Abstract
Agricultural water is closely linked to surface and ground water as well as soil; hence, ensuring its safety is an important endeavor. We used the “quick, easy, cheap, effective, rugged, and safe” (QuEChERS) method to analyze multi-residue pesticides in agricultural water by using a combined-sorbent-based clean-up procedure. Among the various sorbents examined, clean-up using ENVI-Carb combined with a primary secondary amine sorbent delivered the highest recovery of multi-residue pesticides (>93.9%). While the developed method showed satisfactory linearity (R2 > 0.9991), precision, and specificity, recovery was low for pyrazolate (29.1%) and thidiazuron (59.2%). The limits of detection and quantification for the 55 pesticides targeted in this study were in 0.02–3.0 μg L−1 and 0.1–9.9 μg L−1, respectively. The developed method was used to identify and quantify multi-residue pesticides during sample analysis. The results suggest that the QuEChERS method employing a combination of ENVI-Carb and another sorbent can be applied for the effective analysis of multi-residue pesticides in agricultural water.
1. Introduction
Water, an environmental reservoir, has continuously been contaminated by pesticides used for agriculture [1]. Such residual pesticides that remain in agricultural water may have several undesirable effects on soil and crops; these effects tend to vary with water location-ranging from surface and ground water [2,3]. Despite the widespread adverse effects of contaminated water, detection of residual pesticides in agricultural water is difficult because of their low concentrations, wide diversity of chemical classes, and various physicochemical properties and the presence of complex matrices [4]. Therefore, residual pesticides must be strictly controlled to protect the water ecosystem and agricultural produce.
Several analytical methods have been developed for the extraction of residual pesticides from agricultural water; liquid-liquid, solid-phase, and solid-liquid extraction methods are typically used to prepare agricultural water samples [4,5,6]. However, these methods have disadvantages such as the use of large volumes of organic solvents, inefficient clean-up procedures, and long processing times. QuEChERS (quick, easy, cheap, effective, rugged, and safe) was developed as an alternative extraction method to overcome these issues [4,5]. Although the QuEChERS method is very rapid and efficient, improvements for reduction of the interference of complex matrices and achievement of satisfactory recovery from sorbents are still desired [7]. In addition, the QuEChERS method is poorly selective and sensitive under the analysis conditions used for some multi-residue pesticides and samples [8].
In the QuEChERS method, the clean-up procedure is considered as an important factor that influences matrix removal and analyte recovery [8,9]. Primary secondary amine (PSA), graphitized carbon black (GCB), zirconium dioxide bonded to silica (Z-Sep), octadecylsilica (C18), and ENVI-Carb are used as sorbents in the QuEChERS method for multi-residue pesticides [10,11]. Among them, GCB is capable of efficiently eliminating steroids and pigments [12,13]; this sorbent was found to be useful for particular class of pesticide, but was inadequate for the clean-up of matrices and the recovery of a diverse number of pesticides [6,7,8,9,11,12,13,14]. For instance, GCB was known to retain structurally planar pesticides such as chlorothalonil, cyprodinil, fenazaquin, hexachlorobenzene, mepanipyrim, prochloraz, pyrimethanil, quinoxyfen, terbuphos, and thiabendazole [7,8,9,10,14]. The use of high amounts of GCB may lead to low recoveries of some planar pesticides [8,13,14]. Nevertheless, the GCB sorbent has been shown to quantitatively recover polar compounds from various environmental water samples [6,8]; however, a method that uses only GCB as the sorbent is insufficiently optimized to effectively remove co-extractives from agricultural water. The use of only GCB as the sorbent leads to low recovery of residual pesticides because of its high affinity for pesticides with planar structures [12,13]. Therefore, previous studies reported that the low-recovery problem can be solved by combining sorbents, including PSA, Z-Sep, and ENVI-Carb, as alternatives to GCB [2,11,15,16].
