Determination of Genotoxic Azide Impurity in Cilostazol API by Ion Chromatography with Matrix Elimination
Abstract
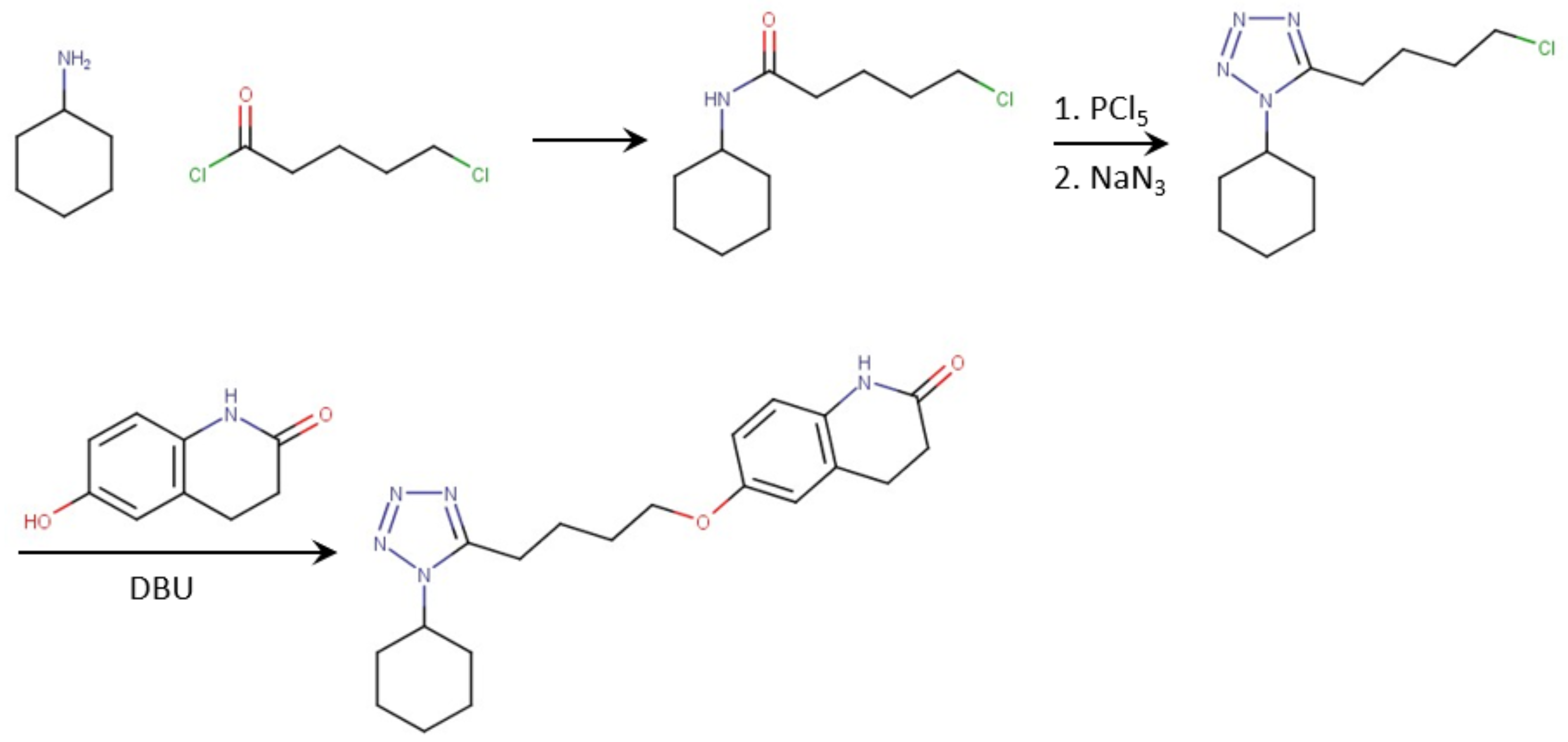
:1. Introduction
2. Materials and Methods
3. Results
3.1. Sample Preparation with Liquid-Liquid Extraction
3.2. Ion Chromatographic Analysis of Sodium Azide
3.2.1. Effect of Flow Rate
3.2.2. Effect of Eluent Concentration
3.2.3. Effect of Column Temperature
3.3. Validation of the Chromatographic Method
3.3.1. Solvent Preparations
- Blank: mixture of 5 mL purified water and 5 mL methylene chloride. It was homogenized by shaking for at least one minute;
- Sodium azide solution: 75 g/mL of sodium azide in dimethyl sulfoxide;
- Sodium azide reference solution: 5 mL of methylene chloride and 50 L of the sodium azide solution was added into a HS vial. After the dissolution, 5 mL of purified water was added. It was homogenized by shaking for at least one minute. For the measurement the upper phase was used;
- Test solution: 100 mg/mL of cilostazol in methylene chloride. After the dissolution 5 mL purified water was added. It was homogenized by shaking for at least one minute. For the measurement the upper phase was used;
