Separation of Benzene/Cyclohexane Mixtures by Pervaporation Using Poly (Ethylene-Co-Vinylalcohol) and Carbon Nanotube-Filled Poly (Vinyl Alcohol-Co-Ethylene) Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. E-VOH and E-VOH/CNT Membranes Preparation
2.3. Membrane Characterization
2.4. Benzene/Cyclohexane Mixture Preparation
2.5. Mass Transfer
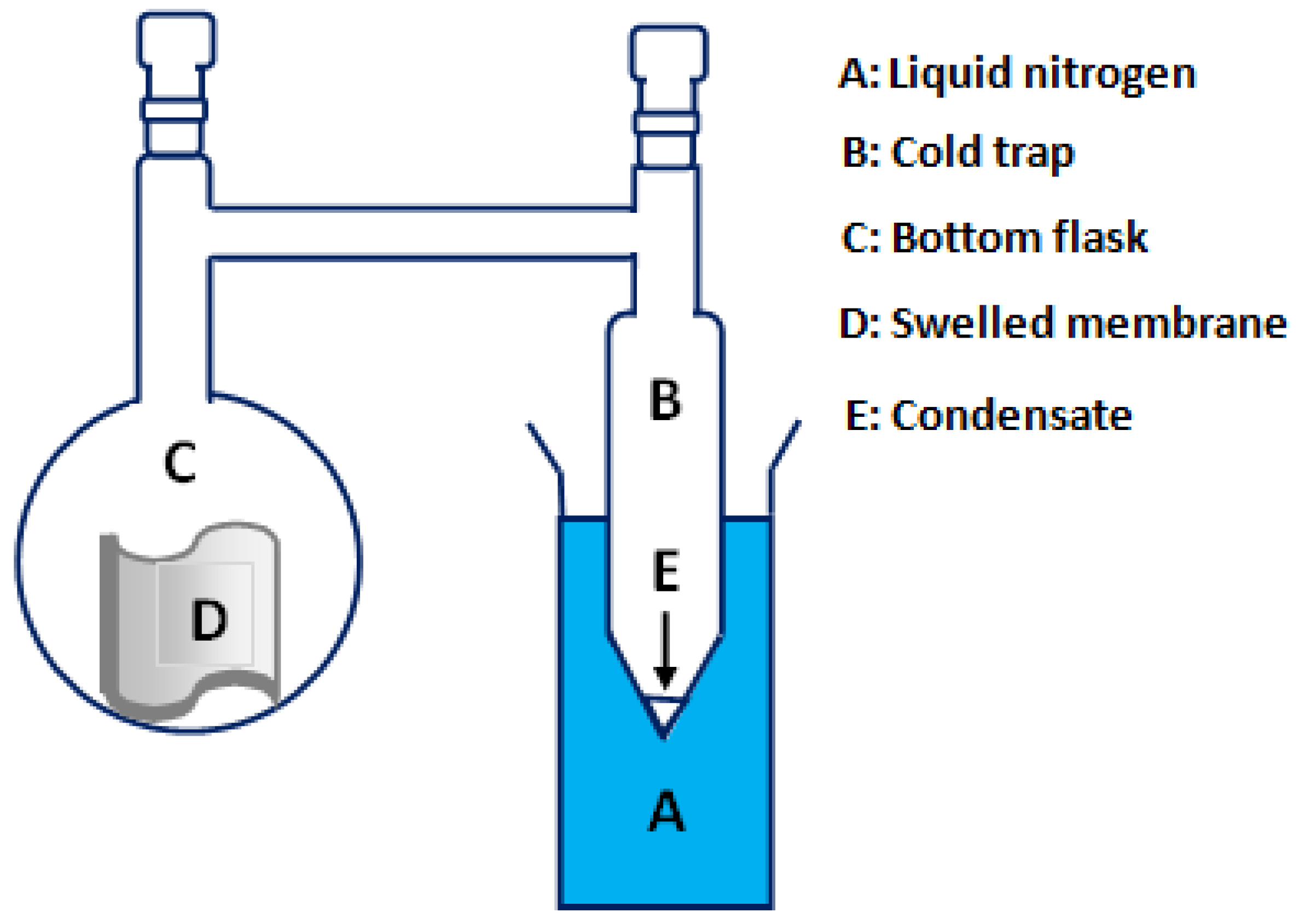
2.5.1. Sorption
2.5.2. Diffusion
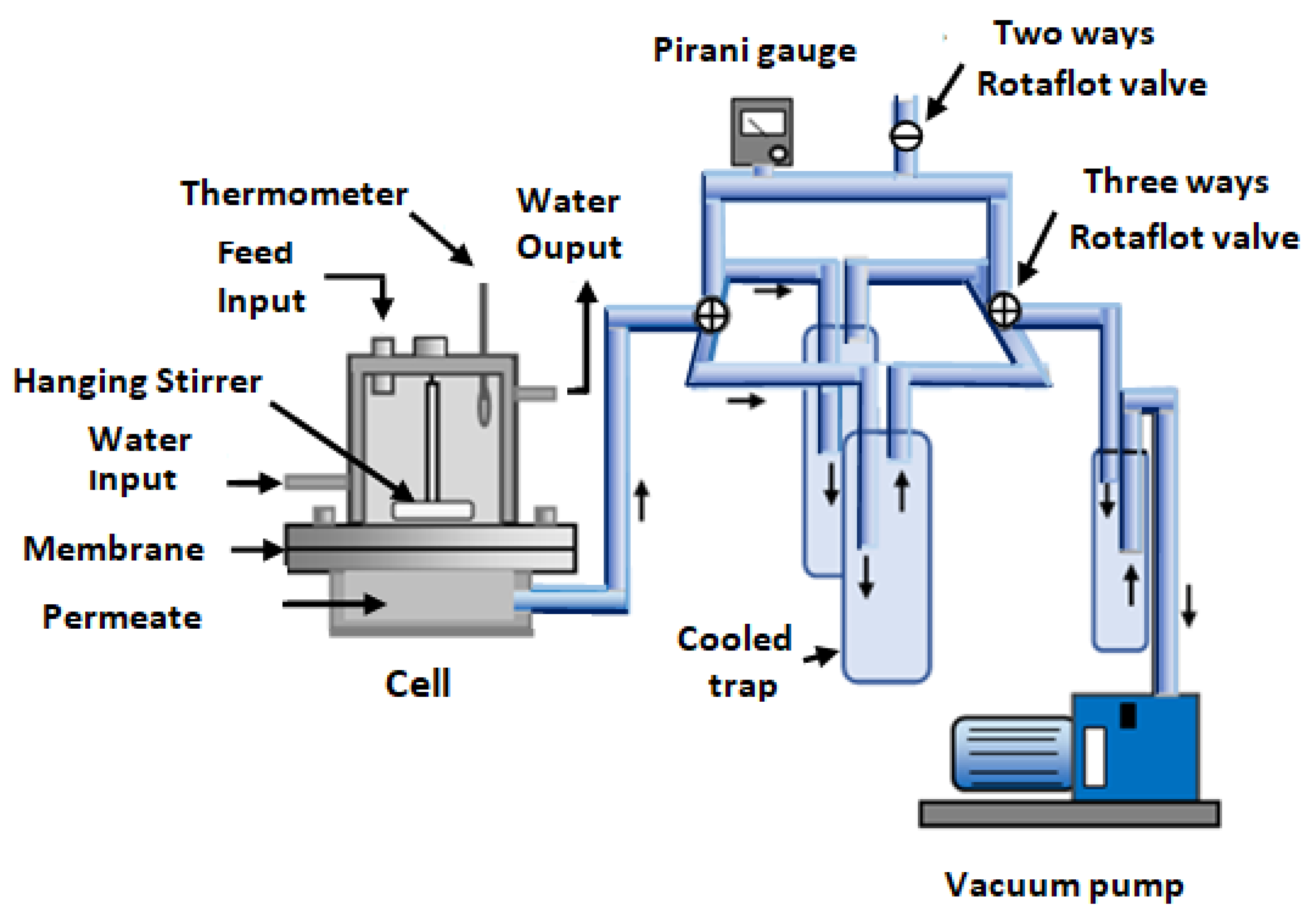
2.6. Pervaporation Experiments
3. Results and Discussion
3.1. Membrane Characterization
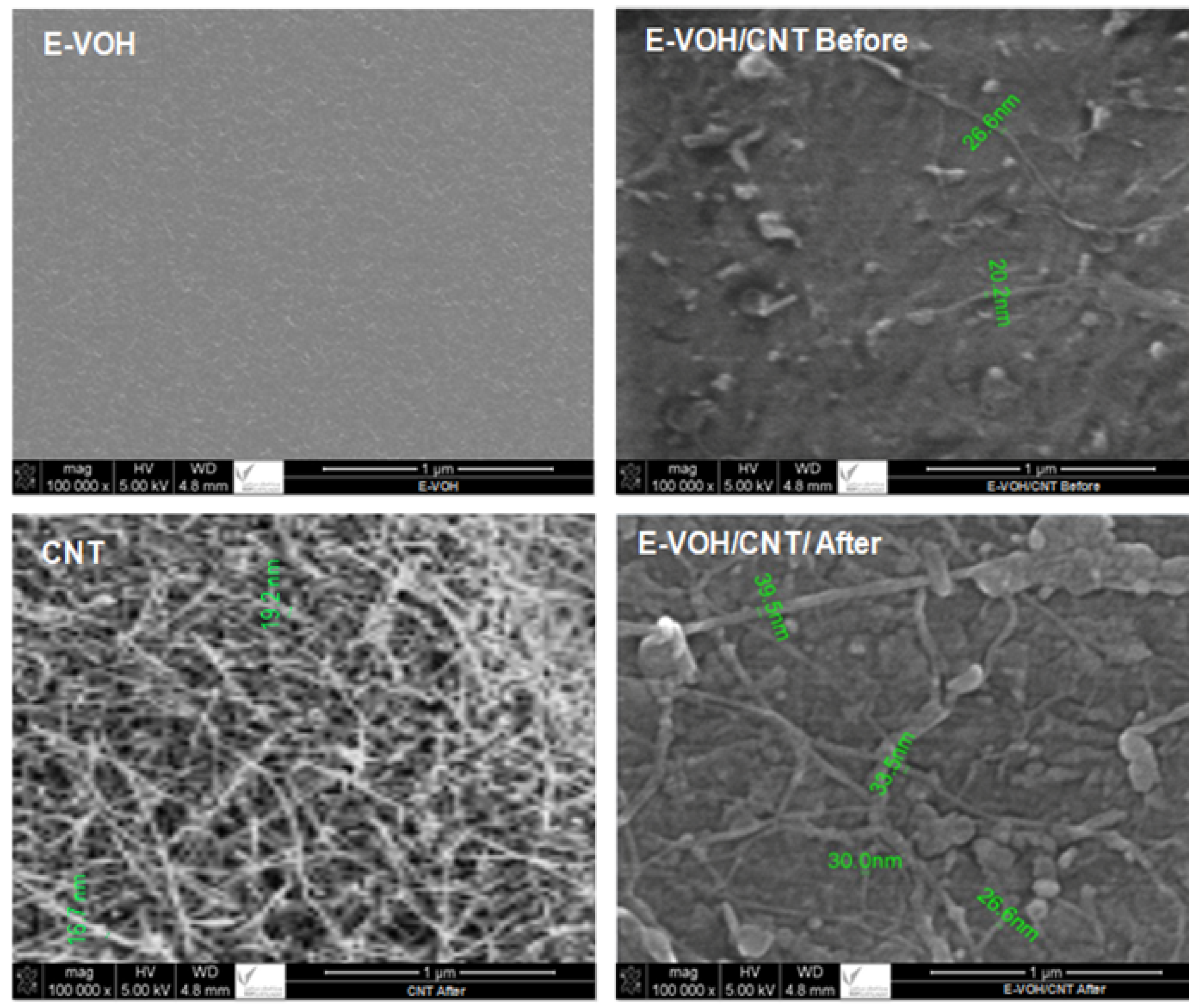
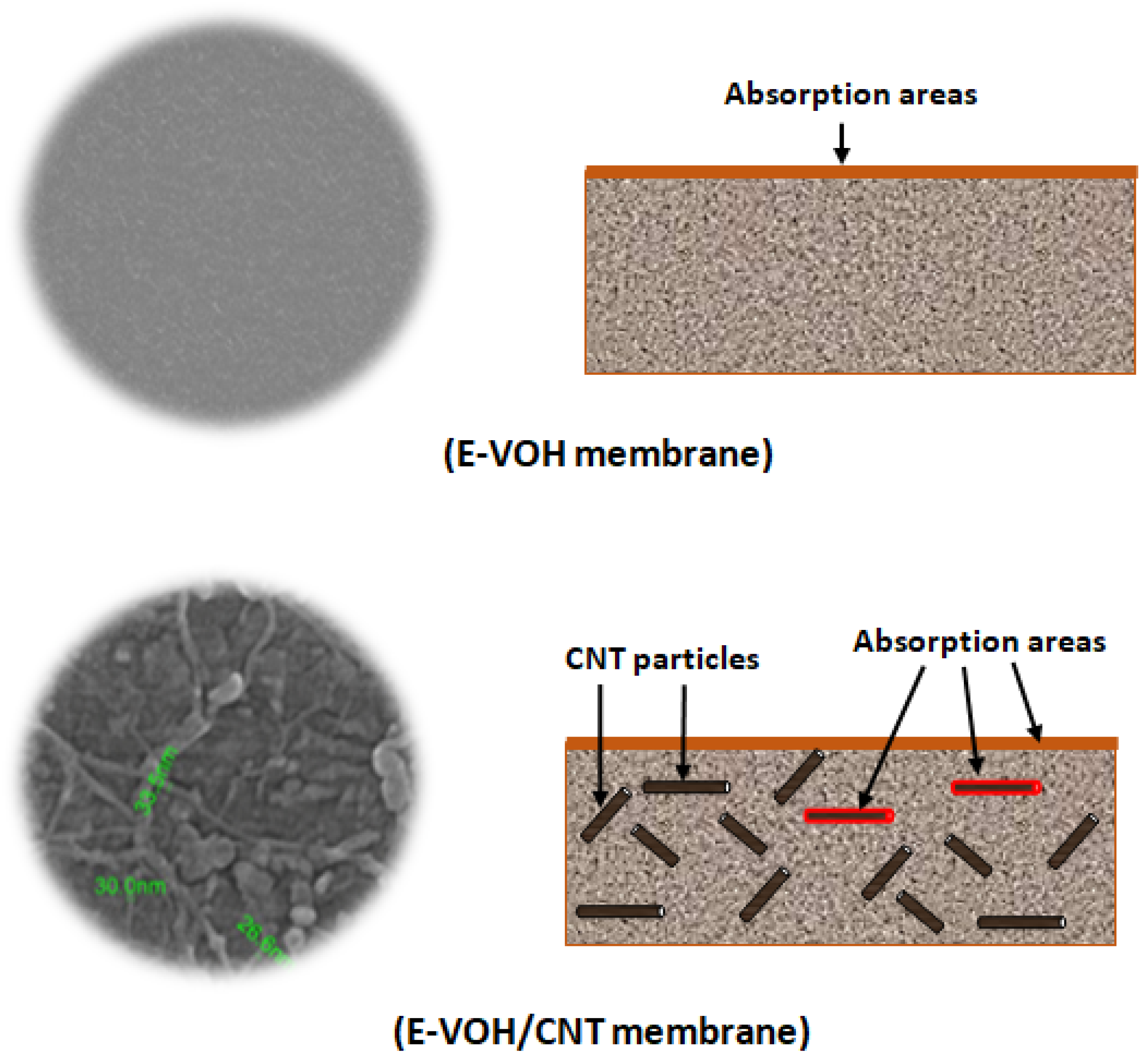
3.1.1. SEM Analysis
3.1.2. DSC Analysis
3.1.3. TGA
3.1.4. XRD Analysis
3.2. Mass Transfer
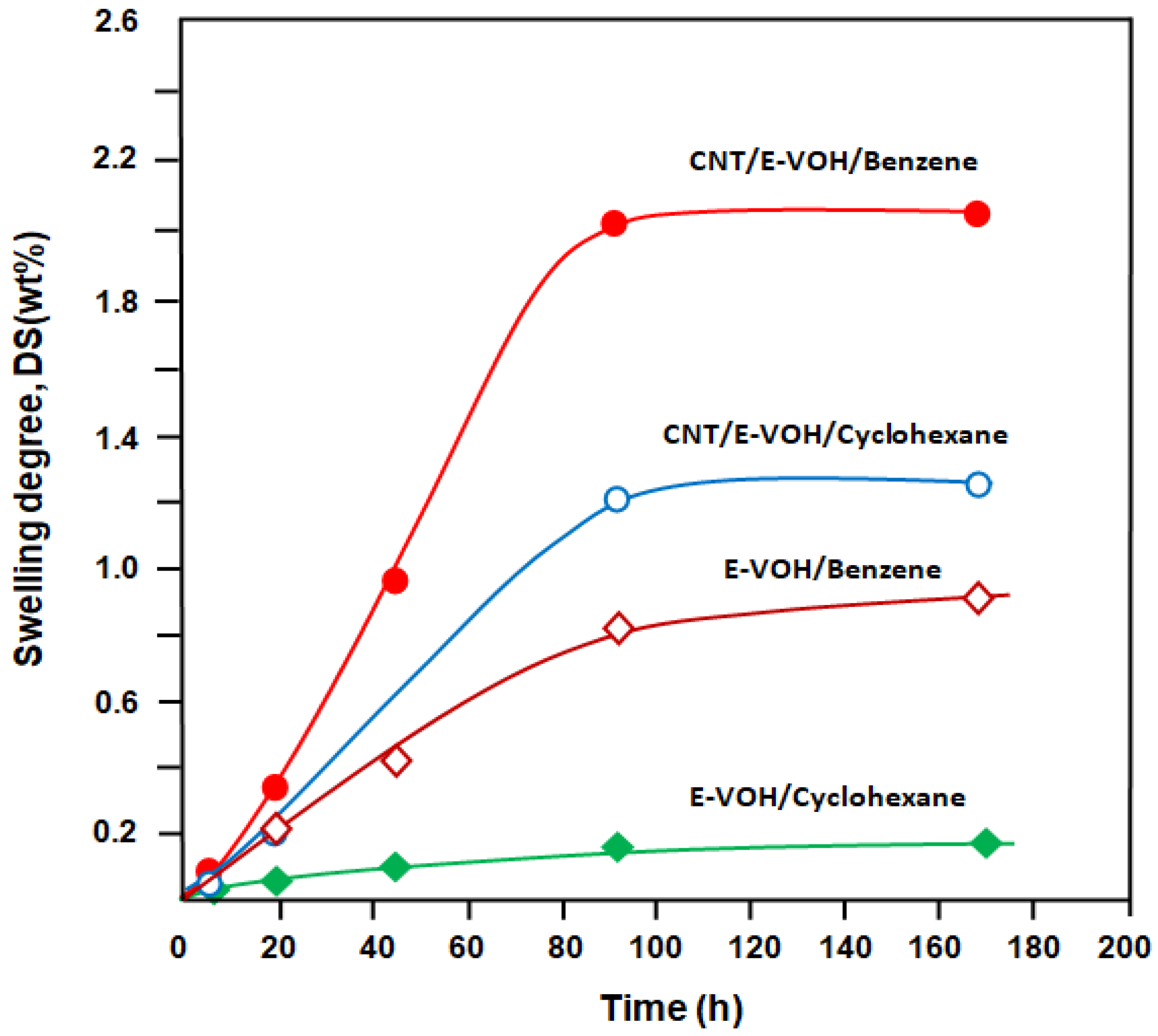
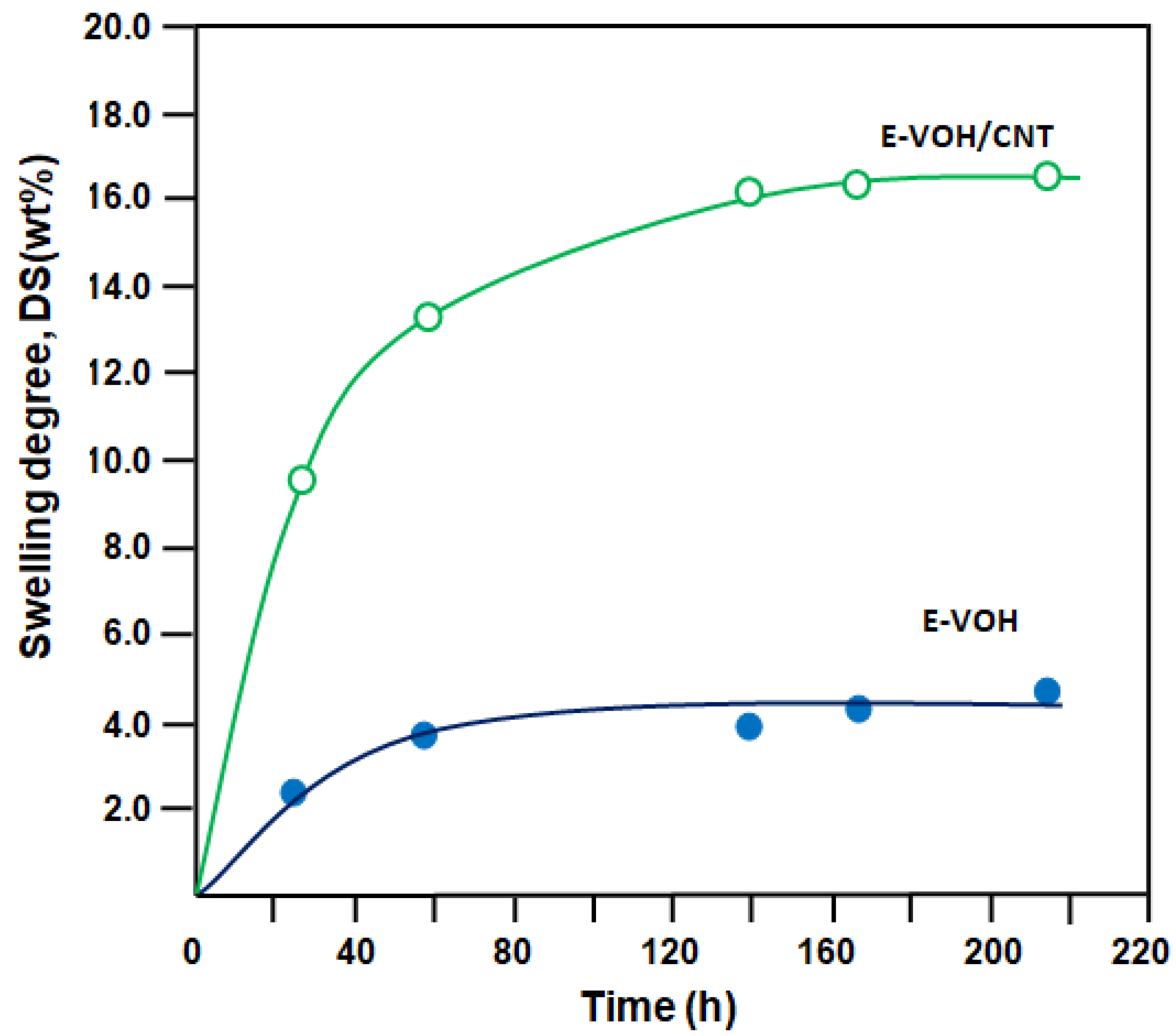
3.2.1. Swelling Degree
3.2.2. Sorption
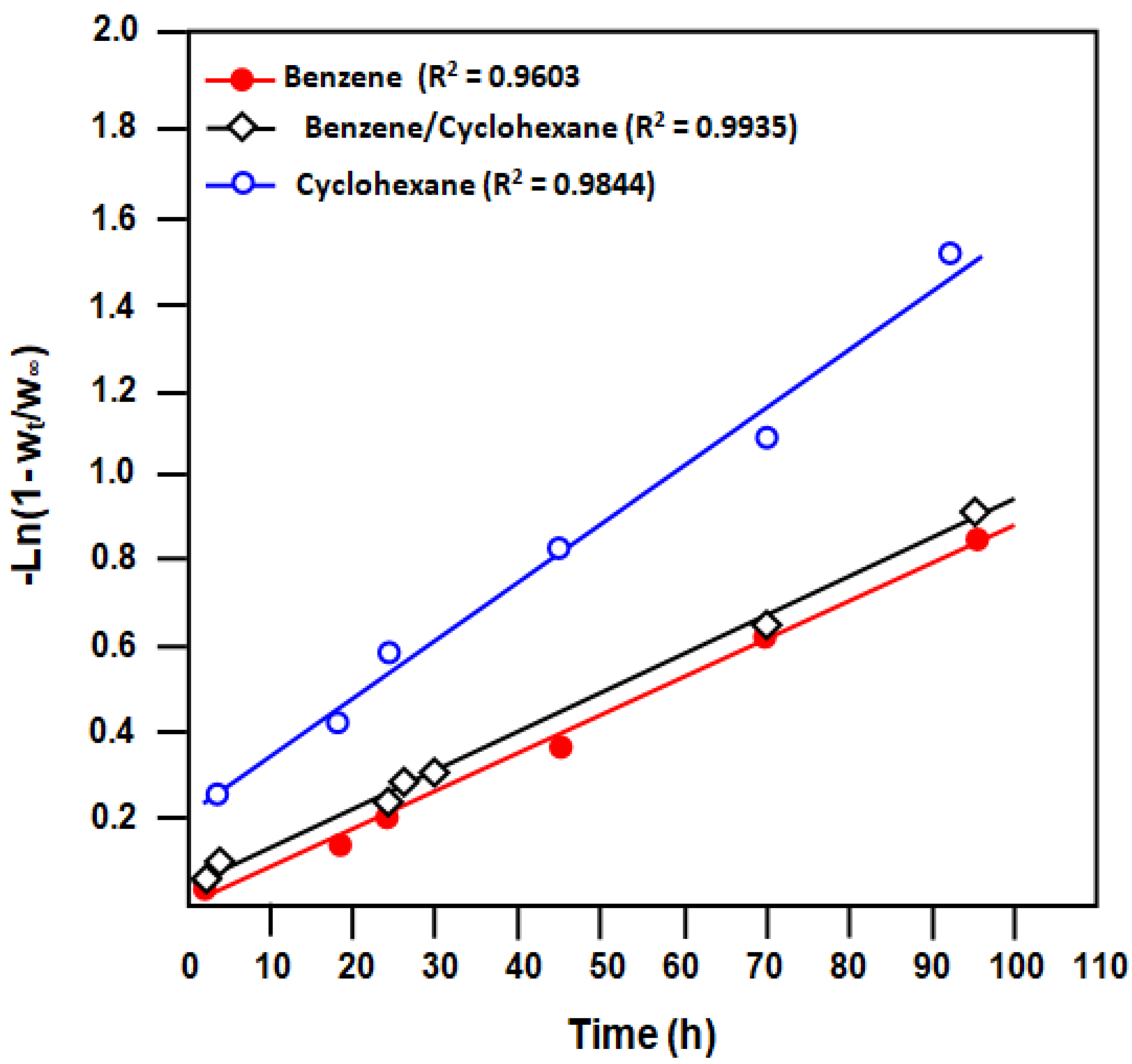
3.2.3. Diffusion
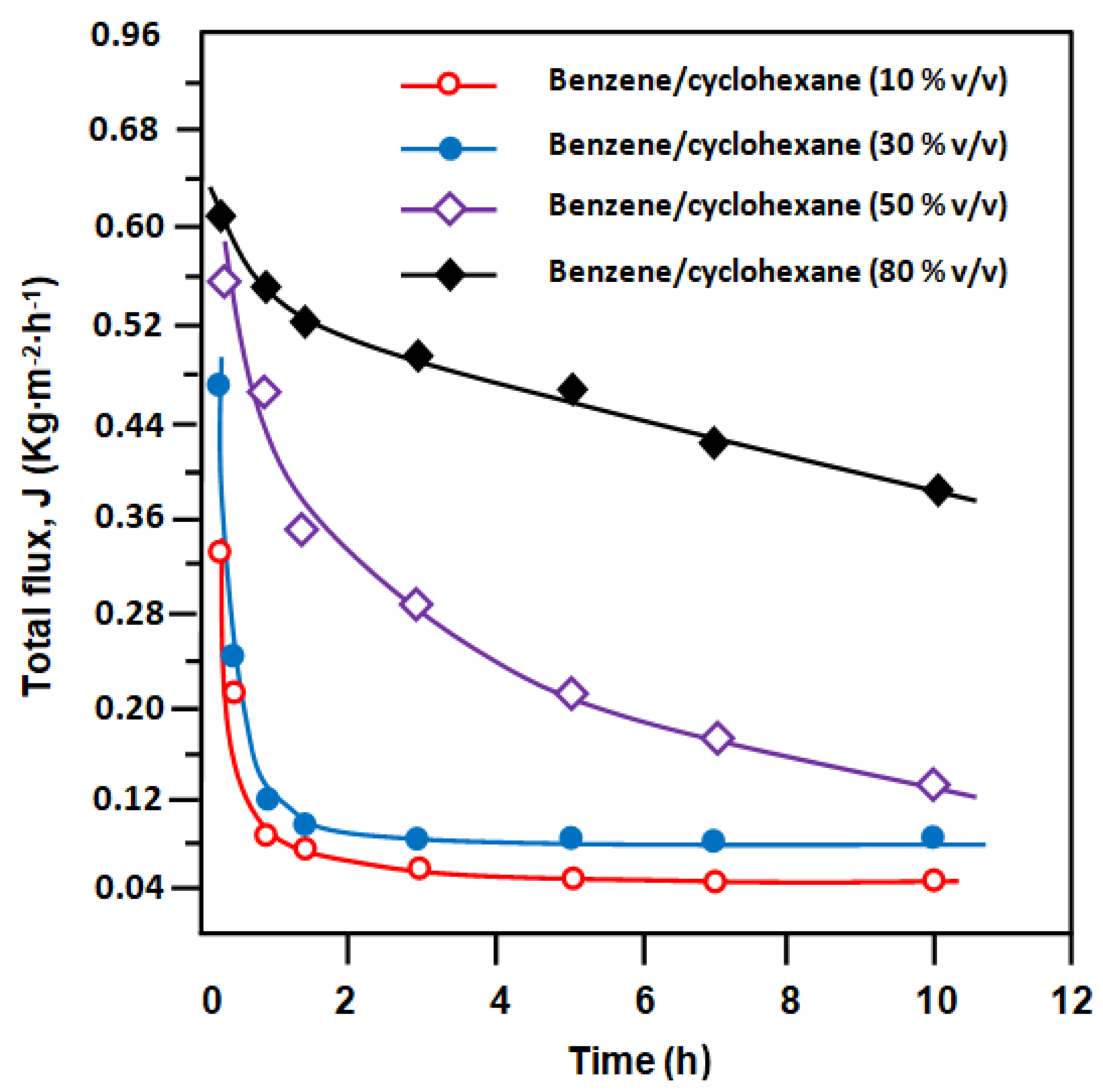
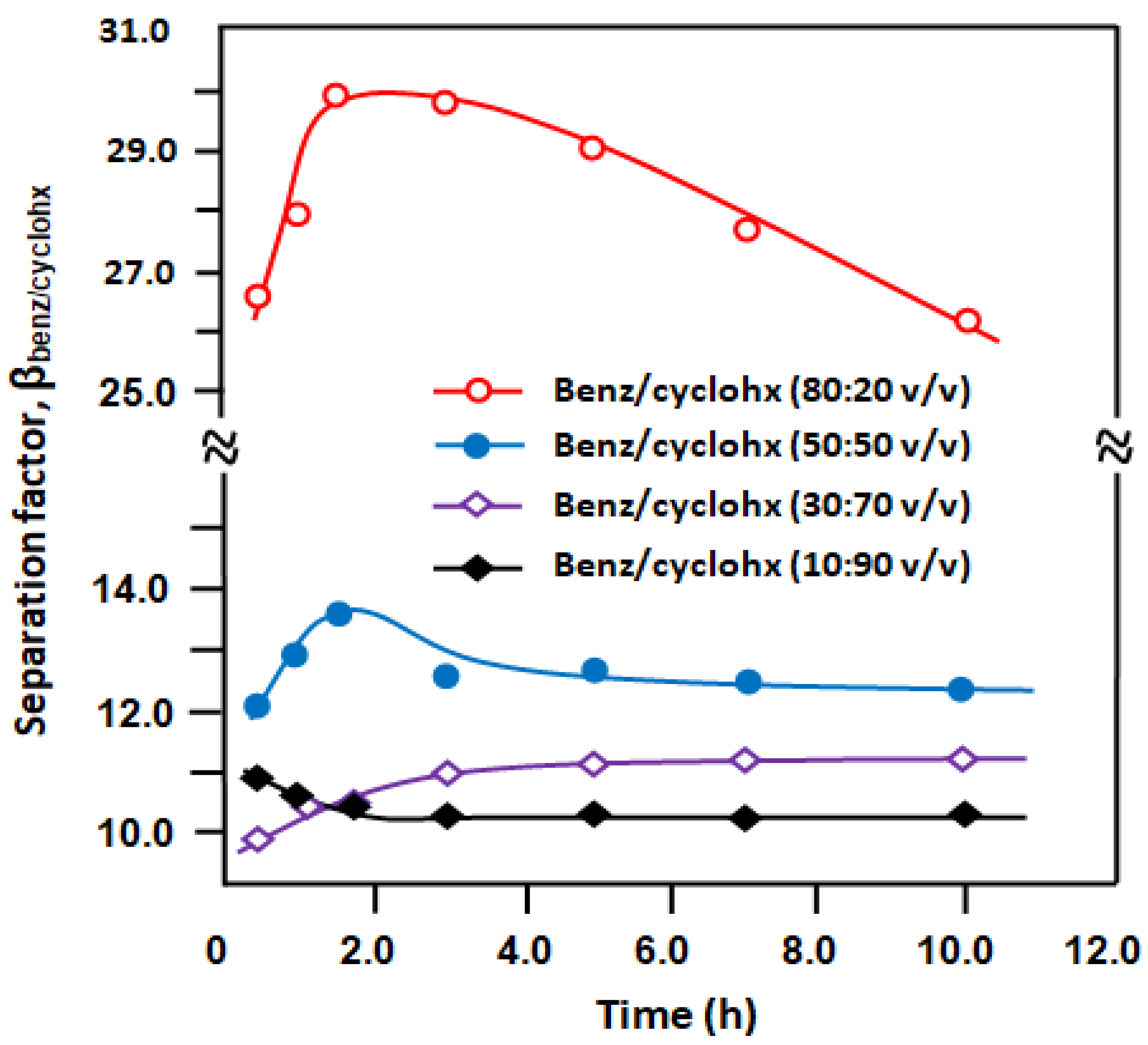
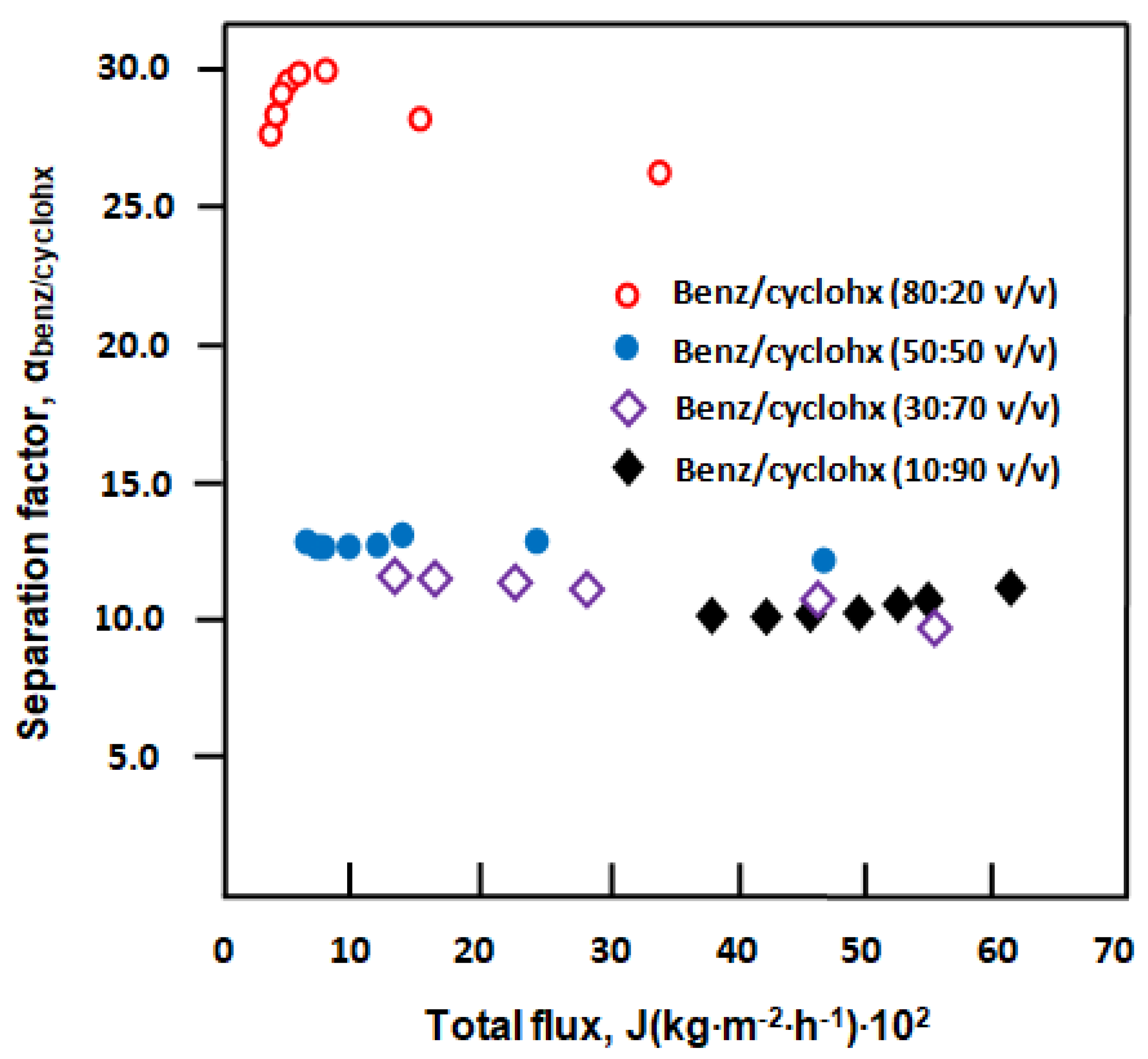
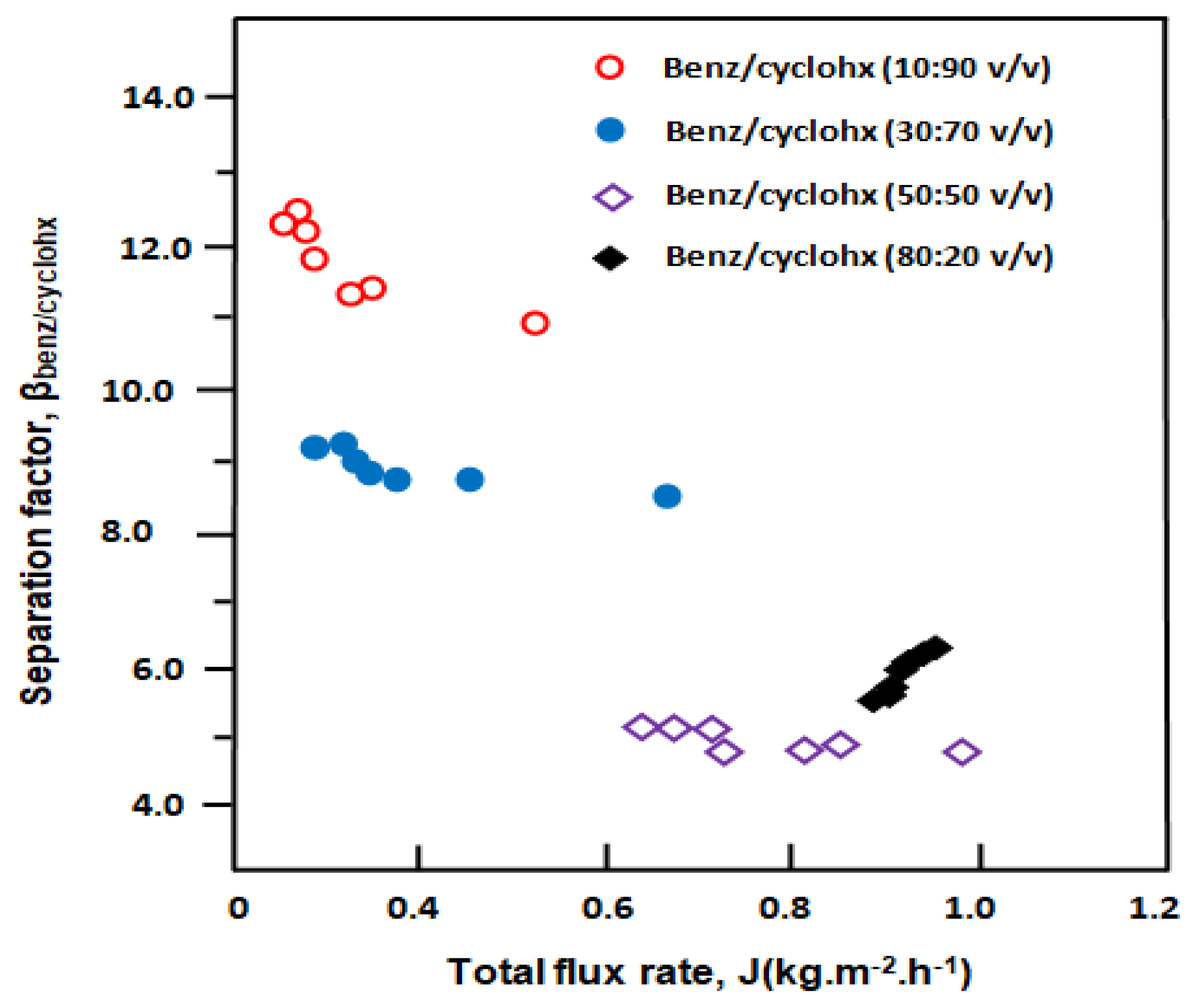
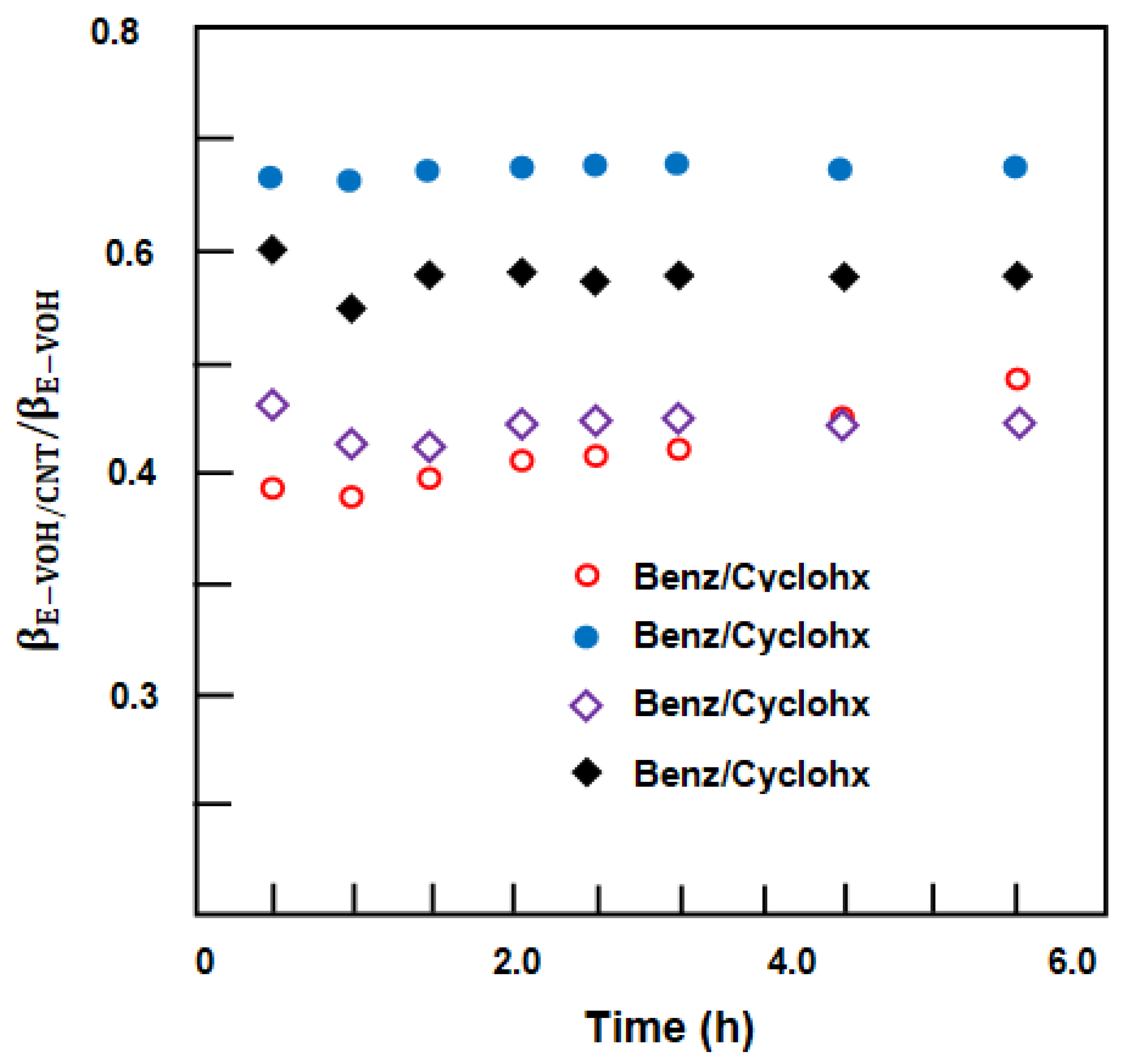
3.3. Separation of Benzene/Cyclohexane Mixture
3.3.1. Separation of Benzene/Cyclohexane by E-VOH Membrane
3.3.2. Separation of Benzene from Benzene/Cyclohexane Mixtures through an E-VOH/CNT-Membrane
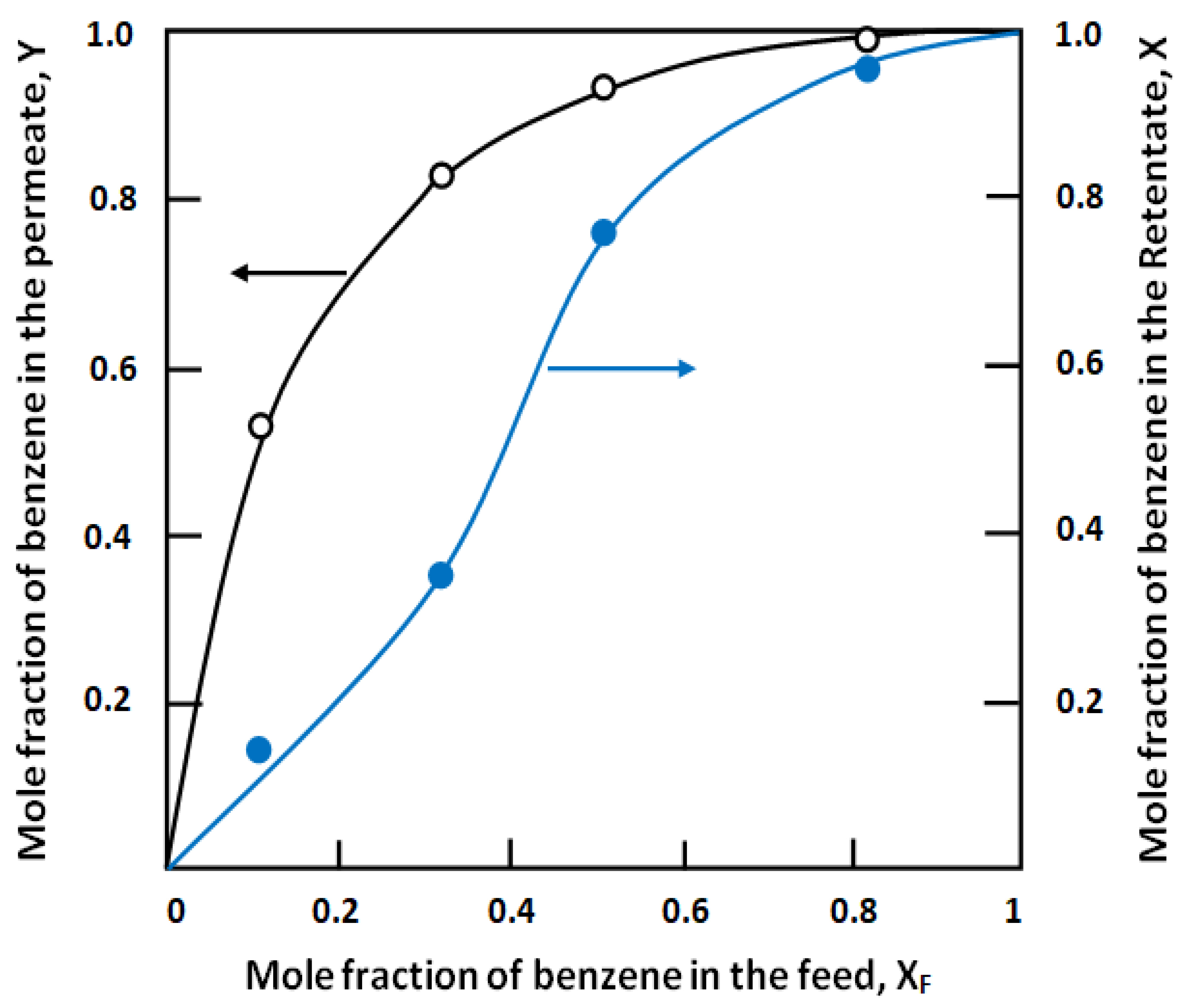
3.3.3. Vapor Liquid Pseudo-Equilibrium (VLPE) Diagrams
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Nomenclature
| γ | Activity coefficient |
| Benz | Benzene |
| Benzene/cyclohexane separation factor | |
| CMSs | Carbon molecular sieves |
| CNTs | Carbon nanotubes |
| MWCNTs | Multi-walled carbon nanotubes |
| CS | Chitosan |
| J | Cumulative total flux |
| JE-VOH | Cumulative total flux obtained by E-VOH membrane |
| JE-VOH/CNT | Cumulative total flux obtained by E-VOH/CNT membrane |
| β-CD | β-cyclodextrin |
| Cyclohx | Cyclohexane |
| DS | Degree of swelling |
| DMF | Dimethylformamid |
| DSC | Differential scanning calorimetry |
| D | Diffusion coefficient |
| β’ | Entropic factor most often eq Blanks et al. and Scott |
| Flory-Huggins interaction parameter between solvent and polymer | |
| GC | Gas chromatography |
| Tg | Glass transition temperature |
| wt | Masses of the sorbed molecules at time t |
| wt∞ | Masses of the sorbed molecules at maximum absorption |
| l | Membrane thickness |
| V1 | Molar volume of solvent |
| Tm | Melting temperature |
| PV | Pervaporation |
| PSI | Pervaporation separation index |
| PE | Polyethylene |
| EV-OH | Poly (ethylene-co-vinyl alcohol) |
