A Sensitive LC–MS/MS Method for the Quantification of 3-Hydroxybenzo[a]pyrene in Urine-Exposure Assessment in Smokers and Users of Potentially Reduced-Risk Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Work-Up for Quantification
2.3. LC–MS/MS
2.4. Calibration
2.5. Method Validation
2.6. Human Study
2.7. Data Evaluation and Statistics
3. Results
3.1. Performance of the Analytical Method
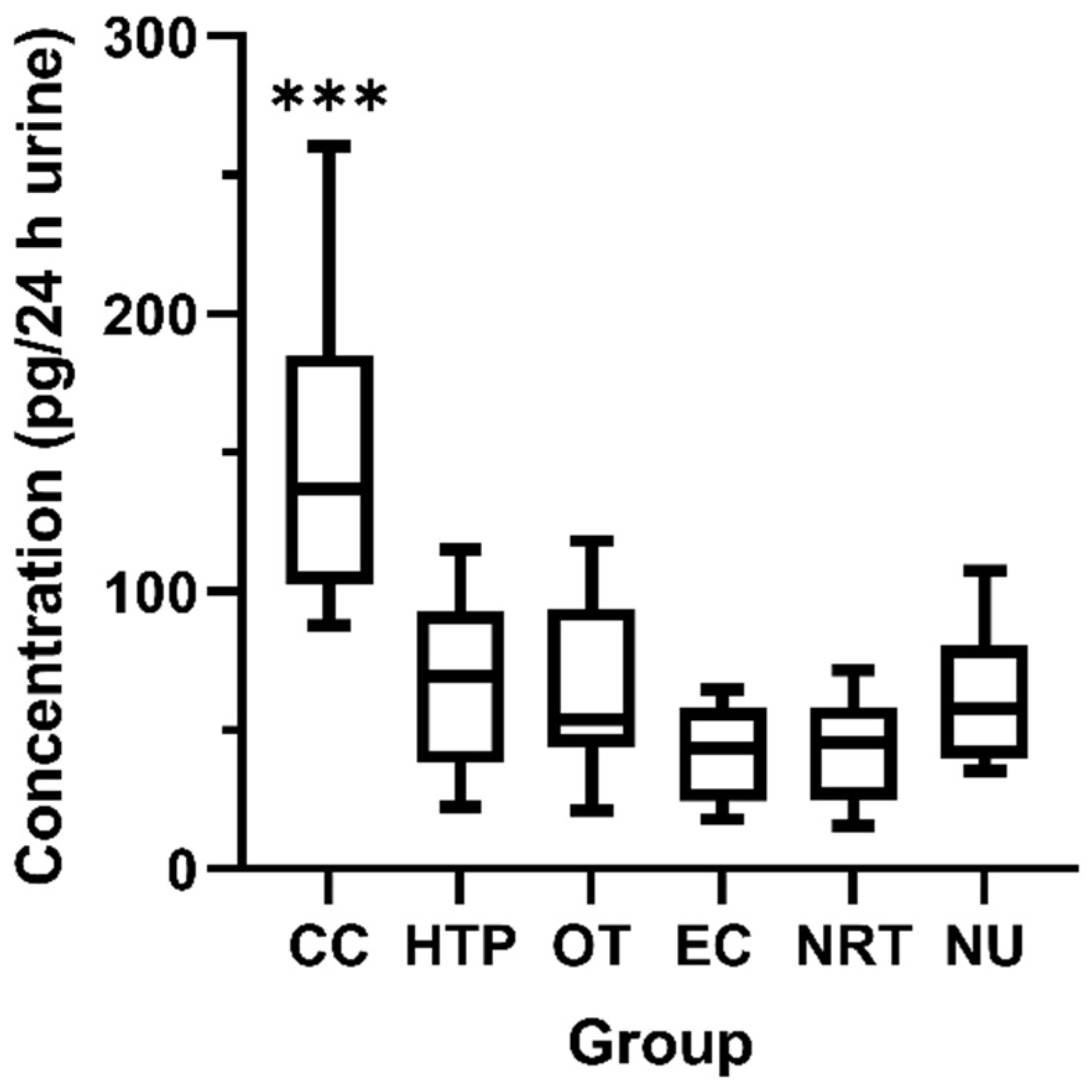
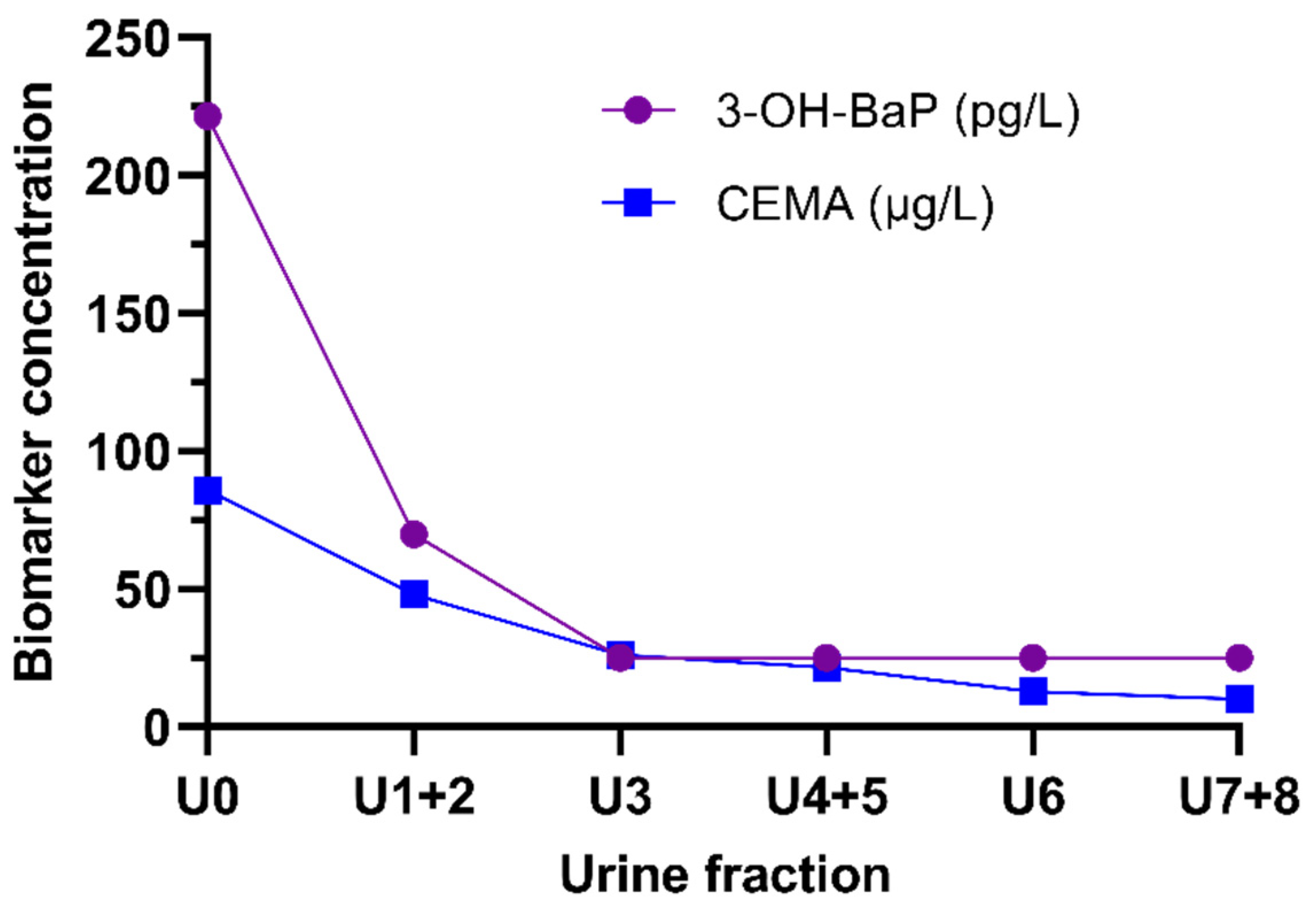
3.2. Human Study—Urinary Excretion of 3-OH-BaP
3.3. Correlation of 3-OH-BaP with Smoking Specific Parameters
4. Discussion
4.1. Analytical Method
4.2. Human Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lijinsky, W. The formation and occurrence of polynuclear aromatic hydrocarbons associated with food. Mutat. Res. Genet. Toxicol. 1991, 259, 251–261. [Google Scholar] [CrossRef]
- Hattemer-Frey, H.A.; Travis, C.C. Benzo-a-pyrene: Environmental partitioning and human exposure. Toxicol. Ind. Health 1991, 7, 141–157. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IRAC: Lyon, France, 2010; Volume 92. [Google Scholar]
- Rodgman, A.; Perfetti, T. The Composition of Cigarette Smoke: A Catalogue of the Polycyclic Aromatic Hydrocarbons. Contrib. Tob. Res. 2006, 22, 13–69. [Google Scholar] [CrossRef] [Green Version]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Polynuclear Aromatic Compounds, Part 1: Chemical, Environmental and Experimental Data; IRAC: Lyon, France, 1983; Volume 32. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Chemical Agents and Related Occupations; IRAC: Lyon, France, 2012; Volume 100 F. [Google Scholar]
- Scherer, G.; Frank, S.; Riedel, K.; Meger-Kossien, I.; Renner, T. Biomonitoring of Exposure to Polycyclic Aromatic Hydrocarbons of Nonoccupationally Exposed Persons. Cancer Epidemiol. Biomark. Prev. 2000, 9, 373–380. [Google Scholar]
- Jacob, J.; Seidel, A. Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2002, 778, 31–47. [Google Scholar] [CrossRef]