Herein, we demonstrate that a clean-up method using ENVI-Carb can be used to detect residual pesticides in agricultural water. We developed and validated the method for the multi-residue analysis of 55 pesticides in agricultural water by using liquid chromatography (LC) with mass spectrometry (MS). The results of this study provide an effective strategy for the analysis of residual pesticides in environmental samples such as agricultural water.
2. Materials and Methods
2.1. Chemicals and Reagents
The pesticides listed in Table 1 (>95%) were purchased from AccuStandard (New Haven, CT, USA). A QuEChERS EN extraction kit (P/N 5982-5650), end-capped C18 adsorbent (P/N 5982-1382), PSA (P/N 5982-8382), and GCB (AT-5982-4482) were supplied by Agilent Technologies (Santa Clara, CA, USA). Supel™QuE PSA/ENVI-Carb™ (P/N 55176-U), Z-Sep+ (55299-U), ammonium acetate (>99.0%), triphenyl phosphate, MgSO4, and formic acid were obtained from Sigma–Aldrich (St. Louis, MO, USA). All solvents were of analytical or high-performance liquid chromatography (HPLC) grade. Each standard stock solutions were prepared to a concentration of 100 μg mL−1 using acetonitrile as the solvent. A working standard mixture was prepared from the stock solutions by serial dilution. Stock and working solutions were stored at −20 °C until required for analysis.

Table 1.
LC-MS/MS parameter values used during the detection of 55 pesticides.
2.2. Sample Preparation
The QuEChERS extraction procedure was performed as follows: sample (10 mL) was placed in a 50-mL centrifuge tube, and 5.0 mL of acetonitrile was added as the extraction solvent. Triphenyl phosphate (1.0 µg mL−1) was spiked directly into the centrifuge tube as the internal standard. Extraction was performed using the QuEChERS EN extraction kit containing 4.0 g of MgSO4, 1.0 g of sodium chloride, 1.0 g of sodium citrate dehydrate, and 0.5 g of sodium hydrogen citrate sesquihydrate. The centrifuge tube was shaken for 1.0 min, charged with the QuEChERS EN extraction kit, shaken vigorously for 10 min, and centrifuged at 4000× g for 10 min at 4 °C.
Various dispersive solid-phase extraction clean-up methods were applied for analyte extraction. The analyte recovery by various clean-up procedures at spiked levels of 10, 50, and 100 μg L−1 was evaluated. The following clean-up procedures were used: Clean-up I (50 mg C18, 50 mg PSA, and 150 mg MgSO4); Clean-up II (50 mg C18, 50 mg PSA, 50 mg GCB, and 150 mg MgSO4); Clean-up III (50 mg GCB, 50 mg PSA, and 150 mg MgSO4); Clean-up IV (50 mg Z-Sep, 50 mg PSA, and 150 mg MgSO4); and Clean-up V (7.5 mg ENVI-Carb, 25 mg PSA, and 150 mg MgSO4). A 1-mL aliquot of the extract was transferred to a 2-mL tube and each tube was strongly vortexed for 1 min and then centrifuged at 4000× g for 5 min at 4 °C. A 0.5-mL aliquot of the upper layer was transferred to a glass tube and the solvent was evaporated by purging with N2 gas. After reconstituting with 0.1 mL of acetonitrile, the extract was filtered through a syringe with a 0.22-µm nylon membrane filter and transferred into an autosampler vial for analysis by LC-MS/MS.