| PHB | Poly (3-hydroxybutyrate) |
| PVA | Poly(vinylalcohol) |
| RhB-G | Rhodamine B-decorated grapheme |
| SEM | Scanning electronic microscopy |
| Selective absorption of the membrane factor | |
| βE-VOH | Separation factor obtained by E-VOHmembrane |
| βE-VOH/CNT | Separation factor obtained by E-VOH/CNT membrane |
| δp | Solubility parameter of the polymer |
| δ1 | Solubility parameter of the solvent |
| ϕ1 | Volume fraction of solvent |
| X | The molar fraction of benzene in the retentate |
| XF | The molar fraction of benzene in the feed |
| XRD | X-Ray diffraction |
| Y | The molar fraction of benzene in the permeate |
References
- Villaluenga, J.P.G.; Tabe-Mohammadi, A. A review on the separation of benzene/cyclohexane mixtures by pervaporation processes. J. Membr. Sci. 2000, 169, 159–174. [Google Scholar] [CrossRef]
- Cabasso, I.; Jagur-Grodzinski, J.; Vofsi, D. A study of permeation of organic solvents through polymeric membranes based on polymeric alloys of polyphosphonates and acetyl cellulose. II. Separation of benzene, cyclohexene, and cyclohexane. J. Appl. Polym. Sci. 1974, 18, 2137–2147. [Google Scholar] [CrossRef]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of organic–organic mixtures by pervaporation—A review. J. Membr. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Z.; Hu, C.; Wang, Y.; Lu, L.; Wu, H.J.D. Pervaporation of benzene/cyclohexane mixtures through poly (vinyl alcohol) membranes with and without β-cyclodextrin. Desalination 2006, 193, 182–192. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Kuila, S.B.; Ray, S.K.J.S.; Technology, P. Separation of benzene–cyclohexane mixtures by filled blend membranes of carboxymethyl cellulose and sodium alginate. Sep. Purif. Technol. 2014, 123, 45–52. [Google Scholar] [CrossRef]
- Yildirim, A.E.; Hilmioglu, N.D.; Tulbentci, S. Separation of benzene/cyclohexane mixtures by pervaporation using PEBA membranes. Desalination 2008, 219, 14–25. [Google Scholar] [CrossRef]
- Ye, H.; Li, J.; Lin, Y.; Chen, J.; Chen, C.J.P.i.C. Pervaporation Membranes for Separation of Aromatic/Aliphatic Mictures. Prog. Chem. 2008, 20, 288. [Google Scholar]
- Zhang, X.; Qian, L.; Wang, H.; Zhong, W.; Du, Q. Pervaporation of benzene/cyclohexane mixtures through rhodium-loaded β-zeolite-filled polyvinyl chloride hybrid membranes. Sep. Purif. Technol. 2008, 63, 434–443. [Google Scholar] [CrossRef]
- Sun, H.L.; Lu, L.Y.; Peng, F.B.; Wu, H.; Jiang, Z.Y. Pervaporation of benzene/cyclohexane mixtures through CMS-filled poly(vinyl alcohol) membranes. Sep. Purif. Technol. 2006, 52, 203–208. [Google Scholar] [CrossRef]