- Ramsauer, B.; Sterz, K.; Hagedorn, H.W.; Engl, J.; Scherer, G.; McEwan, M.; Errington, G.; Shepperd, J.; Cheung, F. A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in urine of non-smokers and smokers. Anal. Bioanal. Chem. 2011, 399, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Dor, F.; Dab, W.; Empereur-Bissonnet, P.; Zmirou, D. Validity of biomarkers in environmental health studies: The case of PAHs and benzene. Crit. Rev. Toxicol. 1999, 29, 129–168. [Google Scholar] [CrossRef]
- Deutsche Forschungsgemeinschaft (DFG). Benzo[a]pyrene. In MAK Collection: Occupational toxicants, Part 1; Commission, M., Ed.; Wiley-VCH Verlag: Heidelberg, Germany, 2012; Volume 27. [Google Scholar]
- Conney, A.H.; Chang, R.L.; Jerina, D.M.; Caroline Wei, S.J. Studies on the Metabolism of Benzo[a]Pyrene and Dose-Dependent Differences in the Mutagenic Profile of Its Ultimate Carcinogenic Metabolite. Drug Metab. Rev. 1994, 26, 125–163. [Google Scholar] [CrossRef]
- Gelboin, H.V. Benzo[a]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [Green Version]
- Andreas, L.; William, M.B. Metabolic Activation and Detoxification of Polycyclic Aromatic Hydrocarbons. In The Carcinogenic Effects of Polycyclic Aromatic Hydrocarbons; World Scientific: London, UK, 2005; pp. 19–96. [Google Scholar]
- Verma, N.; Pink, M.; Rettenmeier, A.W.; Schmitz-Spanke, S. Review on proteomic analyses of benzo[a]pyrene toxicity. Proteomics 2012, 12, 1731–1755. [Google Scholar] [CrossRef]
- Zhong, Y.; Carmella, S.G.; Hochalter, J.B.; Balbo, S.; Hecht, S.S. Analysis of r-7,t-8,9,c-10-Tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in Human Urine: A Biomarker for Directly Assessing Carcinogenic Polycyclic Aromatic Hydrocarbon Exposure Plus Metabolic Activation. Chem. Res. Toxicol. 2011, 24, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter-Brockmann, S.; Dettbarn, G.; Jessel, S.; John, A.; Seidel, A.; Achten, C. GC-APLI-MS as a powerful tool for the analysis of BaP-tetraol in human urine. J. Chromatogr. B 2018, 1100–1101, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Gao, Q.; Hu, J. Determination of 3-Hydroxybenzo[a]pyrene Glucuronide/Sulfate Conjugates in Human Urine and Their Association with 8-Hydroxydeoxyguanosine. Chem. Res. Toxicol. 2019, 32, 1367–1373. [Google Scholar] [CrossRef]
