Abstract
Minor cannabinoid and non-cannabinoid molecules have been proposed to significantly contribute to the pharmacological profile of cannabis extracts. Phytoplant Research has developed highly productive cannabis cultivars with defined chemotypes, as well as proprietary methods for the extraction and purification of cannabinoids. Here, we investigate the effect of solvent selection and decarboxylation on the composition and pharmacological activity of cannabis extracts. A library of forty cannabis extracts was generated from ten different cannabis cultivars registered by Phytoplant Research at the EU Community Plant Variety Office. Plant material was extracted using two different solvents, ethanol and hexane, and crude extracts were subsequently decarboxylated or not. Cannabinoid content in the resulting extracts was quantified, and biological activity was screened in vitro at three molecular targets involved in hypoxia and inflammation (NF-κB, HIF-1α and STAT3). Changes in transcriptional activation were strongly associated to solvent selection and decarboxylation. Two decarboxylated extracts prepared with hexane were the most potent at inhibiting NF-κB transcription, while HIF-1α activation was preferentially inhibited by ethanolic extracts, and decarboxylated extracts were generally more potent at inhibiting STAT3 induction. Our results indicate that solvent selection and proper decarboxylation represent key aspects of the standardized production of cannabis extracts with reproducible pharmacological activity.
1. Introduction
Cannabis sativa L. is an herbal medicine which contains at least 554 identified compounds including 150 phytocannabinoids, terpenophenolic active molecules which are nearly exclusive to this plant, and other phyto-molecular families such as terpenoids, flavonoids and oxylipins [1]. The main two cannabinoids responsible for the pharmacological properties of cannabis extracts are Δ9-tetrahydrocannabinol (THC), the principal active component in drug-type cannabis, and cannabidiol (CBD), the major cannabinoid molecule found in fiber-type cannabis or hemp [2]. These compounds are formed in the cannabis plant as their 2-carboxylic precursors, Δ9-tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), whom rapidly decarboxylate to render the neutral forms upon heating [3]. Pharmacological interest has been attributed to THCA and CBDA, as well as to other less-abundant, non-psychoactive cannabinoids such as cannabigerol (CBG), the molecular precursor of THC and CBD, and Δ9-tetrahydrocannabivarin (THCV) and cannabidivarin (CBDV), their propylic analogs [4]. THC was originally characterized as a partial agonist of G protein-coupled receptors, namely cannabinoid receptors type-1 and type-2 (CB1 and CB2 receptors, respectively). CBD is a rather “promiscuous” molecule, which interacts with a variety of physiological targets such as CB1 and CB2, as a negative allosteric modulator [5,6,7] producing biased agonism at both receptors and CB1-CB2 heteromers [8], G protein-coupled receptor 55 (GPR55), and serotonin receptor 5-HT1A among others [9]. Although the mechanisms of their actions are not completely understood, THC and CBD remain the two cannabinoids available in the clinical practice, either in their synthetic form (dronabinol) or as a standardized cannabis extracts (Sativex, Epidiolex), for the management of nausea and vomiting associated to chemotherapy, spasticity secondary to multiple sclerosis, and epileptic seizures in pediatric syndromes such as Dravet or Lennox-Gastaut [10]. Although adverse effects of cannabis are primarily due to THC, many patients report cannabis extracts having better therapeutic activity and less side effects compared to dronabinol, such as dysphoria, depersonalization, anxiety, panic reactions, and paranoia [11]. Some authors contend that whole-plant cannabis extracts provide better pharmaceutical and mood-altering outcomes compared to isolated cannabinoids owing to (i) an increased therapeutic index due to synergistic actions and (ii) mitigated side effects of the primary active cannabinoid by other active or silent compounds within the phytocomplex [12,13]. Although the molecular underpinnings of this theoretical “entourage effect” remain to be elucidated, recent data seem to indicate that such synergistic interaction does not occur at cannabinoid receptors [14]. At present, many countries have passed legislation permitting the use of cannabis for medical purposes, generally bypassing the strict regulatory procedures that apply to pharmaceutical development [15]. Therefore, much research is required to guide regulation and to fully understand the implications of current industrial practices in the manufacturing of cannabis and hemp extracts for human consumption.
Since 2012, Phytoplant Research SL has developed Cannabis sativa L. cultivars with distinct cannabinoid profiles, including varieties rich in minor cannabinoids, such as CBG, THCV and CBDV [16]. To date, 13 of these cannabis varieties have been registered at the Community Plant Variety Office (CVPO), the European Union agency that manages plant variety rights covering the 28 Member States (https://cpvo.europa.eu). Phytoplant Research SL has also patented proprietary methods for the purification of CBD, CBG and CBGA by crystallization [17] and for the purification of THC, THCA, THCV, CBDA, CBDV and CBGV by liquid-liquid chromatography [18]. Due to the relative high amounts of these cannabinoids in certain registered varieties (Table 1), it is possible to purify them by direct crystallization from a primary cannabis extract obtained by percolation of plant material with an organic solvent followed by a step of crystallization and recrystallization in the same solvent. This method of purification is cost-effective and easily scalable, as it reduces the use of solvents and eliminates the need for chromatographic steps, while yielding isolated cannabinoids with a purity over 98%, compatible with the Good Manufacturing Practices (GMP) certification required for pharmaceutical production. Ethanol and supercritical carbon dioxide (CO2) are usually the preferred options, as polar and non-polar solvents, in the preparation of cannabis extracts for direct human consumption without further purification steps at pilot-industrial scale [19]. We hypothesized that cannabis extracts from the same plant variety obtained with two very different solvents, a polar alcohol and a non-polar alkane, and subject or not to complete decarboxylation might not only differ in their chemical composition but, more importantly, in their overall pharmacological properties. Cannabinoids are soluble in both polar and non-polar solvents, but the rest of cannabis components, such as terpenes, terpenoids, flavonoids and polyphenols, will have different extraction ratios depending on which solvent is used. It seems plausible to speculate that a polar solvent will favor the extraction of more polar molecules, like flavonoids and polyphenols, and disfavors the extraction of non-polar terpenes, and vice versa. The process of decarboxylation further modifies the chemical composition of extracts, not only by transforming acid cannabinoids into neutral, but also potentially affecting other organic acids and thermally-labile substances that may be present in the extract. In support of this hypothesis, a very recent publication describes the differential pharmacological activity of cannabis extracts varying in their decarboxylation degree at cannabinoid receptors [20]. In the present study we broadened our scope to focus on non-classical cannabinoid targets that could mediate cellular responses elicited by cannabis extracts. A plant extract library (PHYRESCEL) was prepared by systematically processing 10 distinct cultivars developed by Phytoplant Research SL and characterizing their cannabinoid profile (Figure 1).

Table 1.
Summary of Cannabis sativa L. varieties used in the present study. ND: Not determined.

Figure 1.
Schematic workflow for the preparation and characterization of Phytoplant Research Cannabis Extract Library (PHYRESCEL). Composition of all 40 extracts was analyzed by GCMS. Ten cannabinoids (Δ9-THC, Δ9-THCA, Δ9-THCV, CBD, CBDA, CBDV, CBG, CBGA, CBC and CBN) were identified and quantified. PHYRESCEL entries were screened in vitro for their ability to inhibit the transcriptional activation of NF-kB, HIF1a and STAT3, three molecular targets involved in inflammation and hypoxia.