- Yamasaki, A.; Shinbo, T.; Mizoguchi, K. Pervaporation of benzene/cyclohexane and benzene/n-hexane mixtures through PVA membranes. J. Appl. Polym. Sci. 1997, 64, 1061–1065. [Google Scholar] [CrossRef]
- Park, C.K.; Oh, B.-K.; Choi, M.J.; Lee, Y.M. Separation of benzene/cyclohexane by pervaporation through chelate poly (vinyl alcohol)/poly (allyl amine) blend membrane. Polym. Bull. 1994, 33, 591–598. [Google Scholar] [CrossRef]
- Lu, L.; Sun, H.; Peng, F.; Jiang, Z. Novel graphite-filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexane mixtures. J. Membr. Sci. 2006, 281, 245–252. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N.R. Polymeric nanocomposite membranes for next generation pervaporation process: Strategies, challenges and future prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, R.; Albrecht, R. Separation of Organic Binary-Mixtures by Pervaporation. J. Membr. Sci. 1980, 7, 203–223. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Lin, V.J.C. Separation of Liquid Mixtures by Using Polymer Membranes. I. Permeation of Binary Organic Liquid Mixtures through Polyethylene. J. Appl. Polym. Sci. 1968, 12, 2615–2631. [Google Scholar] [CrossRef]
- Ketels, H.H.T.M. Synthesis, Characterization and Applications of Ethylene Vinylalcohol Copolymers; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 1989. [Google Scholar]
- Wang, C.; Takei, K.; Takahashi, T.; Javey, A.J.C.S.R. Carbon nanotube electronics–moving forward. Chem. Soc. Rev. 2013, 42, 2592–2609. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef]
- Argente-García, A.I.; Moliner-Martínez, Y.; López-García, E.; Campíns-Falcó, P.; Herráez-Hernández, R. Application of carbon nanotubes modified coatings for the determination of amphetamines by in-tube solid-phase microextraction and capillary liquid chromatography. Separations 2016, 3, 7. [Google Scholar]
- Fontanals, N.; Marcé, R.M.; Borrull, F. Materials for solid-phase extraction of organic compounds. Separations 2019, 6, 56. [Google Scholar] [CrossRef]
- Yu, L.; Shearer, C.; Shapter, J. Recent Development of Carbon Nanotube Transparent Conductive Films. Chem. Rev. 2016, 116, 13413–13453. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Islam, N. Carbon Nanotubes-Properties andApplications. Org. Med. Chem. Int. J. 2018, 7, 1–6. [Google Scholar]
- Gupta, N.; Gupta, S.M.; Sharma, S. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned multiwalled carbon nanotube membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef]
