Abstract
A highly specific, accurate, and simple RP-HPLC technique was developed for the real-time quantification of domperidone (DOMP) and lansoprazole (LANS) in commercial formulations. Chromatographic studies were performed using a Luna C8(2), 5 μm, 100Å, column (250 × 4.6 mm, Phenomenex) with a mobile phase composed of acetonitrile/2 mM ammonium acetate (51:49 v/v), pH 6.7. The flow rate was 1 mL·min−1 with UV detection at 289 nm. Linearity was observed within the range of 4–36 µg·mL−1 for domperidone and 2–18 µg·mL−1 for lansoprazole. Method optimization was achieved using Box-Behnken design software, in which three key variables were examined, namely, the flow rate (A), the composition of the mobile phase (B), and the pH (C). The retention time (Y1 and Y3) and the peak area (Y2 and Y4) were taken as the response parameters. We observed that slight alterations in the mobile phase and the flow rate influenced the outcome, whereas the pH exerted no effect. Method validation featured various ICH parameters including linearity, limit of detection (LOD), accuracy, precision, ruggedness, robustness, stability, and system suitability. This method is potentially useful for the analysis of commercial formulations and laboratory preparations.
1. Introduction
Domperidone (DOMP), a peripherally selective dopamine D2 receptor antagonist, is commonly used as an antiemetic known to facilitate rapid gastric emptying while accelerating the bowel transit time [1]. Additionally, it strengthens gastric peristalsis, which promotes faster passage of food through the digestive system and aids in lowering esophageal sphincter pressure. The drug is rapidly absorbed in the stomach and the upper gastrointestinal tract via active transportation processes after oral administration. However, many unpleasant side effects are associated with its use, including dry mouth, abdominal cramps, diarrhea, nausea, rash, itching, hives, and hyperprolactinemia [2,3]. Lansoprazole (LANS) inhibits the proton pump system and can be used for short-term treatment of erosive reflux esophagitis as well as gastric and duodenal ulcer as it retards the manufacture of gastric acid in the body. It is also active against Helicobacter pylori, the bacterial agent widely recognized as the cause of gastric ulcers. Research has shown that lansoprazole is well tolerated with a minimum incidence of side effects when compared to similar drugs such as omeprazole [2,4,5,6,7,8]. Non-aqueous titration is the official method listed in the British Pharmacopeia and European Pharmacopeia [9].
Several methods can also be utilized, either alone or in combinations, for the quantification of domperidone, namely, UV–VIS spectrophotometry, high-performance liquid chromatography (HPLC) and high-performance thin-liquid chromatography (HPTLC) [10,11,12]. Domperidone has been quantified simultaneously using lansoprazole [9]. The quantification of lansoprazole, both in its commercial dosage form and in biological samples, was conducted using HPLC [13], HPTLC, and LC–MS [14,15,16,17], whereas multiple groups have reported simultaneously quantifying both domperidone and lansoprazole via the UV–VIS spectrophotometry method [18], HPLC [19], HPTLC, and ion-pair complex formation reactions [20]. Optimization of the respective quantification methods is complicated as it requires the synchronous measurement of several unique parameters, including the type and component of the organic phase, analyte retention time, peak area, intensity, column temperature, flow rate, pH, and the nature of the stationary phase. On the basis of analyzing the above parameters, the best quality attributes are selected for the optimization purpose. However, the method developed by Janardhanan et al. in 2011 [21] and by Patel et al. in 2009 [22] is different from this method in terms of mobile phase, calibration range as well as Quality by Design (QbD)-based optimization and validation of the method. HPLC separation techniques based on the “trial and error” methodology are time-consuming and can only provide information on the responsiveness of various critical analytical parameters. Additionally, these techniques offer minimal data on how the different parameters listed above interact with, and ultimately influence, each other. In the past, research has been conducted on the development and application of retaliation surface methodologies for the quantification of various compounds [23,24,25,26,27,28,29,30,31,32,33,34]. Therefore, this research was focused on the development of a Box–Behnken-optimized RP-HPLC method for the quantification of domperidone and lansoprazole in new and unusual solvent systems. The optimization of these Box–Behnken-based (BBD) chromatographic conditions was conducted using the Design-Expert 8.0.7.1 software (Stat-Ease Inc., Minneapolis, MN, USA). Previous studies focused on the validation of BBD-optimized analytical techniques [35].
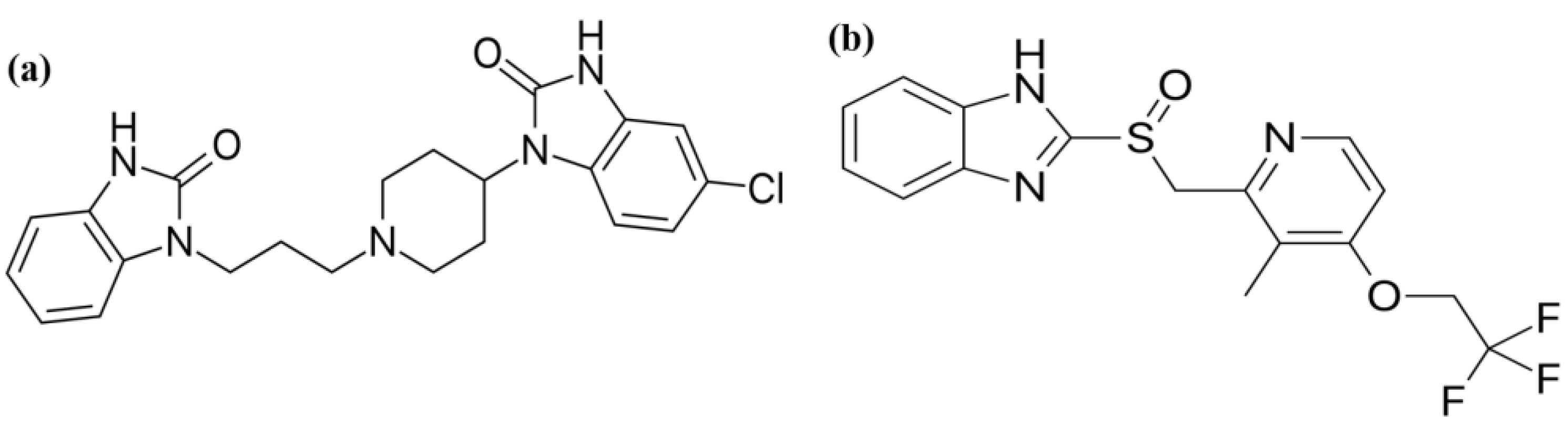
Herein, the development of statistically optimized analytical techniques containing different solvent mixtures for the simultaneous quantification of domperidone and lansoprazole (Figure 1a,b) in commercial dosage forms is described. The application of a response surface methodology (RSM) enabled us to design and successfully optimize a highly sensitive analytical protocol that could be validated in accordance with ICH guidelines using criteria such as linearity, limit of detection (LOD), accuracy, precision, ruggedness, robustness, stability, and system suitability [29]. Using this quantification technique, we were able to generate a mixture with a specified combined dose of domperidone and lansoprazole.
