Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation Using Experimental Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Analytes and Reagents
2.3. Preparation of Standard Solutions
2.4. Preparation of Sample Solutions
2.5. Chromatographic Conditions
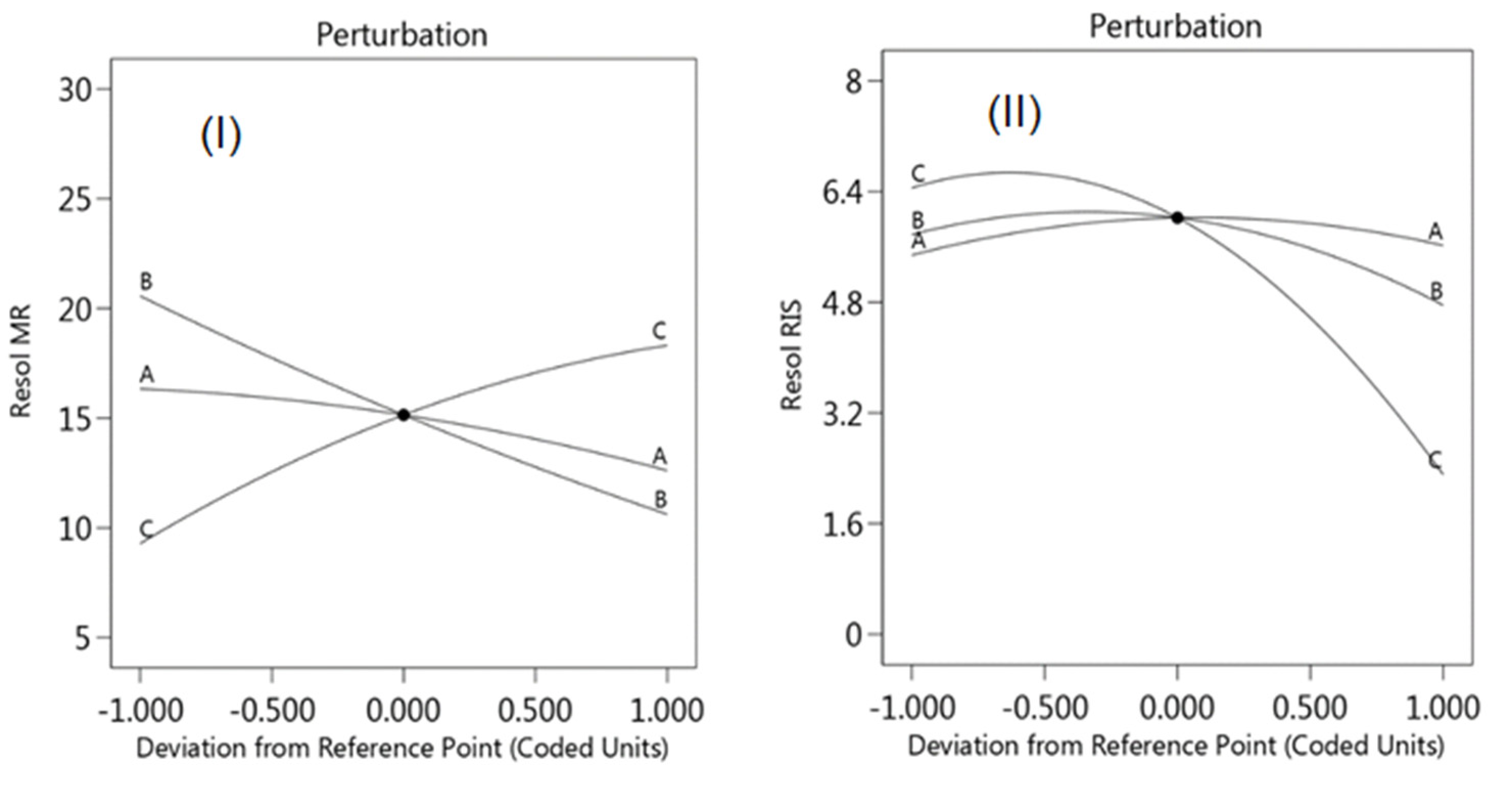
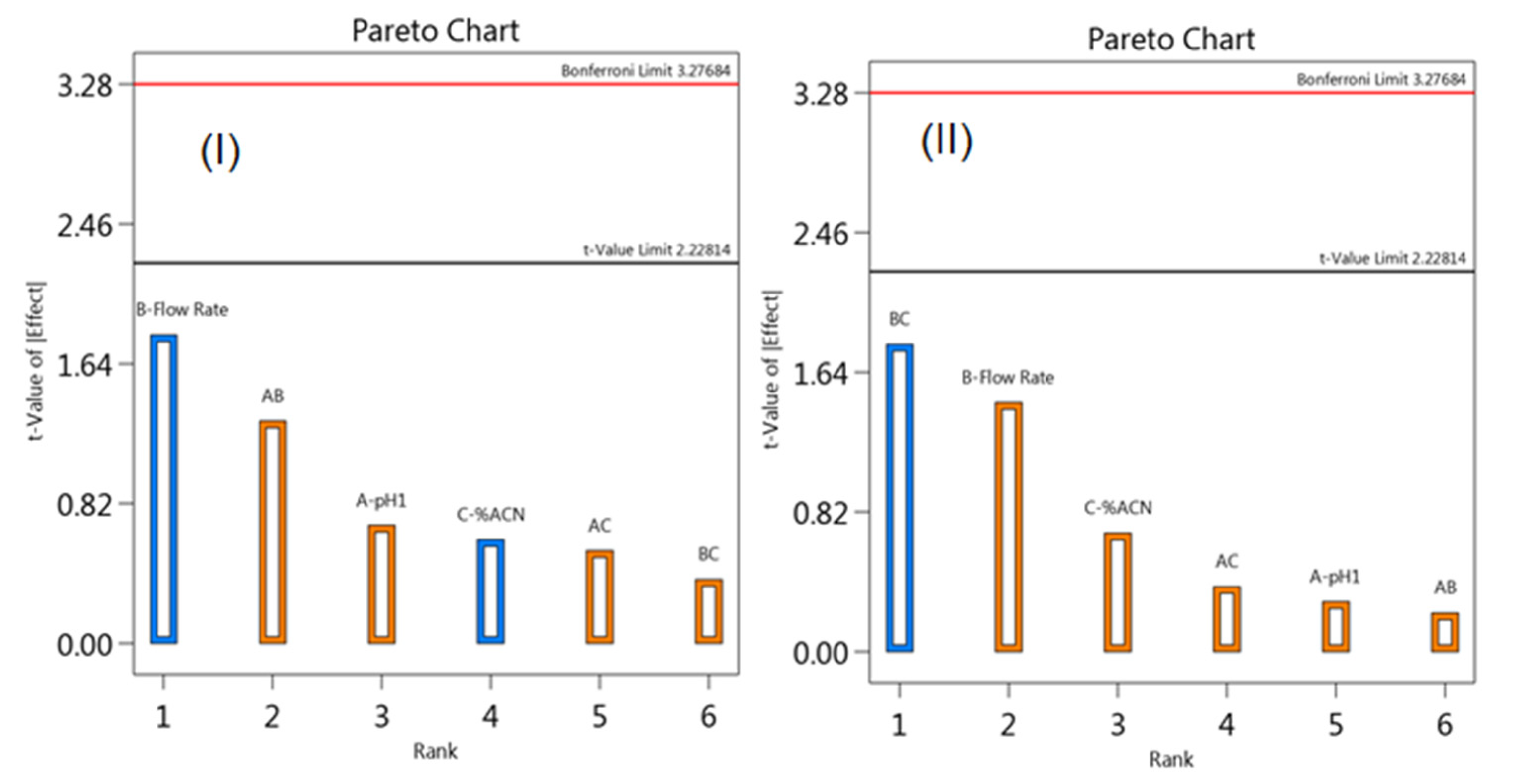
2.6. Optimization of Chromatographic Conditions by Experimental Design
2.7. Method Validation
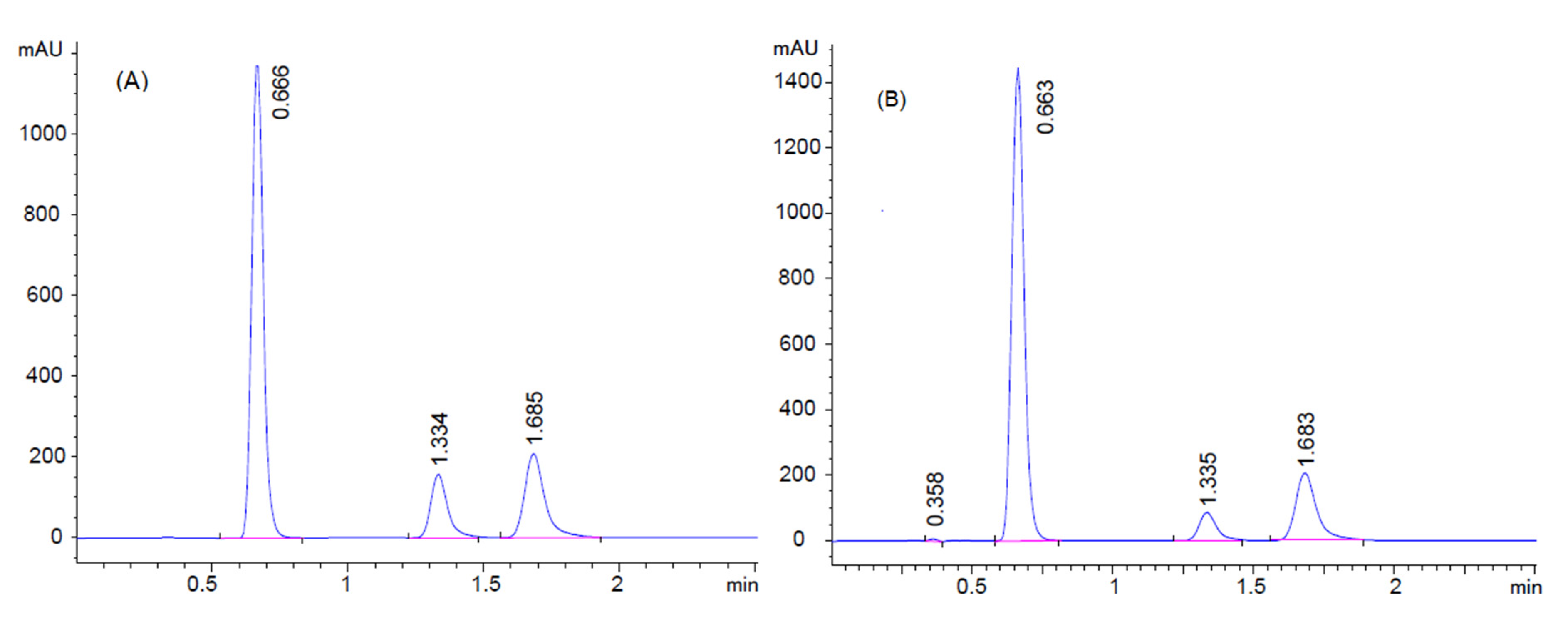
2.7.1. System Suitability for HPLC Method
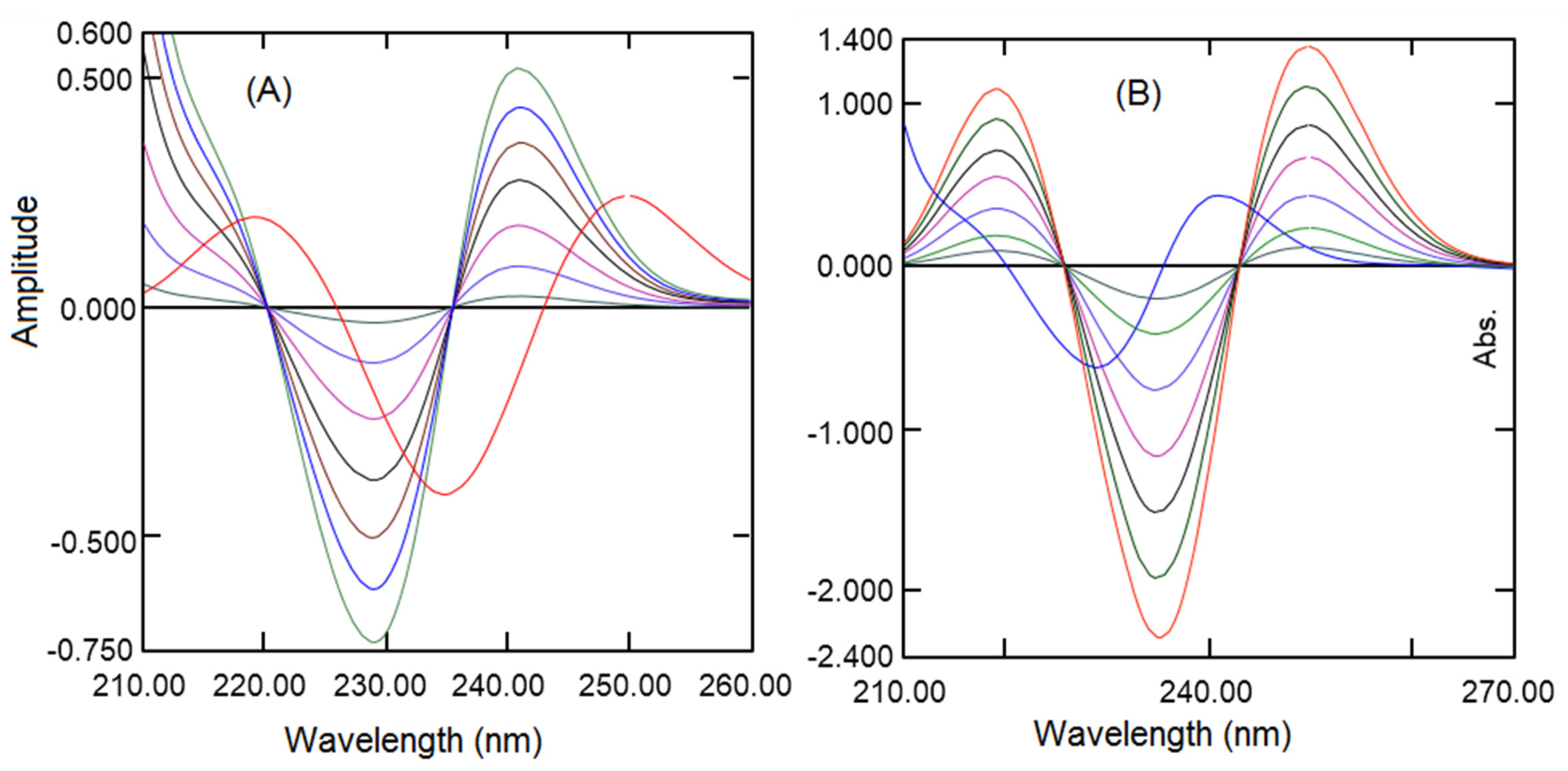
2.7.2. Linearity
Second-derivative Spectroscopic Method
HPLC Method
2.7.3. Limit of Detection and Quantification
2.7.4. Precision and Accuracy
2.7.5. Recovery Studies
2.7.6. Robustness Using Experimental Design
2.8. Analysis of Laboratory Mixed Solutions and Formulations
3. Results and Discussion
3.1. Optimization of HPLC Method
3.2. Validation
3.2.1. System Suitability Tests for the HPLC Method
3.2.2. Linearly
3.2.3. Limit of Detection and Quantification
3.2.4. Precision and Accuracy
3.2.5. Recovery Study
3.2.6. Robustness
3.3. Analysis of Laboratory Mixed Solutions and Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar] [CrossRef]
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus—Implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020, 16, 21–31. [Google Scholar] [CrossRef]
- Layla, A.; Anjali, B.; Azeem, M. Prevalence’s of overweight, obesity, hyperglycaemia, hypertension and dyslipidaemia in the Gulf: Systematic review. JRSM Short Rep. 2011, 2, 55. [Google Scholar] [CrossRef]
- Moon, M.K.; Hur, K.Y.; Ko, S.H.; Park, S.O.; Lee, B.W.; Kim, J.H.; Rhee, S.Y.; Kim, H.; Choi, K.M.; Kim, N.H. Committee of Clinical Practice Guidelines of the Korean Diabetes Association. Combination therapy of oral hypoglycemic agents in patients with type 2 diabetes mellitus. Korean J. Intern. Med. 2017, 32, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2010, 204, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Jabbour, S.; Ziring, B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med. 2011, 123, 15–23. [Google Scholar] [CrossRef]
- Setter, S.; Iltz, J.; Thams, J.; Campbell, R. Metformin Hydrochloride in the Treatment of Type 2 Diabetes Mellitus: A Clinical Review with a Focus on Dual Therapy. Clin. Ther. 2004, 25, 2991–3026. [Google Scholar] [CrossRef]
- Mohan, V.; Mithal, A.; Joshi, S.R.; Aravind, S.R.; Chowdhury, S. Remogliflozin Etabonate in the Treatment of Type 2 Diabetes: Design, Development, and Place in Therapy. Drug Des. Dev. Ther. 2020, 14, 2487–2501. [Google Scholar] [CrossRef]
- Markham, A. Remogliflozin Etabonate: First global approval. Drugs 2019, 79, 1157–1161. [Google Scholar] [CrossRef]
- Simes, B.C.; MacGregor, G.G. Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: A Clinician’s Guide. Diabetes Metab. Syndr. Obes. 2019, 12, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Pharmacodynamics, efficacy and safety of sodium–glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, Y.; Katsuno, K.; Nakashima, I.; Ishikawa-Takemura, Y.; Fujikura, H.; Isaji, M. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J. Pharmacol. Exp. Ther. 2008, 327, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, M.; Aravind, S.R.; Thacker, H.; Paramesh, S.; Mohan, B.; Chawla, M.; Asirvatham, A.; Goyal, R.; Shembalkar, J.; Balamurugan, R.; et al. Efficacy and safety of Remogliflozin Etabonate, a new sodium glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus: A 24-week, randomized, double-blind, active-controlled trial. Drugs 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.; Bonaca, M.P. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Hussey, E.K.; Kapur, A.; O’Connor-Semmes, R.; Tao, W.; Rafferty, B.; Polli, J.W.; James, C.D.; Dobbins, R.L. Safety, pharmacokinetics and pharmacodynamics of remogliflozin etabonate, a novel SGLT2 inhibitor, and metformin when co-administered in subjects with type 2 diabetes mellitus. BMC Pharmacol. Toxicol. 2013, 14, 25. [Google Scholar] [CrossRef]
- Al-Janabi, W.S.K.; Mahmood, A.K.; Luaibi, H.M. Determination of the Dissociation Constants of Metformin from a Second Derivative UV Spectrum. Int. J. Res. Pharm. Sci. 2020, 11, 790–796. [Google Scholar] [CrossRef]
- Chhetri, H.P.; Thapa, P.; Schepdael, A.V. Simple HPLC-UV method for the quantification of metformin in human plasma with one step protein precipitation. Saudi Pharmaceut. J. 2014, 22, 483–487. [Google Scholar] [CrossRef]
- Chaudhari, K.; Wang, J.; Xu, Y.; Winters, A.; Wang, L.; Dong, X.; Cheng, E.Y.; Liu, R.; Yang, S.H. Determination of metformin bio-distribution by LC-MS/MS in mice treated with a clinically relevant paradigm. PLoS ONE 2020, 15, e0234571. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y.; Gupta, V.K. Analysis of metformin dosage formulations by capillary electrophoresis at nano scale detection. Comb. Chem. High Throughput Screen. 2007, 10, 611–615. [Google Scholar] [CrossRef]
- Majithia, R.H.; Khodadiya, A.; Patel, V.B. Spectrophotometric method development and validation for simultaneous estimation of Anagliptin and Metformin HCl BY Q—Absorption ratio method in synthetic mixture. Heliyon 2020, 6, e03855. [Google Scholar] [CrossRef]
- Attimarad, M.V.; Nair, A.B.; Aldhubaib, B.E. Development of liquid chromatographic method for the simultaneous determination of metformin and miglitol in human plasma: Application to pharmacokinetic studies. J. Iranian Chem. Soc. 2015, 12, 1629–1636. [Google Scholar] [CrossRef]
- Neelima, K.; Prasad, Y.R. Analytical method development and validation of metformin, voglibose, glimepiride in bulk and combined tablet dosage form by gradient RP-HPLC. Pharmaceut. Meth. 2014, 5, 27–33. [Google Scholar] [CrossRef]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and gliclazide. J. Food Drug Anal. 2019, 27, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sebaiy, M.M.; El-Adl, S.M.; Baraka, M.M.; Hassan, A.A. Rapid RP-HPLC method for simultaneous estimation of metformin, pioglitazone, and glimepiride in human plasma. Acta Chromatogr. 2020, 32, 16–21. [Google Scholar] [CrossRef]
- Shirode, A.; Maduskar, P.; Deodhar, M.; Kadam, V. RP-HPLC and HPTLC methods for simultaneous estimation of metformin hydrochloride and vildagliptin from bulk and marketed formulation: Development and validation. Br. J. Pharmaceut. Res. 2014, 4, 2370–2386. [Google Scholar] [CrossRef]
- Antonopoulos, N.; Machairas, G.; Migias, G.; Vonaparti, A.; Brakoulia, V.; Pistos, C.; Gennimata, D.; Panderi, I. Hydrophilic Interaction Liquid Chromatography-Electrospray Ionization Mass Spectrometry for Therapeutic Drug Monitoring of Metformin and Rosuvastatin in Human Plasma. Molecules 2018, 23, 1548. [Google Scholar] [CrossRef]