- Noy, A.; Park, H.G.; Fornasiero, F.; Holt, J.K.; Grigoropoulos, C.P.; Bakajin, O. Nanofluidics in carbon nanotubes. Nano Today 2007, 2, 22–29. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.M.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Chen, H.; Sholl, D.S.J.J.o.M.S. Predictions of selectivity and flux for CH4/H2 separations using single walled carbon nanotubes as membranes. J. Membr. Sci. 2006, 269, 152–160. [Google Scholar] [CrossRef]
- Choi, J.H.; Jegal, J.; Kim, W.N.; Choi, H.S. Incorporation of multiwalled carbon nanotubes into poly (vinyl alcohol) membranes for use in the pervaporation of water/ethanol mixtures. J. Appl. Polym. Sci. 2009, 111, 2186–2193. [Google Scholar] [CrossRef]
- Buekenhoudt, A.; Bisignano, F.; De Luca, G.; Vandezande, P.; Wouters, M.; Verhulst, K. Unravelling the solvent flux behaviour of ceramic nanofiltration and ultrafiltration membranes. J. Membr. Sci. 2013, 439, 36–47. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Sudesh, K.; Tan, S.H. Poly (3-hydroxybutyrate)-functionalised multi-walled carbon nanotubes/chitosan green nanocomposite membranes and their application in pervaporation. Sep. Purif. Technol. 2011, 76, 419–427. [Google Scholar] [CrossRef]
- Yeang, Q.W.; Zein, S.H.S.; Sulong, A.B.; Tan, S.H. Comparison of the pervaporation performance of various types of carbon nanotube-based nanocomposites in the dehydration of acetone. Sep. Purif. Technol. 2013, 107, 252–263. [Google Scholar] [CrossRef]
- Peng, F.; Pan, F.; Sun, H.; Lu, L.; Jiang, Z. Novel nanocomposite pervaporation membranes composed of poly (vinyl alcohol) and chitosan-wrapped carbon nanotube. J. Membr. Sci. 2007, 300, 13–19. [Google Scholar] [CrossRef]
- Amirilargani, M.; Ghadimi, A.; Tofighy, M.A.; Mohammadi, T. Effects of poly (allylamine hydrochloride) as a new functionalization agent for preparation of poly vinyl alcohol/multiwalled carbon nanotubes membranes. J. Membr. Sci. 2013, 447, 315–324. [Google Scholar] [CrossRef]
- Blanks, R.F.; Prausnitz, J.M. Thermodynamics of Polymer Solubility in Polar + Nonpolar Systems. Ind. Eng. Chem. Fundam. 1964, 3, 1–8. [Google Scholar] [CrossRef]
- Scott, R.L.; Magat, M. Thermodynamics of high-polymer solutions. III. Swelling of cross-linked rubber. J. Polym. Sci. 1949, 4, 555–571. [Google Scholar] [CrossRef]
- Comyn, J. Introduction to polymer permeability and the mathematics of diffusion. In Polymer Permeability; Springer: Berlin, Germany, 1985; pp. 1–10. [Google Scholar]
- Dong, Y.Q.; Guo, H.B.; Su, Z.X.; Wei, W.J.; Wu, X.Q. Pervaporation separation of benzene/cyclohexane through AAOM-ionic liquids/polyurethane membranes. Chem. Eng. Process. Process Intensif. 2015, 89, 62–69. [Google Scholar] [CrossRef]