Figure 1.
Chemical structures of (a) domperidone and (b) lansoprazole.
2. Experimental Section
2.1. Reagents and Pharmaceutical Preparations
Analytical grade samples of domperidone (>98% pure) and lansoprazole (>98% pure) were procured from Sigma-Aldrich (Kuala Lumpur, Malaysia) and used without further purification. All solvents used in the analysis were acquired as HPLC grade. A mixture containing 30 mg of domperidone and lansoprazole was produced for the proposed simultaneous quantification protocol.
2.2. Instrumentation
The HPLC system used in this study (Hitachi, Chiyoda, Japan) consisted of a binary pump (CM5110 with a maximum flow rate range of 9.999 mL·min−1), an autosampler with an injection volume of 0.1–100 μL, a column oven (CM 5310 with a temperature range of 1–85 °C), and an ultraviolet detector (L-2455 with limits of detection between 190 and 900 nm) operating at a wavelength of 280 nm. The reversed-phase C8 column was 250 × 4.6 mm in dimension with a particle size of 5 μm used for separation. Acquisition, evaluation, and storage of the chromatographic data collection were performed using the Chromaster System Manager software D-2000 Elite.
2.3. Preparation of the Standard Solutions
Domperidone and lansoprazole (25 mg each) were dissolved separately in a minimum amount of a mobile phase mixture containing acetonitrile and 2 mM ammonium acetate (51:49 v/v, pH 6.7) in a 25 mL volumetric flask. From these stock solutions, aliquots of domperidone (5 mL) and lansoprazole (2.5 mL) were transferred to a 50 mL volumetric flask, and the volume was adjusted using a mobile phase solution to maintain the concentration of domperidone and lansoprazole at 100 µg·mL−1 and 50 µg·mL−1, respectively.
2.4. Mobile Phase Preparation
A 2 mM ammonium acetate buffer was prepared by dissolving ammonium acetate (0.0154 g) in 100 mL of HPLC grade water before filtration using a 0.22 μm membrane filter. The mobile phase used was acetonitrile to 2 mM ammonium acetate buffer in a ratio of 51:49 (v/v). The experiments were conducted using a binary pump.
2.5. Preparation of the Pharmaceutical Sample Solutions
Domperidone (10 mg) and lansoprazole (30 mg) were dissolved separately in 60 mL of the mobile phase (i.e., in a 2 mM ammonium acetate solution). After complete dissolution was achieved via sonication, the samples were transferred to a 100 mL volumetric flask and filtered through a 0.45 μm membrane filter. The remaining undissolved particles were washed repeatedly with 10 mL of the mobile phase; the eluent was collected, and the final volume of the resulting solution, which was composed of the same solvent as the mobile phase, was 100 mL. This solution was then subjected to a two-step dilution process. Here, 100 mL of the stock solution were first diluted by a factor of 1:10 in step one, and an aliquot of this 100 mL solution was then diluted by a factor of 2:10 with the mobile phase in step two. Finally, the concentration of each component in the formulation was determined using the proposed RP-HPLC method.
2.6. Method Development and Experimental Design
The composition of the mobile phase was examined with different ratios of acetonitrile and the ammonium acetate buffer using Design-Expert 8.0.7.1 statistical software. The mobile phase was selected based on previous investigations involving a combination of acetonitrile and the ammonium acetate buffer. Three independent variables were chosen for statistical analysis, namely, flow rate (variable A), ammonium acetate buffer (variable B), and pH (variable C). Response variables such as peak area (represented as Y1 for domperidone and Y3 for lansoprazole) and retention time (represented as Y2 for domperidone and Y4 for lansoprazole) were selected as parameters of robustness. Optimization of the developed method was achieved via the response surface methodology in which Box-Behnken Design (BBD) was employed using Design-Expert. A series of seventeen experimental runs were conducted, and the resulting data from the design matrix are shown in Table 1. The design matrix used to generate the reaction variables (i.e., Y1, Y2, Y3, and Y4) are shown in Table 2. The noteworthy variations in the design matrix were computed with the help of analysis of variance (ANOVA). The effects exerted by the independent variables on the response outcomes were examined using the response surface methodology (RSM) to determine the method’s robustness and the composition of the optimized mobile phase.

Table 1.
Design summary.

Table 2.
Linear regression data for DOMP and LANS.