PHYRESCEL contained 40 different entries that were further screened for their pharmacological activity towards NF-κB, HIF-1α and STAT3, three molecular targets known to mediate cellular responses to inflammation and hypoxia.
2. Materials and Methods
2.1. Plant Material
Plant material consisted on female inflorescences with or without the attached leaves from ten different, asexually-propagated, medicinal cannabis varieties registered by Phytoplant Research at the CPVO (https://cpvo.europa.eu), namely: Aida, 2016/0167; Beatriz, 2017/0146; Juani, 2016/0117; Magda, 2017/0145; Mati, 2017/0147; Moniek, 2016/0114; Octavia, 2017/0148; Pilar, 2016/0115; Sara, 2015/0098 and Theresa, 2016/0116 (see Table 1).
2.2. Reagents for Plant Material Extraction, Purification of Cannabinoids and Analysis of Cannabinoid Content
n-hexane and absolute ethanol (Scharlab, Barcelona, Spain) were used as solvents for the extraction of plant material. Petroleum ether bp 40–60 °C (Scharlab, Barcelona, Spain) or n-hexane were used for the purification of cannabinoids by crystallization (CBD, CBG and CBGA), and purified water were used in addition to the n-hexane and absolute ethanol in the purification of cannabinoids by liquid-liquid chromatography (THC, THCA, CBDA, CBDV and CBGV) used in this study. LC–MS-grade ammonium formate from Sigma–Aldrich (San Luis, MO, USA) and acetonitrile, formic acid and methanol from Scharlab (Barcelona, Spain) were used for preparation of chromatographic mobile phases and working solutions. Standards (purity > 98%) of cannabinoids THC, THCA, THCV, CBG, CBGA, CBDV, CBD, CBDA, cannabidivarinic acid (CBDVA), cannabichromene (CBC), Δ8-tetrahydrocannabinol (Δ8-THC), cannabinol (CBN) and cannabicyclol (CBL) were used to make the corresponding calibration curves. All standards were from Cerilliant (Round Rock, TX, USA), commercialized as a solution in methanol (1 mg·mL−1).
2.3. Extraction Procedure
100 g of plant material were extracted, at room temperature, three times in organic solvent (n-hexane or ethanol) under constant stirring at 190 rpm in a mini OVAN OS10E S orbital shaker (Ovan, Barcelona, Spain). Extracts were then filtered under vacuum (Laboxact KNF, Trenton, NJ, USA) through a glass plate porous filter (Pobel, Madrid, Spain) with a pore size of 16–40 μm. Extracts were then evaporated to dryness in a RS-3000V rotaevaporator from Selecta (Barcelona, Spain) at 40 and 50 °C for hexane and ethanol, respectively. This process yielded a total of 20 extracts from Phytoplant varieties, 10 extracted with hexane, and 10 with ethanol. Dry extracts were then divided into two glass vials and one was placed into a “Conterm” oven (Selecta, Barcelona, Spain) and incubated at 150 °C for 60 min to induce decarboxylation of acid cannabinoids to their neutral forms. In this way, a total of 40 extracts were obtained: 20 decarboxylated (DEC) and 20 non-decarboxylated (NDC) (see Figure 1).
2.4. Cannabinoid Purification
CBD, CBG and CBGA cannabinoids were purified (>95%) by direct crystallization of the n-hexane extracts of petroleum ether extracts, and 3 recrystallizations in the same solvent in a 1L reactor and 1L jacketed filter (Vidrafoc, Barcelona, Spain) chilled to −18 °C or 4 °C with Digiterm 30 (Selecta) and filtered under vacuum (Laboxact KNF, Trenton, NJ, USA) [17]. THC, THCA, CBDA and CBDV cannabinoids were purified (>95%) by liquid-liquid chromatography with CPC-1000-PRO and PLC 250 systems from Gilson (Middleton, WI, USA), as previously described [18].
2.5. Analysis of Cannabinoid Content
For LC-DAD analysis, extracts (40 mg) were diluted with 5 mL of methanol, placed in an ultrasound bath for 25 min, and centrifuged for 5 min at 3000 rpm. Then, the supernatant containing cannabinoids was collected and further diluted 1:200 prior to analysis. An 1260 Agilent liquid chromatography (Santa Clara, CA, USA) set-up consisting of a binary pump, a vacuum degasser, a column oven, an autosampler and a diode array detector (DAD) equipped with a 150 mm length × 2.1 mm internal diameter, 2.1 μm pore size Raptor ARC-18 column with a Raptor ARC-18 EXP guard column cartridge 2.7 μm, both from Restek (Bellefonte, PA, USA), was used for the quantification of cannabinoids. The device was controlled by OpenLAB CBS software (Agilent, Santa Clara, CA, USA). Full spectra were recorded in the range of 200–400 nm. The analysis was performed in isocratic mode using water with 5 mM ammonium formate and 0.1% formic acid and acetonitrile with 0.1% formic acid as mobile phases. Flow rate was set at 0.375 mL·min−1, the injection volume at 3 μL and the injector needle was washed between injections with methanol. Chromatographic peaks were recorded at 225 nm. All determinations were carried out at 35 °C. All samples were analyzed in duplicate. The concentration of cannabinoids was calculated by peak area versus a calibration curve prepared with a commercial standard. The results for each cannabinoid were calculated as relative weight (%) in the extract.
2.6. Sample Preparation for Biological Activity
Stock solutions of extracts and purified cannabinoids were prepared in DMSO at a concentration of 100 mg·mL−1 and stored at −80 °C until used. Experimental solutions were freshly prepared on the day of the experiment by diluting stock solutions in DMSO. Final concentrations tested were 10, 25 and 50 μg·mL−1 in the case of plant extracts and 5, 12.5 and 25 μg·mL−1 for purified cannabinoids (THC, THCA, CBD, CBDA, CBDV, CBG, CBGA). Dosage range was determined to allow for direct comparison between the biological activity of purified cannabinoids and plant extracts, anticipating a 50% w/w concentration of the main cannabinoid in the extract.
2.7. Cell Lines
Anti-NF-kB activity was determined using the Raw-KBF-Luc cell line, stably transfected with the plasmid KBF-Luc, which contains three copies of the NF-kB binding site from the major histocompatibility complex promoter, fused to a fragment of the simian virus 40 (SV40) promoter driving the firefly luciferase reporter gene. The increase in NF-κB levels activates the pCRE-Luc system, inducing the expression of the luciferase reporter gene. The determination of STAT-3 activity was performed using the Raw-STAT3-Luc cell line, which is stably transfected with the plasmid 4xM67 pTATA TK-Luc that contains STAT3 binding sites and whose activation induces the expression of the firefly luciferase reporter gene. The determination of HIF-1α activity was performed using the cell line NIH-3T3-Epo-Luc. This cell line is stably transfected with the plasmid Epo-Luc, which contains three tandem copies of the hypoxia response element (HRE) of the erythropoietin (EPO) promoter and whose activation induces the expression of the firefly reporter gene. The determination of cytotoxicity was performed using the mouse brain neuroblastoma cell line N2a obtained from ATCC (Manassas, VA, USA).
