Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Honey Sample
2.3. Preparation of Honey Extracts
2.4. HPLC–DAD–MS/MSn Analysis of Phenolic Compounds
2.5. ROS- and RNS-Scavenging Assays
2.5.1. O2•ˉ-Scavenging Assay
2.5.2. H2O2-Scavenging Assay
2.5.3. HOCl-Scavenging Assay
2.5.4. 1O2-Scavenging Assay
2.5.5. •NO-Scavenging Assay
2.5.6. ONOOˉ-Scavenging Assay
2.6. In Vitro Evaluation of the ROO•-Induced Oxidative Damage in Erythrocytes from Human Blood
2.6.1. Inhibition of ROO•-Induced Hemoglobin Oxidation
2.6.2. Inhibition of Hemolysis
2.6.3. Inhibition of Lipid Peroxidation
3. Results and Discussion
3.1. Phenolic Compounds and Abscisic Acids
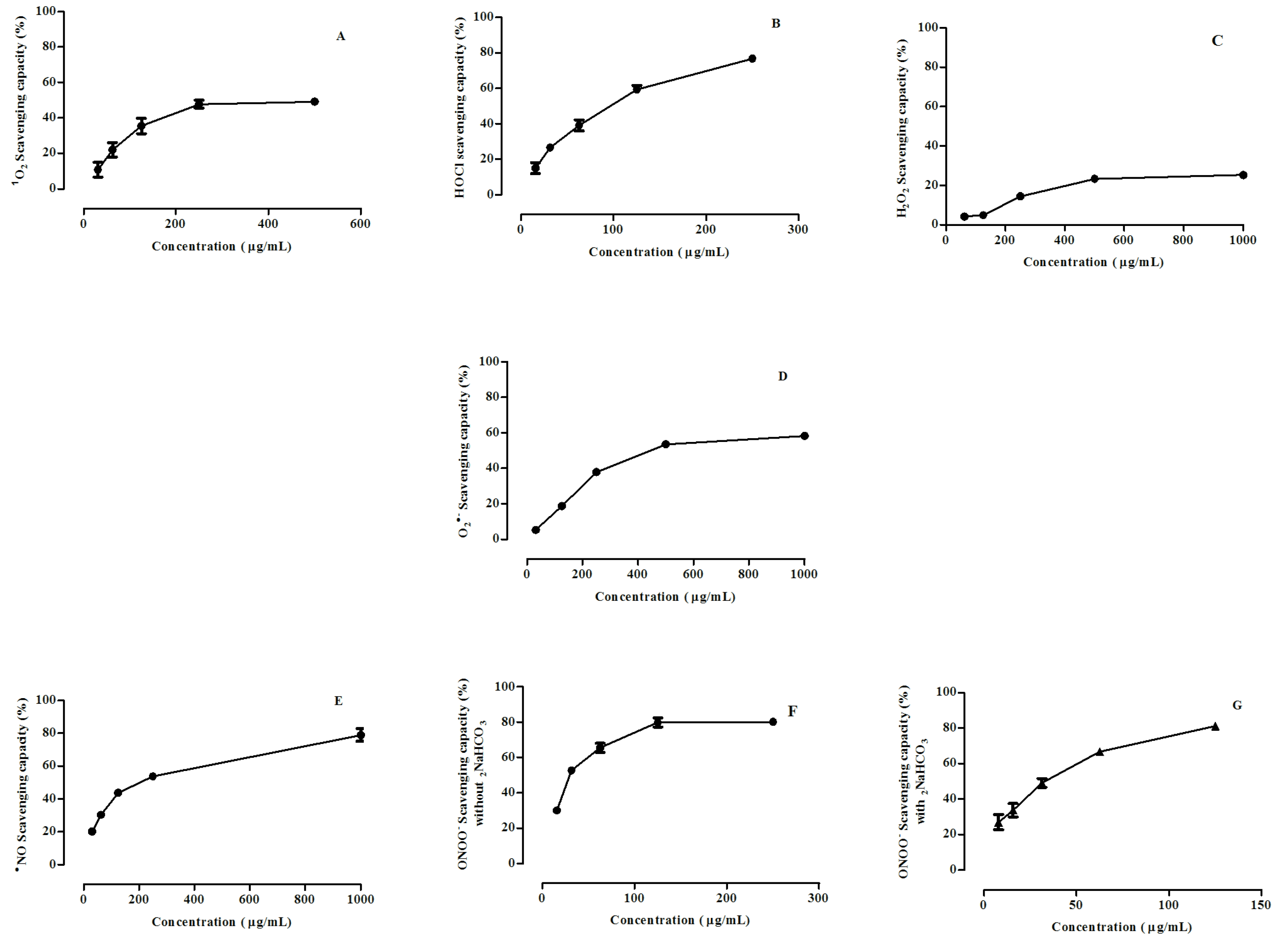
3.2. Scavenging of ROS and RNS by Calluna vulgaris Honey
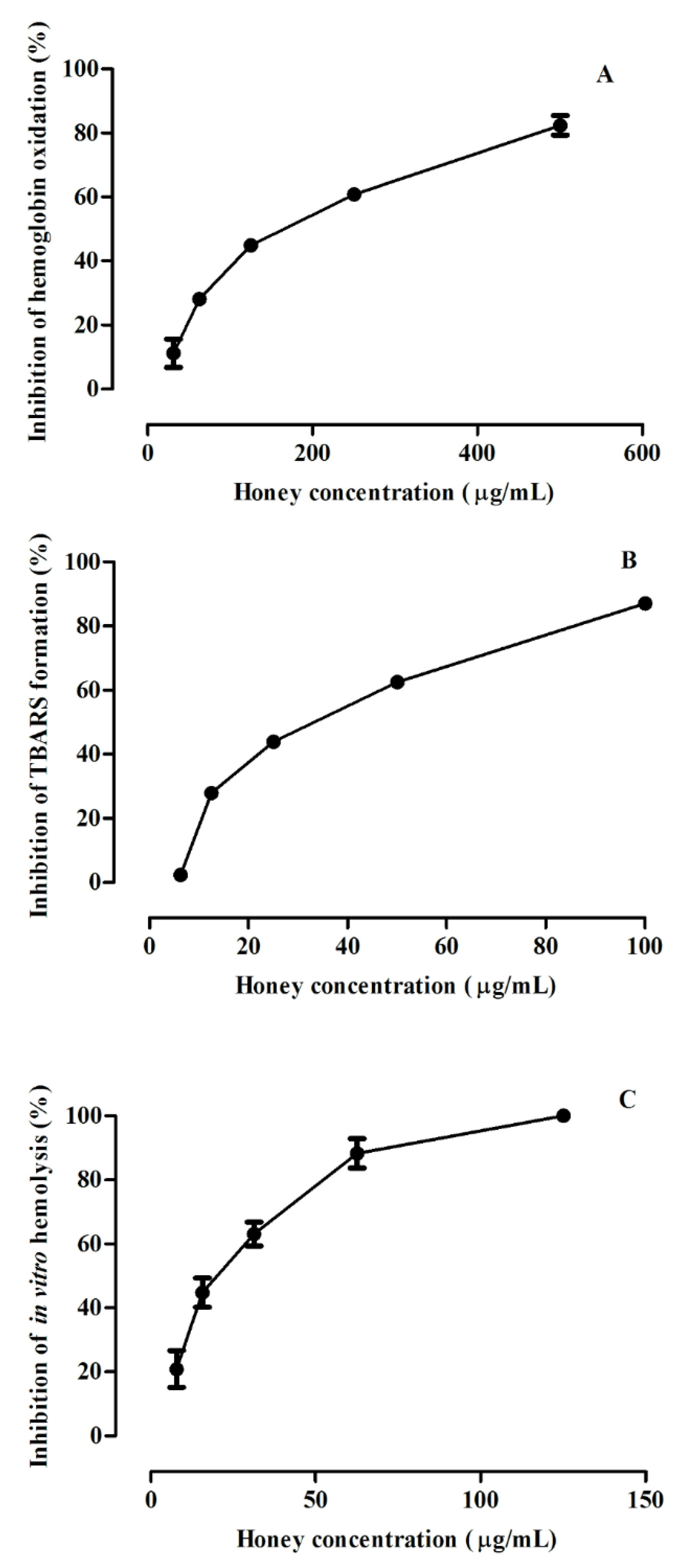
3.3. Protective Effect against ROO•-Induced Oxidative Damage in Human Erythrocytes
3.3.1. Inhibition of Hemoglobin Oxidation in Human Erythrocytes
3.3.2. Inhibition of Lipid Peroxidation in Human Erythrocytes
3.3.3. Inhibition of Hemolysis in Human Erythrocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filip, G.A.; Postescu, I.D.; Tatomir, C.; Muresan, A.; Clichici, S. Calluna vulgaris extract modulates NF-kappaB/ERK signaling pathway and matrix metallo-proteinase expression in SKH-1 hairless mice skin exposed to ultraviolet B irradiation. J. Physiol. Pharmacol. 2002, 63, 423–432. [Google Scholar]
- Chie, R. Encyclopaedia of Medicinal Plants; MacDonald Press: London, UK, 1984. [Google Scholar]
- Mabey, R. Plants with a Purpose; Fontana Presshouse: London, UK, 1979. [Google Scholar]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Simon, A.; Najid, A.; Chulia, A.J.; Delage, C.; Rigaud, M. Inhibition of lipoxygenase activity and HL60 leukemic cell proliferation by ursolic acid isolated from heather flowers (Calluna vulgaris). Biochim. Biophys. Acta Lipids Lipid Metab. 1992, 1125, 68–72. [Google Scholar] [CrossRef]
