Abstract
This study investigates the feasibility of producing ethanol from date palm seeds. The chemical compositions of three varieties of date seeds were first studied, showing mainly the presence of cellulose and hemicellulose. Ethanol was produced after a pre-treatment of date seeds using acid hydrolysis to extract the cellulosic fraction and to remove the lignin. Producing ethanol by fermentation was performed using the yeast Saccharomyces cerevisiae for 24 h, during which ethanol yield, biomass concentration, and total reducing sugars were recorded. The results obtained showed that the sugar content decreased over time, while ethanol production increased. Indeed, date seeds gave the highest ethanol concentration of 21.57 g/L after 6 h of alcoholic fermentation. These findings proved the feasibility of producing ethanol from date seeds.
1. Introduction
Renewable energy corresponds to the energy provided from renewable resources and constitutes one of the worldwide headlines to meet the global energy requirements. The rapid depletion of fossil resources has incited researchers to develop alternative, renewable, low cost-effective, and environmentally friendly fuels [1]. The conversion of natural resources (e.g., trees, agricultural and forestry waste, crops, etc.) to energy (e.g., fuel and electricity) is already in progress in many countries [2]. Several environmental issues are associated with the use of petroleum resources, which requires developing alternative systems to produce renewable energy [3,4,5,6,7]. Bioethanol, as a renewable energy, has a high octane number and could be blended with gasoline to reduce the emission of CO2, NOx, and hydrocarbons after combustion. It has a high compression ratio and showed adequacy to be used in the combustion engines [3,8,9]. Bioethanol has become a major biotechnology product, and the needs in the market are continuously increasing. Various agricultural waste products are rich in sugars, which could be efficiently fermented to ethanol. The main feedstock for bioethanol production could be classified into starchy materials (e.g., corn, barley, wheat, etc.), sucrose-rich materials (e.g., sugar cane, sugar beet, sweet sorghum, etc.), and lignocellulosic materials (e.g., grasses, forest “waste”, wood straw, etc.) [10].
In this regard, bioethanol has been produced from different kinds of biomasses that include rice hulls [11], sugar cane leaves [12], industrial waste [13], agriculture residue [14], sweet sorghum [15], and lignocellulose from wood and agricultural residues [16], among others, have been reported. Bioethanol production needs usually pre-treatment steps (chemically and or enzymatically) of the feedstock to produce fermentescible sugars before their conversion by alcoholic fermentation to bioethanol. Different strains of yeast and bacteria have been described in the literature for the production of ethanol [17,18,19]. Many factors should be considered to select the strains such as the productivity, the resistance to ethanol, the presence of inhibitors in the medium, and the range of pH and temperature [20]. The yeast Saccharomyces cerevisiae is used in most of the alcoholic fermentation processes, mainly because of its high ethanol resistance, low optimal pH range, and anaerobic conditions requirement [21]. However, it should be noted that S. cerevisiae yeast is not suitable for ethanol production from xylose, which needs modified strains, or the pre-treatment of xylose by bacterial enzymes [22].
Information in the literature about ethanol production from date seeds is scarce, and research studies were mainly focused on their chemical composition [23,24,25], even though many industries transforming date fruits generate the seeds as a by-product. In fact, date palm (Phoenix dactylifera L.) represents one of the oldest plants cultivated mainly in the arid and semiarid regions [26]. Date fruit (a fleshy part and one seed) production was estimated to 8.52 million tons in 2018 [27]. Considering that the date seed represents ≈10% of the fruit mass, ≈852,000 tons of date seeds are then generated [26]. Tunisia has more than 4 million date palm trees that produce annually ≈100,000 tons of fruits [28]. Most of the seeds generated are usually disposed of or used as animal feed. Therefore, their valorization to higher-added value products, such as ethanol, is of paramount importance. In this line, this work aims to investigate the enzymatic treatment of date seeds for the generation of fermentescible sugars, which is followed by their conversion into ethanol by fermentation.
2. Materials and Methods
2.1. Date Seed Material
Three cultivars of date seeds (Deglet Nour, Ghars Souf, and Allig) (Figure 1) were separated from date fruits (≈50 kg) cultivated in Douz city (Tunisia). Date fruits were harvested at the full ripening stage, which is also called the “Tamr stage”. The collected seeds were first soaked in water and then washed using ultrapure water for the removal of the adhering date flesh. Then, the seeds were air-dried before oven-drying at 50 °C during 48 h. Date seeds from each variety were then grinded using a heavy-duty grinder until passing 200 µm sieve pore size and stored at −20 °C until use.
Figure 1.
Photos of the different date seed varieties.
2.2. Chemical Characterization of Date Seeds
2.2.1. Determination of Extractives Content
The extractives (E, %) include mainly carbohydrates of low molecular weight, salts, non-volatile hydrocarbons, waxes, and fats. Removing them from the date seeds was performed by placing 4.0 ± 0.1 g of date seed powder in a pre-weighed extraction thimble and heating in a Soxhlet apparatus under reflux for 8 h in the presence of a 150 mL ethanol/toluene (2:1) mixture. After oven drying during 24 h at 105 °C, the extraction thimble was cooled to room temperature and then weighed. Then, the free of extractives sample obtained was placed in a desiccator for analysis.
2.2.2. Determination of Insoluble and Soluble Lignin Content
The free of extractives sample (m0 = 1 g) was introduced in a 1-L flask and mixed with 15 mL of sulfuric acid (72%) to determine the insoluble lignin content. The mixture was first placed in a water bath for 1 h at 20 °C, then supplemented by 575 mL ultrapure water, and finally brought to boiling under reflux during 4 h. Separation between the soluble and the insoluble fraction was made by filtration using a pre-weighed (m1) fritted glass filter (16–40 µm porosity). The insoluble fraction kept in the filter was washed with hot water until neutrality and then oven-dried at 105 °C for 24 h. After cooling to room temperature in a desiccator, the weight (m2) (filter + insoluble matter) was measured, and the insoluble lignin content (in % dry basis) was determined according to Equation (1).