3. Results and Discussion
3.1. Chromatographic Analysis
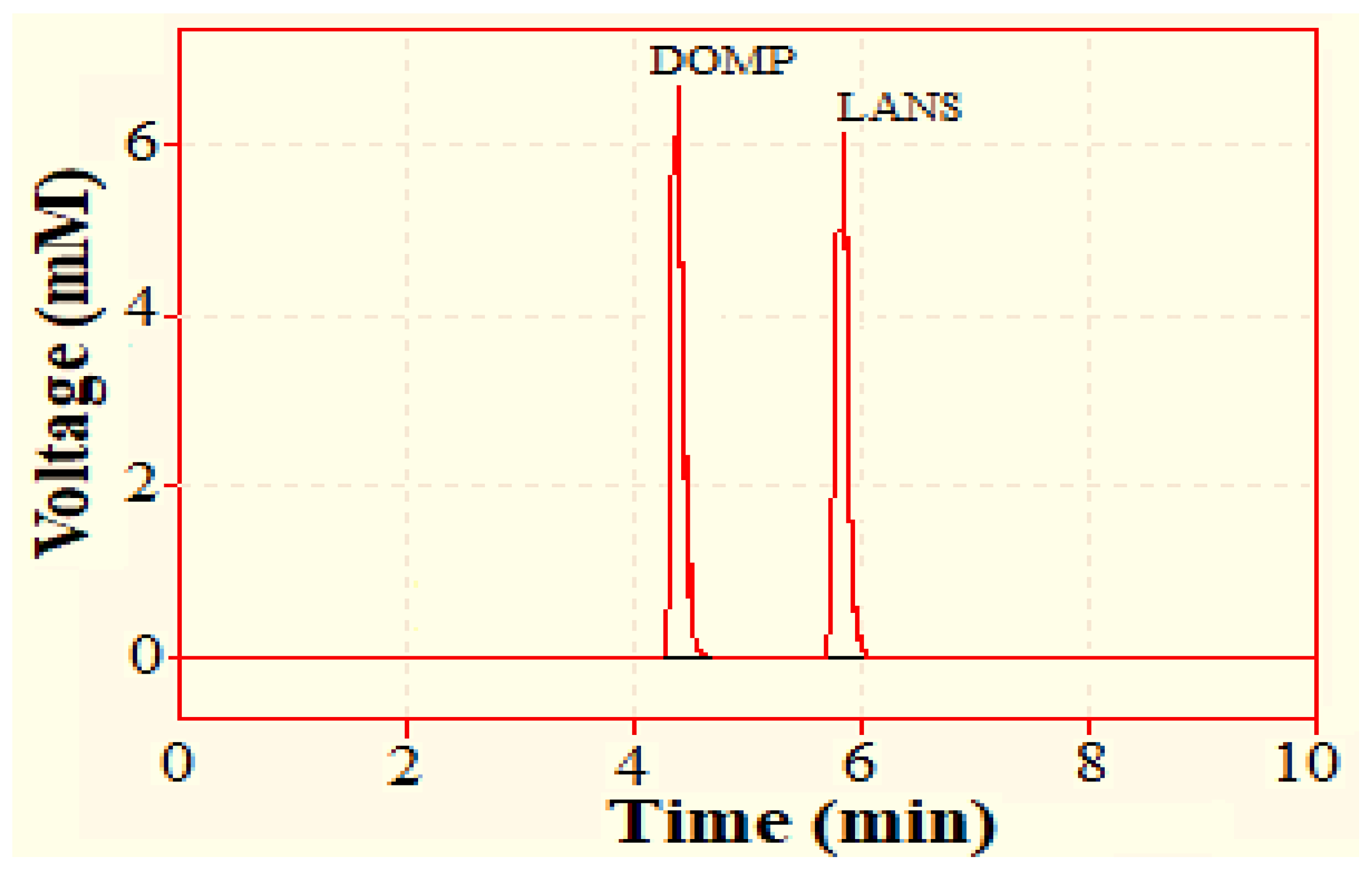
The mobile phase composed of acetonitrile and 2 mM ammonium acetate buffer (51:49 v/v, pH 6.7) was chosen due to its polar composition for the efficient separation of our analytes DOMP and LANS. The resulting chromatographs displayed separate peaks with retention times of 4.375 and 5.762 min for domperidone and lansoprazole, respectively (Figure 2).
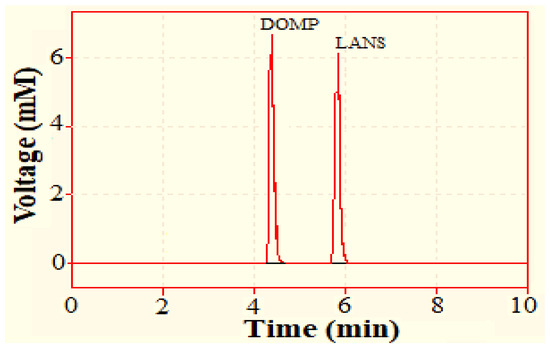
Figure 2.
Chromatogram showing retention time (Rt) of domperidone (DOMP) (4.375 min) and lansoprazole (LANS) (5.726 min).
3.2. Validation of the Analytical Method
Method validation is the process of quantitatively verifying that an analytical test system is suitable for its intended cause and, thus, is capable of providing accurate data. The plausibility of the standardization test’s design was determined by simultaneously quantifying various concentrations of domperidone and lansoprazole using a prediction set containing calibrated standards. In our proposed system for the mean retrieval of data, reasonable standard variations were fitted to the relevant data models, and the results were statistically acceptable due to the high-quality recovery values obtained. As a result, our system was recognized as statistically valid. Parameters, such as linearity, selectivity, sensitivity, stability, ruggedness, and robustness, were subsequently validated.
3.3. Linearity
To establish the linearity of the proposed HPLC method, a series of dilutions ranging from 4 to 36 µg·mL−1 for DOMP and from 2 to 18 µg·mL−1 LANS were set individually. Linearity was determined using the ratio of the peak area response variable of the internal standard to the pure analytes. The peak area was plotted graphically as a function of the analyte concentration. The regression line, which provided information on the correlation coefficient for the calibration curves, showed that there was a proportionate relationship between the peak area and the concentration of domperidone and lansoprazole (Table 2). The correlation coefficient, the slope, and the intercept values, which were used to authenticate the linearity of the calibration graphs, were plotted against peak area and concentration. A straight-line match was observed in the concentration ranges of 4–36 µg·mL−1 and 2–18 µg·mL−1 for domperidone and lansoprazole, respectively.
3.4. Limits of Detection and Quantification
The ICH standard recommendations were used to determine the limits of detection and quantification. Here, known analytes in low concentrations were compared with the signals of the blank samples, and the resulting chromatographs were analyzed. Signal-to-noise ratios of 3:1 and 10:1 were calculated for the limits of detection and quantification, respectively. As seen in Table 2, the method had LOD and LOQ values of 0.020 μg·mL−1 and 0.068 μg·mL−1, respectively, for domperidone and 0.018 μg·mL−1 and 0.066 μg·mL−1, respectively, for lansoprazole. Basically, the LOD and LOQ indicated by Patel et al. were found to be 0.03 and 0.09 μg·mL−1, respectively, for LANS and 0.23 and 0.72 μg·mL−1, respectively, for DOM. However, Janardhanan et al. reported 1.76 and 5.36 μg·mL−1, respectively, for LANS and 2.8 and 8.42 μg·mL−1, respectively, for DOM. In comparison to these methods, the present method is superior in terms of LOD and LOQ. The experimental results indicated that the composition of the selected mobile phase was responsible for the higher sensitivity.