2.8. Determination of Biological Activity
100 µL of a cell suspension were seeded in 96-well plates (500,000 cells·mL−1) and incubated for 24 h. Cells were then treated for 30 min either with cannabis extracts or purified cannabinoids and then stimulated with either LPS (1 μg·mL−1, in the case of NF-κB and STAT-3) [21] or deferoxamine (150 µM, in the case of HIF-1α) for 6 h [22]. Deferoxamine, a potent hypoximimetic compound acting as an iron chelating agent, or LPS were used alone as positive control and cells without stimulus were used as negative control. Cells were then washed twice with PBS 1X and lysed in 100 µL of lysis buffer (25 mM Tris-phosphate pH 7.8, 8 mM MgCl2, 1 mM DTT, 1% Triton X-100 and 7% glycerol) for 15 min at room temperature under orbital shaking. Luciferase activity was determined with a TriStar LB 942 plate multi-reader (Berthold Technologies, GmbH & Co. KG, Bad Wildbad, Germany) following the instructions of the Luciferase Assay system from Promega. Resulting RLU (Relative Light Units) were normalized to positive control (deferoxamine or LPS) in order to calculate the percentage of activity left. Percentage of inhibition was calculated as:
where T are the RLU of cells treated with cannabis extracts, C are the RLU of cells challenged with LPS of deferoxamine, and B are the RLU of un-stimulated control cells.
2.9. Determination of Cytotoxicity
100 µL of a cell suspension were seeded in 96-well plates (40,000 cells·mL−1) and incubated for 24 h. Cells were then treated for 24 h either with cannabis extracts or purified cannabinoids and then the media removed and 50 μL of MTT was added (1.25 mg·mL−1) and incubated for 4 h. Then the supernatant was removed and 100 μL of DMSO were added and the absorbance recorded at 550 nm with a TriStar LB 941 plate multi-reader (Berthold Technologies, GmbH & Co. KG, Bad Wildbad, Germany). Resulting absorbances were normalized to control (untreated cells) and blank (DMSO) to calculate the percentage of cell viability according to the following formula:
where T is the absorbance of the cannabis extract treated cells, C is the absorbance of the untreated cells and B is the absorbance of the blank.
2.10. Statistical Analysis
Cannabinoid concentration is reported as percentage (w/w). Biological activity is expressed as the mean ± standard deviation of two or three independent assays. Dose-response inhibition of NF-κB activity was analyzed by a one-way ANOVA followed by a Dunnett’s multiple comparisons test with significance set at p < 0.05, using GraphPad Prism (Version 8.1.1) (San Diego, CA, USA).