- Spiked test solution: 500 mg of cilostazol and 50 L of the sodium azide solution was measured into 5.0 mL methylene chloride. After the dissolution 5 mL purified water was added. It was homogenized by shaking for at least one minute. For the measurement the upper phase ws used;
- Limit of detection: 5 mL of methylene chloride and 50 L of 22.5 g/mL of sodium azide solution was added into a HS vial. After the dissolution 5 mL purified water was measured. It was homogenized by shaking for at least one minute. For the measurement the upper phase was used.
3.3.2. Ion Chromatographic Method for Azide Determination
3.4. Validation Measurements
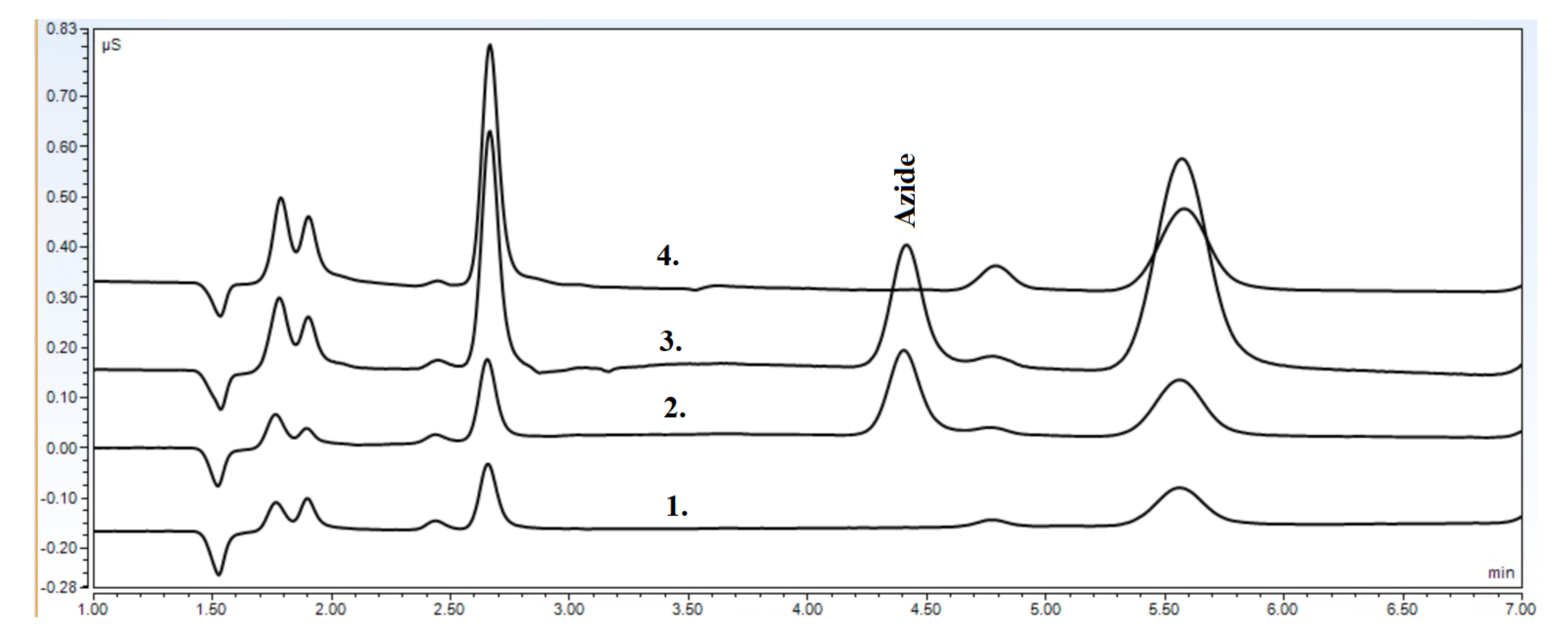
- Specificity. The specificity test has to be made for the limit validation. It can verify that the method is specific and selective for sodium azide;
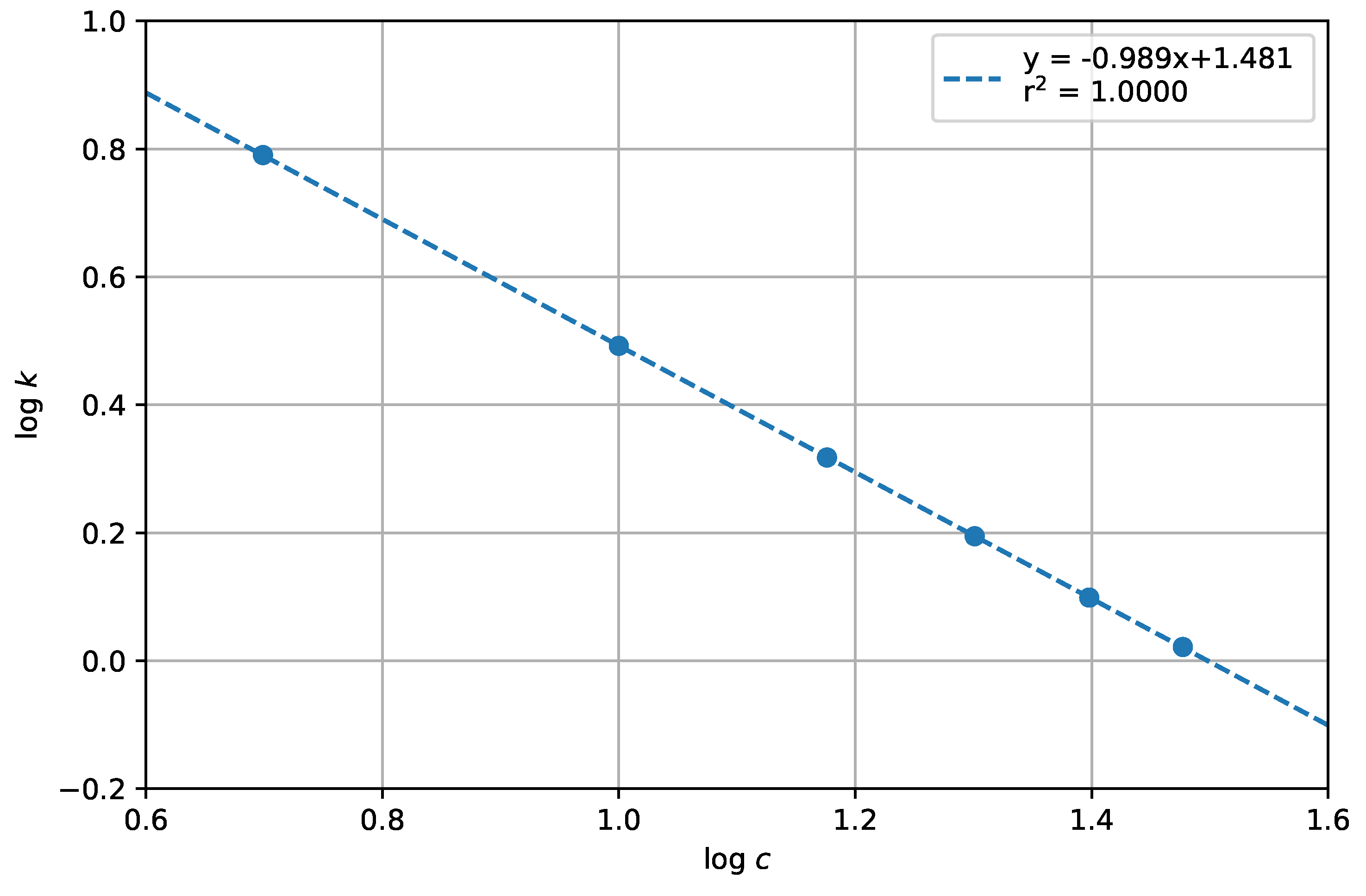
- Limit of quantification (LQ) and limit of detection (LD). Limit of detection (LD) and limit of quantification (LQ) were specified as the minimum concentration, at which the signal of the investigated component was at least three times (LD) and ten times (LQ) greater than the noise level;
- System precision. System precision was demonstrated by calculating the repeatability of six replicate injections of the reference solution at limit level;