3.5. Selectivity
The proposed procedure for determining the selectivity of this method was evaluated by examining the recovery of the mean percentage and the percentage relative standard deviation using five duplicate samples at a concentration of 10 μg·mL−1 (Table 3). The recovery percentage of domperidone was 99.77%, and the percentage relative standard deviation was 0.38%, both of which were clear evidence of the high selectivity of this procedure.

Table 3.
Summary of validation parameters for the proposed HPLC methods.
3.6. Precision
The precision of the method was estimated by analyzing domperidone and lansoprazole at various concentrations for different time intervals on the same day and repeating the same analysis on three different days to obtain the intraday and interday precision values, respectively. The proposed protocols were precise, and the percentage relative standard deviation for the intraday and interday precision values ranged from 0.41% to 1.53% and from 0.68% to 1.24%, respectively. These values were within the 2% prescribed limits dictated by the ICH guidelines (Table 3).
3.7. Accuracy
The standard addition equated technique was used to determine the accuracy of the analytical findings. Here, the percentage recovery and the percentage relative standard deviation values were within the range of 99.26–99.89% and 0.42–0.83% (domperidone) and 98.9–100.2% and 0.07–0.59% (lansoprazole), respectively, thereby satisfying the acceptance criterion dictated by the ICH guidelines (Table 3).
3.8. Stability
Stability studies showed that the samples were stable in a benchtop bioreactor at ± 2 °C for 22 h (short-term stability) and at −40 °C for up to 42 days (long-term stability) at low temperatures. On inspection, we also determined that the samples did not decrease below the acceptance range of 90–110% (Table 4).

Table 4.
Short-term and long-term stability of DOMP and LANS.
3.9. Ruggedness
The ruggedness of a method is defined by the size and strength of the samples that can be analyzed. Here, analysis of aliquots from a homogenous mixture was performed by investigating the sample’s functional and environmental circumstances, and ensuring that the defined parameters remained within the specified limits of the test. A fixed-dose combination assay was executed under various experimental conditions, using different analysts and experiment dates. The resulting parameters for measuring the ruggedness of the procedure were 101.01% (domperidone) and 99.31% (lansoprazole) in the measured samples, which were within the defined acceptance limit.
3.10. Robustness of the Test Using the Box-Behnken Experimental Design
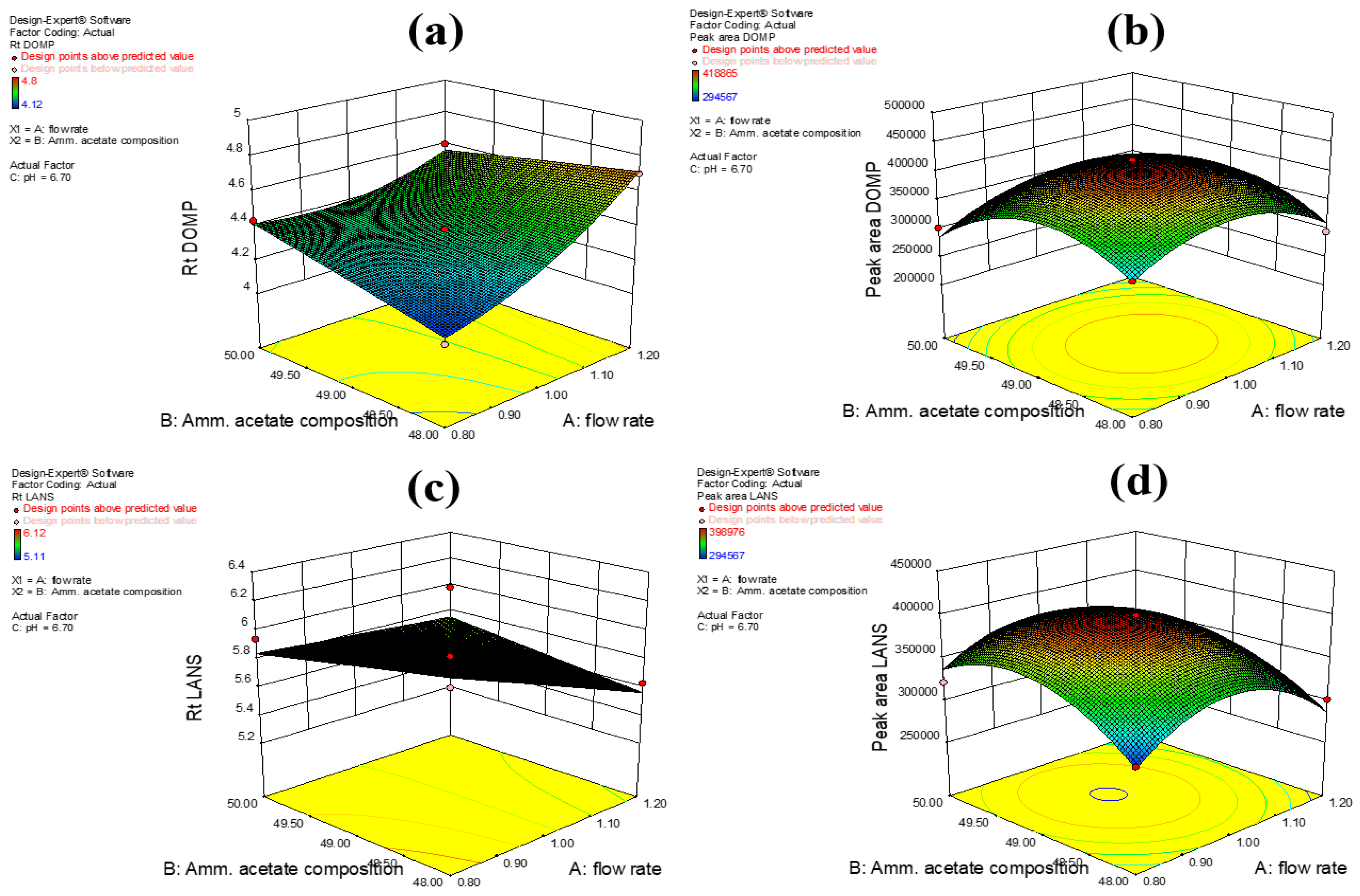
A chromatographic method’s insusceptibility to small, premeditated variations related to factors such as the mobile phase composition, flow rate, and column temperature is a measure of the method’s robustness. In our study, the robustness of our method was established via Box-Behnken design using variables such as the flow rate (A), the mobile phase (51:49 v/v acetonitrile/2 mM ammonium acetate buffer) composition (B), and pH (C). Similarly, the peak area (cm2) (Y2 and Y4 for domperidone and lansoprazole, respectively) and the retention time (min) (Y1 and Y3 for domperidone and lansoprazole, respectively) were also considered as variables for judging the method’s robustness. After several runs were conducted during the experimental analysis, the proposed sample mixtures were monitored on the system, as summarized in Table 1. From the multiple runs, the average of three duplicate samples was chosen for a specific chromatographic range and criteria (Table 5). The experiments were performed in series to determine the associated error deviation for each test run and to check the predictive rationality of the method’s design. The proposed model derived from the Box–Behnken-based design was finally implemented, and response outcomes such as retention time and peak area were noted (Figure 3a–d).