- Andrade, P.; Ferreres, F.; Gil, M.I.; Tomás-Barberán, F.A. Determination of phenolic compounds in honeys with different floral origin by capillary zone electrophoresis. Food Chem. 1997, 60, 79–84. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Flavonoids from Portuguese heather honey. Z Lebensm Unters Forsch 1994, 1991, 32–37. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural occurrence of abscisic acid in heather honey and floral nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Ferreres, F.; Paula, A.; Gil, M.I.; Tomás-Barberán, F.A. Floral nectar phenolic as biochemical markers of the botanic origin of heather honey. Z. Lebensm Unters Forsch 1996, 202, 40–44. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Pérez-Coello, M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Seisonen, S.; Kivima, E.; Vene, K. Characterisation of the aroma profiles of different honeys and corresponding flowers using solid-phase microextraction and gas chromatography-mass spectrometry/olfactometry. Food Chem. 2015, 169, 34–40. [Google Scholar] [CrossRef]
- Guyot, C.; Scheirman, V.; Collin, S. Floral origin markers of heather honeys: Calluna vulgaris and Erica arborea. Food Chem. 1999, 64, 3–11. [Google Scholar] [CrossRef]
- Andrade, P.B.; Amaral, M.T.; Isabel, P.; Carvalho, J.C.M.F.; Seabra, R.M.; Proença da Cunha, A. Physicochemical attributes and pollen spectrum of Portuguese heather honeys. Food Chem. 1999, 66, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Martins, R.C.; Lopes, V.V.; Valentão, P.; Carvalho, J.C.; Isabel, P.; Amaral, M.T.; Batista, M.T.; Andrade, P.B.; Silva, B.M. Relevant principal component analysis applied to the characterisation of Portuguese heather honey. Nat. Prod. Res. 2008, 22, 1560–1582. [Google Scholar] [CrossRef] [PubMed]
- Lutier, P.M.; Vaissière, B.E. An improved method for pollen analysis of honey. Rev. Palaeobot. Palynol. 1993, 78, 129–144. [Google Scholar] [CrossRef]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z. Carotenoids are effective inhibitors of in vitro hemolysis of human erythrocytes, as determined by a practical and optimized cellular antioxidant assay. J. Agric. Food Chem. 2012, 60, 5884–5892. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679. [Google Scholar] [CrossRef] [Green Version]