- Al Bratty, M.; Alhazmi, H.A.; Javed, S.A.; Lalitha, K.G.; Asmari, M.; Wölker, J.; El Deeb, S. Development and Validation of LC–MS/MS Method for Simultaneous Determination of Metformin and Four Gliptins in Human Plasma. Chromatographia 2017, 80, 891–899. [Google Scholar] [CrossRef]
- Attimarad, M. Multivariate optimization of a capillary zone electrophoresis assay method for simultaneous quantification of metformin and vildagliptin from a formulation. J. Liq. Chrom. Relat. Technol. 2016, 39, 401–407. [Google Scholar] [CrossRef]
- Alnajjar, A.O.; Idris, A.M.; Attimarad, M.V.; Elgorashe, R.E.E. Quadruple Response Factorial Design Optimization of Capillary Zone Electrophoresis Assay Procedure for Metformin and Sitagliptin Combination. J. Liq. Chrom. Relat. Technol. 2015, 38, 1379–1383. [Google Scholar] [CrossRef]
- Mohamed, D.; Elshahed, M.S.; Nasr, T.; Aboutaleb, N.; Zakaria, O. Novel LC-MS/MS method for analysis of metformin and canagliflozin in human plasma: Application to a pharmacokinetic study. BMC Chem. 2019, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Munde, M.K.; Kulkarni, N.S.; Khiste, R.H.; Sen, D.B. Development and Validation of Novel Analytical Method for Empagliflozin and Metformin Hydrochloride in Bulk and Pharmaceutical Dosage Form by Four Different Simultaneous Estimation Approaches using UV Spectroscopy. Res. J. Pharm. Technol. 2020, 13, 1236–1242. [Google Scholar] [CrossRef]
- Tayade, A.B.; Patil, A.S.; Shirkhedkar, A.A. Development and Validation of Zero Order UV-Spectrophotometric Method by Area under Curve Technique and High Performance Thin Layer Chromatography for the Estimation of Remogliflozin Etabonate in Bulk and In-House Tablets. Invent. Rapid Pharm. Anal. Qual. Assur. 2019, 3, 1–5. [Google Scholar]
- Sigafoos, J.F.; Bowers, G.D.; Castellino, S.; Culp, A.G.; Wagner, D.S.; Reese, M.J.; Humphreys, J.E.; Hussey, E.K.; Semmes, R.L.C.; Kapur, A.; et al. Assessment of the drug interaction risk for remogliflozin etabonate, a sodium-dependent glucose cotransporter-2 inhibitor: Evidence from in vitro, human mass balance, and ketoconazole interaction studies. Drug Metab. Dispos. 2012, 40, 2090–2101. [Google Scholar] [CrossRef]
- Tammisetty, M.; Challa, B.R.; Puttagunta, S.B. A novel analytical method for the simultaneous estimation of remogliflozin and metformin hydrochloride by UPLC/PDA in bulk and formulation. Application to the estimation of product traces. Turk. J. Pharm. Sci. 2020, 39699. [Google Scholar] [CrossRef]
- Kamal, A.H.; El-Malla, S.F.; Hammad, S.F. A Review on UV spectrophotometric methods for simultaneous multicomponent analysis. Eur. J. Pharm. Med. Res. 2016, 3, 348–360. [Google Scholar]
- Salinas, F.; Nevado, J.J.B.; Mansilla, A.E. A new spectrophotometric method for quantitative multicomponent analysis resolution of mixtures of salicylic and salicyluric acids. Talanta 1990, 37, 347–351. [Google Scholar] [CrossRef]
- Chohan, M.S.; Elgorashe, R.E.E.; Balgoname, A.A.; Attimarad, M.; SreeHarsha, N.; Venugopala, K.N.; Nair, A.B.; Pottathil, S. Eco-friendly Derivative UV Spectrophotometric Methods for Simultaneous Determination of Diclofenac Sodium and Moxifloxacin in Laboratory Mixed Ophthalmic Preparation. Indian J. Pharm. Edu. Res. 2019, 53, 166–174. [Google Scholar] [CrossRef]
