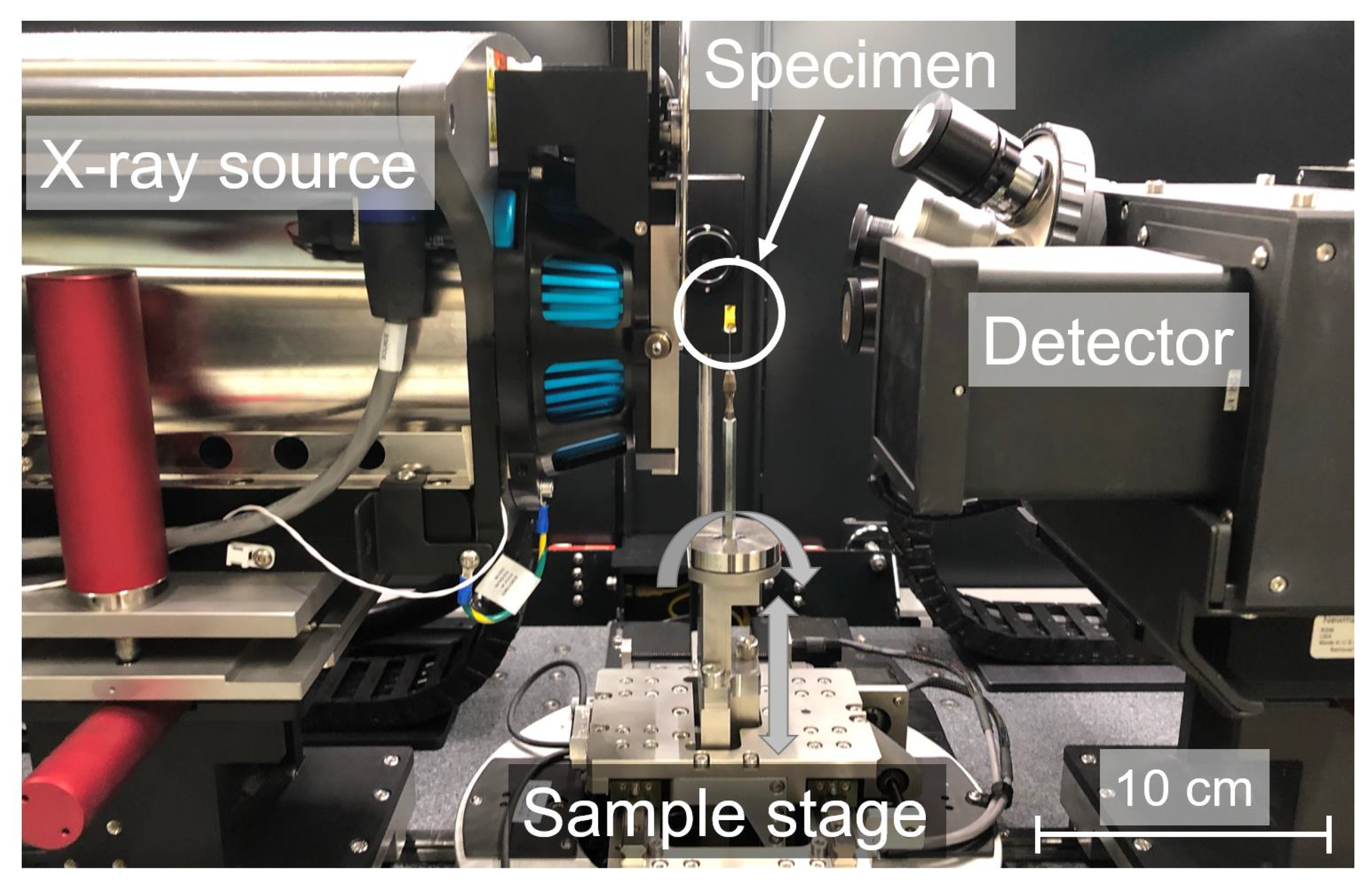
2.3. -CT Scanning
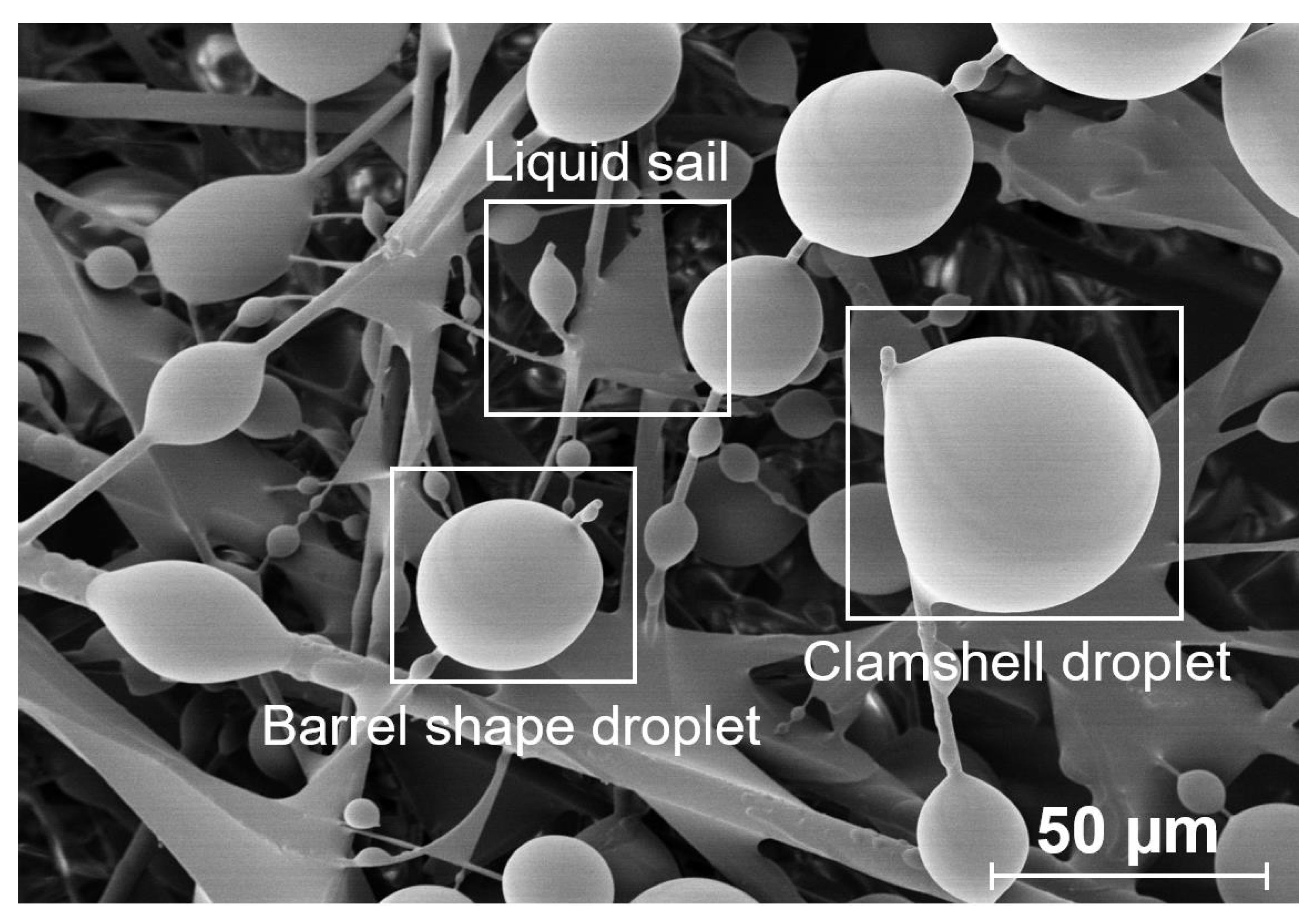
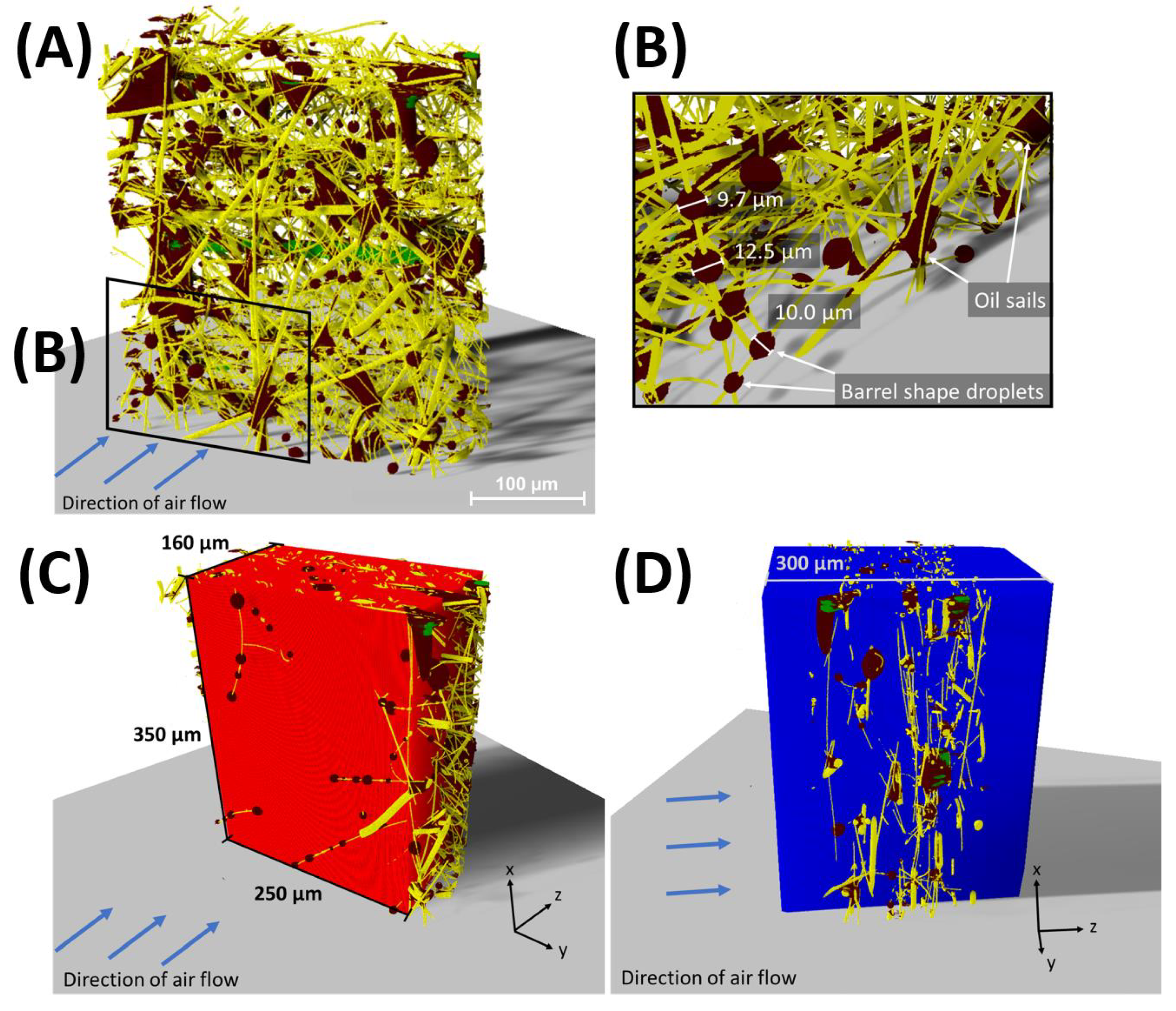
3. Results and Discussion
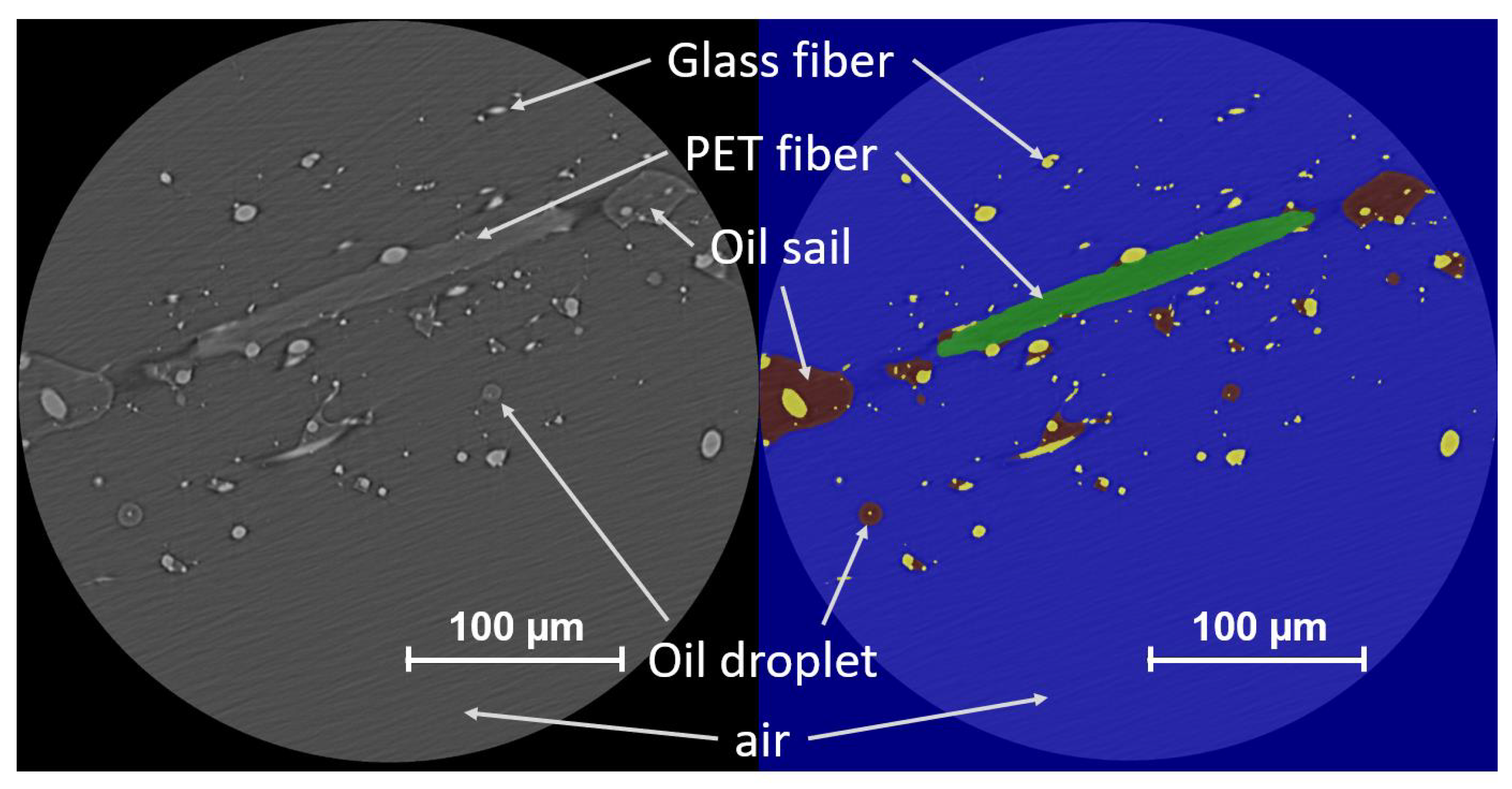
3.1. Segmentation Using a Deep Learning Tool
3.2. Porosity of the Filter Medium
3.3. Saturation of the Filter Media
3.4. PET and Micro Glass Fiber Ratio
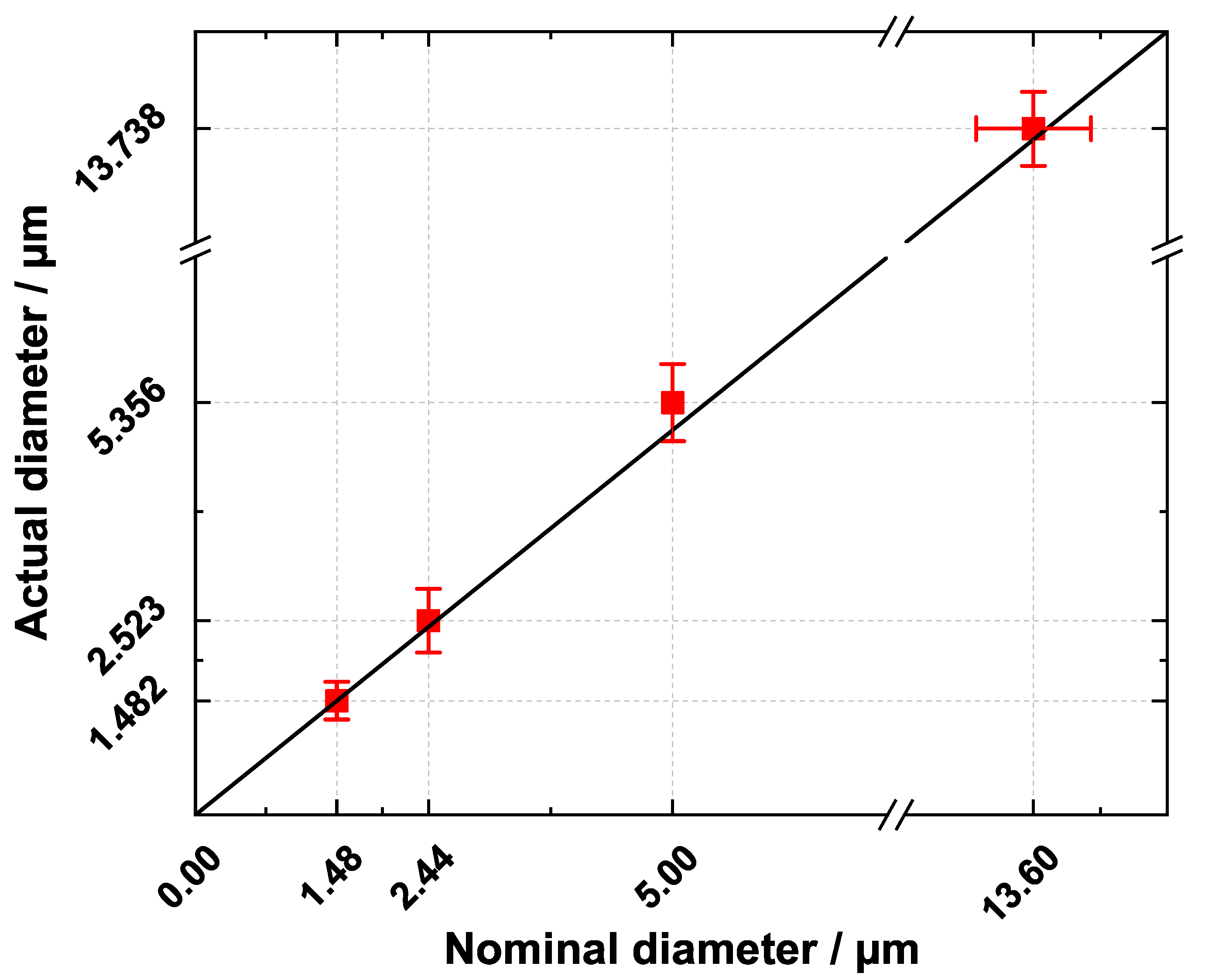
3.5. Comparing Fiber Diameters of the -CT Scan with the Manufacturer’s Specifications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| µ-CT | X-ray microtomography |
| AI | Artificial intelligence |
| MPPS | Most penetrating particle size |
| PET | Polyethylene terephthalate |
| PPS | Polyphenylene sulfide |
| ROI | Region of interest |
| SEM | Scanning electron microscope |
| UV | Ultraviolet |
References
- Mead-Hunter, R.; King, A.J.; Mullins, B.J. Aerosol-mist coalescing filters—A review. Sep. Purif. Technol. 2014, 133, 484–506. [Google Scholar] [CrossRef]