Table 5.
Chromatographic conditions.
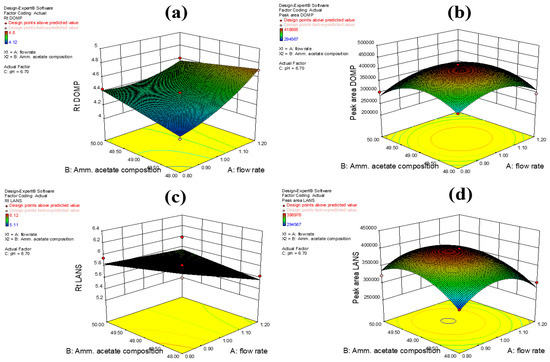
Figure 3.
3D surface responses showing (a) retention time of DOMP (Y1), (b) peak area of DOMP (Y2), (c) retention time of LANS (Y3), and (d) peak area of LANS (Y4).
All experiments were executed in random order to minimize the risk of introducing bias in the results. From the experimental model, a second-degree quadratic equation was recommended with the highest least squares regression (r2) values of 0.9815 (for Y1), 0.8936 (for Y2), 0.8462 (for Y3), and 0.9606 (for Y4); these values were compared with other models. The lack-of-fit on response variables were considered to be insignificant due to the high p-value obtained relative to the model’s F-value. The statistical significance of the peak area and retention times obtained from our method was established using the analysis of variance (ANOVA) test, and the results indicated that both response outcomes were statistically significant. The analysis also showed that response outcomes exhibited significant differences in their values.
The projected F-value was 41.34 for all the variables of interest (i.e., the flow rate, the mobile phase composition, and the pH), indicating that the model was statistically significant with a p-value of 0.0001. In other words, there was only a 0.01% chance that the F-value derived for the variables tested was due to excessive noise in the signal-to-noise ratio. Thus, the F-value associated with our method was within the acceptable limits defined by the ICH guidelines. The results obtained from the ANOVA test were satisfactory for the Y2 response outcome with an F-value of 25.75 for our method, indicating that there was only a 1.08% chance that the predicted F-value was due to excessive noise in the signal-to-noise ratio. For the Y3 response, the F-value was 9.17, which was indicative of statistical significance (i.e., p = 0.0014), meaning that there was only a 0.14% possibility that the F-value predicted by the software was caused by a large noise signal. The Y4 response outcome exhibited a similar trend; here, the predicted F-value of 18.95 represented only a 0.04% possibility of noise interference (p = 0.0004).
The predicted outcomes of our statistical model closely matched the experimental values and were indicative of the high degree of accuracy and precision associated with our method (Table 6, Table 7, Table 8 and Table 9). For domperidone and lansoprazole, the factors that influence both response outcomes are described by the equations listed below.

Table 6.
ANOVA for response surface for Rt DOMP.

Table 7.
ANOVA for response surface for peak area of DOMP.

Table 8.
ANOVA for response surface for Rt LANS.

Table 9.
ANOVA for response surface for peak area of LANS.
The final equation used to predict the retention time of domperidone is as follows:
Rt DOMP (Y1) = + 4.38 + 0.18 × A + 0.026 × B − 0.13 × C − 0.10 × A × B + 0.17 × A × C − 0.15 × B × C + 0.084 × A2 − 1.250 × 10−3 × B+0.17 × C2
The final equation for the actual retention time of domperidone is as follows:
Rt DOMP (Y1) = +316.63625−33.46875 × flow rate +10.71125 × amm. acetate solution − 165.55000 × pH−0.51250 × flow rate × amm. acetate solution+8.25000 × flow rate × pH − 1.50000 × amm. acetate solution × pH +2.09375 × flow rate2 − 1.25000 × 103 × amm. acetate solution2 +17.12500 × pH2