- Gündel, J.; Angerer, J. High-performance liquid chromatographic method with fluorescence detection for the determination of 3-hydroxybenzo[a]pyrene and 3-hydroxybenz[a]anthracene in the urine of polycyclic aromatic hydrocarbon-exposed workers. J. Chromatogr. B Biomed. Sci. Appl. 2000, 738, 47–55. [Google Scholar] [CrossRef]
- Gündel, J.; Schaller, K.H.; Angerer, J. Occupational exposure to polycyclic aromatic hydrocarbons in a fireproof stone producing plant: Biological monitoring of 1-hydroxypyrene, 1-, 2-, 3- and 4-hydroxyphenanthrene, 3-hydroxybenz(a)anthracene and 3-hydroxybenzo(a)pyrene. Int. Arch. Occup. Environ. Health 2000, 73, 270–274. [Google Scholar] [CrossRef]
- Raponi, F.; Bauleo, L.; Ancona, C.; Forastiere, F.; Paci, E.; Pigini, D.; Tranfo, G. Quantification of 1-hydroxypyrene, 1- and 2-hydroxynaphthalene, 3-hydroxybenzo[a]pyrene and 6-hydroxynitropyrene by HPLC-MS/MS in human urine as exposure biomarkers for environmental and occupational surveys. Biomarkers 2017, 22, 575–583. [Google Scholar] [CrossRef]
- Förster, K.; Preuss, R.; Rossbach, B.; Bruning, T.; Angerer, J.; Simon, P. 3-Hydroxybenzo[a]pyrene in the urine of workers with occupational exposure to polycyclic aromatic hydrocarbons in different industries. Occup. Environ. Med. 2008, 65, 224–229. [Google Scholar] [CrossRef]
- Gendre, C.; Lafontaine, M.; Morele, Y.; Payan, J.-P.; Simon, P. Relationship Between Urinary Levels of 1-Hydroxypyrene and 3-Hydroxybenzo[a]pyrene for Workers Exposed to Polycyclic Aromatic Hydrocarbons. Polycyclic Aromat. Compd. 2002, 22, 761–769. [Google Scholar] [CrossRef]
- Luo, K.; Gao, Q.; Hu, J. Derivatization method for sensitive determination of 3-hydroxybenzo [a] pyrene in human urine by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2015, 1379, 51–55. [Google Scholar] [CrossRef]