- Tuberoso, C.I.G.; Bifulco, E.; Caboni, P.; Cottiglia, F.; Cabras, P.; Floris, I. Floral markers of strawberry tree (Arbutus unedo L.) honey. J. Agric. Food Chem. 2010, 58, 384–389. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorg. Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Gomes, A.; Reis, S.; Lima, J.L.; Fernandes, E. Hydrogen peroxide scavenging activity by non-steroidal anti-inflammatory drugs. Life Sci. 2005, 76, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Fernandes, E.; Santos, J.L.M.; Pinto, D.C.G.A.; Silva, A.M.S.; Lima, J.L.F.C. New noncellular fluorescence microplate screening assay for scavenging activity against singlet oxygen. Anal. Bioanal. Chem. 2007, 387, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Chisté, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 2014, 99, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic acid: A novel nutraceutical for glycemic control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Truchado, P.; Ferreres, F.; Bortolotti, L.; Sabatini, A.G.; Tomás-Barberán, F.A. Nectar flavonol rhamnosides are floral markers of Acacia (Robinia pseudacacia) honey. J. Agric. Food Chem. 2008, 56, 8815–8824. [Google Scholar] [CrossRef]
- Falcão, S.I.; Vilas-Boas, M.; Estevinho, L.M.; Barros, C.; Domingues, M.R.M.; Cardoso, S.M. Phenolic characterization of Northeast Portuguese propolis: Usual and unusual compounds. Anal. Bioanal. Chem. 2010, 396, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Silva, L.R.; Valentão, P.; Andrade, P.B. Phenolic compounds in honey as health promoters. In Honey: Produxtion, Consumption and Health Benefits; Bondurand, G., Bosch, H., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 81–111. [Google Scholar]