- Accuracy and stability. In the study of accuracy, the sample was spiked with sodium azide at limit level (7.5 ppm). The concentrations were determined, and the recoveries of the spiked quantities were calculated in each case.The stability was determined by analyzing the prepared solutions over a period of 24 h in closed plastic vials in the sampler holder.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| API | Active pharmaceutical ingredient |
| CD | Conductivity detector |
| EMA | European medicine agency |
| EGC | Eluent generator cartridge |
| HPIC | High-performance ion chromatography |
| HPLC | High-performance liquid chromatography |
| IC | Ion chromatography |
| ICH | International Council for Harmonization |
| LD | Limit of detection |
| LQ | Limit of quantification |
| LLE | Liquid–liquid extraction |
| QbD | Quality by design |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| UV | Ultra-violet |
References
- Cilostazol Monograph for Professionals. American Society of Health-System Pharmacists. Available online: https://www.drugs.com/monograph/cilostazol.html (accessed on 5 February 2021).
- Gysi, D.; Do Valle, I.; Zitnik, M.; Ameli, A.; Gan, X.; Varol, O.; Ghiassian, S.; Patten, J.; Davey, R.; Loscalzo, J.; et al. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2025581118. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.; Money, S.; Brass, E. Long-term safety of cilostazol in patients with peripheral artery disease: The CASTLE study (Cilostazol: A Study in Long-term Effects). J. Vasc. Surg. 2008, 47, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleemann, A. Cardiovascular Drugs. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: Atlanta, GA, USA, 2008. [Google Scholar] [CrossRef]
- Chang, S.; Lamm, S. Human health effects of sodium azide exposure: A literature review and analysis. Int. J. Toxicol. 2003, 22, 175–186. [Google Scholar] [CrossRef] [PubMed]
- ICH Guidance for Industry M7(R1), Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. 2017. Available online: https://database.ich.org/sites/default/files/M7_R1_Guideline.pdf (accessed on 27 July 2021).
- European Medicines Agency, Cilostazol-Containing Medicines, Cilostazol-Containing Medicines—Article-31 referral—Annex III (Cilostazol), Published: 11 September 2013. Available online: https://www.ema.europa.eu/en/documents/referral/cilostazol-containing-medicines-article-31-referral-annex-iii-cilostazol_en.pdf (accessed on 27 July 2021).