- Sarkar, M.; Liu, J.; Koval, T.; Wang, J.; Feng, S.; Serafin, R.; Jin, Y.; Xie, Y.; Newland, K.; Roethig, H.J. Evaluation of biomarkers of exposure in adult cigarette smokers using Marlboro Snus. Nicotine Tob. Res. 2010, 12, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yang, J.; Liu, B.; Zheng, S.; Wang, W.; Zhu, X.; Qian, X. Development of a sensitive method for the quantification of urinary 3-hydroxybenzo[a]pyrene by solid phase extraction, dansyl chloride derivatization and liquid chromatography-tandem mass spectrometry detection. Anal. Methods 2014, 6, 6488–6493. [Google Scholar] [CrossRef]
- Hu, H.; Liu, B.; Yang, J.; Lin, Z.; Gan, W. Sensitive determination of trace urinary 3-hydroxybenzo[a]pyrene using ionic liquids-based dispersive liquid-liquid microextraction followed by chemical derivatization and high performance liquid chromatography-high resolution tandem mass spectrometry. J. Chromatogr. B 2016, 1027, 200–206. [Google Scholar] [CrossRef]
- Barbeau, D.; Maître, A.; Marques, M. Highly sensitive routine method for urinary 3-hydroxybenzo[a]pyrene quantitation using liquid chromatography-fluorescence detection and automated off-line solid phase extraction. Analyst 2011, 136, 1183–1191. [Google Scholar] [CrossRef]
- Simon, P.; Lafontaine, M.; Delsaut, P.; Morele, Y.; Nicot, T. Trace determination of urinary 3-hydroxybenzo[a]pyrene by automated column-switching high-performance liquid chromatotgraphy. J. Chromatogr. B Biomed. Sci. Appl. 2000, 748, 337–348. [Google Scholar] [CrossRef]
- Lafontaine, M.; Champmartin, C.; Simon, P.; Delsaut, P.; Funck-Brentano, C. 3-Hydroxybenzo[a]pyrene in urine of smokers and non-smokers. Toxicol. Lett. 2006, 162, 181–185. [Google Scholar] [CrossRef]
- Richter-Brockmann, S.; Dettbarn, G.; Jessel, S.; John, A.; Seidel, A.; Achten, C. Ultra-high sensitive analysis of 3-hydroxybenzo[a]pyrene in human urine using GC-APLI-MS. J. Chromatogr. B 2019, 1118–1119, 187–193. [Google Scholar] [CrossRef]
- Sibul, F.; Burkhardt, T.; Kachhadia, A.; Pilz, F.; Scherer, G.; Scherer, M.; Pluym, N. Identification of biomarkers specific to five different nicotine product user groups: Study protocol of a controlled clinical trial. Contemp. Clin. Trials Commun. 2021, 22, 100794. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Bioanalytical Method Validation—Guidance for Industry. FDA-2013-D-1020; 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 6 September 2021).