- Kazerouni, N.; Thomas, T.L.; Petralia, S.A.; Hayes, R.B. Mortality among workers exposed to cutting oil mist: Update of previous reports. Am. J. Ind. Med. 2000, 38, 410–416. [Google Scholar] [CrossRef]
- Kampa, D.; Wurster, S.; Buzengeiger, J.; Meyer, J.; Kasper, G. Pressure drop and liquid transport through coalescence filter media used for oil mist filtration. Int. J. Multiph. Flow 2014, 58, 313–324. [Google Scholar] [CrossRef]
- Kampa, D.; Wurster, S.; Meyer, J.; Kasper, G. Validation of a new phenomenological “jump-and-channel” model for the wet pressure drop of oil mist filters. Chem. Eng. Sci. 2015, 122, 150–160. [Google Scholar] [CrossRef]
- Penner, T.; Heikamp, W.; Meyer, J.; Dittler, A. Einfluss ausgewählter Medienstrukturparameter auf das Betriebsverhalten von Ölnebelfiltern. Chem. Ing. Tech. 2019, 91, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Contal, P.; Simao, J.; Thomas, D.; Frising, T.; Callé, S.; Appert-Collin, J.; Bémer, D. Clogging of fibre filters by submicron droplets. Phenomena and influence of operating conditions. J. Aerosol Sci. 2004, 35, 263–278. [Google Scholar] [CrossRef]