- Xi, T.; Tang, L.; Hao, W.T.; Yao, L.L.; Cui, P. Morphology and pervaporation performance of ionic liquid and waterborne polyurethane composite membranes. RSC Adv. 2018, 8, 7792–7799. [Google Scholar] [CrossRef]
- Ping, Z.H.; Nguyen, Q.T.; Clement, R.; Neel, J. Pervaporation of Water Ethanol Mixtures through a Poly(Acrylic Acid) Grafted Polyethylene Membrane—Influence of Temperature and Nature of Counter-Ions. J. Membr. Sci. 1990, 48, 297–308. [Google Scholar] [CrossRef]
- Sampranpiboon, P.; Jiraratananon, R.; Uttapap, D.; Feng, X.; Huang, R. Pervaporation separation of ethyl butyrate and isopropanol with polyether block amide (PEBA) membranes. J. Membr. Sci. 2000, 173, 53–59. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Ikehara, T.; Nishi, T. Miscibility and crystallization in crystalline/crystalline blends of poly(butylene succinate)/poly(ethylene oxide). Polymer 2003, 44, 2799–2806. [Google Scholar] [CrossRef]
- Costache, M.C.; Heidecker, M.J.; Manias, E.; Camino, G.; Frache, A.; Beyer, G.; Gupta, R.K.; Wilkie, C.A. The influence of carbon nanotubes, organically modified montmorillonites and layered double hydroxides on the thermal degradation and fire retardancy of polyethylene, ethylene–vinyl acetate copolymer and polystyrene. Polymer 2007, 48, 6532–6545. [Google Scholar] [CrossRef]
- Omastová, M.; Mičušík, M.; Fedorko, P.; Pionteck, J.; Kovářová, J.; Chehimi, M.M. The synergy of ultrasonic treatment and organic modifiers for tuning the surface chemistry and conductivity of multiwalled carbon nanotubes. Surf. Interface Anal. 2014, 46, 940–944. [Google Scholar] [CrossRef]
- Alvarez, V.; Ruseckaite, R.; Vázquez, A. Kinetic analysis of thermal degradation in poly (ethylene–vinyl alcohol) copolymers. J. Appl. Polym. Sci. 2003, 90, 3157–3163. [Google Scholar] [CrossRef]
- Holland, B.; Hay, J. The thermal degradation of poly (vinyl alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Aouak, T.; Deraz, N.M.; Alarifi, A.S. Synthesis, non-isothermal crystallization and magnetic properties of Co 0· 75 Zn 0· 25 Fe 2 O 4/poly (ethylene-co-vinyl alcohol) nanocomposite. Bull. Mater. Sci. 2013, 36, 417–427. [Google Scholar] [CrossRef][Green Version]
- Caufield, J.H.; Zhou, Y.; Garlid, A.O.; Setty, S.P.; Liem, D.A.; Cao, Q.; Lee, J.M.; Murali, S.; Spendlove, S.; Wang, W.; et al. A reference set of curated biomedical data and metadata from clinical case reports. Sci. Data 2018, 5, 180258. [Google Scholar] [CrossRef]