- Bhatt, N.M.; Chavada, V.D.; Sanyal, M.; Shrivastav, P.S. Manipulating ratio spectra for the spectrophotometric analysis of diclofenac sodium and pantoprazole sodium in laboratory mixtures and tablet formulation. Sci. World J. 2014, 495739. [Google Scholar] [CrossRef]
- Attimarad, M.; Chohan, M.S.; Balgoname, A.A. Simultaneous Determination of Moxifloxacin and Flavoxate by RP-HPLC and Ecofriendly Derivative Spectrophotometry Methods in Formulations. Int. J. Environ. Res. Public Health 2019, 16, 1196. [Google Scholar] [CrossRef]
- Ganorkar, S.B.; Shirkhedkar, A.A. Design of experiments in liquid chromatography (HPLC) analysis of pharmaceuticals: Analytics, applications, implications and future prospects. Rev. Anal. Chem. 2017, 36, 20160025. [Google Scholar] [CrossRef]
- Hashem, H.; El-Sayed, H.M. Quality by design approach for development and validation of a RP-HPLC method for simultaneous determination of co-administered levetiracetam and pyridoxine HCl in prepared tablets. Microchem. J. 2018, 143, 55–63. [Google Scholar] [CrossRef]
- Hashem, H.; El-Sayed, H.M. Quality by Design Strategy for Simultaneous HPLC Determination of Bromhexine HCl and Its Metabolite Ambroxol HCl in Dosage Forms and Plasma. Chromatographia 2020, 83, 123–129. [Google Scholar] [CrossRef]
- Peraman, R.; Bhadraya, K.; Reddy, Y.P.; Reddy, C.S.; Lokesh, T. Analytical Quality by Design Approach in RP-HPLC Method Development for the Assay of Etofenamate in Dosage Forms. Indian J. Pharm. Sci. 2015, 77, 751–757. [Google Scholar] [CrossRef]
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. Development and Optimization of Liquid Chromatography Analytical Methods by Using AQbD Principles: Overview and Recent Advances. Org. Process Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef]
- Shao, J.; Cao, W.; Qu, H.; Pan, J.; Gong, X. A novel quality by design approach for developing an HPLC method to analyze herbal extracts: A case study of sugar content analysis. PLoS ONE 2018, 13, e0198515. [Google Scholar] [CrossRef]
- Patel, K.G.; Shah, P.M.; Shah, P.A.; Gandhi, T.R. Validated high-performance thinlayer chromatographic (HPTLC) method for simultaneous determination of nadifloxacin, mometasone furoate, and miconazole nitrate cream using fractional factorial design. J. Food Drug Anal. 2016, 24, 610–619. [Google Scholar] [CrossRef]
- Patel, K.G.; Patel, A.T.; Shah, P.A.; Gandhi, T.R. Multivariate optimization for simultaneous determination of aspirin and simvastatin by reverse phase liquid chromatographic method using AQbD approach. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 293–301. [Google Scholar] [CrossRef]
- Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S. Box–Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 2020, 10, 3595. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Tony, M.A.; Mansour, S.A. Application of Box–Behnken factorial design for parameters optimization of basic dye removal using nano-hematite photo-Fenton tool. Appl. Water Sci. 2018, 8, 138. [Google Scholar] [CrossRef]
- ICH Harmonized Tripartite Guideline; Text on Validation of Analytical Procedures, Text and Methodology, Q2 (R1), International Conference on Harmonization; ICH Secretariat, c/o IFPMA, 30 rue de St-Jean: Geneva, Switzerland, 2005; pp. 1–17.