- Blaszkewicz, M.; Liesenhoff-Henze, K. Creatinine in urine [Biomonitoring Methods, 2010]. In The MAK-Collection for Occupational Health and Safety; Wiley-VCH: Weinheim, Germany, 2010; pp. 169–184. [Google Scholar]
- Piller, M.; Gilch, G.; Scherer, G.; Scherer, M. Simple, fast and sensitive LC-MS/MS analysis for the simultaneous quantification of nicotine and 10 of its major metabolites. J. Chromatogr. B 2014, 951–952, 7–15. [Google Scholar] [CrossRef]
- Barbeau, D.; Persoons, R.; Marques, M.; Hervé, C.; Laffitte-Rigaud, G.; Maitre, A. Relevance of urinary 3-hydroxybenzo (a) pyrene and 1-hydroxypyrene to assess exposure to carcinogenic polycyclic aromatic hydrocarbon mixtures in metallurgy workers. Ann. Occup. Hyg. 2014, 58, 579–590. [Google Scholar] [CrossRef] [Green Version]
- Lafontaine, M.; Gendre, C.; Delsaut, P.; Simon, P. Urinary 3-hydroxybenzo[a]pyrene as a biomarker of exposure to polycyclic aromatic hydrocarbons: An approach for determining a biological limit value. Polycycl. Aromat. Compd. 2004, 24, 441–450. [Google Scholar] [CrossRef]
- Li, Z.; Romanoff, L.C.; Trinidad, D.A.; Pittman, E.N.; Hilton, D.; Hubbard, K.; Carmichael, H.; Parker, J.; Calafat, A.M.; Sjödin, A. Quantification of 21 metabolites of methylnaphthalenes and polycyclic aromatic hydrocarbons in human urine. Anal. Bioanal. Chem. 2014, 406, 3119–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Pluym, N.; Gilch, G.; Scherer, G.; Scherer, M. Analysis of 18 urinary mercapturic acids by two high-throughput multiplex-LC-MS/MS methods. Anal. Bioanal. Chem. 2015, 407, 5463–5476. [Google Scholar] [CrossRef] [PubMed]