3. Results
Extraction yield was found to be cultivar-dependent, ranging from the less-producing variety, Octavia (main cannabinoid, CBG), to the largest producer, Moniek (main cannabinoid, THC). Although not significant differences were found, hexane extraction yields were slightly lower compared to ethanol: 4.9% vs. 6.8% for Octavia and 28.4% vs. 28% for Moniek (Table 1). This may be indicative of a richer phytocomplex with more polar molecules like flavonoids and polyphenols in the case of ethanol whom, contrary to hexane, is nor cancerous nor toxic for the environment. Accordingly, average weight loss during decarboxylation ranged from 0.37% and 6.37% for hexane extracts and between 8.65% and 16.67% in the case of ethanol, likely due to the amount of water extracted by ethanol from the plant material. All extracts pass the residual solvents analysis accordance of European Pharmacopoeia 9.0. (2.4.24, Identification and control of Residual Solvents using Methodology system A) for Class I solvents the HEX cannabis extracts and for Class III solvents the ETH cannabis extracts. Of note, the salient carbon dioxide molecule generated during decarboxylation represents approximately 12% of the molecular weight of THCA, CBDA and CBGA [3]. Decarboxylation did not produce significant changes in the color or physical appearance of extracts.
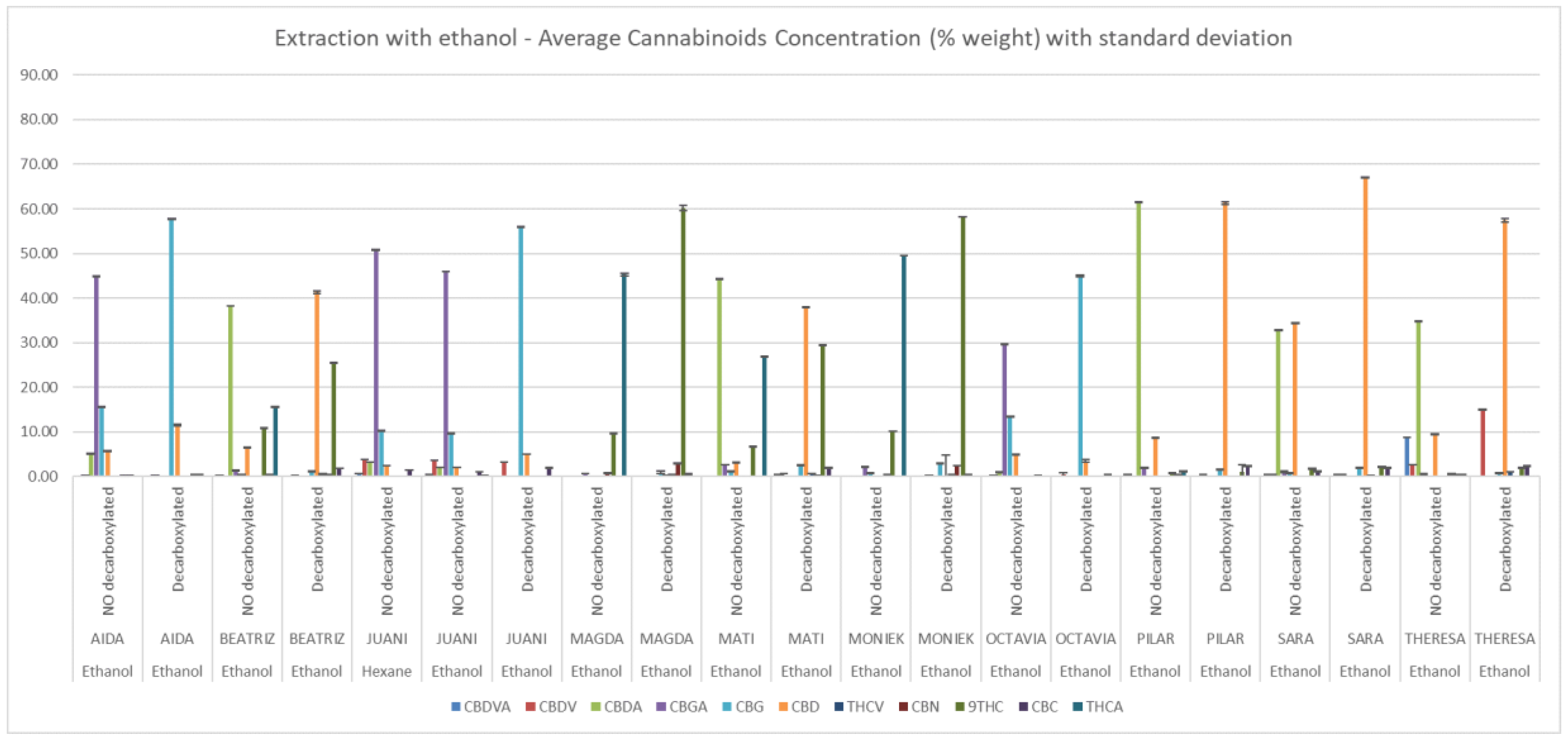
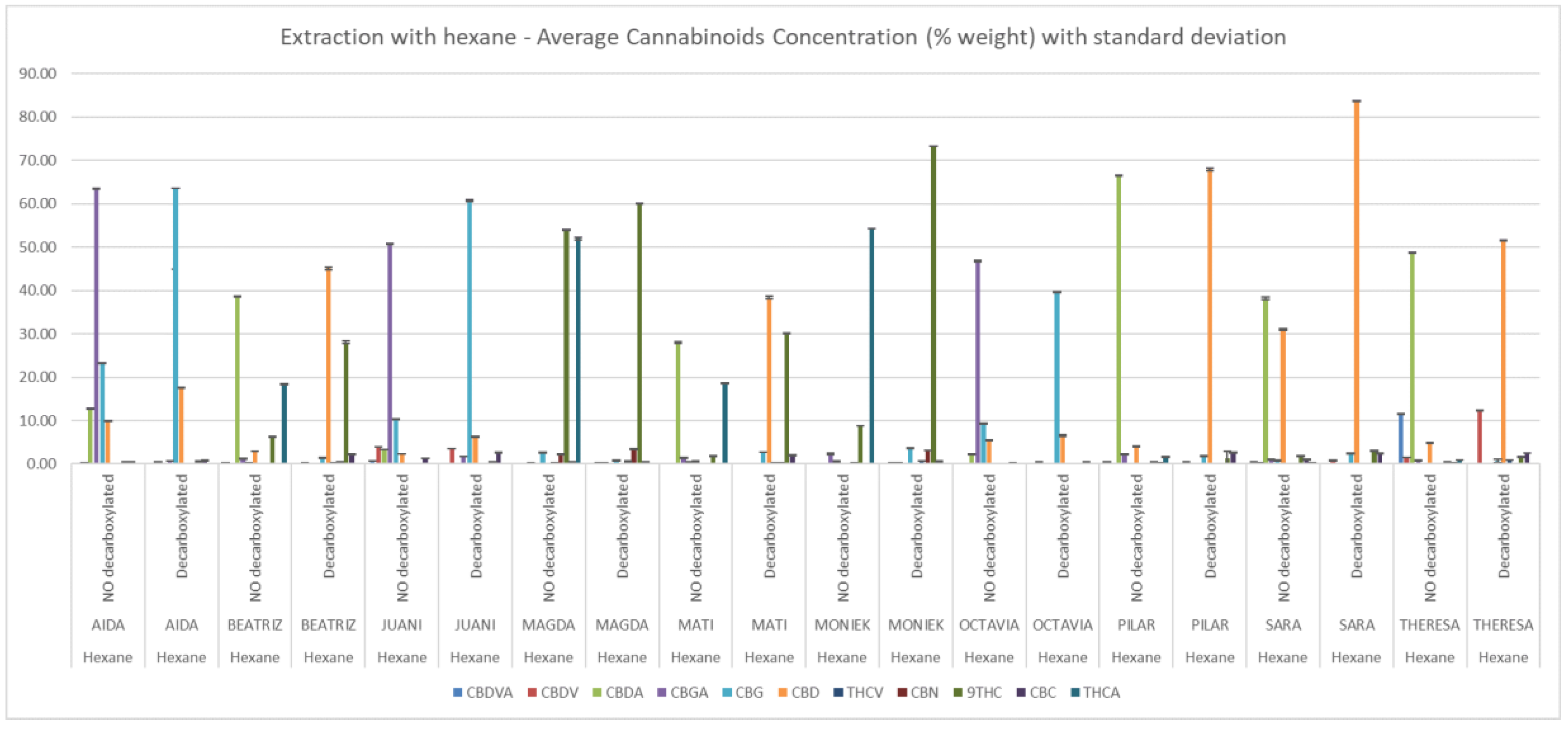
No significant differences were appreciated between solvents in the average total content of cannabinoids among extracts, which was 71.1 ± 15.7 % for hexane and 68.0 ± 10.0% for ethanol. In the case of non-decarboxylated extracts the average of total content in hexane and ethanol extracts were 74.30 ± 12.15 % and 68.1 ± 7.9% respectively (Supplementary Table S1). Figure 2 and Figure 3 depict the relative concentrations of major and minor cannabinoids, a total of 13 is reported. Because of their low quantity and similar relative abundance across extracts, CBL, Δ8-THC, CBN, CBC and THCV were not tested in the in vitro assays.
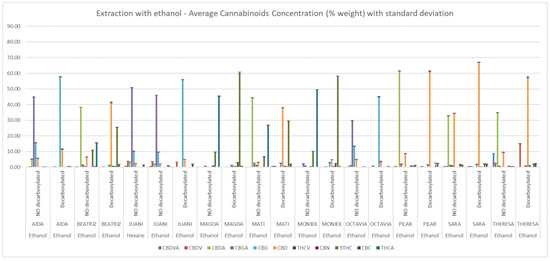
Figure 2.
Cannabinoid composition of ethanolic extracts determined by HPLC-DAD. Concentrations (% w/w) are expressed as mean ± standard deviation.
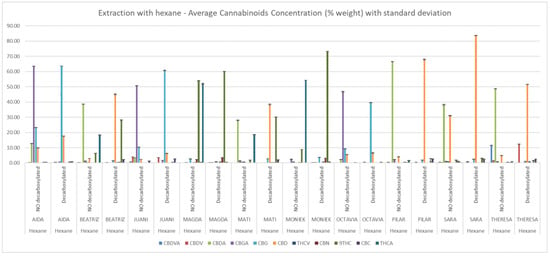
Figure 3.
Cannabinoid composition of hexane extracts determined by HPLC-DAD. Concentrations (% w/w) are expressed as mean ± standard deviation.