- Attimarad, M.; Sreeharsha, N.; Aldhubaib, B.E.; Nair, A.B.; Venugopala, K.N. Simultaneous determination of metformin and three gliptins in pharmaceutical formulations using RPHPLC: Application to stability studies on linagliptin tablet formulation. Indian J. Pharm. Edu. Res. 2014, 48, 45–53. [Google Scholar] [CrossRef]
- Lee, D.H.; Jeong, I.J. A desirability function method for optimizing mean and variability of multiple responses using a posterior preference articulation approach. Qual. Reliab. Eng. Int. 2018, 34, 360–376. [Google Scholar] [CrossRef]
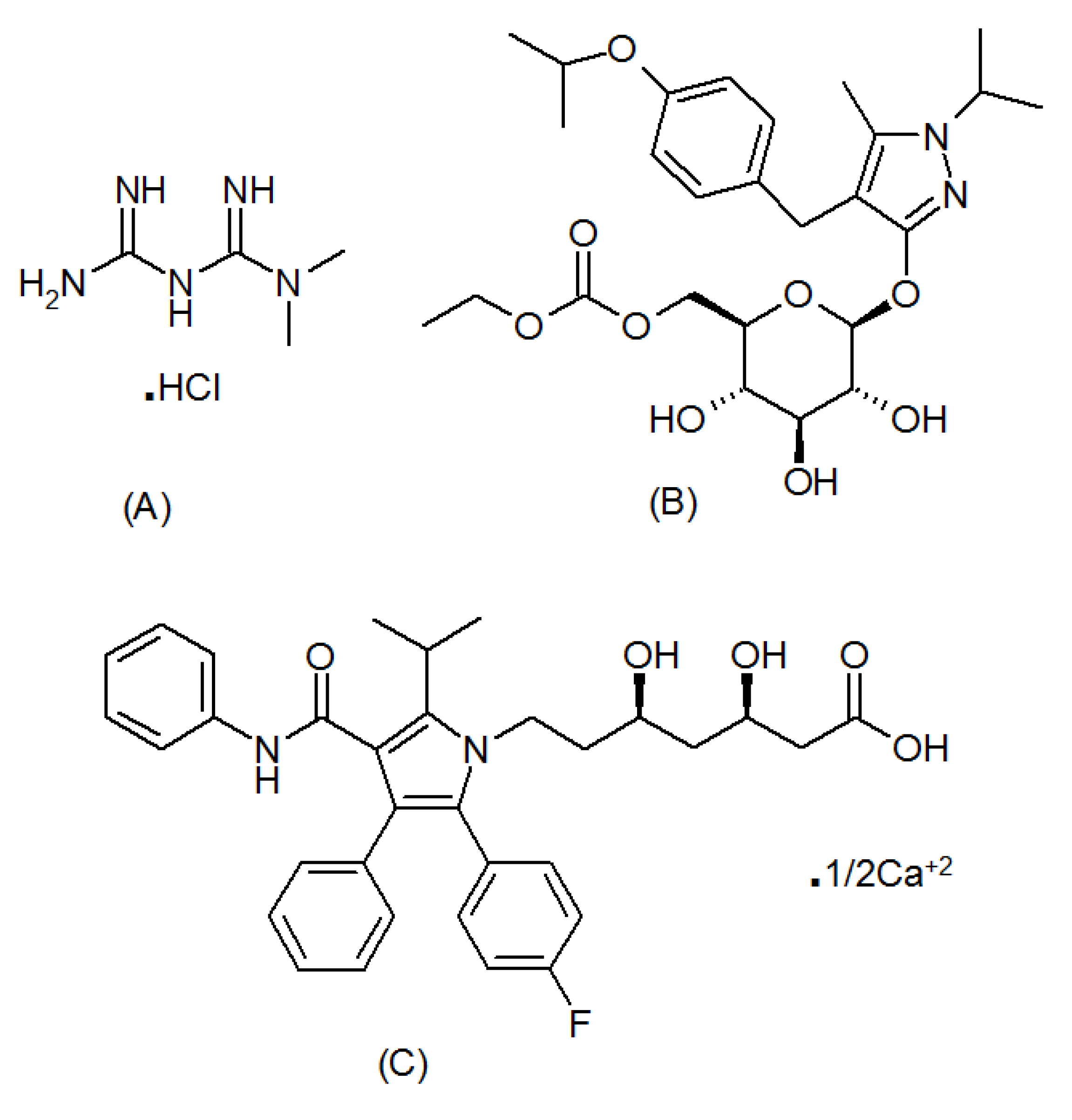
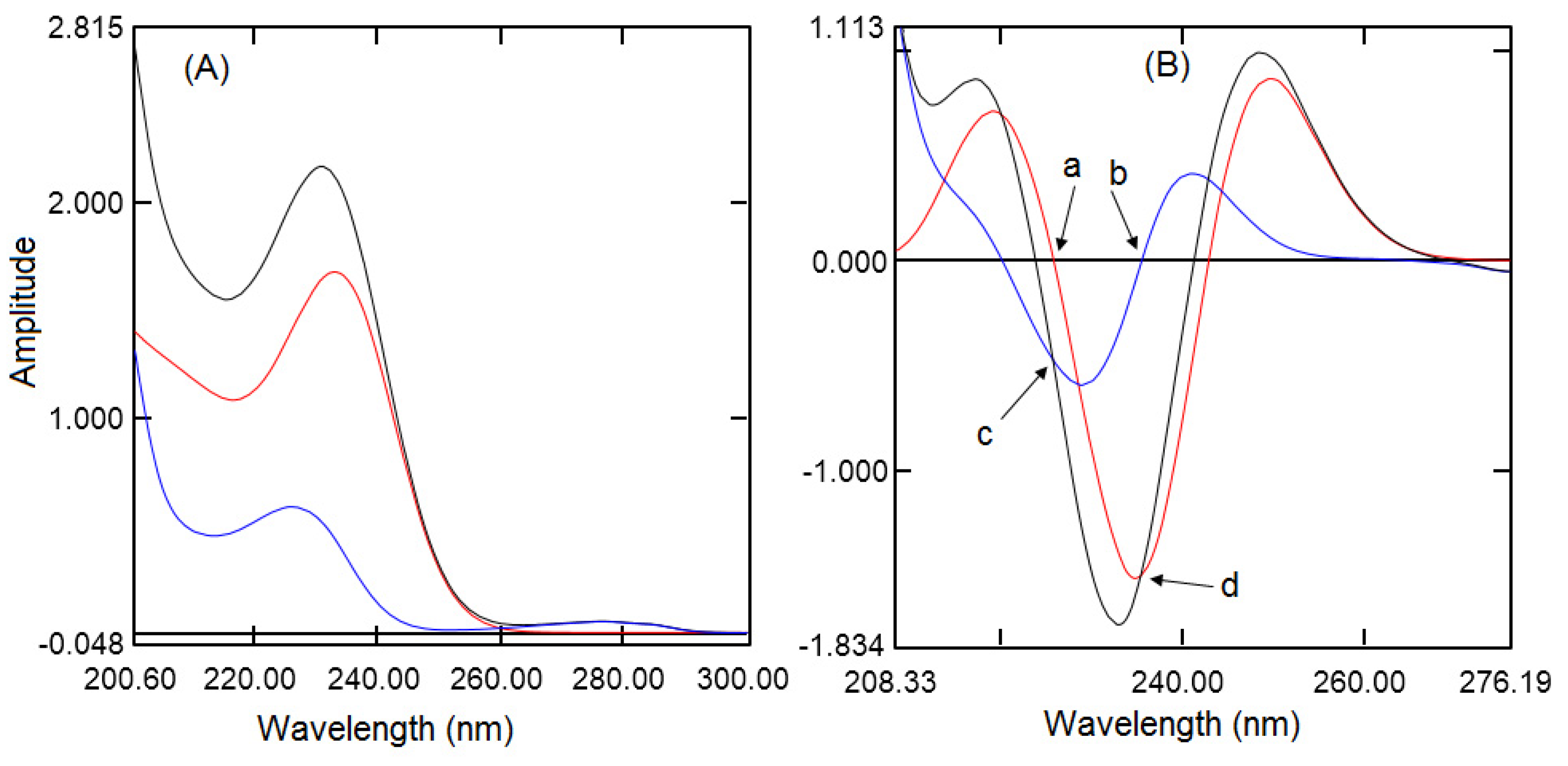
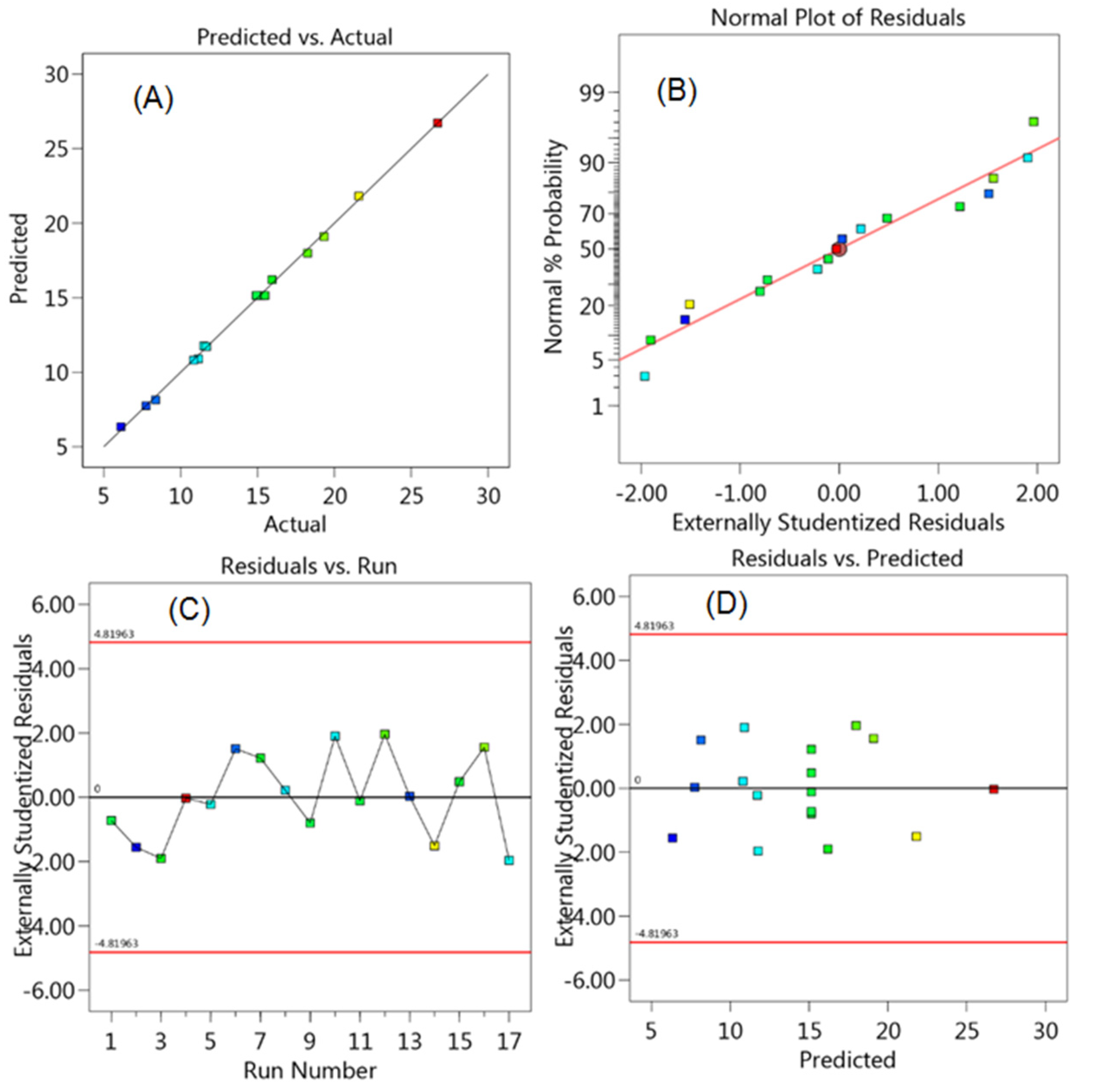
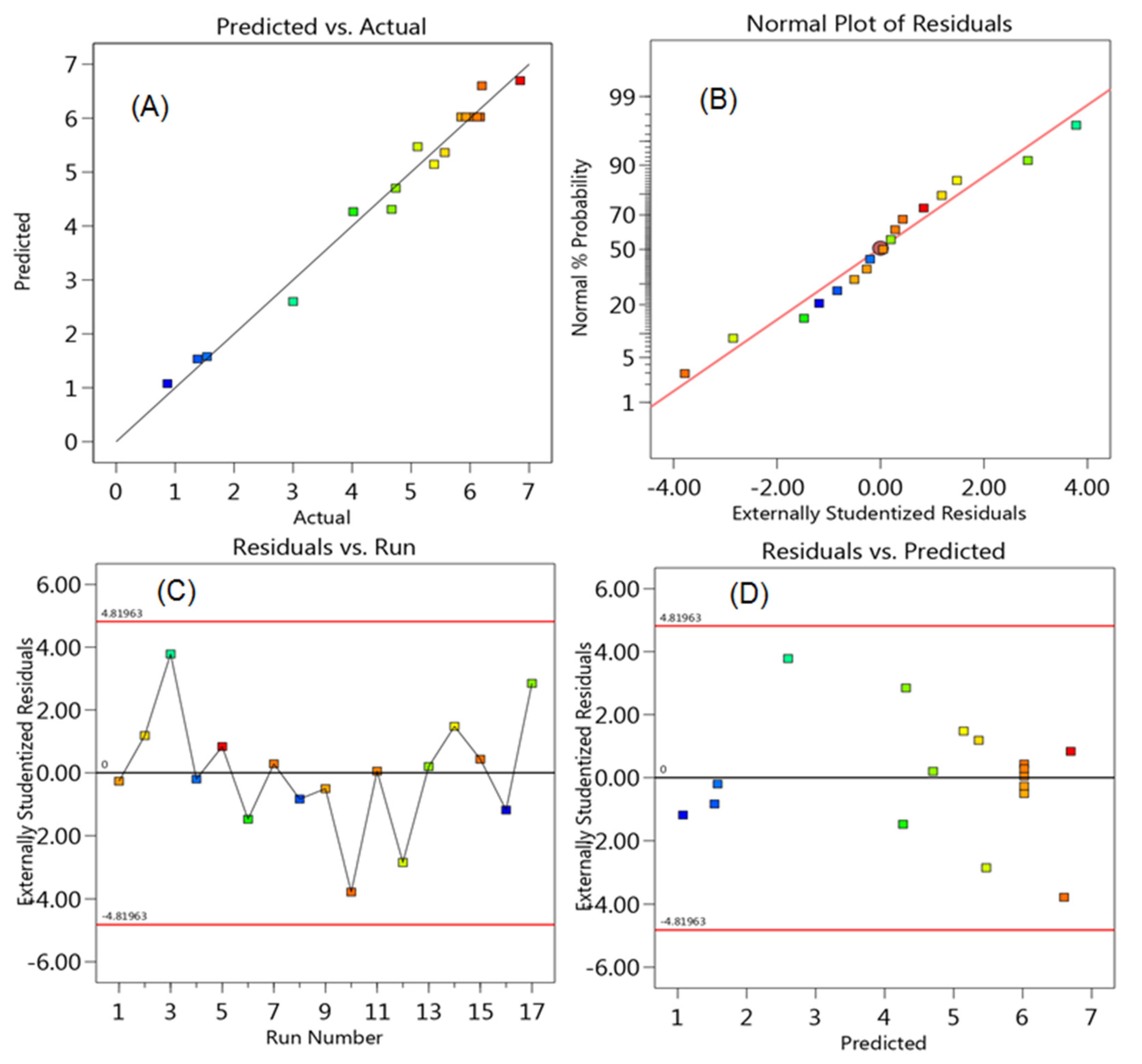
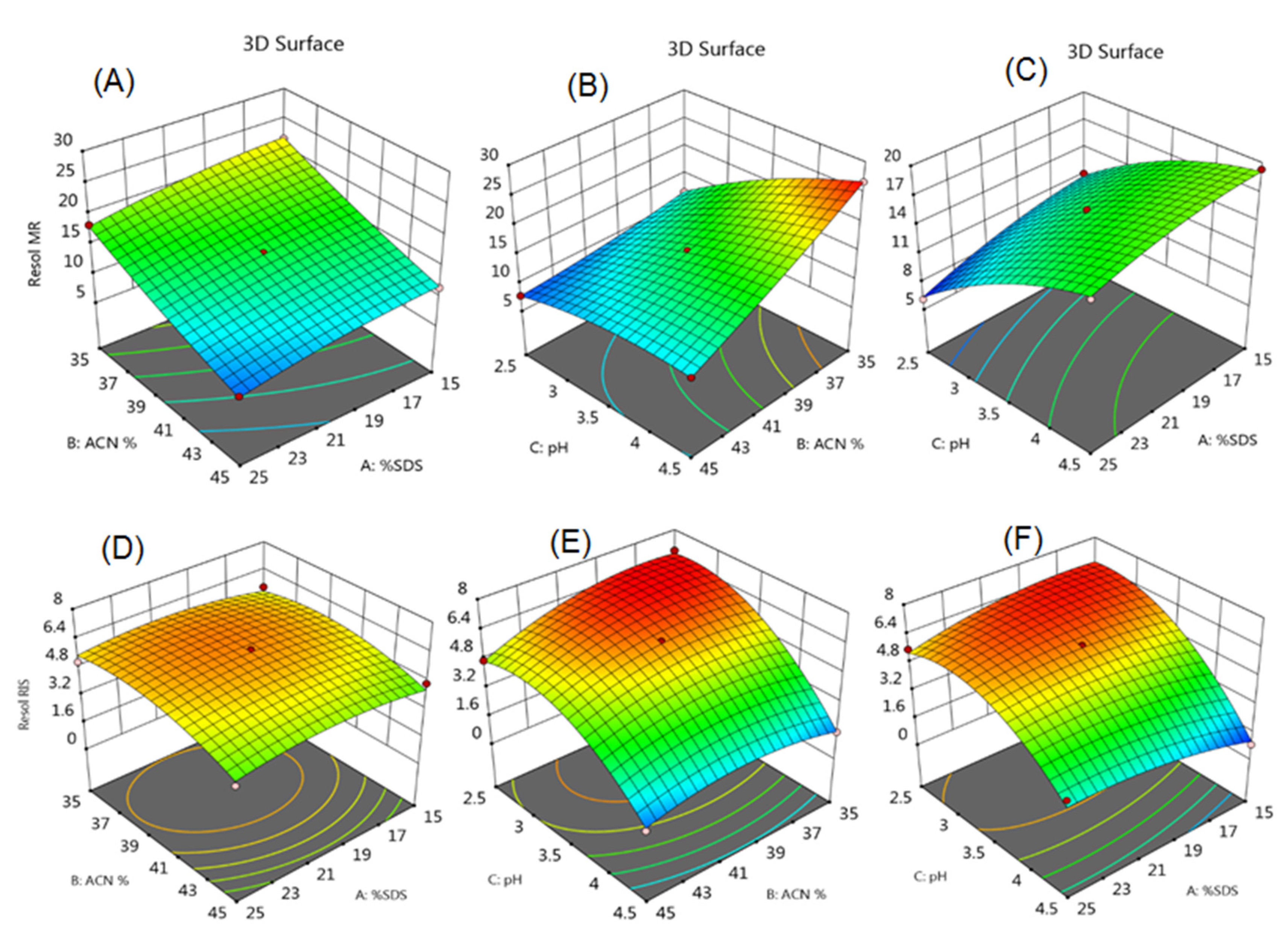

| Experiment Number | Percent SDS | Percent Acetonitrile | pH | Resolution of MFH to RGE | Resolution of RGE to IS |
|---|---|---|---|---|---|
| 1 | 20 | 40 | 3.5 | 14.93 | 5.93 |
| 2 | 25 | 40 | 2.5 | 6.11 | 5.57 |
| 3 | 25 | 40 | 4.5 | 15.94 | 3.00 |
| 4 | 20 | 35 | 4.5 | 26.72 | 1.54 |
| 5 | 20 | 35 | 2.5 | 11.68 | 6.85 |
| 6 | 25 | 45 | 3.5 | 8.36 | 4.02 |
| 7 | 20 | 40 | 3.5 | 15.48 | 6.12 |
| 8 | 20 | 45 | 4.5 | 10.84 | 1.38 |
| 9 | 20 | 40 | 3.5 | 14.91 | 5.85 |
| 10 | 15 | 40 | 2.5 | 11.15 | 6.2 |
| 11 | 20 | 40 | 3.5 | 15.11 | 6.04 |
| 12 | 25 | 35 | 3.5 | 18.25 | 5.11 |
| 13 | 20 | 45 | 2.5 | 7.75 | 4.74 |
| 14 | 15 | 35 | 3.5 | 21.59 | 5.39 |
| 15 | 20 | 40 | 3.5 | 15.29 | 6.17 |
| 16 | 15 | 40 | 4.5 | 19.32 | 0.87 |
| 17 | 20 | 40 | 3.5 | 14.93 | 5.93 |
| Parameters | MFH | RGE | IS | |
|---|---|---|---|---|
| Retention Time ± SD | 0.66 ± 0.019 | 1.31 ± 0.022 | 1.69 ± 0.012 | |
| Peak area ± SD | 4294.15 ± 41.48 a | 363.12 ± 6.95 b | 1070.01 ± 25.57 c | |