The absorbance of the soluble fraction was then measured at 280 nm (TAPPI UM250 standards), and the content of soluble lignin was calculated using Equation (2).
2.2.3. Determination of Holocellulose Content
The content of holocellulose (cellulose and hemicellulose) was determined by placing 2.5 g of free of extractives sample (m0) in an Erlenmeyer flask and adding 80 mL hot water. Then, the suspension was placed in a water bath at 70 °C and supplemented each hour by 2.6 mL sodium chlorite (25%) and 0.5 mL of glacial acetic acid until 8 h. Separation between soluble and insoluble fraction was performed by filtration using a pre-weighed (m1) crucible filter (40–100 µm porosity). Then, the set of insoluble fraction/filter was oven-dried during 24 h at 105 °C, cooled in a desiccator to room temperature, and finally weighed (m2). The content of holocellulose (HOL) was calculated according to Equation (3) and was expressed in % dry basis.
2.2.4. Determination of Cellulose and Hemicellulose Contents
The extracted holocellulose was fractionated into cellulose and hemicellulose. This experiment was performed in a 250 mL glass beaker by mixing 2 g (m0) of holocellulose sample and 10 mL of NaOH (17.5%). The total volume was made up to 25 mL by adding 5 mL NaOH (17.5%) every 5 min. Then, the mixture was supplemented with 33 mL ultrapure water and further stirred for 1 h. Separation of the solid fraction (cellulose) from the mixture was performed by vacuum filtration using a pre-weighed (m1) fritted glass filter (40–100 µm porosity). Then, the cellulose fraction was washed by 100 mL NaOH (8.3%) followed by 500 mL of ultrapure water. After 3 min soaking in 15 mL acetic acid (10%), it was washed with ultrapure water until reaching a neutral pH. The weight (m2) of the set filter/cellulose was determined after oven drying during 24 h at 105 °C and cooling to room temperature. Then, the content of cellulose (CEL) was calculated based on Equation (4) and expressed on a percentage dry basis.
Then, the content of hemicellulose (HEM) was calculated based on the difference between the content of holocellulose and cellulose, as shown in Equation (5).
2.3. Infrared Spectroscopy Analyses
Each seed variety sample (1 mg) was mixed with 100 mg KBr and then scanned in a wavenumber range of 650–4000 cm−1 to determine the Fourier Transform Infrared (FTIR) spectra [29]. A Shimadzu spectrometer (UV 1240, Beijing, China) was used to record the sample transmission (%).
2.4. Extraction of Cellulose from Date Seeds
After characterization, impurities and lipophilic molecules were removed from date seed powder (50 g of each variety) using 95% ethanol during 24 h and under agitation at 4 °C. Then, the powder obtained was mixed with 20 volumes of ultrapure water and brought to boiling during 2 h. Separation of the solid residue was performed by filtration using Whatman no. 4 filter paper. The filtrate containing the soluble polysaccharides was discarded, and the solid residue was brought to boiling during 2 h in the presence of 500 mL NaOH (1 N). Then, the mixture was filtered using Whatman n° 4 filter paper to separate the filtrate (containing hemicellulose) and the solid residue (containing cellulose) that was further bleached in the presence of NaClO2 at 80 °C for 2 h and under continuous stirring [30,31,32]. Then, the mixture was cooled down to room temperature and neutralized by adding ultrapure water. The cellulose obtained was left to dry at room temperature and then stored at 4 °C until hydrolysis. The cellulose yield calculation is given by Equation (6).
2.5. Microbial Strains and Culture Conditions
2.5.1. Penicillium occitanis Pol6
Enzymatic hydrolysis of date seed cellulose was performed using an enzymatic mixture produced by Penicillium occitanis Pol6 mutant (Cayla company, Toulouse, France) [33]. The strain was growing in 1000 mL flasks containing 200 mL of a modified Mandels liquid medium. The composition of the medium was as follows: date seed powder (2%) acting as carbon substrate, yeast extract (1 g/L), NaNO3 (5 g/L), KH2PO4 (2 g/L), CaCl2 (0.3 g/L), MgSO4 7 H2O (0.3 g/L), Tween 80 (0.1%), and 1 mL of oligoelements (CoCl2 (2 g/L), MnSO4 H2O (1.6 g/L), ZnSO4 H2O (1.4 g/L), and FeSO4 7 H2O (5 g/L)) [34,35]. The fermentation was conducted in a shaker for 9 days at 30 °C, pH 5.5, and 250 rpm agitation. The separation between the microbial biomass and the liquid medium containing the secreted enzymes (cellulase and endoglucanase) was performed by centrifugation at 4 °C, 7000 rpm, and during 20 min.
2.5.2. Saccharomyces cerevisiae Strain Culture
The strain Saccharomyces cerevisiae used for ethanol production from the hydrolyzed date seed cellulose was provided from the American Type Culture Collection (Manassas, VA, USA). A yeast extract peptone dextrose (YPD) medium (dextrose (20 g/L), yeast extract (10 g/L), peptone (20 g/L), and agar (18 g/L)) was used to prepare the inoculum (50 mL in 250 mL Erlenmeyer flasks). After incubation at 30 °C during 24 h, 2% of the inoculum was used to start the ethanolic fermentation.
2.6. Enzymatic Hydrolysis
2.6.1. Enzyme Activity Measurements
A standard filter paper assay was used to determine the cellulose activity [36]. The experiment consisted of mixing 0.4 mL of enzymatic extract, 0.6 mL of sodium acetate buffer (50 mM, pH 5.5), and 50 mg Whatman (No. 1) filter paper. Hydrolysis was performed in a water bath at 50 °C during 1 h, and the results were expressed as filter paper units (FPU). One FPU was defined as the amount of enzyme releasing 1 µmol of glucose/min [36].
Carboxymethyl cellulose (CMC) was used as substrate to determine the endoglucanase activity [36]. The reaction consisted of mixing 0.1 mL of enzymatic extract, 0.4 mL sodium acetate buffer (50 mM, pH 5.5), and 0.5 mL CMC (1%). Hydrolysis was performed in a water bath at 50 °C during 30 min. Results were expressed in international unit of endoglucanase, in which one unit corresponds to the amount of enzyme releasing 1 µmol of glucose/min [37].