2.3. LC-MS/MS
Pesticides were analyzed by using an Agilent LC 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA) consisted of a 4000 QTRAP mass spectrometer coupled with a turbo ion-spray ionization source (AB SCIEX, Foster City, CA, USA). Chromatographic separation was carried out at 40 °C with an Zorbax Eclipse Plus C18 column (2.1 × 100 mm, 3.5 μm) supplied by Agilent (Santa Clara, CA, USA). The separation was performed with a mobile phase A (5.0 mM ammonium acetate and 0.1 vol % formic acid in water) and mobile phase B (5.0 mM ammonium acetate and 0.1 vol % formic acid in methanol), at a flow rate 0.4 mL/min; the injection volume was 2.0 μL. A linear gradient was performed as follows: 0–8 min, 60% A; 8–1.5 min, 40% A; 1.5–2.5 min, 30% A; 2.5–9 min 20% A; 9 to 12 min 0% A; 12–15 min, 95% A. The mass analysis using the positive ESI mode was operated in the scheduled multiple reaction monitoring (MRM) mode with the following parameters: curtain gas (30 psi), ion-source gas 1 (50 psi), ion-source gas 2 (55 psi), source temperature (400 °C), and ion-spray voltage, which was set at 5500 V. Table 1 summarized the MRM transitions for each pesticide.
2.4. Method Validation and Matrix Effects
Method validation was carried out according to the European Commission SANTE/12682/2019 [17] guidelines for linearity, matrix effects (MEs), limits of detection (LODs), limits of quantification (LOQs), accuracy (recovery percentage), and precision (relative standard deviation, RSD%). The linearity was determined by analyzing matrix-matched calibration curves with spiked blank samples at concentrations of 5, 10, 20, 50, and 100 μg L−1.
The accuracy and precision were determined by analyzing blank samples spiked with standard solutions at three concentrations: 10, 50, and 100 μg L−1. Repeatability and reproducibility were expressed as intra-day (same day) and inter-day (three different days) RSDs%, respectively, by assaying at least six replicates. LODs and LOQs were determined from five independently spiked concentrations of pesticide (5, 10, 20, 50, and 100 μg L−1). LODs and LOQs were calculated based on standard deviations of response and slope, and are expressed as: LOD = 3.3 σ/s, LOQ = 10 σ/s (where σ = standard deviation of the response, s = slope of the matrix-matched calibration curve).