- Tang, Z.H.; Lei, Y.D.; Guo, B.C.; Zhang, L.Q.; Jia, D.M. The use of rhodamine B-decorated graphene as a reinforcement in polyvinyl alcohol composites. Polymer 2012, 53, 673–680. [Google Scholar] [CrossRef]
- Awadallah-f, A.; Al-Muhtaseb, S. Carbon nanoparticles-Decorated carbon nanotubes. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Khoiruddin; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef]
- Matsuyama, H.; Kobayashi, K.; Maki, T.; Tearamoto, M.; Tsuruta, H. Effect of the ethylene content of poly (ethylene-co-vinyl alcohol) on the formation of microporous membranes via thermally induced phase separation. J. Appl. Polym. Sci. 2001, 82, 2583–2589. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E. Polymer Handbook, Solubility Parameter Values; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Uragami, T.; Tsukamoto, K.; Miyata, T.; Heinze, T. Permeation and separation characteristics for benzene/cyclohexane mixtures through tosylcellulose membranes in pervaporation. Cellulose 1999, 6, 221–231. [Google Scholar] [CrossRef]
- Shen, J.N.; Chu, Y.X.; Ruan, H.M.; Wu, L.G.; Gao, C.J.; Van der Bruggen, B. Pervaporation of benzene/cyclohexane mixtures through mixed matrix membranes of chitosan and Ag+/carbon nanotubes. J. Membr. Sci. 2014, 462, 160–169. [Google Scholar] [CrossRef]
- Okeowo, O.; Nam, S.Y.; Dorgan, J.R. Nonequilibrium nanoblend membranes for the pervaporation of benzene/cyclohexane mixtures. J. Appl. Polym. Sci. 2008, 108, 2917–2922. [Google Scholar] [CrossRef]
- An, Q.F.; Qian, J.W.; Zhao, Q.; Gao, C.J. Polyacrylonitrile-block-poly(methyl acrylate) membranes 2: Swelling behavior and pervaporation performance for separating benzene/cyclohexane. J. Membr. Sci. 2008, 313, 60–67. [Google Scholar] [CrossRef]
- Wang, N.X.; Wu, T.; Wang, L.; Li, X.T.; Zhao, C.; Li, J.; Ji, S.L. Hyperbranched polymer composite membrane using water as solvent for separating aromatic/aliphatic hydrocarbon mixtures. Sep. Purif. Technol. 2017, 179, 225–235. [Google Scholar] [CrossRef]
- Maji, S.; Banerjee, S. Preparation of new semifluorinated aromatic poly (ether amide)s and evaluation of pervaporation performance for benzene/cyclohexane 50/50 mixture. J. Membr. Sci. 2010, 349, 145–155. [Google Scholar] [CrossRef]
- Banerjee, A.; Ray, S.K. Synthesis of novel composite membranes by in-situ intercalative emulsion polymerization for separation of aromatic-aliphatic mixtures by pervaporation. J. Membr. Sci. 2020, 597, 117729. [Google Scholar] [CrossRef]