2.6.2. Date Seed Cellulose Hydrolysis
Once the cellulase and endoglucanase activities were determined, different concentrations of date seed cellulose, from 2.5 to 20 mg/mL, were used to optimize the substrate concentration for cellulose hydrolysis. Different samples were taken from the mixture over time, centrifuged during 10 min at 8000 rpm, and the supernatant was analyzed for reducing sugars using the 3,5-dinitrosalicylic acid (DNS) method [38].
2.7. Ethanol Fermentation of Date Seed Cellulose Hydrolysates
Hydrolyzed date seed cellulose was fermented using S. cerevisiae in 250 mL Erlenmeyer flasks to produce ethanol. The culture medium was composed of yeast extract (1%), peptone (2%), and the hydrolyzates (1, 2, and 4% w/v). It was sterilized by autoclaving (121 °C for 20 min) prior to fermentation and then inoculated by a 20-h-old culture of S. cerevisiae. Ethanolic fermentation was conducted at 30 °C temperature, 100 rpm agitation, and pH 5. Sampling was performed periodically until 48 h fermentation, and each sample was analyzed to determine the reducing sugar content as above described. Ethanol content in each sample was determined using the ethanol FS kit (Diagnostic System International). The reaction consisted first of measuring the absorbance A1 at 376 nm of a mixture of 10 µl sample and 1 mL of reagent R1 (buffer pH 9) incubated at 37 °C for 5 min. Then, this mixture was supplemented by 250 µl reagent R2 (buffer pH 6.6, NAD, alcohol dehydrogenase), incubated for 5 min at 37 °C, and its absorbance A2 was measured at 376 nm. In this reaction mixture, ethanol is converted in the presence of NAD+ to acetaldehyde by alcohol dehydrogenase. A standard curve was established using a solution of ethanol (3 g/L). The ethanol concentration in each sample was determined according to Equation (7).
The yield of ethanol was calculated according to Equation (8). It corresponds to the yield obtained using the hydrolyzed cellulose divided by the theoretical one, when pure glucose is used (0.51 kg ethanol/kg glucose) [39].
2.8. Statistical Analysis
Results in the graphs correspond to the average of three biological replicates ± standard deviation (SD). Significant differences (p < 0.05) were determined using SPSS software 17.0 by Duncan’s multiple range test.
3. Results and Discussion
3.1. Date Seed Characterization
The results of the chemical compositions of date seeds are summarized in Table 1. The moisture content in the three varieties was low; the highest value recorded (8.02 ± 0.18%) was for the Deglet Nour variety and the lowest one (4.76 ± 0.2%) was for the Allig variety. The results obtained concur with those found previously [24]. Low moisture contents allow the storage of date seeds at room temperature for subsequent analysis. The variation observed in the moisture content of date seeds could be related to the differences in the varieties studied.

Table 1.
Physicochemical composition of three varieties of date seeds (Allig, Deglet Nour, Ghars).