Additionally, as described in SANTE/12682/2019, matrix-matched standards were used to compensate for MEs. Matrix-matched calibration curves were used to overcome quantification accuracy problems. MEs (%ME) were evaluated by comparing the slopes of the calibration curves in acetonitrile with the matrix-matched calibration curves in the extracts using the following equation: % ME = ((slope of calibration curve in the extract/slope of calibration curve in solvent)−1) × 100 [8].
3. Results and Discussion
3.1. Optimization of LC-MS/MS Conditions
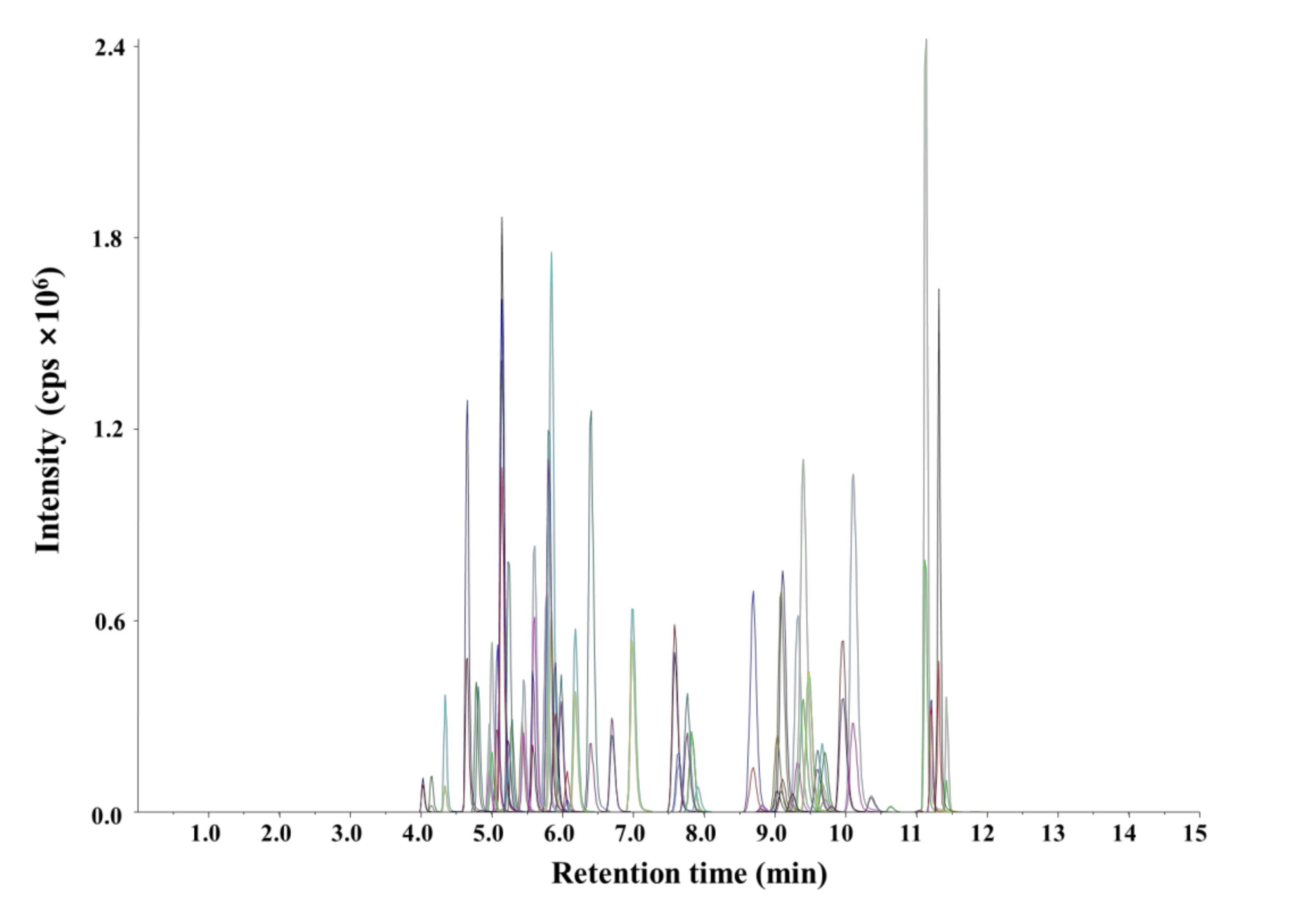
The conditions for the ionization and fragmentation of 55 pesticides were established prior to method validation. The conditions for the quantitative and qualitative analyses of the target pesticides were determined by MRM on the basis of retention times and qualitative and quantitative ion abundances. The target pesticides had previously been successfully quantified and qualified in the positive ESI mode [18,19] under the optimized LC-MS/MS conditions. The MRM transitions were confirmed through direct infusion with a syringe pump at a flow rate of 10 μL min−1. The most intense product ion was chosen as the quantifier ion and the second most selective as the qualifier ion. The optimized LC-MS/MS conditions that afforded the best MRM transitions, including retention times, quantifier and qualifier ion transitions, declustering potentials, and collision energies, are listed in Table 1. The mobile phases used in this study were composed of water (containing 0.1% formic acid and 5 mM ammonium acetate) and methanol (containing 0.1% formic acid); satisfactory separation and resolution were achieved for all pesticides. The analyte peaks eluted separately and exhibited good shapes at a spiked level of 100 μg L−1 in an agricultural water sample (Figure 1).
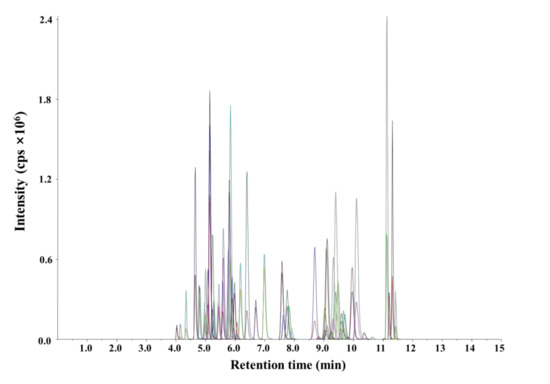
Figure 1.