- Mullins, B.J.; Agranovski, I.E.; Braddock, R.D.; Ho, C.M. Effect of fiber orientation on fiber wetting processes. J. Colloid Interface Sci. 2004, 269, 449–458. [Google Scholar] [CrossRef]
- Ojaghlou, N.; Tafreshi, H.V.; Bratko, D.; Luzar, A. Dynamical insights into the mechanism of a droplet detachment from a fiber. Soft Matter 2018, 14, 8924–8934. [Google Scholar] [CrossRef]
- Mullins, B.J.; Braddock, R.D.; Agranovski, I.E.; Cropp, R.A.; O’Leary, R.A. Observation and modelling of clamshell droplets on vertical fibres subjected to gravitational and drag forces. J. Colloid Interface Sci. 2005, 284, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mullins, B.J.; Braddock, R.D.; Agranovski, I.E.; Cropp, R.A. Observation and modelling of barrel droplets on vertical fibres subjected to gravitational and drag forces. J. Colloid Interface Sci. 2006, 300, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raynor, P.C.; Leith, D. The influence of accumulated liquid On fibrous filter performance. J. Aerosol Sci. 2000, 31, 19–34. [Google Scholar] [CrossRef]
- Kolb, H.E.; Watzek, A.K.; Zaghini Francesconi, V.; Meyer, J.; Dittler, A.; Kasper, G. A mesoscale model for the relationship between efficiency and internal liquid distribution of droplet mist filters. J. Aerosol Sci. 2018, 123, 219–230. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Goldstein, S.A.; Parfitt, A.M.; Jesion, G.; Kleerekoper, M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1989, 4, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef]
- Mehdikhani, M.; Breite, C.; Swolfs, Y.; Wevers, M.; Lomov, S.V.; Gorbatikh, L. Combining digital image correlation with X-ray computed tomography for characterization of fiber orientation in unidirectional composites. Compos. Part A Appl. Sci. Manuf. 2021, 142, 106234. [Google Scholar] [CrossRef]
- Bordelon, A.C.; Roesler, J.R. Spatial distribution of synthetic fibers in concrete with X-ray computed tomography. Cem. Concr. Compos. 2014, 53, 35–43. [Google Scholar] [CrossRef]