- Stief, T.W. Physiology and pharmacology of singlet oxygen. Med. Hypotheses 2003, 60, 567–572. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltaci, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Inoue, K.; Murayama, S.; Seshimo, F.; Takeba, K.; Yoshimura, Y.; Nakazawa, H.J. Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. Sci. Food Agric. 2005, 85, 872–878. [Google Scholar] [CrossRef]
- Dor, G.O.L.M.; Mahomoodally, M.F. Chemical profile and in vitro bioactivity of tropical honey from Mauritius. Asian Pac. J. Trop. Dis. 2014, 4, S1002–S1013. [Google Scholar] [CrossRef]
- Kassim, M.; Achoui, M.; Mansor, M.; Yusoff, K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia 2010, 81, 1196–1201. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.; Liaudet, S.L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Pannala, A.; Razaq, R.; Halliwell, B.; Singh, S.; Rice-Evans, C.A. Inhibition of peroxynitrite dependent tyrosine nitration by hydroxycinnamates: Nitration or electron donation? Free Radic. Biol. Med. 1998, 24, 594–606. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; González-Paramás, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Piatti, E. Honey flavonoids as protection agents against oxidative damage to human red blood cells. Food Chem. 2007, 104, 1635–1640. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int. J. Biol. Macromol. 2007, 41, 42–48. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian fruit Byrsonima crassifolia effectively scavenges reactive oxygen and nitrogen species and protects human erythrocytes against oxidative damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Shukitt-Hale, B.; MacKinnon, S.; Kalt, W.; Joseph, J.A. Polyphenolics enhance red blood cell resistance to oxidative stress: In vitro and in vivo. Biochim. Biophys Acta Gen. Subj. 2000, 1523, 117–122. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Zaid, M.A.; Anis, R.; Mishra, N. Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes. Clin. Exp. Pharmacol. Physiol. Suppl. 2005, 32, 70–75. [Google Scholar] [CrossRef] [PubMed]
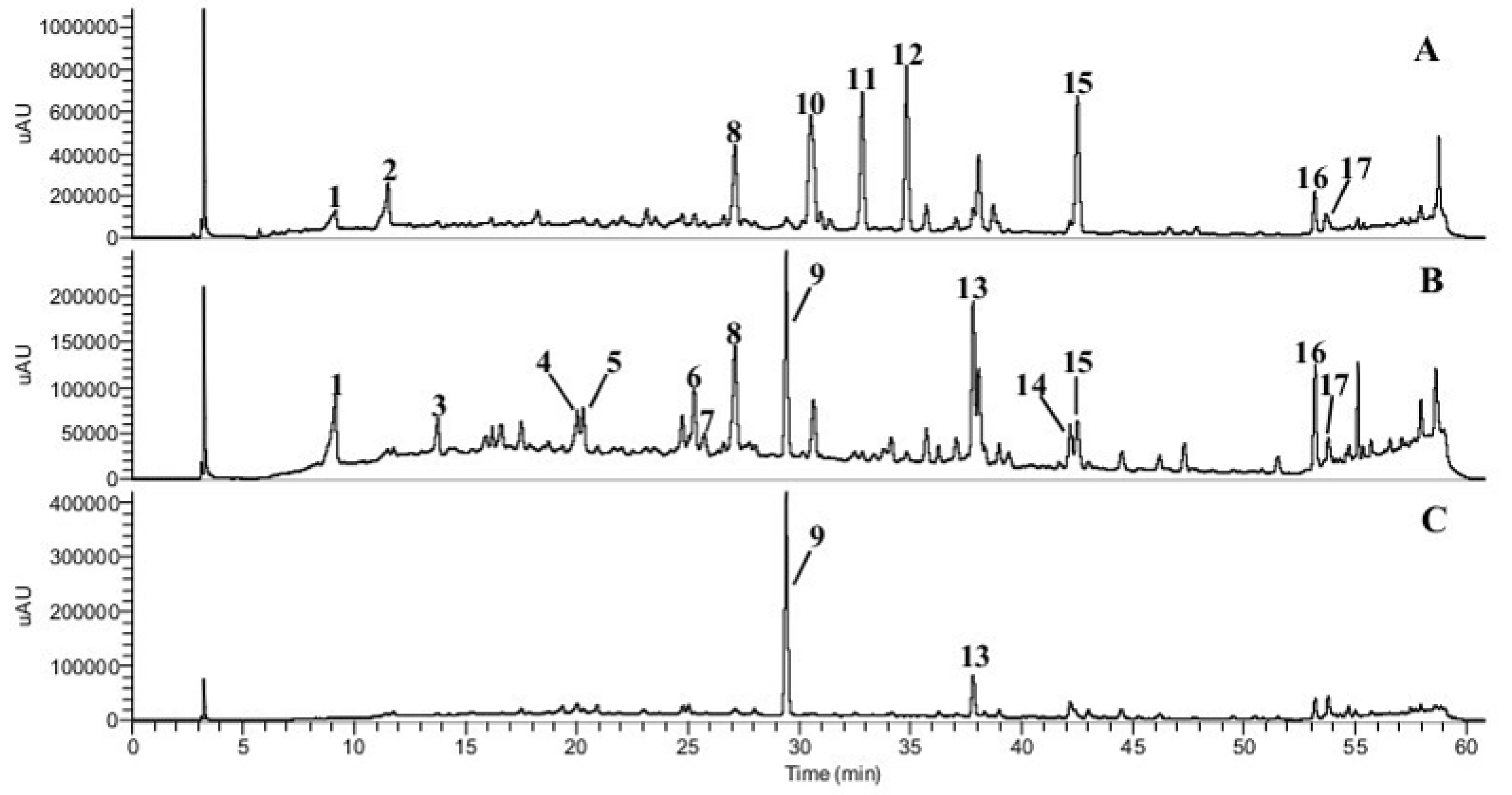