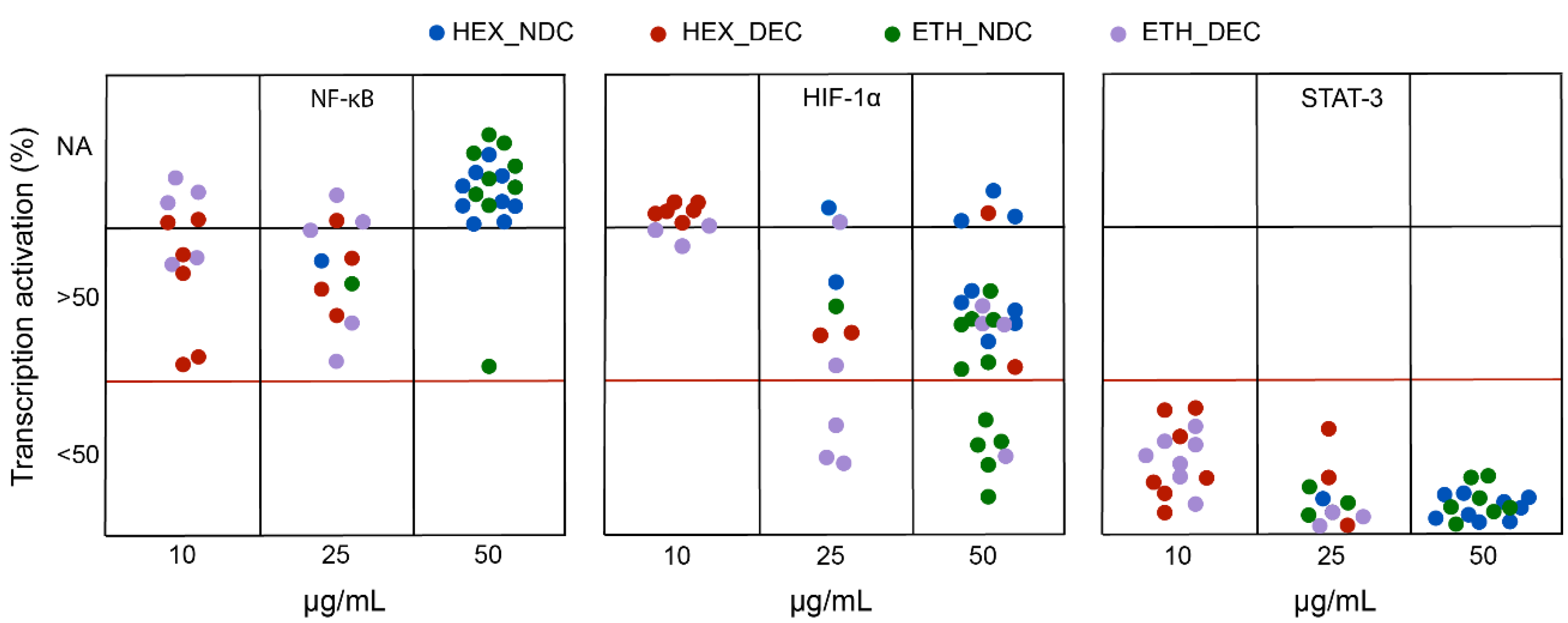
Figure 4 displays the degree of transcriptional inhibition (as % of activation) at the most effective, non-toxic dose for each cannabis extract in PHYRESCEL at the three targets investigated, NF-κB (left panel), HIF-1α (middle panel) and STAT3 (right panel), following incubation with LPS or deferoxamine. First, our results indicate that decarboxylated (DEC) extracts, rich in neutral cannabinoids such as THC, CBD and CBG, were consistently more toxic for the cell lines used in the assay, which share some proliferative features of cancerous cells, than their non-decarboxylated (NDC) counterparts irrespectively of the solvent used. Accordingly, most DEC extracts (>95%) were toxic at a dose of 50 μg·mL−1, with less than 20% of NDC extracts exhibiting cytotoxicity at the same dose (Figure 4). This observation agrees with the literature, where the cytotoxic effect of neutral cannabinoids on immortalized cell lines has been thoroughly noted as a limitation of cell-based assays in the pharmacological characterization of both cannabinoids [23,24] and cannabis extracts [25]. Therefore, we were not able to obtain complete dose-response information regarding the potency of most entries in PHYRESCEL, particularly in the case of either DEC_HEX or DEC_ETH groups (Figure 4). However, despite this toxicity issue, we were able to draw relevant information regarding the differential effects of solvent selection and cannabinoid decarboxylation on the biological activity of cannabis extracts. Although this not always correlated with cannabinoid content, the overall results suggest that DEC extracts displayed higher potency, with a majority of non-toxic ID50 values in the range of 10–25 μg·mL−1, while NDC extracts were generally most effective at 50 μg·mL−1 with a few exceptions (Figure 4).
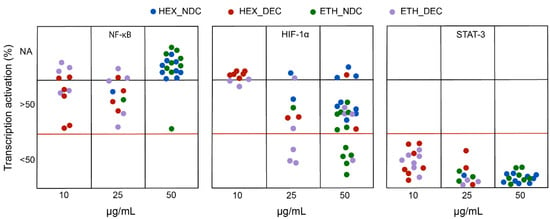
Figure 4.
Screening of the biological activity of PHYRESCEL. The ability of cannabis extracts to inhibit the activation of transcription factors NF-κB, HIF-1α and STAT-3 was measured in vitro. Degree of inhibition is expressed as reduction of transcription activation in percentage. Color legend indicates solvent used and decarboxylation treatment. Blue: Hexane/non-decarboxylated; Red: Hexane/decarboxylated; Green: Ethanol/non-decarboxylated; Purple: Ethanol/decarboxylated. NA: not active.
The molecular mechanism by which cannabinoids modulate NF-κB signaling remains controversial. For example, CBD but not THC has been shown to reduce the activity of the NF-κB pathway in BV-2 microglial cells [26]. However, synthetic cannabinoid dexanabinol, non-antioxidant CB1 receptor agonist WIN 55,212-2 and phytocannabinoid CBN, which weakly binds to CB1 receptors, inhibited NF-κB transcriptional activity and apoptosis in a neuron-like cell line, which suggests that neither the antioxidant properties of cannabinoids nor binding to cannabinoid receptors are responsible for this effect [27]. Our results show how most extracts did not block more than 50% the transcriptional activation of NF-κB in macrophages induced by LPS (Figure 4). Accordingly, THC and CBD displayed cellular toxicity at higher doses and were inactive towards NF-κB at the dose of 10 μg·mL−1 (Table 2). Discrepancies with cited literature may be due to differences in the cell lines used as well as in the experimental design [25]. Nevertheless, we were able to identify four cannabis extracts capable of inhibiting NF-κB activation more than 40% at non-toxic doses (Table 2).

Table 2.
Inhibition of LPS-induced NF-κB activation in Raw-KBF-Luc macrophages by cannabis extracts. Activity values for the most effective extracts (transcription activation < 60%) identified in the screening assay, paired with their cannabinoid composition. Activity values of major cannabinoids present in those extracts (THC, THCA, CBD and CBDA) are also presented.