Similarly, equations that describe the peak area are listed below. The final equation, which describes the predicted peak area for domperidone, is as follows:
Peak area DOMP (Y2) = +4.189 × 105 + 316.38 × A − 12413.25 × B − 1165.87 × +5547.25 × A × B + 21741.50 × A × C + 23657.25 × B × C − 53089.50 × A2 − 62171.25 × B2 − 35131.50 × C2
In contrast, the final equation that describes the actual peak area values for domperidone is as follows:
Peak area DOMP (Y2) = −2.20893 × 108 − 5.98642 × 106 × flow rate + 4.46760 × 106× amm. acetate solution + 3.43854 × 107 × pH + 27736.25000 × flow rate × amm. acetate solution + 1.08707 × 106 × flow rate × pH + 2.36572 × 105 × amm. acetate solution × pH − 1.32724 × 106 × flow rate2 − 62171.25000 × amm. acetate solution2 − 3.51315 × 106 × pH2
Similar equations were applied to determine the retention time for lansoprazole. Here, the final equation that describes the predicted coded factors is as follows:
Rt LANS (Y3) = +5.81 − 0.15 × A − 0.01 × B − 0.24 × C +0.10 × A × B − 0.18 × A× C + 0.10 × B × C
On the other hand, the actual retention time observed for lansoprazole is described by the following:
Rt LANS (Y3) = +323.94441 + 34.66875 × flow rate − 7.40375 × amm. acetate solution − 43.45000 × pH + 0.52500 × flow rate × amm. acetate solution − 9.12500 × flow rate × pH + 1.02500 × amm. acetate solution × pH
For the peak area associated with lansoprazole, the final equation for the predicted variable is as follows:
Peak area LANS (Y4) = +3.990 × 105 − 11788.87 × A + 13592.63 × B − 15057.50 × C − 5061.25 × A × B + 21922.50 × A × C − 21805.00 × B × C − 51345.88 × A2 − 41021.87 × B2 − 27650.63 × C2
On the other hand, the final equation for the actual peak area observed for lansoprazole is described by the following:
Peak area LANS (Y4) = −2.88582×108 − 3.59568 × 106 × flow rate + 5.51998 × 106 × amm. acetate solution + 4.64896 × 107 × pH − 25306.25000 × flow rate × amm. acetate solution + 1.09612 × 106 × flow rate × pH − 2.18050 × 105 × amm. acetate solution × pH − 1.28365 × 106 × flow rate2 − 41021.87500 × amm. acetate solution − 2.76506 × 106 × pH2
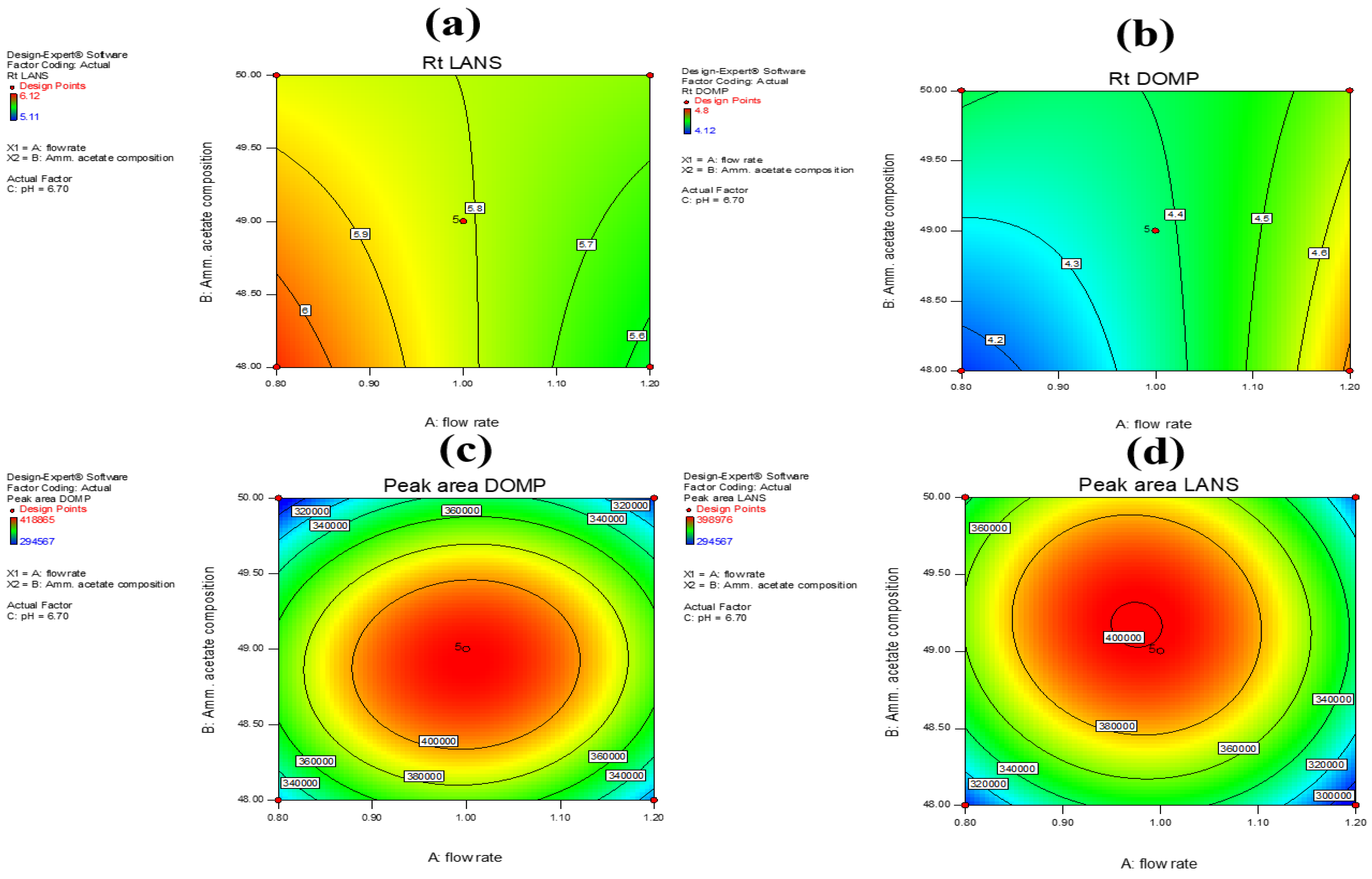
Contour plots representing the response surface variables for Y1, Y2, Y3, and Y4 (Figure 4a–d) were used to present the results of the response outcomes graphically. The influence exerted by the flow rate and mobile phase composition was evident in the plots, whereas pH had little to no effect on the response outcome of interest. We were able to examine the interactions and influence exerted by various external factors on the response outcomes as a means of validating the results obtained using this analysis method. From the results of the aforementioned study on the influence exerted by certain response factors on the retention time and peak area of the drug samples, it was clear that the flow rate of the mobile phase was significantly influenced by the previously mentioned interaction processes. The composition of the mobile phase was affected by multiple interaction processes that were clearly noted in the contour plots (Figure 4a–d). Variable C, i.e., pH, had no effect on either of the response outcomes. These were referred to as the actual factors.
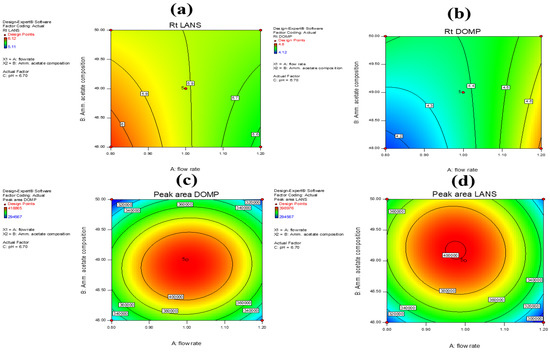
Figure 4.