The results of lignin content analysis in date seeds show that the Allig variety had the highest content of lignin (24.06%), while the Deglet Nour variety had the lowest one (21.2 ± 0.062%).
A high content of hemicellulose was obtained in date seeds with the respective values of 42.3 ± 0.3%, 34.29 ± 0.241%, and 31.97 ± 0.26% in Allig, Ghars, and Deglet Nour varieties. Similarly, high cellulose contents were obtained in the three varieties with the highest value of 33.92 ± 0.075% for the Allig variety. These results were higher than those found previously, reporting a cellulose content between 15.18% and 19.26% [40].
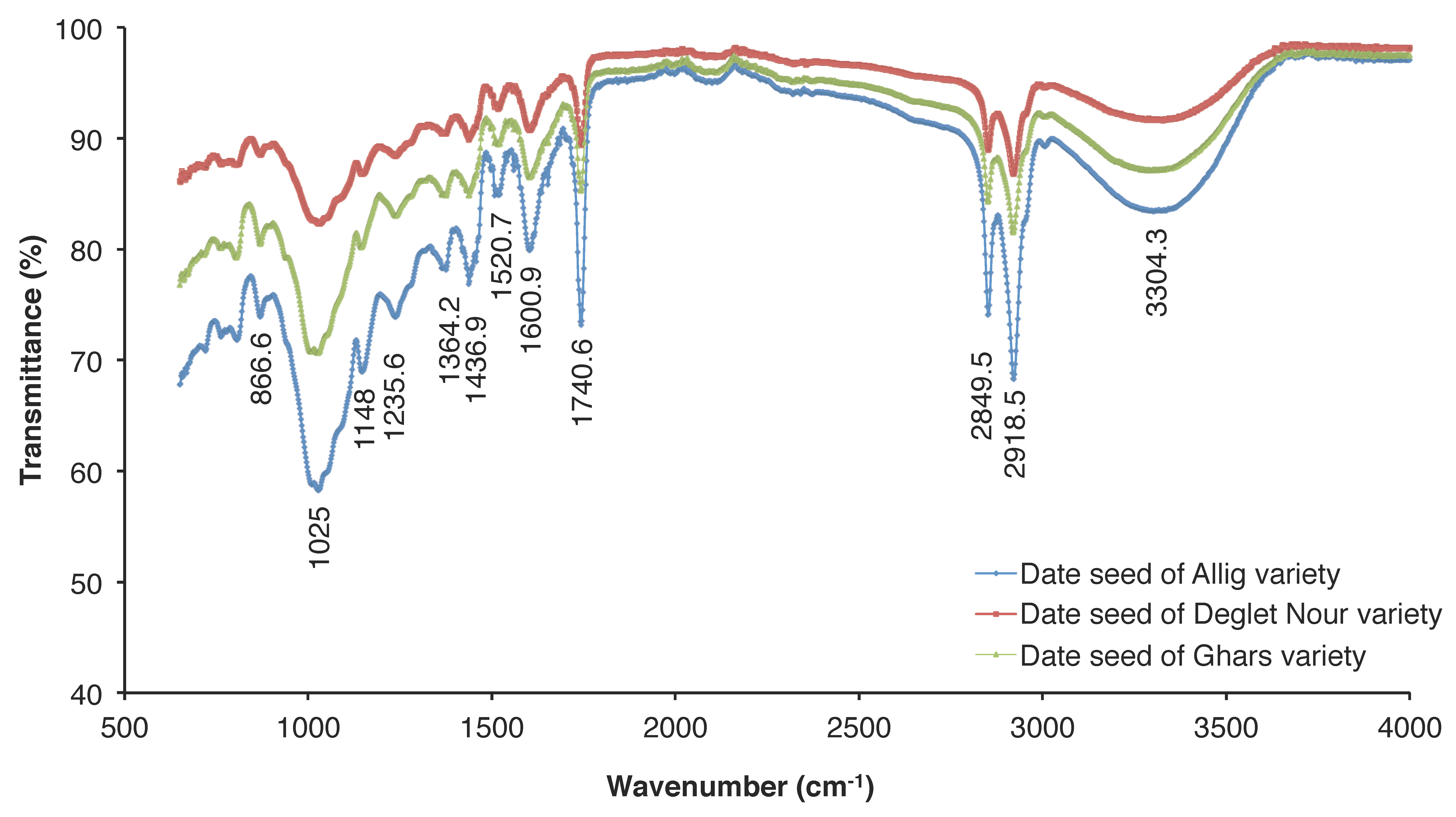
The functional groups of the three varieties of date seeds analyzed using infrared spectroscopy showed the presence of characteristic absorption peaks observed around 2800–3000 cm−1 and 3304.28 cm−1 for the respective stretching vibrations of C-H and O-H (Figure 2), which concur with the results found by Koubaa et al. [29]. The spectrum analysis shows also the presence of a band at 1600 cm−1 corresponding to the symmetrical deformation of -CH2 and -CH3 [41], a band at 1002 cm−1 corresponding to the vibration of C-O-H deformation, a band at 963 cm−1 corresponding to C-H bending [42], and the bands at 1072–1080 cm−1 corresponding to the presence in the main chain of galactopyranose units [43,44].
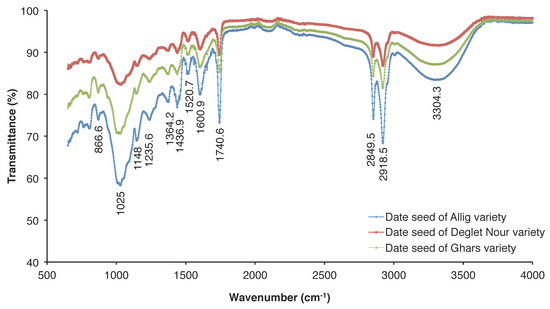
Figure 2.
Fourier Transform Infrared (FTIR) spectra of three date seed varieties (Allig, Ghars, and Deglet Nour).
3.2. Extraction of Cellulose from Date Seeds
The results of cellulose content extracted from the date seed powders of the three varieties are presented in Figure 3. The extraction yields were of 24 ± 0.08%, 30.25 ± 0.08%, and 28.77 ± 0.09% for Allig, Deglet Nour, and Ghars varieties, respectively. Thus, the date seed of Deglet Nour gave the best yield of cellulose and was selected for ethanol production.

Figure 3.
Photos of cellulose extracted from date seed powders.
3.3. Enzymatic Hydrolysis of Date Seed Cellulose
3.3.1. Optimization of Enzyme Production
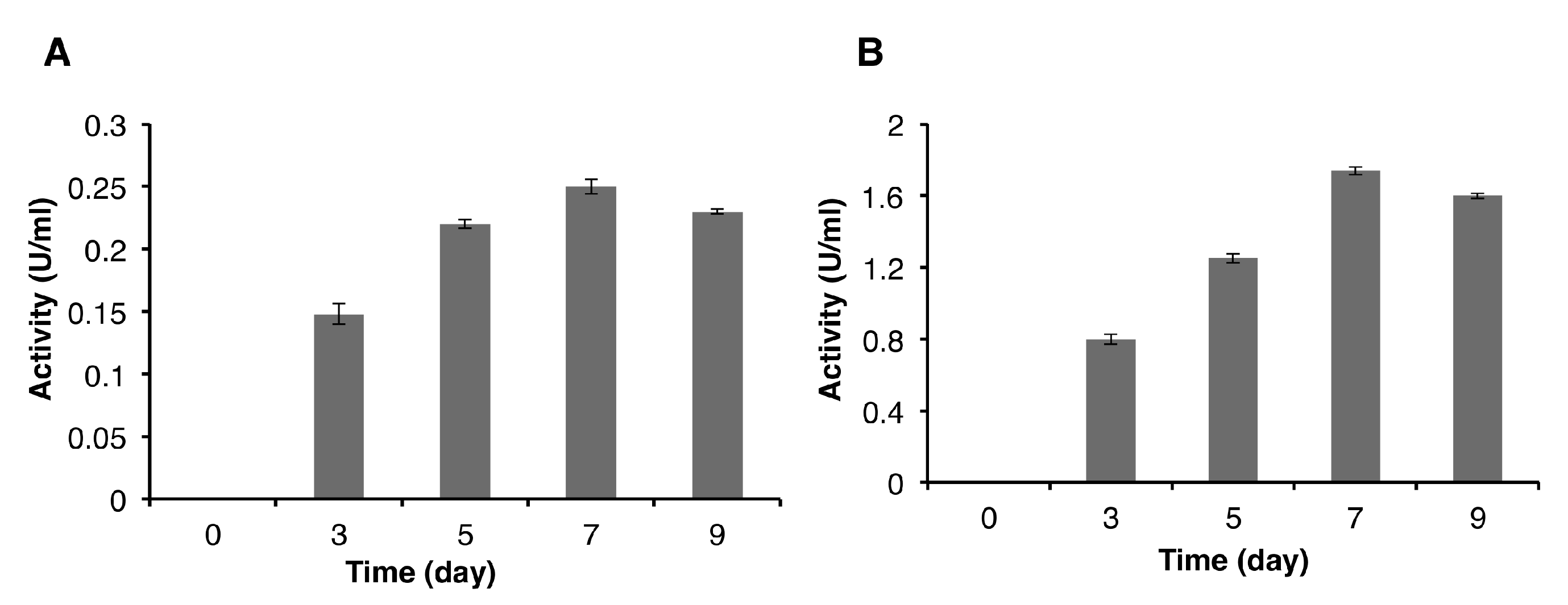
Deglet Nour date seed powder, used as carbon substrate, was used at different concentrations to optimize the production of cellulase and endoglucanase by P. occitanis Pol6. The different samples taken at days 3, 5, 7, and 9 were analyzed for their FPase (filter-paperase) and CMCase (carboxymethyl-cellulase) activities. The results presented in Figure 4A show the proportional increase of the FPase activity with the concentration of substrate up to the 7th day of culture, reaching a maximum of 0.25 ± 0.0058 U/mL (using 2% (w/v) date seed). Beyond the 7th day, the FPase activity decreased, which was probably due to the cell lysis, the release of protease, and the enzyme degradation. Similar results were observed for the CMCase activity, with the highest value (1.74 ± 0.022 U/mL) found at the 7th day (using 2% (w/v) date seed) (Figure 4B), which decreased beyond this point.