| Resolution ± SD | - | 11.77 ± 0.65 d | 5.14 ± 0.25 e | |
| Theoretical plate ± SD | 748.54 ± 15.83 | 1003.62 ± 25.71 | 1151.91 ± 47.28 | |
| Tailing factor ± SD | 0.97 ± 0.034 | 0.89 ± 0.013 | 0.81 ± 0.024 | |
| HPLC Method | UV Spectroscopic Method | |||
|---|---|---|---|---|
| Parameters | MFH | RGE | MFH | RGE |
| Linearity | ||||
| Wavelength (nm) | 230.0 | 230.0 | 235.5 | 243.0 |
| Linearity range (µg/mL) | 5–200 | 2–150 | 2–30 | 1–24 |
| Slope | 0.0353 | 0.0163 | 0.0753 | 0.0248 |
| Intercept | +0.355 | +0.019 | +0.015 | +0.0035 |
| Regression coefficient (r2) | 0.9989 | 0.9993 | 0.9997 | 0.9991 |
| Sensitivity | ||||
| LOD (µg/mL) | 1.49 | 0.48 | 0.54 | 0.24 |
| LOQ (µg/mL) | 4.53 | 1.56 | 1.68 | 0.76 |
| Drug | Amount of Drug [µg/mL] | Inter Day | Intra Day | ||||
|---|---|---|---|---|---|---|---|
| Amount Found Mean a ± SD | %RSD | %RE | Amount Found Mean b ± SD | %RSD | %RE | ||
| UV spectroscopic method | |||||||
| RGE | 2 | 2.01 ± 0.02 | 1.00 | 0.50 | 1.99 ± 0.03 | 1.51 | −0.50 |
| 8 | 7.86 ± 0.14 | 1.78 | −1.75 | 7.93 ± 0.11 | 1.39 | −0.88 | |
| 15 | 14.9 ± 0.25 | 1.68 | −0.67 | 15.07 ± 0.25 | 1.66 | 0.47 | |
| 20 | 19.76 ± 0.27 | 1.37 | −1.20 | 19.8 ± 0.12 | 0.61 | −1.00 | |
| MFH | 5 | 4.93 ± 0.07 | 1.42 | −1.40 | 5.06 ± 0.09 | 1.78 | 1.20 |
| 15 | 15.09 ± 0.13 | 0.86 | 0.60 | 14.79 ± 0.17 | 1.15 | −1.40 | |
| 20 | 19.72 ± 0.31 | 1.57 | −1.40 | 19.75 ± 0.16 | 0.81 | −1.25 | |
| 30 | 29.87 ± 0.36 | 1.21 | −0.43 | 29.68 ± 0.34 | 1.15 | −1.07 | |
| HPLC Method | |||||||
| RGE | 5 | 5.01 ± 0.07 | 1.40 | 0.20 | 4.87 ± 0.08 | 1.64 | −2.60 |
| 50 | 49.69 ± 0.43 | 0.87 | −0.62 | 50.13 ± 0.32 | 0.64 | 0.26 | |
| 100 | 101.81 ± 1.45 | 1.42 | 1.81 | 98.69 ± 0.93 | 0.94 | −1.31 | |