- Schettgen, T.; Musiol, A.; Alt, A.; Ochsmann, E.; Kraus, T. A method for the quantification of biomarkers of exposure to acrylonitrile and 1,3-butadiene in human urine by column-switching liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 393, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Carmella, S.G.; Chen, M.; Jensen, J.A.; Wilkens, L.R.; Le Marchand, L.; Hatsukami, D.K.; Murphy, S.E.; Hecht, S.S. Urinary Cyanoethyl Mercapturic Acid, a Biomarker of the Smoke Toxicant Acrylonitrile, Clearly Distinguishes Smokers From Nonsmokers. Nicotine Tob. Res. 2020, 22, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
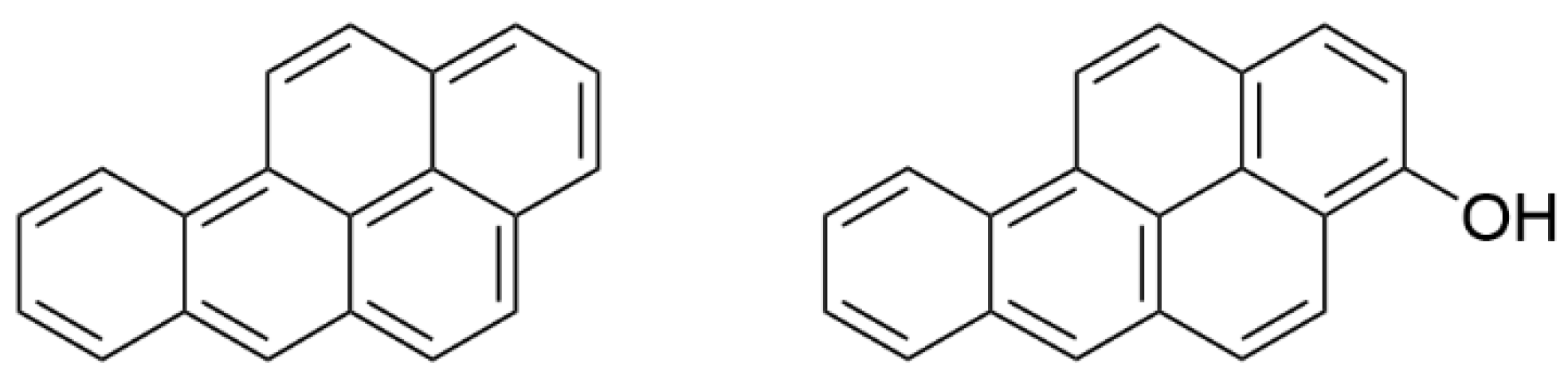
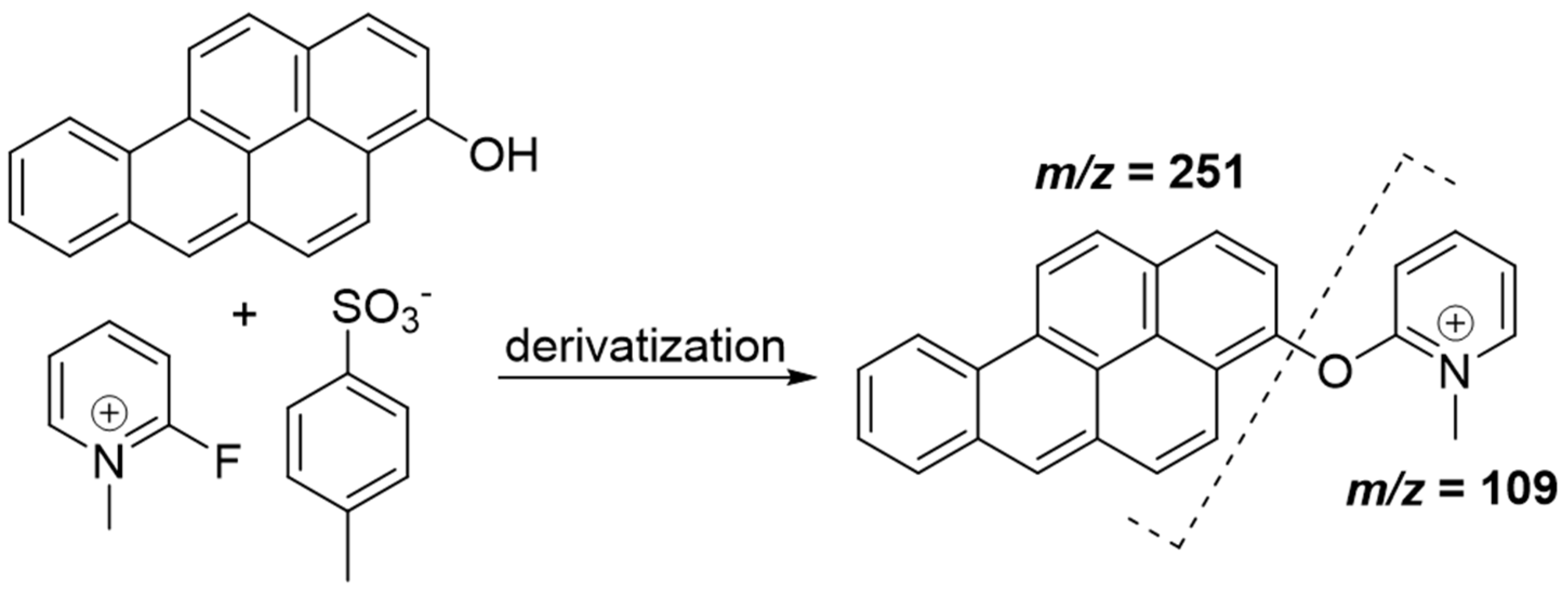
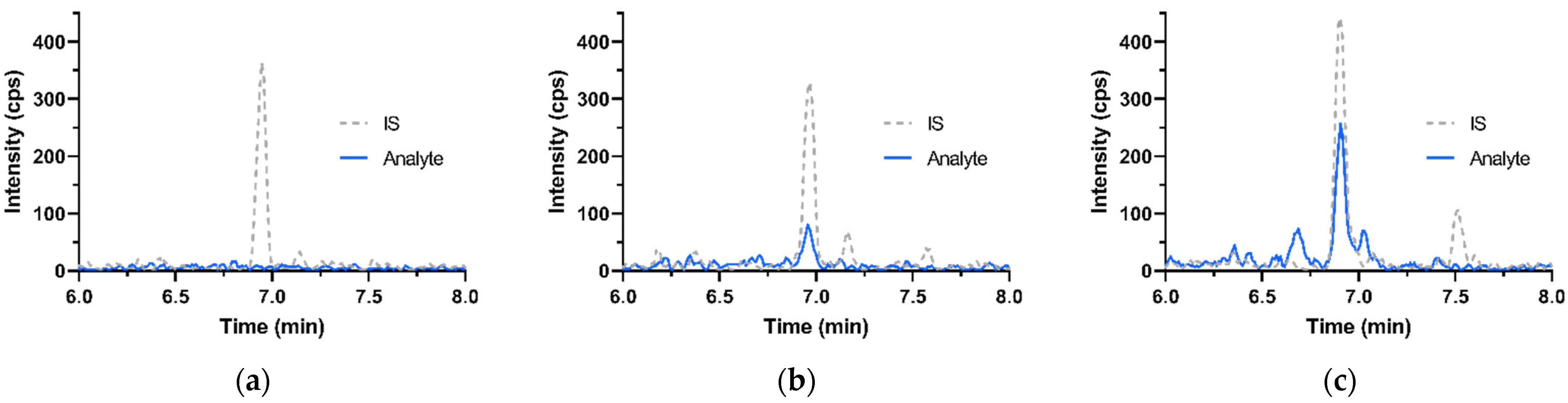

| Analyte or IS | Retention Time (min) | Mass Transitions (m/z) | Role | Dwell Time (msec) | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| 3-OH-BaP | 6.9 | 360 → 251 | Quantifier | 150 | 161 | 45 | 18 |
| 3-OH-BaP | 6.9 | 360 → 267 | Qualifier | 150 | 161 | 45 | 18 |
| 13C6-3-OH-BaP | 6.9 | 366 → 257 | IS | 150 | 161 | 45 | 18 |
| User Groups 1 | N (m/f) | Age (Years) | BMI | 24 h Urine Volume (mL) | |||
|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | ||
| CC | 10 (6/4) | 35.1 | ±9.1 | 26.0 | ±3.9 | 2891 | ±828 |
| HTP | 10 (6/4) | 36.1 | ±12 | 25.5 | ±3.2 | 2685 | ±1300 |
| OT | 10 (9/1) | 28.1 | ±8.2 | 25.9 | ±4.2 | 2638 | ±1290 |
| EC | 10 (6/4) | 38.4 | ±14 | 23.5 | ±2.7 | 1627 | ±664 |