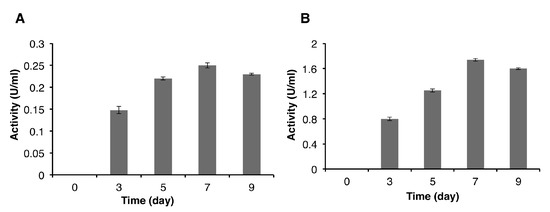
Figure 4.
FPase (A) and carboxymethyl cellulose (CMC)ase (B) activities produced by P. occitanis Pol6 taken at the 3rd, 5th, 7th, and 9th day of culture.
3.3.2. Cellulose Hydrolysis
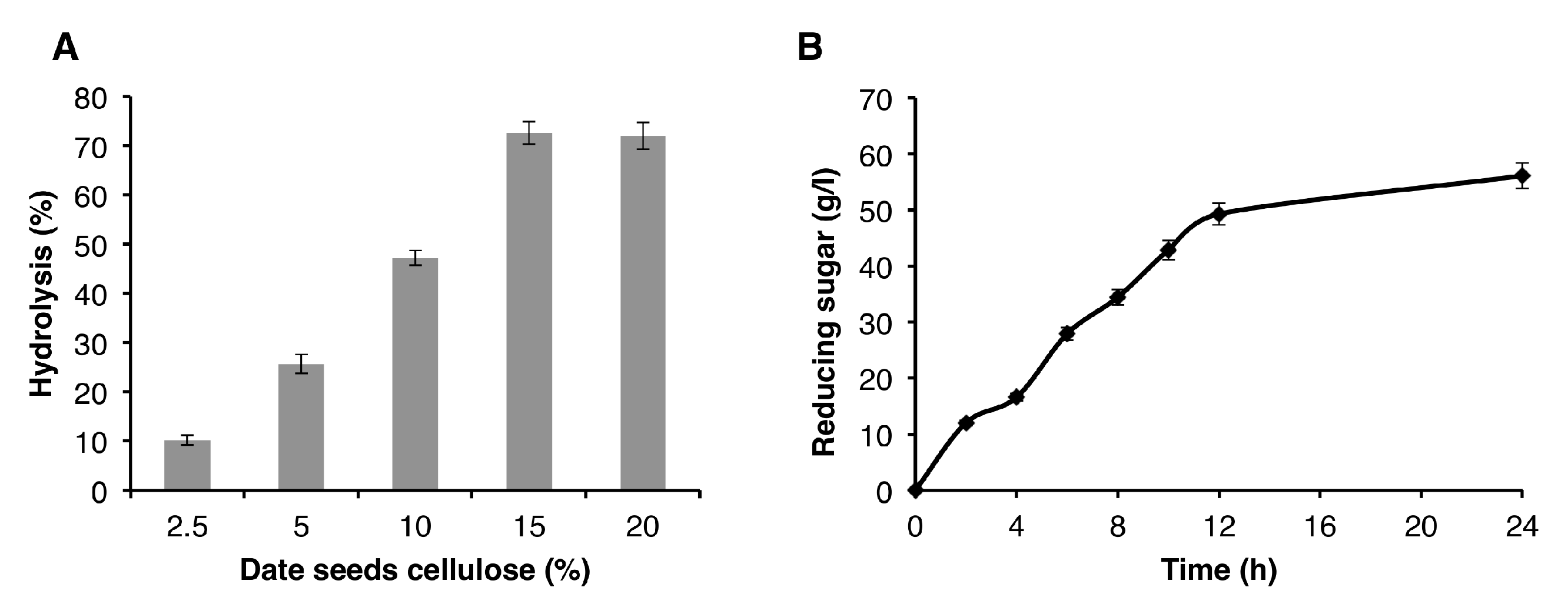
The cellulose fraction from seeds of the Deglet Nour variety was hydrolyzed using the enzymes produced by P. occitanis Pol6 strain, which were collected at the 7th day of culture. Different concentrations of date seed cellulose were used to optimize the substrate concentration for cellulose hydrolysis. The obtained results (Figure 5) showed that the maximum hydrolysis was obtained using a 15% substrate concentration. The hydrolysis of date seed cellulose was performed over 24 h using 1.74 ± 0.022 U/mL CMCase and 0.25 ± 0.0058 U/mL PFase. After incubation for 20 h, the amount of reducing sugars released was 56.1 mg/mL, and the hydrolysis yield recorded was 37.4%.