Multiple reaction monitoring chromatogram of 55 pesticides under the optimized LC–MS/MS conditions.
3.2. Clean-Up Sorbent Determination
Since its introduction in 2003 by Anastassiades, QuEChERS has been modified and optimized by many researchers [11,20,21,22]. Acetonitrile is commonly selected as the extraction solvent for multi-residue pesticide analysis because of its high eluent strength and versatility for both liquid chromatography- and gas chromatography-amenable pesticides [5,11]. In the partitioning process, MgSO4 and NaCl (4:1) are mainly used as salts, resulting in high recovery and fewer co-extractives [11]. However, the sorbents used to remove the co-extractives during the extraction process require appropriate modification and effective approaches because of the presence of various matrices (lipids, flavonoids, etc.) in the samples [11,16]. In this study, the recovery of the sorbents commonly used in the QuEChERS method was evaluated for the analysis of multi-residue pesticides in the samples that are widely exposed in nature, such as agricultural water [10]. The PSA sorbent is a weak anion-exchange agent that removes co-extractives such as sugars and fatty acids [16]. The GCB sorbent helps to remove pigments such as carotenoids and chlorophyll as well as steroids [9,11], while the octadecyl (C18) reverse phase clean-up sorbent retains fats, vitamins, and minerals based on van der Waals interactions or dispersion [11,21].
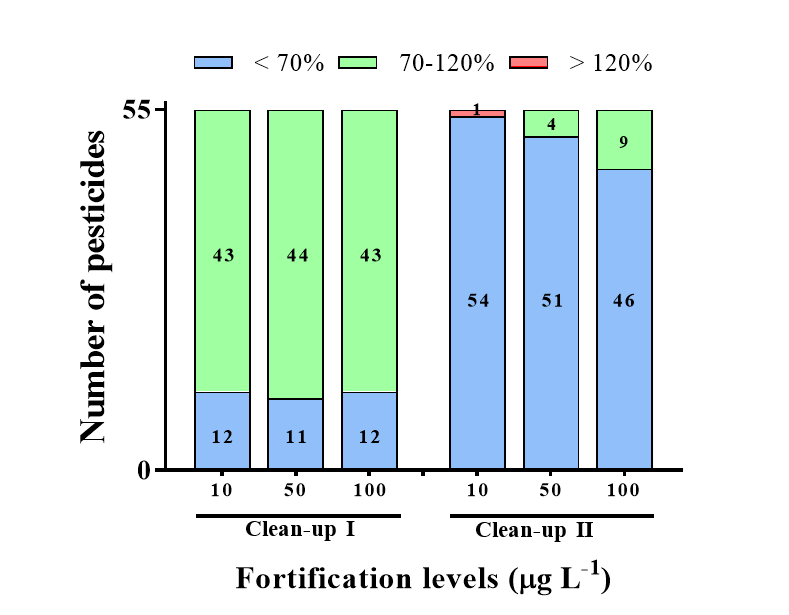
In a preliminary study, universal sorbents, including PSA, GCB, and C18 were used to remove the co-extractives (Figure 2). Almost all pesticides exhibited low recoveries (<70%) when GCB was used as the clean-up sorbent with C18 and PSA (Clean-up II), and some pesticides showed low recovery in the absence of GCB (Clean-up I). The low recovery observed for Clean-ups I and II is attributed to the disadvantages of the GCB sorbent, i.e., its high affinity for planar compounds, and the limitations of the PSA sorbent, which is incapable of removing excessive interferents in a complex sample [9,11]. According to Tolosa et al. [6], the GCB sorbent affects pesticide stability through three hydrolysis mechanisms; non-catalyzed hydrolysis by residual water, hydrolysis catalyzed by the chemical heterogeneity of the GCB surface, and chemisorption by surface-active sites.