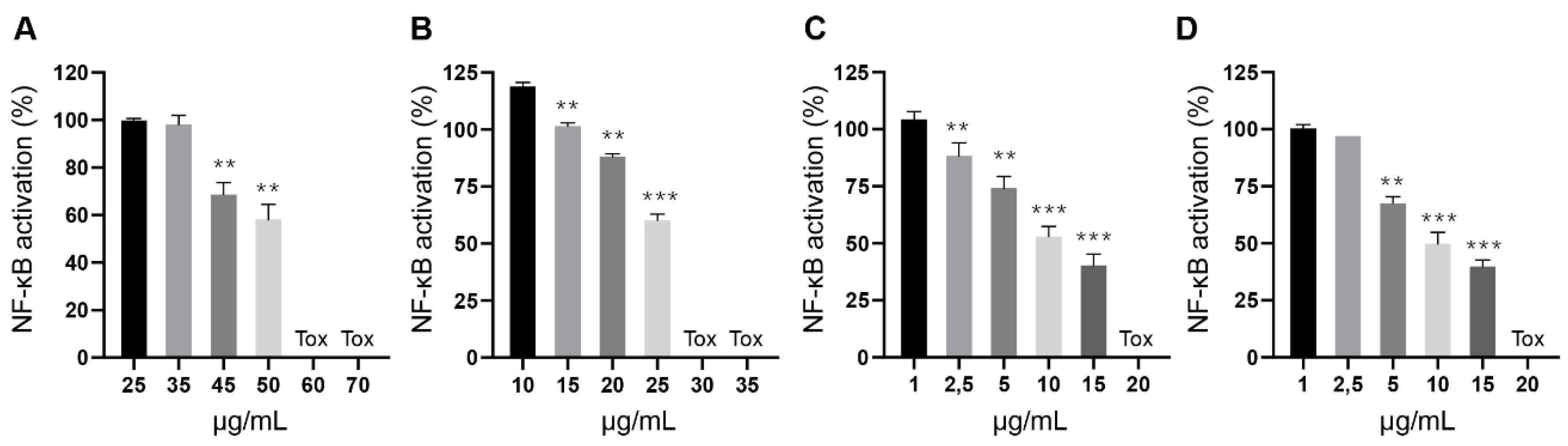
Contrary to their toxic potential, the inhibitory activity of these extracts did not seem to correlate with their cannabinoid content. To further characterize this finding, we conducted a refined dose-response experiment with extracts of Magda (ETH_NDC, Figure 5A), Beatriz (ETH_DEC, Figure 5B), Pilar (HEX_DEC, Figure 5C) and Sara (HEX_DEC, Figure 5D) and were able to confirm their potency and toxic range, with all of them inhibiting NF-κB activation in a dose-dependent manner before reaching toxic concentrations. Such effect that was not found when the same plant varieties were subjected to different processing conditions or with pure cannabinoids. Therefore, it is plausible to speculate that these extracts may contain additional molecules capable of blocking NF-κB transcriptional activation, either acting alone or as a part of a phytocomplex. For example, flavonoid Cannflavin A and a phenanthrenequinone derivative, Denbinobin, present in the cannabis variety “Carma”, elicit synergistic effects in combination with CBD and CBG to inhibit NF-κB activation [28]. Denbinobin alone also inhibits NF-κB [29].
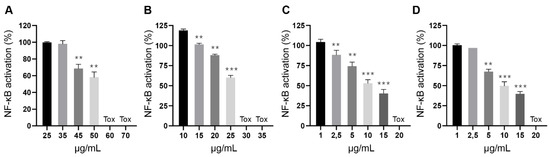
Figure 5.
Inhibition of LPS-induced NF-κB activation in Raw-KBF-Luc macrophages by cannabis extracts. Plant extracts from (A) Magda (ETH_NDC), (B) Beatriz (ETH_DEC), (C) Pilar (HEX_DEC) and (D) Sara (HEX_DEC) were tested at different concentrations to further investigate their inhibitory effect and toxicity. Values are expressed as the mean ± standard deviation of 2–3 independent assays. ** p < 0.01; *** p < 0.001 vs. LPS alone.
Hypoxia-inducible factor-1α (HIF-1α) has been identified as a key regulator of the hypoxic response with pleiotropic effects, such as promotion of invasion, angiogenesis, switch to glycolytic metabolism, and up-regulation of cell survival-related molecules [30]. The HIF-1α signaling pathway has been proposed to mediate the neuroprotective role for CB2 receptors in microglia [31]. A single study has reported the ability of CBD to dose-dependently reduce the expression of HIF-1α in glioma cells [32]. Results from our biological screening suggest that among phytocannabinoids, CBDV, CBD and CBG were able to reduce the activation of HIF-1α induced by deferoxamine, with IC50s under 12.5 μg·mL−1 for CBD and CBDV, and 25 μg·mL−1 for CBG (Table 3).

Table 3.
Inhibition of deferoxamine-induced HIF-1α activation in NIH-3T3- Epo-Luc cells by cannabis extracts. Table shows the activity values for the most effective extracts identified in the screening assay, paired with their cannabinoid composition. Activity values of major cannabinoids present in those extracts (THCA, CBD, CBDA, CBDV, CBG and CBGA) are also presented.
Additional studies would be required to confirm and better characterize this finding. As for the extracts, ETH extracts were clearly more potent than HEX, with a total of nine ETH extracts found active at inhibiting HIF-1α (Figure 4, Table 3). Among these, five NDC extracts presented similar potencies, with IC50s in the 25–50 μg·mL−1 range, irrespective of their major cannabinoid component (either CBDA, THCA or both). However, this observation did not correlate with the reported potency for acid cannabinoids in this assay, which were not active at the highest concentration tested (Table 3). This may be suggestive of the presence of non-cannabinoid active molecules, selectively extracted by the polar solvent ethanol and not by the non-polar solvent hexane, occurring in five different cannabis varieties (Moniek, Magda, Mati, Pilar and Theresa). Several flavonoids have been shown to actively inhibit HIF-1a transcription [33]. Flavonoids are more polar than cannabinoids and, therefore, more likely to be extracted by ethanol than hexane, thus providing a plausible explanation as to why ETH extracts from the same cannabis cultivar are active, but not their HEX counterparts. On the other hand, the degree of inhibition displayed by the remaining four ETH_DEC extracts seems to correlate with their cannabinoid content, dominated by the presence of CBD, CBDV and CBG. However, the reason why their HEX_DEC counterparts were either not active or toxic remains unclear. Theresa and Mati varieties produced ETH_NDC and ETH_DEC extracts active at inhibiting HIF-1a with the ETH_DEC IC50 of <25 μg·mL−1, more active than the ETH_NDC with a IC50 between 25–50 μg·mL−1 Further studies are currently undergoing to help clarifying this finding by employing more powerful analytical and statistical techniques, such as quantitative time-of-flight (QTOF) detection and principal component analysis (PCA), similarly to what recently reported by other authors [25].