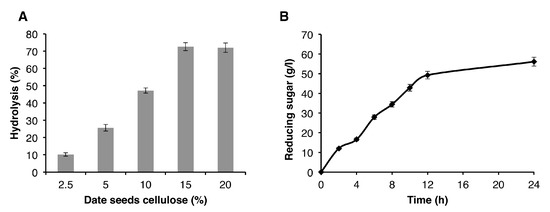
Figure 5.
(A) Optimization of date seed cellulose percentage. (B) Hydrolysis kinetic of date seed cellulose.
3.4. Ethanol Production by Yeast Fermentation
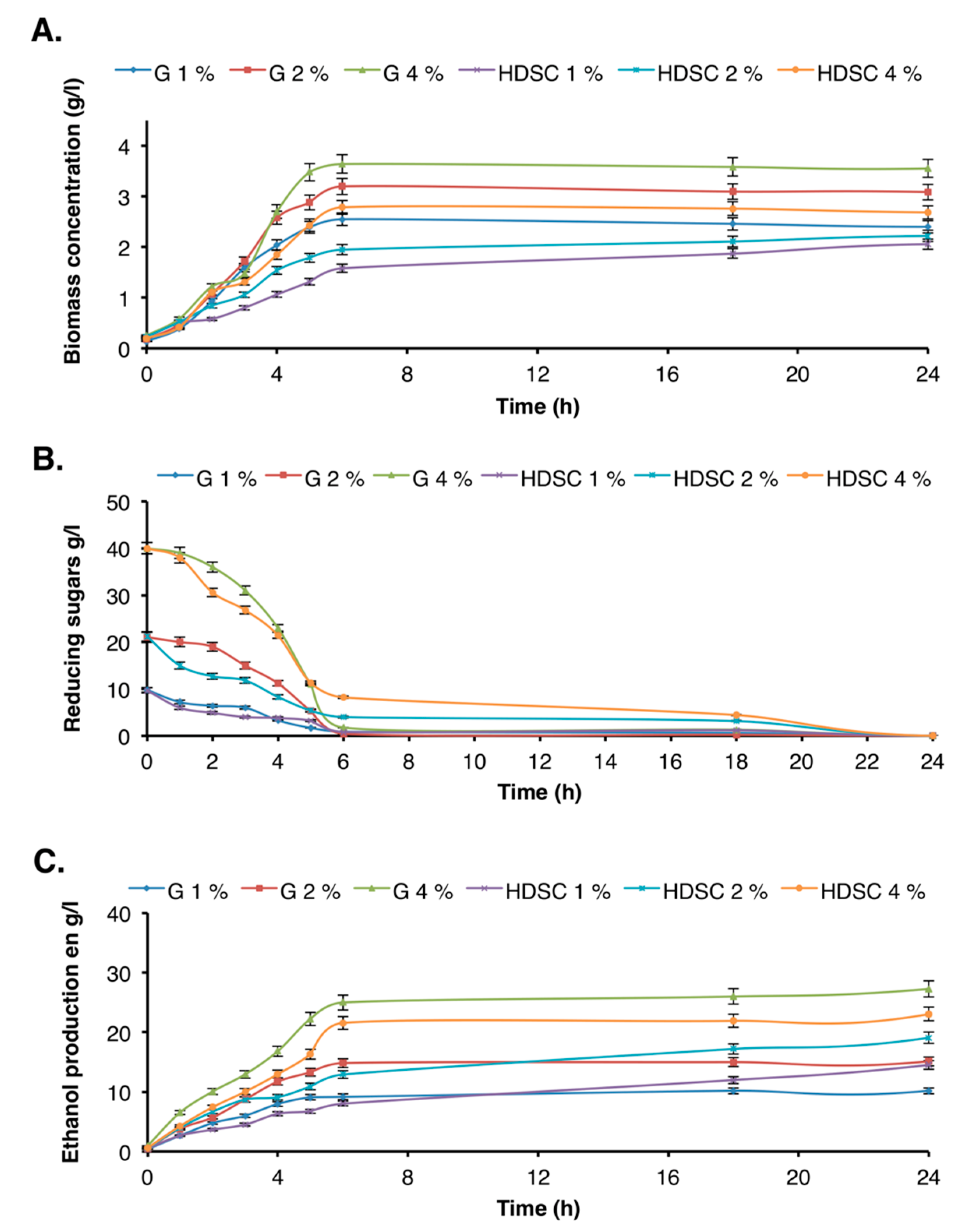
After cellulose hydrolysis, the released glucose was converted to ethanol using S. cerevisiae yeast. Results were compared to those obtained with commercial glucose used at the same concentration range. The biomass concentration, residual reducing sugars amount, and concentration of ethanol were optimized. The results presented in Figure 6 showed that the highest bioethanol production yield was obtained using a mixture of 4% commercial glucose and 4% enzymatic hydrolyzate.
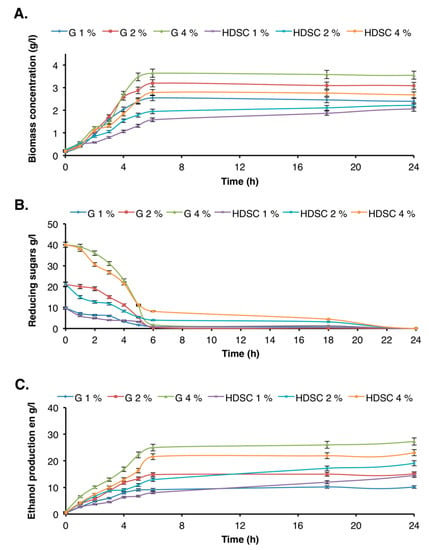
Figure 6.
Determination of fermentation parameters: (A) biomass concentration, (B) reducing sugars, (C) bioethanol production. G 1%, G 2%, and G 4% represent respectively the concentrations of commercial glucose used as control for ethanol production. HDSC denotes hydrolyzate of date seed cellulose.