- Vinković, K.; Drevenkar, V. Ion chromatography of azide in pharmaceutical protein samples with high chloride concentration using suppressed conductivity detection. J. Chromatogr. B 2008, 864, 102–108. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. The National Formulary. Irbesartan. USP 31-NF 26; United States Pharmacopeia: Rockville, MD, USA, 2008; p. 2446. [Google Scholar]
- USP Monographs for Dionex Columns (2008 USP Pharmaciopeia). Available online: http://tools.thermofisher.com/content/sfs/manuals/Man-025203-USP-Monographs-Man025203-EN.pdf (accessed on 27 July 2021).
- Kushwah, D.K.; Kohle, P.Y.; Joshi, R.D.; Rajyaguru, B.; Pandey, R.; Vishwakarma, B. Validated Method for the Quantification of Sodium Azide in a Range of `Sartan’ Drugs by Ion Chromatography. Res. J. Pharm. Tech. 2010, 3, 82–86. [Google Scholar]
- Gričar, M.; Andrenšek, S. Determination of azide impurity in sartans using reversed-phase HPLC with UV detection. J. Pharm. Biomed. Anal. 2016, 125, 27–32. [Google Scholar] [CrossRef]
- ICH Guidance for Industry Q2(R1), Validation of Analytical Procedures: Text and Methodology. 2005. Available online: https://database.ich.org/sites/default/files/Q2(R1)Guideline.pdf (accessed on 27 July 2021).
| Run time | 40 min |
| Eluent | KOH solution |
| Eluent flow rate | 0.50 mL/min |
| Gradient program | |
| 15 mM (5 min) 80 mM (12 min) 15 mM (17 min) | |
| Temperatures | |
| Autosampler temperature | 15C |
| Column temperature | 40 C |
| CD detector cell temperature | 35 C |
| Compartment temperature | 30 C |
| Suppressor | |
| 19 mA (7 min) ⟶ 99 mA(18 min) ⟶ 19 mA (15 min) | |
| Injection volume | 5.0 L |
| Parameters | Results | Requirement |
|---|---|---|
| Specificity (ppm) | 7.5 | 7.5 |
| Retention time (, min) | 4.41 | – |
| Retention factor (k; = 1.5 min) | 2.94 | 1–10 |
| Plate number | 5097 | 2000 |
| Symmetry factor (As) | 1.1 | 1.5 |
| Limit of Detection (S/N = 3) | 0.52 | 2.25 |
| Limit of Quatification (S/N = 10) | 1.73 | 7.5 |
| System precision (at 7.5 ppm) | ||
| Retention time (RSD%) | 0.14 | 5 |
| Peak area (RSD%) | 16.5 | 20 |
| Recovery (%, at 7.5 ppm) | 102.4 | 75–125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páll, B.; Gyenge, Z.; Kormány, R.; Horváth, K. Determination of Genotoxic Azide Impurity in Cilostazol API by Ion Chromatography with Matrix Elimination. Separations 2021, 8, 162. https://doi.org/10.3390/separations8100162
Páll B, Gyenge Z, Kormány R, Horváth K. Determination of Genotoxic Azide Impurity in Cilostazol API by Ion Chromatography with Matrix Elimination. Separations. 2021; 8(10):162. https://doi.org/10.3390/separations8100162
Chicago/Turabian StylePáll, Boglárka, Zsuzsa Gyenge, Róbert Kormány, and Krisztián Horváth. 2021. "Determination of Genotoxic Azide Impurity in Cilostazol API by Ion Chromatography with Matrix Elimination" Separations 8, no. 10: 162. https://doi.org/10.3390/separations8100162
APA StylePáll, B., Gyenge, Z., Kormány, R., & Horváth, K. (2021). Determination of Genotoxic Azide Impurity in Cilostazol API by Ion Chromatography with Matrix Elimination. Separations, 8(10), 162. https://doi.org/10.3390/separations8100162