- Yildirim, A.E.; Hilmioglu, N.D.; Tulbentci, S. Pervaporation separation of benzene/cyclohexane mixtures by poly (vinyl chloride) membranes. Chem. Eng. Technol. 2001, 24, 275–279. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, K.H. A study on desorption resistance in pervaporation of single component through dense membranes. J. Appl. Polym. Sci. 1997, 63, 221–232. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Comprehensive Membrane Science and Engineering; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Heymann, D. Solubility of fullerenes C60 and C70 in water. LPI 1996, 27, 543. [Google Scholar]
- Sarkhel, D.; Roy, D.; Bandyopadhyay, M.; Bhattacharya, P. Studies on separation characteristics and pseudo-equilibrium relationship in pervaporation of benzene–cyclohexane mixtures through composite PVA membranes on PAN supports. Sep. Purif. Technol. 2003, 30, 89–96. [Google Scholar] [CrossRef]









| Mixture | Benzene | Cyclohexane | Composition (wt %) | |||
|---|---|---|---|---|---|---|
| (mL) | (g) | (mL) | (mL) | Benzene | Cyclohexane | |
| Benz/Cycl10 | 2.5 | 2.19 | 22.5 | 17.53 | 11.11 | 88.89 |
| Benz/Cycl30 | 7.5 | 6.57 | 17.5 | 13.63 | 32.52 | 67.48 |
| Benz/Cycl50 | 12.5 | 10.95 | 12.5 | 9.74 | 52.92 | 47.08 |
| Benz/Cycl80 | 20.0 | 17.52 | 5.0 | 3.90 | 81.79 | 18.21 |
| Swelling at Equilibrium, DS (wt %) | |||
|---|---|---|---|
| Membrane | Benzene | Benzene/Cyclohexane (50:50 wt %) | Cyclohexane |
| E-VOH | 0.92 | 4.20 | 0.18 |
| E-VOH/CNT | 2.05 | 16.50 | 1.25 |
| Component | V1 (ml∙mol−1) | δ1 (MPa)0.5 |
|---|---|---|
| E-VOH | 44.02 | 23.1 [54] |
| Benzene | 80.16 | 18.6 [55] |
| Cyclohexane | 147.4 | 16.8 [55] |
| E-VOH/Solvent System | γ1 | δ1- δp (MPa)0.5 | χ1,p |
|---|---|---|---|
| E-VOH/Benzene | 1.40 | −4.47 | 0.689 |
| E-VOH/Cyclohexane | 1.04 | −6.27 | 2.636 |
| Membrane | Initial Feed Composition (wt %) | Mixture Absorbed Composition (wt %) | |||
|---|---|---|---|---|---|
| Benzene | Cyclohexane | Benzene | Cyclohexane | ||
| E-VOH | 10 | 90 | 72.12 | 27.88 | 06.69 |
| 30 | 70 | 80.55 | 19.45 | 17.15 | |
| 50 | 50 | 88.32 | 11.68 | 57.18 | |
| 80 | 20 | 95.02 | 04.98 | 361.0 | |
| E-VOH/CNT | 10 | 90 | 67.56 | 32.44 | 04.34 |
| 30 | 70 | 73.07 | 26.93 | 07.35 | |
| 50 | 50 | 83.13 | 16.87 | 24.28 | |
| 80 | 20 | 92.22 | 7.78 | 140.51 | |
| Membrane. | Benz/Cyclohx Mixture (wt/wt) | n | D (μm2∙s−1)∙102 |
|---|---|---|---|
| E-VOH | 100:0 | 1.0 | 2.83 |
| E-VOH/CNT | 1.0 | 0.18 | |
| E-VOH | 50:50 | 1.0 | 2.94 |
| E-VOH/CNT | 1.0 | 2.60 | |
| E-VOH | 0:100 | 1.0 | 4.43 |
| E-VOH/CNT | 1.0 | 0.10 |
| Benz/Cyhx Mixture (v/v) | Flux (kg∙m−2∙h−1) | Flux Performance | Separation Factor Performance | |||