| Peaks | Compounds | Concentration (μg/g) a | HPLC-DAD-ESI-MSn Characteristics | |||
|---|---|---|---|---|---|---|
| tR b | λmax | [M-H]− (m/z) | Fragments (m/z) c | |||
| 1 | Benzoic acid derivative 1 | 62 ± 5 | 9.1 | 295 | 175.9583 | MS2 [175]: 157, 147, 131, 115, 97, 89. MS3 [175→131]: 119, 103, 87. |
| 2 | Benzoic acid derivative 2 | 170 ± 1 | 11.5 | 284 | 326.1218 | MS2 [326]: 308, 278, 235, 164. MS3 [326→235]: 164. |
| 3 | Hydroxycinnamic acid derivative 1 | 14 ± 2 | 13.7 | 300(sh), 325 | 465.1665 | MS2 [465]: 447, 419, 303. MS3 [465→419]: 391, 359, 257, 221, 179, 161 |
| 4 | Hydroxycinnamic acid derivative 2 | 17 ± 3 | 20.0 | 309, 342(sh) | 327.1117 | MS2 [327]:309, 165, 147 MS3 [327→147]: 147. |
| 5 | Caffeic acid | 13 ± 2 | 20.3 | 290, 320 | 179.0371 | MS2 [179]: 161, 151, 135. MS3 [179→135]: 135, 107, 91. |
| 6 | p-Coumaric acid derivative | <LOQ | 25.3 | 280(sh), 307 | 379.2022 | MS2 [379]: 333, 291, 179. MS3 [379→333]: 315, 241, 161, 143, 101. |
| 7 | p-Coumaric acid | <LOQ | 25.56 | 299, 309 | 163.04 | MS2[163]: 145 MS3[163→145]: 119, 103 |
| 8 | Unknown flavonoid derivative 1 | 711 ± 19 | 27.1 | 254, 270(sh), 310 | 267.1267 | MS2 [267]: 249, 221, 199 MS3 [267→221]: 206, 179, 160. |
| 9 | Quercetin acetyl hexoside | 632 ± 28 | 29.4 | 255, 350, 386 | 505.1408 | MS2: nd MS3: nd |
| 10 | Abscisic acid derivative | 1015 ± 28 | 30.6 | 280(sh) | 249.9634 | MS2 [249]: 231, 205, 153. MS3 [249→205]: 181, 155. |
| 11 | trans-trans-Abscisic acid | 942 ± 32 | 32.8 | 270 | 263.1318 | MS2 [263]: 245, 219, 201, 153. MS3 [263→219]: 201, 186, 163. |
| 265.1434 | MS2 [265]: 247, 229, 208, 205, 181, 151. MS3 [265→208]: 191, 163, 149, 119, 93. | |||||
| 12 | cis-trans-Abscisic acid | 1011 ± 36 | 34.8 | 270 | 263.1318 | MS2 [263]: 245, 219, 201, 153. MS3 [263→153]: 138. |
| 13 | Kaempferol-3-O-rutinoside | 79 ± 6 | 37.8 | 267, 290(sh), 315, 365 | 593.1370 | MS2 [593]: 447, 285, 257, 229. MS3 [593→285]: 257, 241, 213, 151. |