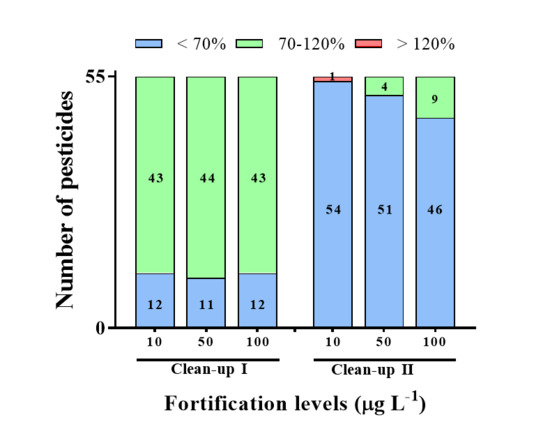
Figure 2.
The recoveries of different clean-up procedures for LC-MS/MS at the spiked level of 10, 50, and 100 μg L−1. The different clean-up procedures used were as follows: Clean-up I (50 mg C18, 50 mg PSA, and 150 mg MgSO4); Clean-up II (50 mg C18, 50 mg PSA, 50 mg GCB, and 150 mg MgSO4).
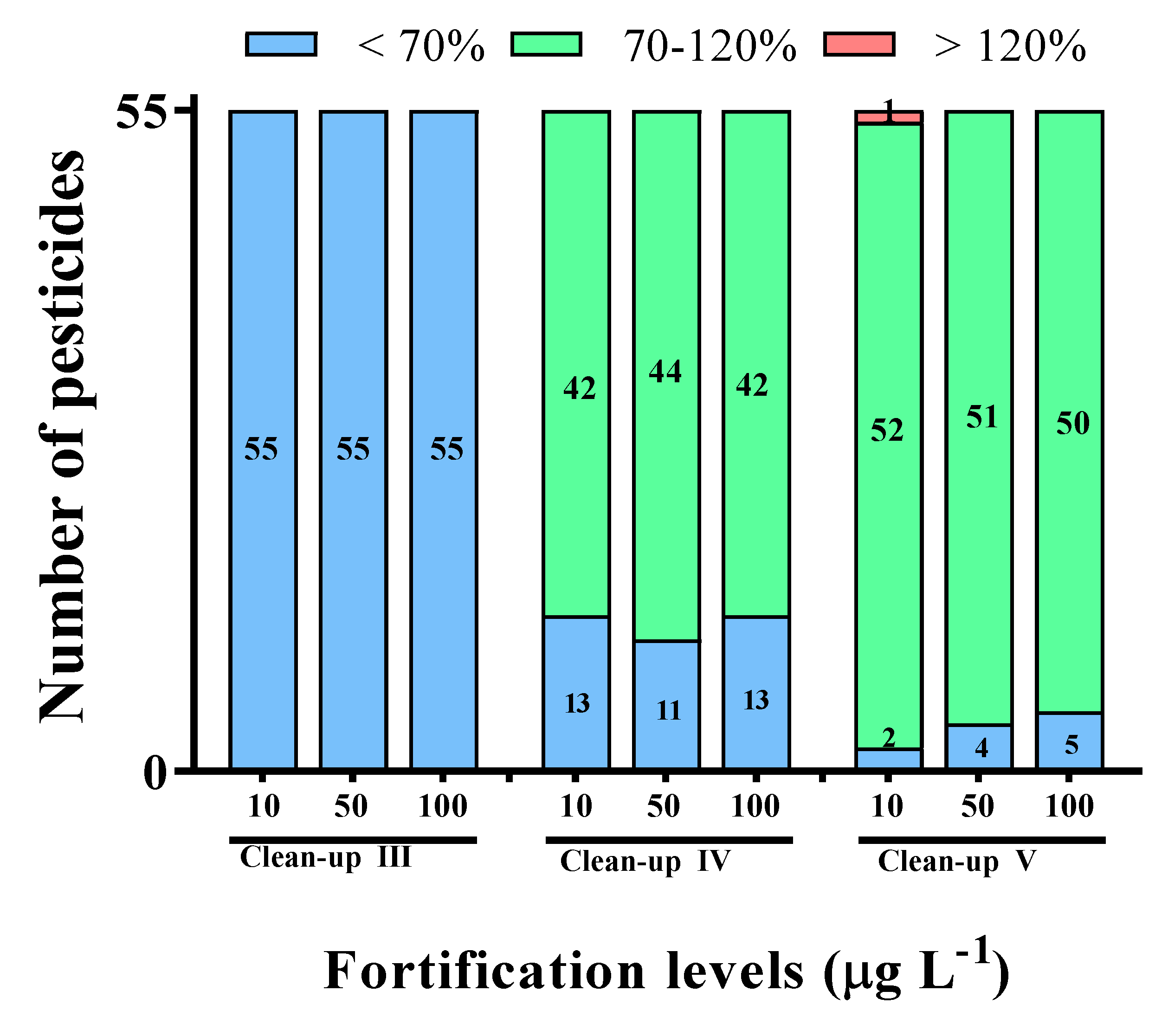
As shown in Figure 3, when Clean-up III (GCB/PSA/MgSO4) was used as the clean-up procedure, all 55 pesticides showed recoveries of less than 70% at all fortification levels. Additional carbon-based sorbents were studied in order to overcome the low recovery issues associated with the Clean-up I and II methods. Z-Sep is a sorbent composed of C18 coated with ZrO2 and has the properties of an amphoteric oxide. As the ZrO2:C18 ratio is 2:5, Z-Sep is expected to exhibit properties identical to those of the