| NRT | 10 (5/5) | 35.3 | ±15 | 25.5 | ±3.5 | 1602 | ±802 |
| NU | 10 (6/4) | 32.9 | ±8.8 | 24.7 | ±3.2 | 2475 | ±936 |
| ∑ all | 60 (38/22) | 34.3 | ±11 | 25.2 | ±3.4 | 2320 | ±1090 |
| Validation Parameter | Level | 3-OH-BaP |
|---|---|---|
| LOD 1 | 16.7 pg/L | |
| LLOQ | 50 pg/L | |
| Calibration range | 50–3221 pg/L | |
| Precision, intra-day, N = 5 | ||
| LLOQ: 50 pg/L | 10.1% CV | |
| Low: 100 pg/L | 12.0% CV | |
| Medium: 400 pg/L | 12.3% CV | |
| High: 1600 pg/L | 3.3% CV | |
| Precision, inter-day, N = 3 × 5 | ||
| LLOQ: 50 pg/L | 7.9% CV | |
| Low: 100 pg/L | 9.0% CV | |
| Medium: 400 pg/L | 8.0% CV | |
| High: 1600 pg/L | 5.8% CV | |
| Accuracy, intra-day, N = 5 | ||
| LLOQ: 50 pg/L | 101.8% | |
| Low: 100 pg/L | 105.1% | |
| Medium: 400 pg/L | 94.0% | |
| High: 1600 pg/L | 98.2% | |
| Accuracy, inter-day, N = 3 × 5 | ||
| LLOQ: 50 pg/L | 105.8% | |
| Low: 100 pg/L | 110.7% | |
| Medium: 400 pg/L | 95.6% | |
| High: 1600 pg/L | 99.6% | |
| Recovery 2,3, N = 6 | ||
| Low: 200 pg/L | 121.3% | |
| Medium: 640 pg/L | 108.9% | |
| High: 1600 pg/L | 89.1% | |
| Matrix effect 3, N = 3 | ||
| Low: 200 pg/L | +31.4% | |
| High: 1600 pg/L | +43.3% | |
| Low: IS | +25.3% | |
| High: IS | +47.9% | |
| Re-injection 3, N = 3 × 3 | ||
| Low: 200 pg/L | 5.0% CV | |
| Medium: 640 pg/L | 4.6% CV | |
| 3-OH-BaP (pg/24 h) 1 | ||||||
|---|---|---|---|---|---|---|
| CC | HTP | OT | EC | NRT | NU | |
| Mean ± SD | 149.0 ± 57.0 | 67.14 ± 32.6 | 65.96 ± 32.3 | 40.68 ± 16.6 | 43.31 ± 19.2 | 61.88 ± 23.4 |