- Ueda, M.; Rozy, M.I.F.; Fukasawa, T.; Ishigami, T.; Fukui, K. Phase-Field Simulation of the Coalescence of Droplets Permeating through a Fibrous Filter Obtained from X-ray Computed Tomography Images: Effect of the Filter Microstructure. Langmuir ACS J. Surf. Colloids 2020, 36, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Fukasawa, T.; Ishigami, T.; Fukui, K. Effect of Surface Wettability on Droplet Coalescence and Pressure Drop in a Fibrous Filter: Direct Numerical Simulation Coordinated with X-ray Computed Tomography Images. Ind. Eng. Chem. Res. 2021, 60, 4168–4179. [Google Scholar] [CrossRef]
- Fang, J.; Davoudi, M.; Chase, G.G. Drop movement along a fiber axis due to pressure driven air flow in a thin slit. Sep. Purif. Technol. 2015, 140, 77–83. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015; Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2015; Volume 9351, pp. 234–241. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Shelhamer, E.; Darrell, T. Fully Convolutional Networks for Semantic Segmentation. Available online: http://openaccess.thecvf.com/content_cvpr_2015/html/Long_Fully_Convolutional_Networks_2015_CVPR_paper.html (accessed on 18 October 2021).
- McHale, G.; Newton, M.I.; Carroll, B.J. The Shape and Stability of Small Liquid Drops on Fibers. Oil Gas Sci. Technol. 2001, 56, 47–54. [Google Scholar] [CrossRef] [Green Version]
- McHale, G.; Newton, M. Global geometry and the equilibrium shapes of liquid drops on fibers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 79–86. [Google Scholar] [CrossRef]
- Chou, T.H.; Hong, S.J.; Liang, Y.E.; Tsao, H.K.; Sheng, Y.J. Equilibrium phase diagram of drop-on-fiber: Coexistent states and gravity effect. Langmuir ACS J. Surfaces Colloids 2011, 27, 3685–3692. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Warm-Up Scan | Main Scan |
|---|---|---|
| Objective | 40× | 40× |
| Binning | 2 | 1 |
| Exposure time [s] | 6 | 25 |
| Tube voltage [kV] | 50 | 50 |
| Source power [W] | 4 | 4 |
| Pictures taken | 1001 | 1671 |
| Voxel size [nm] | 387.1 | 193.5 |
| Voxel count (width) | 912 | 1832 |
| Voxel count (height) | 977 | 1958 |
| Voxel count (depth) | 954 | 1911 |
| Model Architecture | Model Type | Class Count | Patch Size [Pixels] | Batch Size [Patches] |
|---|---|---|---|---|
| U-Net | Multi-label segmentation | 4 | 80 | 128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straube, C.; Meyer, J.; Dittler, A. Identification of Deposited Oil Structures on Thin Porous Oil Mist Filter Media Applying µ-CT Imaging Technique. Separations 2021, 8, 193. https://doi.org/10.3390/separations8100193
Straube C, Meyer J, Dittler A. Identification of Deposited Oil Structures on Thin Porous Oil Mist Filter Media Applying µ-CT Imaging Technique. Separations. 2021; 8(10):193. https://doi.org/10.3390/separations8100193
Chicago/Turabian StyleStraube, Christian, Jörg Meyer, and Achim Dittler. 2021. "Identification of Deposited Oil Structures on Thin Porous Oil Mist Filter Media Applying µ-CT Imaging Technique" Separations 8, no. 10: 193. https://doi.org/10.3390/separations8100193
APA StyleStraube, C., Meyer, J., & Dittler, A. (2021). Identification of Deposited Oil Structures on Thin Porous Oil Mist Filter Media Applying µ-CT Imaging Technique. Separations, 8(10), 193. https://doi.org/10.3390/separations8100193