After 6 h fermentation, the substrate was completely consumed, and the production of biomass and ethanol reached a maximum using both substrates (commercial glucose and date seed cellulose hydrolyzate), irrespective of the concentration. In addition, the obtained result showed that for the same initial concentration of carbon substrate, biomass and ethanol production were higher using a commercial substrate than enzymatic hydrolyzate. This result was not surprising due to the presence of other secondary molecules in the hydrolyzate that may decrease the growth of S. cerevisiae yeast and consequently the production of ethanol. Furthermore, after 6 h of fermentation, the biomass and ethanol concentrations obtained using 4% cellulose hydrolyzate were 2.78 g/L and 21.57 g/L, respectively, whereas these values were of 3.64 g/L and 25 g/L, respectively using commercial glucose. Compared to the other by-products, date seeds are a promising source for bioethanol production. For example, ethanol production using 6% potato peel waste was 21 g/L after 10 h [45], and that obtained using sugar beet molasses and thick juice was in the range of 31.7–109.5 g/L, depending on sugar concentration and the use or not of immobilized cells [46].
Additionally, the maximal specific growth rate (µmax) values increased proportionally to glucose concentrations (Table 2), with similar values obtained using 1% and 2% substrate concentrations. However, the µmax values were higher for the commercial glucose (µmax = 0.427 h−1) than the enzymatic hydrolyzate (µmax = 0.306 h−1). After 6 h fermentation, the productivities YX/S and YP/S using enzymatic hydrolyzate were of 7.16% and 65.84%, respectively, whereas those using commercial glucose were of 8.86% and 62.89, respectively (Table 2).

Table 2.
Fermentation characteristics. µmax represents the maximal specific growth rate, G represents the generation time, YX/S and YP/S represent the yield coefficients of the mass of cells or product formed per unit mass of substrate consumed, respectively. G 1%, G 2%, and G 4% represent respectively the concentrations of commercial glucose used as control for ethanol production. HDSC denotes hydrolyzate of date seed cellulose.
4. Conclusions
Results from this work showed that date seeds represent a potential feedstock for bioethanol production after an appropriate pre-treatment. Ethanol production was performed after the generation of fermentescible sugars using enzymatic hydrolysis of the cellulosic fraction of date seeds, followed by alcoholic fermentation. Interesting ethanol concentration (21.57 g/L) was obtained, which demonstrates the feasibility of adopting this multistage strategy for the valorization of date seed by-product and its possible scaling-up. Limitations to scaling up the process could be related to the relatively high-energy consumption to grind the seeds and the cost associated to enzyme production for cellulose hydrolysis, which could make the process less competitive than others, transforming ready-to-use by-products such as sugar beet and sugar cane molasses.
Author Contributions
Conceptualization, S.E.C. and R.E.G.; methodology, F.B. and A.B.A.; formal analysis, I.K.; investigation, M.K., K.B.J.; writing—original draft preparation, F.B. and M.K.; writing—review and editing, M.K. and F.J.B.; supervision, S.E.C.; funding acquisition, S.E.C. and R.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education and Scientific Research in Tunisia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Awasthi, P.; Shrivastava, S.; Kharkwal, A.C.; Varma, A. Biofuel from agricultural waste: A review. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 470–477. [Google Scholar]
- Shah, N.; Rehan, T. Bioethanol production from biomass. J. Chem. Biochem. 2014, 2, 161–167. [Google Scholar] [CrossRef][Green Version]
- Balan, V.; Chiaramonti, D.; Kumar, S. Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels Bioprod. Biorefin. 2013, 7, 732–759. [Google Scholar] [CrossRef]
- Chandel, A.K.; Chan, E.N.; Rudravaram, R.; Lakshmi Narasu, M.; Venkateswar Rao, L.; Ravindra, P. Economics and environmental impact of bioethanol production technologies: An appraisal. Biotechnol. Mol. Biol. Rev. 2007, 2, 14–32. [Google Scholar]
- Conde-Mejía, C.; Jiménez-Gutiérrez, A.; El-Halwagi, M. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Mood, S.M.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992–5002. [Google Scholar] [CrossRef]
- Hu, G.; Heitmann, J.A.; Rojas, O.J. Feedstock pretreatment strategies for producing ethanol from wood, bark, and forest residues. BioResources 2008, 3, 270–294. [Google Scholar]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Dagnino, E.P.; Chamorro, E.R.; Romano, S.D.; Felissia, F.E.; Area, M.C. Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production. Ind. Crop. Prod. 2013, 42, 363–368. [Google Scholar] [CrossRef]
- Krishna, S.H.; Prasanthi, K.; Chowdary, G.V.; Ayyanna, C. Simultaneous saccharification and fermentation of pretreated sugar cane leaves to ethanol. Process Biochem. 1998, 33, 825–830. [Google Scholar] [CrossRef]
- Kádár, Z.; Szengyel, Z.; Réczey, K. Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind. Crop. Prod. 2004, 20, 103–110. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels securing the planet’s future energy needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- Marta, A.D.; Mancini, M.; Orlando, F.; Natali, F.; Capecchi, L.; Orlandini, S. Sweet sorghum for bioethanol production: Crop responses to different water stress levels. Biomass Bioenergy 2014, 64, 211–219. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Cheng, K.K.; Ge, J.P.; Zhang, J.A.; Ling, H.Z.; Zhou, Y.J.; Yang, M.D.; Xu, J.M. Fermentation of pretreated sugarcane bagasse hemicellulose hydrolysate to ethanol by Pachysolen tannophilus. Biotechnol. Lett. 2007, 29, 1051–1055. [Google Scholar] [CrossRef]