| Median | 136.9 | 69.40 | 53.75 | 43.35 | 45.40 | 57.60 |
| Min–max | 87.70–260.3 | 22.30–115.1 | 21.10–118.0 | 17.90–64.60 | 15.30–71.50 | 35.40–107.3 |
| <LLOQ, N (%) 2 | 8 (40%) | 20 (100%) | 20 (100%) | 20 (100%) | 19 (95%) | 20 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rögner, N.; Hagedorn, H.-W.; Scherer, G.; Scherer, M.; Pluym, N. A Sensitive LC–MS/MS Method for the Quantification of 3-Hydroxybenzo[a]pyrene in Urine-Exposure Assessment in Smokers and Users of Potentially Reduced-Risk Products. Separations 2021, 8, 171. https://doi.org/10.3390/separations8100171
Rögner N, Hagedorn H-W, Scherer G, Scherer M, Pluym N. A Sensitive LC–MS/MS Method for the Quantification of 3-Hydroxybenzo[a]pyrene in Urine-Exposure Assessment in Smokers and Users of Potentially Reduced-Risk Products. Separations. 2021; 8(10):171. https://doi.org/10.3390/separations8100171
Chicago/Turabian StyleRögner, Nadine, Heinz-Werner Hagedorn, Gerhard Scherer, Max Scherer, and Nikola Pluym. 2021. "A Sensitive LC–MS/MS Method for the Quantification of 3-Hydroxybenzo[a]pyrene in Urine-Exposure Assessment in Smokers and Users of Potentially Reduced-Risk Products" Separations 8, no. 10: 171. https://doi.org/10.3390/separations8100171
APA StyleRögner, N., Hagedorn, H.-W., Scherer, G., Scherer, M., & Pluym, N. (2021). A Sensitive LC–MS/MS Method for the Quantification of 3-Hydroxybenzo[a]pyrene in Urine-Exposure Assessment in Smokers and Users of Potentially Reduced-Risk Products. Separations, 8(10), 171. https://doi.org/10.3390/separations8100171