|---|---|---|---|---|---|---|
| E-VOH | E-VOH/CNT | E-VOH | E-VOH/CNT | |||
| 10:90 | 0.06 | 0.18 | 3.00 | 10.20 | 05.00 | 0.49 |
| 30:70 | 0.08 | 0.29 | 3.63 | 11.10 | 06..00 | 0.54 |
| 50:50 | 0.28 | 0.74 | 2.64 | 12.90 | 09.03 | 0.70 |
| 80:20 | 0.44 | 0.93 | 2.11 | 30.00 | 12.22 | 0.41 |
| Membrane | Temperature (°C) | Total Flux (g∙m−2∙h−1) | Separation Factor | PSI (g∙m−2∙h−1) | Reference |
|---|---|---|---|---|---|
| β-CD/PVA/GAa | 20 | 30.9 | 27.0 | 803.4 | [4] |
| Tosylcellulose | 40 | 6.0 | 28.8 | 168.0 | [56] |
| Chitosan/Ag+/CNTb | 20 | 358.0 | 7.9 | 2466.3 | [57] |
| NBR/hydrin/PMMAc | 60 | 160.0 | 7.3 | 1008.0 | [58] |
| P(AN-b-MA)d | 30 | 70.0 | 10.5 | 665.0 | [59] |
| Boltorn W3000/ceramic | 40 | 278.0 | 4.1 | 861.8 | [60] |
| CMS/PVAe | 50 | 72.0 | 20.8 | 1424.7 | [10] |
| AAOM.[C8mim]PF6/PUf | 45 | 12.2 | 34.2 | 405.0 | [40] |
| [emim][PF6]WPUg | 50 | 190.0 | 8.4 | 1406.0 | [41] |
| PEA Ih | 50 | 320.0 | 6.9 | 1888.0 | [61] |
| PEA IIi | 50 | 520.0 | 6.5 | 2860.0 | [61] |
| PEAIIIj | 50 | 570.0 | 5.9 | 2793.0 | [61] |
| PEAIVk | 50 | 550.0 | 7.6 | 3630.0 | [61] |
| PVA + 5:1 poly (ANco- AA) coated (1:1) NaMMT clay 8 wt %l | 30 | 168.6 | 137.2 | - | [62] |
| E-VOH | 25 | 280.0 | 12.9 | 3332.0 | This work |
| E-VOH/CNT | 25 | 740.0 | 9.0 | 5942.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahlan, H.; Saeed, W.S.; Alqahtani, S.; Aouak, T. Separation of Benzene/Cyclohexane Mixtures by Pervaporation Using Poly (Ethylene-Co-Vinylalcohol) and Carbon Nanotube-Filled Poly (Vinyl Alcohol-Co-Ethylene) Membranes. Separations 2020, 7, 68. https://doi.org/10.3390/separations7040068
Zahlan H, Saeed WS, Alqahtani S, Aouak T. Separation of Benzene/Cyclohexane Mixtures by Pervaporation Using Poly (Ethylene-Co-Vinylalcohol) and Carbon Nanotube-Filled Poly (Vinyl Alcohol-Co-Ethylene) Membranes. Separations. 2020; 7(4):68. https://doi.org/10.3390/separations7040068
Chicago/Turabian StyleZahlan, Hala, Waseem Sharaf Saeed, Saad Alqahtani, and Taieb Aouak. 2020. "Separation of Benzene/Cyclohexane Mixtures by Pervaporation Using Poly (Ethylene-Co-Vinylalcohol) and Carbon Nanotube-Filled Poly (Vinyl Alcohol-Co-Ethylene) Membranes" Separations 7, no. 4: 68. https://doi.org/10.3390/separations7040068
APA StyleZahlan, H., Saeed, W. S., Alqahtani, S., & Aouak, T. (2020). Separation of Benzene/Cyclohexane Mixtures by Pervaporation Using Poly (Ethylene-Co-Vinylalcohol) and Carbon Nanotube-Filled Poly (Vinyl Alcohol-Co-Ethylene) Membranes. Separations, 7(4), 68. https://doi.org/10.3390/separations7040068