- Pasha, C.; Kuhad, R.C.; Rao, L.V. Strain improvement of thermotolerant Saccharomyces cerevisiae vs. strain for better utilization of lignocellulosic substrates. J. Appl. Microbiol. 2007, 103, 1480–1489. [Google Scholar] [CrossRef]
- Walfridsson, M.; Bao, X.; Anderlund, M.; Lilius, G.; Bülow, L.; Hahn-Hägerdal, B. Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl. Environ. Microbiol. 1996, 62, 4648–4651. [Google Scholar] [CrossRef]
- Cao, L.; Tang, X.; Zhang, X.; Zhang, J.; Tian, X.; Wang, J.; Xiong, M.; Xiao, W. Two-stage transcriptional reprogramming in Saccharomyces cerevisiae for optimizing ethanol production from xylose. Metab. Eng. 2014, 24, 150–159. [Google Scholar] [CrossRef]
- Martín, C.; Galbe, M.; Wahlbom, C.F.; Hahn-Hägerdal, B.; Jönsson, L.J. Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzym. Microb. Technol. 2002, 31, 274–282. [Google Scholar] [CrossRef]
- Gong, C.S.; Chen, L.F.; Flickinger, M.C.; Chiang, L.C.; Tsao, G.T. Production of ethanol from D-xylose by using D-xylose isomerase and yeasts. Appl. Environ. Microbiol. 1981, 41, 430–436. [Google Scholar] [CrossRef]
- Afiq, M.J.A.; Rahman, R.A.; Man, Y.B.C.; AL-Kahtani, H.A.; Mansor, T.S.T. Date seed and date seed oil. Int. Food Res. J. 2013, 20, 2035–2043. [Google Scholar]
- Hamada, J.S.; Hashim, I.B.; Sharif, F.A. Preliminary analysis and potential uses of date pits in foods. Food Chem. 2002, 76, 135–137. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Taghizadeh, M. Date seed as a new source of dietary fiber: Physicochemical and baking properties. Int. Food Res. J. 2016, 23, 2419–2425. [Google Scholar]
- Bouaziz, F.; Abdeddayem, A.B.; Koubaa, M.; Ghorbel, R.E.; Chaabouni, S.E. Date seeds as a natural source of dietary fibers to improve texture and sensory properties of wheat bread. Foods 2020, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nation FAO, Statistical Databases. Available online: http://www.fao.org/faostat/en/#home (accessed on 17 April 2020).
- Herch, W.; Kallel, H.; Boukhchina, S. Physicochemical properties and antioxidant activity of Tunisian date palm (Phoenix dactylifera L.) oil as affected by different extraction methods. Food Sci. Technol. 2014, 34, 464–470. [Google Scholar] [CrossRef]
- Koubaa, M.; Ktata, A.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Bouaziz, F.; Driss, D.; Chaabouni, S.E. Water-soluble polysaccharides from Opuntia stricta Haw. fruit peels: Recovery, identification and evaluation of their antioxidant activities. Int. Agrophys. 2015, 29, 299–306. [Google Scholar] [CrossRef]
- Bettaieb, F.; Khiari, R.; Dufresne, A.; Mhenni, M.F.; Belgacem, M.N. Mechanical and thermal properties of Posidonia oceanica cellulose nanocrystal reinforced polymer. Carbohydr. Polym. 2015, 123, 99–104. [Google Scholar] [CrossRef]
- Bettaieb, F.; Khiari, R.; Hassan, M.L.; Belgacem, M.N.; Bras, J.; Dufresne, A.; Mhenni, M.F. Preparation and characterization of new cellulose nanocrystals from marine biomass Posidonia oceanica. Ind. Crop. Prod. 2015, 72, 175–182. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2012; ISBN 978-3-11-048041-2. [Google Scholar]
- Jain, S.; Parriche, M.; Durand, H.; Tiraby, G. Production of polysaccharidases by a cellulase-pectinase hyperproducing mutant (Pol6) of Penicillium occitanis. Enzym. Microb. Technol. 1990, 12, 691–696. [Google Scholar] [CrossRef]
- Chaabouni, S.E.; Belguith, H.; Hassairi, I.; M’Rad, K.; Ellouz, R. Optimization of cellulase production by Penicillium occitanis. Appl. Microbiol. Biotechnol. 1995, 43, 267–269. [Google Scholar] [CrossRef]
- Mandels, M.; Weber, J. The production of cellulases. In Cellulases and Their Applications; Hajny, G.J., Reese, E.T., Eds.; American Chemical Society: Washington, DC, USA, 1969; Volume 95, pp. 391–414. ISBN 0-8412-0095-5. [Google Scholar]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bryan, W.L. Solid-state fermentation of sugars in sweet sorghum. Enzym. Microb. Technol. 1990, 12, 437–442. [Google Scholar] [CrossRef]
- Boudechiche, L.; Araba, A.; Tahar, A.; Ouzrout, R. Etude de la composition chimique des noyaux de dattes en vue d’une incorporation en alimentation animale. Livest. Res. Rural Dev. 2009, 21, 69. [Google Scholar]
- Aguirre, M.J.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Zúñiga, E.A. Characterization of a neutral polysaccharide with antioxidant capacity from red wine. Carbohydr. Res. 2009, 344, 1095–1101. [Google Scholar] [CrossRef]
- Sebastian, S.; Sundaraganesan, N.; Manoharan, S. Molecular structure, spectroscopic studies and first-order molecular hyperpolarizabilities of ferulic acid by density functional study. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 312–323. [Google Scholar] [CrossRef]
- Hua, D.; Zhang, D.; Huang, B.; Yi, P.; Yan, C. Structural characterization and DPPH radical scavenging activity of a polysaccharide from Guara fruits. Carbohydr. Polym. 2014, 103, 143–147. [Google Scholar] [CrossRef]
- Sila, A.; Sayari, N.; Balti, R.; Martinez-Alvarez, O.; Nedjar-Arroume, N.; Moncef, N.; Bougatef, A. Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 2014, 148, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, K.B.; Maktouf, S.; Ghazala, I.; Frikha, D.; Ghribi, D.; Ghorbel, R.E.; Nouri-Ellouz, O. Potato peel as feedstock for bioethanol production: A comparison of acidic and enzymatic hydrolysis. Ind. Crop. Prod. 2014, 52, 144–149. [Google Scholar]
- Vučurović, V.M.; Puškaš, V.S.; Miljić, U.D. Bioethanol production from sugar beet molasses and thick juice by free and immobilised Saccharomyces cerevisiae. J. Inst. Brew. 2019, 125, 134–142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).