Effect of individual factors on response to (a) retention time of LANS (Y3), (b) retention time of DOMP (Y1), (c) peak area of DOMP (Y2), and (d) peak area of LANS (Y4).
3.11. Fixed-Dose Combination Analysis
It was theorized that the proposed method was applicable for analyzing fixed-dose combinations of formulated mixtures composed of domperidone and lansoprazole. The percentage resurgence of the above-mentioned mixtures was similar to the label values of these binary formulations (Table 3). These findings demonstrated that the developed method could be employed for the immediate quantification of these commercial drugs in complex matrices such as binary combination samples or human plasma.
4. Conclusions
A straightforward robust RP-HPLC technique was developed that utilized UV–VIS spectrophotometry for the simultaneous identification and quantification of domperidone and lansoprazole in a commercial binary mixture sample. Validation of the proposed analytical method proved that the technique could be applied with consistent, reliable, and accurate data outcomes that could be defined using multiple parameters, including the recovery percentage, the associated degree of uncertainty, the limits of detection and quantification, the detection capability, the level of accuracy, and the degree of precision. After a relatively short run time of ~7 min, this technique could be useful for the efficient quantification of numerous real-world pharmaceutical samples for routine quality control analyses. Other benefits of our method included the reduced costs associated with conducting the analysis and the uptick in sample throughput, a process that was optimized by the experimental design. The Box-Behnken design, which was an extremely effective procedure for conducting method optimization, showed that the outcome of the analysis could be altered by tweaking the mobile phase composition and the flow rate. On the other hand, pH exerted no effect. As a result, strict monitoring of the two above-mentioned factors is crucial during chromatographic testing. We concluded that the results provided proof that the proposed method was suitable for a rapid, yet simple sample preparation technique for routine drug analysis in a binary mixture. It could be concluded that the assigned mode of the proposed method adhered to international guidelines and could be used for obtaining reliable results during routine evaluation of drug samples in pharmaceutical formulations.
Author Contributions
A.A., M.A., and M.M. designed the study protocol, supervised the experimental work and finished the draft of the manuscript. M.T.A. provided the compound of domperidone and lansoprazole and visualized and interpreted all of the obtained data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors extend their sincere appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia, for funding this research work through the project number IFKSURG–1440–076. The authors also thank the RSSU at King Saud University for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ANOVA, analysis of variance; BBD, Box-Behnken design; DOMP, domperidone; HPLC, high-performance liquid chromatography; HPTLC, high-performance thin-liquid chromatography; LANS, lansoprazole; LOD, limit of detection; LOQ, limit of quantification; RP-HPLC, reversed-phase high-performance liquid chromatography; RSM, response surface methodology.
References
- Barone, J.A. Domperidone: A Peripherally Acting Dopamine 2-Receptor Antagonist. Ann. Pharmacother. 1999, 33, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F. Lansoprazole Oro-Dispersible Tablet. Drugs 2005, 65, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Stedman, C.A.M.; Barclay, M.L. Review article: Comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment. Pharmacol. Ther. 2000, 14, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Alai, M. Application of nanoparticles for oral delivery of acid-labile lansoprazole in the treatment of gastric ulcer: In vitro and in vivo evaluations. Int. J. Nanomedicine 2015, 10, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Barradell, L.B.; Faulds, D.; McTavish, D. Lansoprazole. Drugs 1992, 44, 225–250. [Google Scholar] [CrossRef]
- Brogden, R.N.; Carmine, A.A.; Heel, R.C.; Speight, T.M.; Avery, G.S. Domperidone. Drugs 1982, 24, 360–400. [Google Scholar] [CrossRef]
- Reddymasu, S.C.; Soykan, I.; McCallum, R.W. Domperidone: Review of Pharmacology and Clinical Applications in Gastroenterology. Am. J. Gastroenterol. 2007, 102, 2036–2045. [Google Scholar] [CrossRef]
- Singh, I.; Arora, S.; Rana, V.; Arora, G.; Malik, K. Formulation and evaluation of controlled release matrix mucoadhesive tablets of domperidone using Salvia plebeian gum. J. Adv. Pharm. Technol. Res. 2011, 2, 163. [Google Scholar] [CrossRef]
- British Pharmacopoeia Commission, Secretariat of the Medicines and Healthcare Products Regulatory Agency (MHRA). British Pharmacopoeia; British Pharmacopoeia Commission: Norwich, UK, 2013.