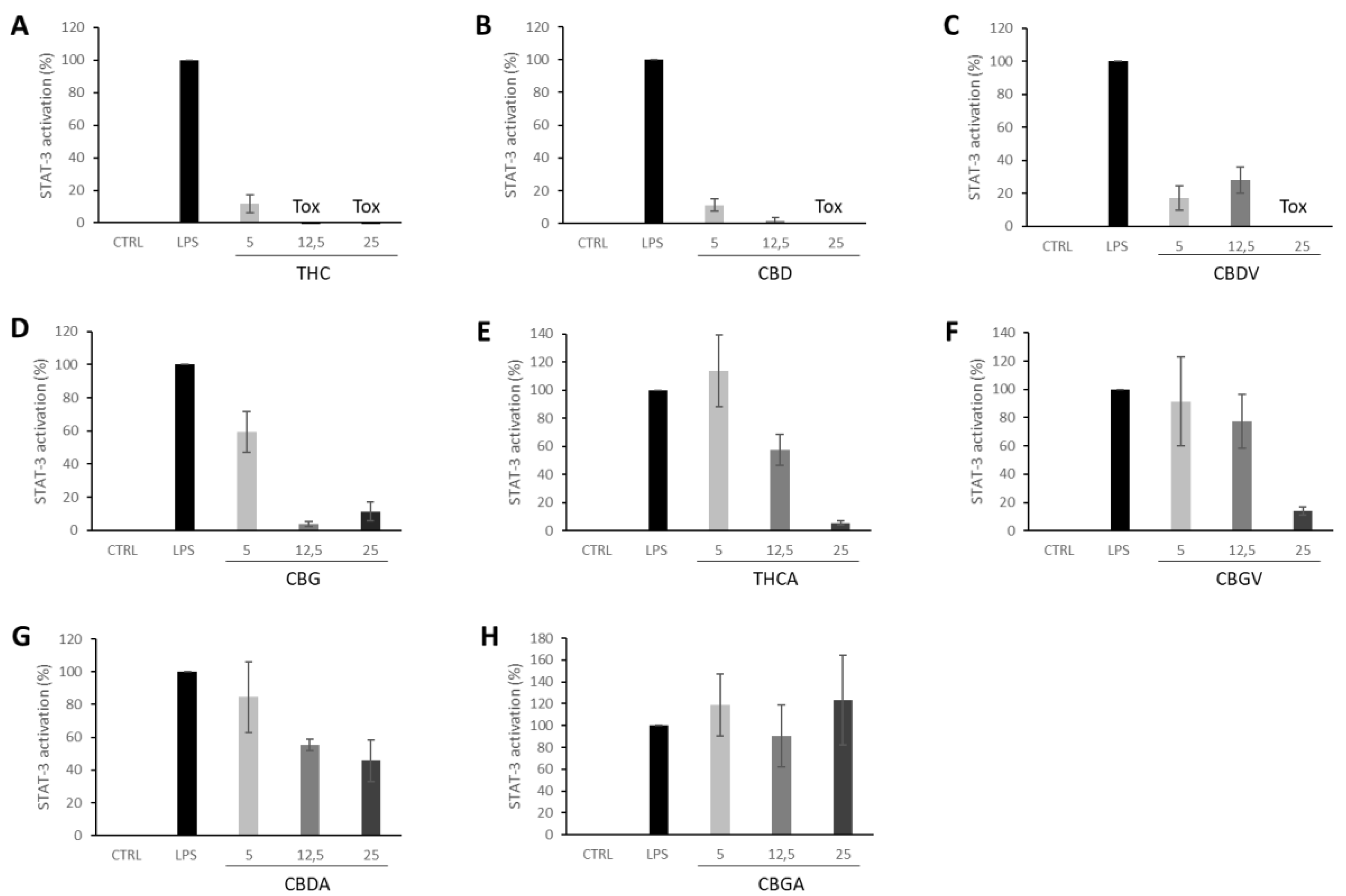
Signal transducer and activator of transcription 3 (STAT3), a member of the STAT families, is involved in regulating essential cellular functions in cancer, such as proliferation, differentiation, survival, invasion, angiogenesis, and metastasis [34]. STAT3 activation can be effectively modulated by several key receptors of the endocannabinoid system such as CB1 [35,36], CB2 [37] and G protein-coupled receptor 55 (GPR55), which has been postulated as a novel cannabinoid receptor [38]. Further, THC induces trophoblast dysfunction in pregnant mice by suppressing STAT3 signaling [39]. Indeed, our results show how THC nearly abolished STAT3 activation at the lower dose tested (5 μg·mL−1). All other cannabinoids tested were also able to suppress the activation of STAT3 induced by LPS except for CBGA (Figure 6). Accordingly, all PHYRESCEL entries resulted active at, at least, one non-toxic dose, with DEC extracts being about five-times more potent than NDC extracts (Figure 4). However, major differences between solvents were not observed, probably due to the efficacy of the cannabinoid components present in the extracts. While remarkable, more directed studies will be required to confirm and better characterize this inhibitory effect displayed by cannabinoids over LPS-induced STAT3 activation.
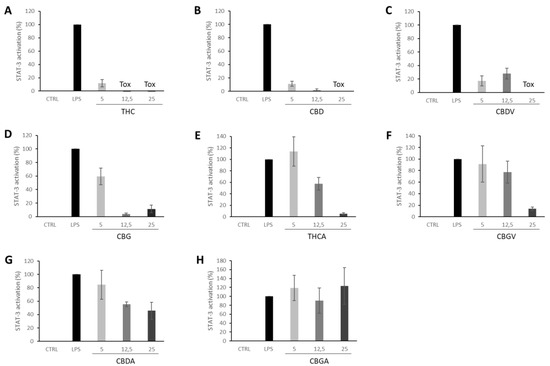
Figure 6.
Inhibition of LPS-induced STAT-3 activation in Raw-STAT3-Luc macrophages by pure isolated cannabinoids (A) THC, (B) CBD, (C) CBDV, (D) CBG, (E) THCA, (F) CBGV, (G) CBDA, (H) CBGA, were tested at different concentrations of 5, 12.5 and 25 μg·mL−1. Values are expressed as the mean ± standard deviation of 2–independent assays.
4. Conclusions
Here we disclose the preparation and preliminary characterization of PHYRESCEL, a botanical drug library containing 40 cannabis extracts with varying cannabinoid content. By screening the pharmacological activity of PHYRESCEL in vitro, we were able to pin-point several cannabis extracts that differentially inhibited the transcriptional activation of NF-κB, HIF-1α and STAT3, three molecular targets known to mediate cellular responses to inflammation and hypoxia. The efficacy of isolated minor cannabinoids at inhibiting these transcription factors is also reported. Our results support the notion that cannabis extracts possess differential pharmacological properties compared to isolated cannabinoid molecules. The composition and pharmacological activity of different cannabis extracts obtain from a single cultivar differed largely depending on the organic solvent used and the subsequent decarboxylation process. Although the translation value of these findings is unclear, we expect these functional coordinates to aid in the identification of novel, natural modulators of cellular responses to pro-inflammatory and hypoxic stimuli present in certain varieties of Cannabis sativa L. developed by Phytoplant Research.
Supplementary Materials
The following are available online at https://www.mdpi.com/2297-8739/7/4/56/s1, Table S1: Cannabinoid composition of all extracts determined by HPLC-DAD. Concentrations are expressed as mean ± standard deviation.
Author Contributions
Conceptualization, X.N.R. and V.S.d.M.B.; methodology, X.N.R., V.S.d.M.B., C.F.V., C.S.-C.; validation, G.M.-S., X.N.R., C.F.V. and V.S.d.M.B.; formal analysis, G.M.-S., C.F.V. and C.S.-C.; investigation, X.N.R., C.F.V. and V.S.d.M.B.; data curation, V.S.d.M.B.; writing—original draft preparation, G.M.-S.; writing—review and editing, X.N.R., C.F.V., G.M.-S. and V.S.d.M.B.; supervision, V.S.d.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Technical assistance and scientific support from Juan Diego Unciti and Eduardo Muñoz from Emerald Health Biosciences España is gratefully acknowledged.