| 150 | 148.89 ± 2.08 | 1.40 | −0.74 | 148.82 ± 1.99 | 1.34 | −0.79 | |
| MFH | 25 | 24.67 ± 0.39 | 1.58 | −1.32 | 25.15 ± 0.17 | 0.68 | 0.60 |
| 50 | 49.11 ± 0.94 | 1.91 | −1.78 | 49.02 ± 0.69 | 1.41 | −1.96 | |
| 100 | 99.03 ± 1.34 | 1.35 | −0.97 | 98.73 ± 1.52 | 1.54 | −1.27 | |
| 200 | 201.59 ± 1.83 | 0.91 | 0.80 | 197.78 ± 1.59 | 0.80 | −1.11 | |
| Drug | UV Spectroscopic Method | HPLC Method | ||||
|---|---|---|---|---|---|---|
| Amount Added in [µg/mL] | %Recovery | %RE | Amount Added in [µg/mL] | %Recovery | %RE | |
| RGE | 1 | 101.00 | 1.00 | 5 | 100.40 | 0.40 |
| 2 | 98.50 | −1.50 | 10 | 99.50 | −0.50 | |
| 3 | 99.33 | −0.67 | 15 | 98.80 | −1.20 | |
| Across Mean | 99.61 | 99.57 | ||||
| %RSD | 1.27 | 0.80 | ||||
| MFH | 5 | 98.40 | −1.60 | 25 | 99.24 | −0.76 |
| 10 | 100.10 | 0.10 | 50 | 98.06 | −1.94 | |
| 15 | 99.07 | −0.93 | 75 | 99.59 | −0.41 | |
| Across Mean | 99.19 | 98.96 | ||||
| %RSD | 0.85 | 0.81 | ||||
| Ratios a RGE: MFH | UV Method (%Recovory ± SD) | Ratiosa RGE: MFH | HPLC Method (%Recovory ± SD) | ||
|---|---|---|---|---|---|
| Remogliflozin | Metformin | Remogliflozin | Metformin | ||
| 2:05 | 101.23 ± 0.01 | 99.50 ± 0.02 | 5:25 | 100.90 ± 0.07 | 98.29 ± 0.09 |
| 5:30 | 99.68 ± 0.04 | 98.58 ± 0.03 | 5:100 | 99.29±0.19 | 99.04 ± 0.17 |
| 10:05 | 100.98 ± 0.15 | 99.71 ± 0.08 | 75:200 | 101.06 ± 0.21 | 101.35 ± 0.06 |
| 10:20 | 98.82 ± 0.09 | 101.32 ± 0.12 | 75:100 | 99.18 ± 0.11 | 99.77 ± 0.15 |
| 20:20 | 99.16 ± 0.32 | 100.94 ± 0.14 | 150:200 | 101.35 ± 0.24 | 98.51 ± 0.23 |
| 20:30 | 101.39 ± 0.21 | 99.30 ± 0.18 | 150:25 | 98.13 ± 0.35 | 98.39 ± 0.31 |
| UV Spectroscopic Method | HPLC Method | |||
|---|---|---|---|---|
| RGE | MFH | RGE | MFH | |