| 14 | Pinobanksin | 1.5 ± 0.1 | 42.2 | 270, 290, 325 | 271.0642 | MS2 [271]: 253, 225, 197. MS3 [271→253]: 225, 207, 197. |
| 15 | Unknown flavonoid derivative 2 | 10,370 ± 422 | 42.5 | 281 | 329.2370 | MS2 [329]: 311, 293, 229, 211, 171. MS3 [329→229]: 211, 165. |
| 16 | Chrysin + pinocembrin? | 238 ± 9 | 53.2 | 270, 283, 325 | 253.0536 255.0690 | MS2 [253]: 235, 225, 209, 181, 165, 151, 143. MS3 [253→209]: 181, 165, 153, 139, 121 MS2 [255]: 213, 187, 169, 151, 145, 107. MS3 [255→213]: 195, 185, 169, 145. |
| 17 | Galangin | 171 ± 6 | 53.77 | 225, 245, 276 | 269.05 | MS2 [269]: 241, 197 MS3 [269→241]: 197, 169, 141 |
| Total sum of identified phenolic compounds | 15,446.5 | |||||
| Reactive Species | IC50 (µg/mL) | |
|---|---|---|
| Honey Extract | Ascorbic Acid (Positive Control) | |
| ROS | ||
| 1O2 | 48 ± 1% at 250 µg/mL | 1.1 ± 0.1 |
| HOCl | 95 ± 2 | 0.05 ± 0.03 |
| H2O2 | 23 ± 2% at 500 µg/mL | 188 ± 2 |
| O2•˗ | 394 ± 3 | NA |
| RNS | ||
| •NO | 181 ± 2 | 0.28 ± 0.02 |
| ONOO˗ | 29 ± 2 | 1.7 ± 0.1 |
| ONOO˗ * | 32 ± 1 | 2.1 ± 0.4 |
| Erythrocytes Assay | IC50 (µg/mL) a |
|---|---|
| Inhibition of hemoglobin oxidation | 158 ± 2 |
| Inhibition of lipid peroxidation | 36 ± 1 |
| Inhibition of hemolysis | 19 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues da Silva, L.; Campos Chisté, R.; Fernandes, E. Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris. Separations 2021, 8, 177. https://doi.org/10.3390/separations8100177
Rodrigues da Silva L, Campos Chisté R, Fernandes E. Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris. Separations. 2021; 8(10):177. https://doi.org/10.3390/separations8100177
Chicago/Turabian StyleRodrigues da Silva, Luís, Renan Campos Chisté, and Eduarda Fernandes. 2021. "Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris" Separations 8, no. 10: 177. https://doi.org/10.3390/separations8100177
APA StyleRodrigues da Silva, L., Campos Chisté, R., & Fernandes, E. (2021). Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris. Separations, 8(10), 177. https://doi.org/10.3390/separations8100177