Conflicts of Interest
C.F.V. and V.S.d.M.B. are employees of Phytoplant Research. X.N.R. and C.S.-C. are former employees of Phytoplant Research. G.M.-S. is a paid consultant to Phytoplant Research.
Abbreviations
CBC: cannabichromene; CBD: cannabidiol; CBDA: cannabidiolic acid; CBDV: cannabidivarin; CBDVA: cannabidivarinic acid; CBG: cannabigerol; CBGA: cannabigerolic acid; CBN: cannabinol; CPVO: Community Plant Variety Office; HIF-1α: Hypoxia-inducible factor-1α; HPLC-DAD: high-performance liquid chromatography-diode array detection; IC50: inhibitory concentration 50; LPS: lipopolysaccharide; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PCA: principal component analysis; PHYRESCEL: Phytoplant Research Cannabis Extract Library; QTOF: quantitative time-of-flight; STAT3: signal transducer and activator of transcription 3; THC: Δ9-tetrahydrocannabinol; THCA: Δ9-tetrahydrocannabinolic acid; THCV: Δ9-tetrahydrocannabivarin.
References
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sanz, G. Can you pass the acid test? Critical review and novel therapeutic perspectives of δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of cannabis sativa L. In Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–36. [Google Scholar]
- LaPrairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; LaPrairie, R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef]
- Navarro, G.; Reyes-Resina, I.; Rivas-Santisteban, R.; De Medina, V.S.; Morales, P.; Casano, S.; Ferreiro-Vera, C.; Lillo, A.; Aguinaga, D.; Jagerovic, N.; et al. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem. Pharmacol. 2018, 157, 148–158. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2017; pp. 67–134. [Google Scholar]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef]
- Grinspoon, L.; Bakalar, J.B.; Zimmer, L.; Morgan, J.P.; McGregor, I.S.; Di Chiaro, G.; De Fonseca, F.R.; Navarro, M.; Carrera, M.R.A.; Koob, G.F.; et al. Marijuana addiction. Science 1997, 277, 749–752. [Google Scholar] [CrossRef]
- Russo, E.B.; McPartland, J.M. Cannabis is more than simply Δ9-tetrahydrocannabinol. Psychopharmacology 2003, 165, 431–432. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of entourage: Terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1and CB2 receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Eisenberg, E. Medical cannabis: A forward vision for the clinician. Eur. J. Pain 2018, 22, 485–491. [Google Scholar] [CrossRef]
- Callado, C.S.-C.; Núñez-Sánchez, N.; Casano, S.; Ferreiro-Vera, C. The potential of near infrared spectroscopy to estimate the content of cannabinoids in Cannabis sativa L.: A comparative study. Talanta 2018, 190, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Roura, X.N. Methods of Purifying Cannabinoids, Compositions, and Kits Thereof. WO Patent 2016116628, 28 July 2016. [Google Scholar]
- Roura, X.N. Methods of Purifying Cannabinoids Using Liquid: Liquid Chromatography. WO Patent 2019145552A1, 1 August 2019. [Google Scholar]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of medical cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef]
- Del Prete, D.; Taglialatela-Scafati, O.; Minassi, A.; Sirignano, C.; Cruz, C.; Bellido, M.L.; Muñoz, E.; Appendino, G. Electrophilic triterpenoid enones: A comparative thiol-trapping and bioactivity study. J. Nat. Prod. 2017, 80, 2276–2283. [Google Scholar] [CrossRef]
- Minassi, A.; Rogati, F.; Cruz, C.; Prados, M.E.; Galera, N.; Jiménez, C.; Appendino, G.; Bellido, M.L.; Calzado, M.A.; Caprioglio, D.; et al. Triterpenoid hydroxamates as HIF prolyl hydrolase inhibitors. J. Nat. Prod. 2018, 81, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef]
- Brown, I.; Cascio, M.G.; Rotondo, D.; Pertwee, R.G.; Heys, S.D.; Wahle, K.W.J. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 2013, 52, 80–109. [Google Scholar] [CrossRef] [PubMed]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091–4106. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ 9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and Interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Jüttler, E.; Potrovita, I.; Tarabin, V.; Prinz, S.; Dong-Si, T.; Fink, G.; Schwaninger, M. The cannabinoid dexanabinol is an inhibitor of the nuclear factor-kappa B (NF-κB). Neuropharmacology 2004, 47, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.M.; Appendino, G.; Fiebich, B.L.; Grassi, G. A Composition Containing Non-Psychotropic Cannabinoids for the Treatment of Inflammatory Diseases. WO Patent 2009043836A1, 9 April 2007. [Google Scholar]
- Sánchez-Duffhues, G.; Calzado, M.A.; de Vinuesa, A.G.; Appendino, G.; Fiebich, B.L.; Loock, U.; Lefarth-Risse, A.; Krohn, K.; Muñoz, E. Denbinobin inhibits nuclear factor-kappaB and induces apoptosis via reactive oxygen species generation in human leukemic cells. Biochem. Pharmacol. 2009, 77, 1401–1409. [Google Scholar] [CrossRef]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. NeuroOncology 2005, 7, 134–153. [Google Scholar] [CrossRef]
- Kossatz, E.; Maldonado, R.; Robledo, P. CB2 cannabinoid receptors modulate HIF-1α and TIM-3 expression in a hypoxia-ischemia mouse model. Eur. Neuropsychopharmacol. 2016, 26, 1972–1988. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Peng, Y.; Zhao, J.; Lei, X.; Zheng, X.; Xie, Z.-Z.; Tang, G. Anticancer activity of natural flavonoids: Inhibition of HIF-1α signaling pathway. Curr. Org. Chem. 2019, 23, 2945–2959. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Wei, H.; Wang, F.; Guo, F.; Gao, Z.; Marsicano, G.; Wang, Q.; Xiong, L. Activation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in rats. Brain Res. 2013, 1529, 154–164. [Google Scholar] [CrossRef]
- Ciaglia, E.; Torelli, G.; Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Malfitano, A.M.; Fiore, D.; Zottola, A.C.P.; Proto, M.C.; et al. Cannabinoid receptor CB1 regulates STAT3 activity and its expression dictates the responsiveness to SR141716 treatment in human glioma patients’ cells. Oncotarget 2015, 6, 15464–15481. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Lenglet, S.; Braunersreuther, V.; Burger, F.; Pelli, G.; Bertolotto, M.B.; Mach, F.; Steffens, S. CB (2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J. Mol. Cell. Cardiol. 2009, 46, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Stančić, A.; Jandl, K.; Hasenöhrl, C.; Reichmann, F.; Marsche, G.; Schuligoi, R.; Heinemann, A.; Storr, M.A.; Schicho, R. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol. Motil. 2015, 27, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Bian, Y.; He, Q.; Yao, J.; Zhu, J.; Wu, J.; Wang, K.; Duan, T. Suppression of STAT3 signaling by Δ9-Tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cell. Physiol. Biochem. 2017, 42, 537–550. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).