| Label Claim (mg) | 100 | 500 | 100 | 500 |
| Amount taken [µg/mL] | 4 | 20 | 20 | 100 |
| Amount found [µg/mL] | 3.95 | 20.04 | 19.83 | 98.48 |
| Label Claim (mg) | 100 | 1000 | 100 | 1000 |
| Amount taken [µg/mL] | 3 | 30 | 15 | 150 |
| Amount found [µg/mL] | 2.97 | 29.78 | 15.12 | 148.24 |
| Mean %estimation a | 99.51 | 99.60 | 99.80 | 100.07 |
| %RSD | 1.34 | 0.89 | 0.78 | 1.08 |
| n | 6 | 6 | ||
| Student t-test (2.306) b | 0.711 | 0.450 | ||
| F (6.388) c | 3.521 | 1.436 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attimarad, M.; Elgorashe, R.E.E.; Subramaniam, R.; Islam, M.M.; Venugopala, K.N.; Nagaraja, S.; Balgoname, A.A. Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation Using Experimental Design. Separations 2020, 7, 59. https://doi.org/10.3390/separations7040059
Attimarad M, Elgorashe REE, Subramaniam R, Islam MM, Venugopala KN, Nagaraja S, Balgoname AA. Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation Using Experimental Design. Separations. 2020; 7(4):59. https://doi.org/10.3390/separations7040059
Chicago/Turabian StyleAttimarad, Mahesh, Rafea Elamin Elgack Elgorashe, Rajasekaran Subramaniam, Mohammed Monirul Islam, Katharigatta N. Venugopala, Sreeharsha Nagaraja, and Abdulmalek Ahmed Balgoname. 2020. "Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation Using Experimental Design" Separations 7, no. 4: 59. https://doi.org/10.3390/separations7040059
APA StyleAttimarad, M., Elgorashe, R. E. E., Subramaniam, R., Islam, M. M., Venugopala, K. N., Nagaraja, S., & Balgoname, A. A. (2020). Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation Using Experimental Design. Separations, 7(4), 59. https://doi.org/10.3390/separations7040059