- Patel, B.H.; Suhagia, B.N.; Patel, M.M.; Patel, J.R. HPTLC Determination of Rabeprazole and Domperidone in Capsules and its Validation. J. Chromatogr. Sci. 2008, 46, 304–307. [Google Scholar] [CrossRef][Green Version]
- Shirwaikar, A.; Shirwaikar, A.; Kumar, C.D.; Joseph, A.; Kumar, R.; Prabu, S.L. Simultaneous estimation of esomeprazole and domperidone by UV spectrophotometric method. Indian J. Pharm. Sci. 2008, 70, 128. [Google Scholar] [CrossRef]
- Thanikachalam, S.; Rajappan, M.; Kannappan, V. Stability-Indicating HPLC Method for Simultaneous Determination of Pantoprazole and Domperidone from their Combination Drug Product. Chromatographia 2008, 67, 41–47. [Google Scholar] [CrossRef]
- Ali, M.S.; Ghori, M.; Khatri, A.R. Stability indicating simultaneous determination of domperidone (DP), methylparaben (MP) and propylparaben by high performance liquid chromatography (HPLC). J. Pharm. Biomed. Anal. 2006, 41, 358–365. [Google Scholar] [CrossRef]
- Avgerinos, A.; Karidas, T.; Potsides, C.; Axarlis, S. Determination of lansoprazole in biological fluids and pharmaceutical dosage by HPLC. Eur. J. Drug Metab. Pharmacokinet. 1998, 23, 329–332. [Google Scholar] [CrossRef]
- Oliveira, C.H.; Barrientos-Astigarraga, R.E.; Abib, E.; Mendes, G.D.; da Silva, D.R.; de Nucci, G. Lansoprazole quantification in human plasma by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B 2003, 783, 453–459. [Google Scholar] [CrossRef]
- Pandya, K.; Mody, V.; Satia, M.; Modi, I.; Modi, R.; Chakravarthy, B.; Gandhi, T. High-performance thin-layer chromatographic method for the detection and determination of lansoprazole in human plasma and its use in pharmacokinetic studies. J. Chromatogr. B Biomed. Sci. Appl. 1997, 693, 199–204. [Google Scholar] [CrossRef]
- Wahbi, A.-A.M.; Abdel-Razak, O.; Gazy, A.A.; Mahgoub, H.; Moneeb, M.S. Spectrophotometric determination of omeprazole, lansoprazole and pantoprazole in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2002, 30, 1133–1142. [Google Scholar] [CrossRef]
- Sherje, A.; Kasture, A.; Gujar, K.; Yeole, P. Simultaneous spectrophotometric determination of lansoprazole and domperidone in capsule dosage form. Indian J. Pharm. Sci. 2008, 70, 102. [Google Scholar] [CrossRef]
- Janardhanan, V.S.; Manavalan, R.; Valliappan, K. Stability-indicating HPLC method for the simultaneous determination of pantoprazole, rabeprazole, lansoprazole and domperidone from their combination dosage forms. Int. J. Drug Dev. Res. 2011, 3, 323–335. [Google Scholar]
- Ravi, T.; Susheel, J.; Lekha, M. High performance thin layer chromatographic estimation of lansoprazole and domperidone in tablets. Indian J. Pharm. Sci. 2007, 69, 684. [Google Scholar] [CrossRef]
- Devi, O.Z.; Basavaiah, K.; Vinay, K.B. Quantitative determination of lansoprozole in capsules and spiked human urine by spectrophotometry through ion-pair complex formation reaction. J. Saudi Chem. Soc. 2013, 17, 387–396. [Google Scholar] [CrossRef]
- Patel, B.; Dedania, Z.; Dedania, R.; Ramolia, C.; Sagar, G.V.; RS, M. Simultaneous Estimation of Lansoprazole and Domperidone in Combined Dosage Form by RP-HPLC. Asian J. Res. Chem. 2009, 2, 210–212. [Google Scholar]
- Rapalli, V.K.; Singhvi, G.; Gorantla, S.; Waghule, T.; Dubey, S.K.; Saha, R.N.; Hasnain, M.S.; Nayak, A.K. Stability indicating liquid chromatographic method for simultaneous quantification of betamethasone valerate and tazarotene in in vitro and ex vivo studies of complex nanoformulation. J. Sep. Sci. 2019, 42, 3413–3420. [Google Scholar] [CrossRef]
- Panda, S.S.; Ravi Kumar Bera, V.V.; Beg, S.; Mandal, O. Analytical Quality by Design (AQbD)-oriented RP-UFLC method for quantification of lansoprazole with superior method robustness. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 479–485. [Google Scholar] [CrossRef]
- Sivakumar, T.; Manavalan, R.; Muralidharan, C.; Valliappan, K. Multi-criteria decision making approach and experimental design as chemometric tools to optimize HPLC separation of domperidone and pantoprazole. J. Pharm. Biomed. Anal. 2007, 43, 1842–1848. [Google Scholar] [CrossRef]
- Khan, A.; Iqbal, Z.; Khadra, I.; Ahmad, L.; Khan, A.; Khan, M.I.; Ullah, Z. Ismail Simultaneous determination of domperidone and Itopride in pharmaceuticals and human plasma using RP-HPLC/UV detection: Method development, validation and application of the method in in-vivo evaluation of fast dispersible tablets. J. Pharm. Biomed. Anal. 2016, 121, 6–12. [Google Scholar] [CrossRef]
- Beg, S.; Kohli, K.; Swain, S.; Hasnain, M.S. Development and validation of rp-hplc method for quantitation of amoxicillin trihydrate in bulk and pharmaceutical formulations using box-behnken experimental design. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 393–406. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Rao, S.; Singh, M.; Vig, N.; Singh, M.; Budakoti, S.; Ansari, A. Development and validation of an improved LC-MS/MS method for the quantification of desloratadine and its metabolite in human plasma using deutrated desloratadine as internal standard. J. Pharm. Bioallied Sci. 2013, 5, 74–79. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Rao, S.; Singh, M.K.; Vig, N.; Gupta, A.; Ansari, A.; Sen, P.; Joshi, P.; Ansari, S.A. Development and validation of LC–MS/MS method for the quantitation of lenalidomide in human plasma using Box-Behnken experimental design. Analyst 2013, 138, 1581–1588. [Google Scholar] [CrossRef]
- Lewis, J.A.; Snyder, L.R.; Dolan, J.W. Initial experiments in high-performance liquid chromatographic method development II. Recommended approach and conditions for isocratic separation. J. Chromatogr. A 1996, 721, 15–29. [Google Scholar] [CrossRef]
- Bhatt, P.; Saquib Hasnain, M.; Nayak, A.K.; Hassan, B.; Beg, S. Development and Validation of QbD-Driven Bioanalytical LC-MS/MS Method for the Quantification of Paracetamol and Diclofenac in Human Plasma. Anal. Chem. Lett. 2018, 8, 677–691. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Siddiqui, S.; Rao, S.; Mohanty, P.; Jahan Ara, T.; Beg, S. QbD-Driven Development and Validation of a Bioanalytical LC–MS Method for Quantification of Fluoxetine in Human Plasma. J. Chromatogr. Sci. 2016, 54, 736–743. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ansari, S.A.; Rao, S.; Tabish, M.; Singh, M.; Abdullah, M.S.; Ansari, M.T. QbD-Driven Development and Validation of Liquid Chromatography Tandem Mass Spectrometric Method for the Quantitation of Sildenafil in Human Plasma. J. Chromatogr. Sci. 2017, 55, 587–594. [Google Scholar] [CrossRef]
- Sivakumar, T.; Manavalan, R.; Muralidharan, C.; Valliappan, K. An improved HPLC method with the aid of a chemometric protocol: Simultaneous analysis of amlodipine and atorvastatin in pharmaceutical formulations. J. Sep. Sci. 2007, 30, 3143–3153. [Google Scholar] [CrossRef]
- Hubert, C.; Houari, S.; Rozet, E.; Lebrun, P.; Hubert, P. Towards a full integration of optimization and validation phases: An analytical-quality-by-design approach. J. Chromatogr. A 2015, 1395, 88–98. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).