C18 sorbent [15]. Figure 3 shows that the pesticide recovery achieved for Clean-up IV (Z-Sep/PSA/MgSO4) is similar to that for Clean-up I. While the Z-Sep sorbent effectively removed matrices that contain carboxylic acid groups, such as proteins, its strong Lewis-acidic properties result in the very slow desorption of basic substances [15]. Therefore, we investigate that clean-up with Z-Sep would not satisfy the desired pesticide-recovery criteria.
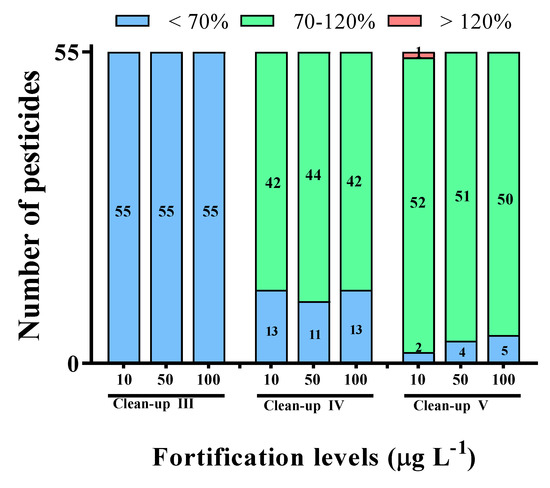
Figure 3.
The recoveries of different clean-up procedures for LC-MS/MS at the spiked level of 10, 50, and 100 μg L−1. The different clean-up procedures used were as follows: Clean-up Ⅲ (50 mg GCB, 50 mg PSA, and 150 mg MgSO4); Clean-up Ⅳ (50 mg Z-Sep, 50 mg PSA, and 150 mg MgSO4); Clean-up Ⅴ (7.5 mg ENVI-Carb, 25 mg PSA, and 150 mg MgSO4).
Figure 3 reveals that Clean-up V (ENVI-Carb/PSA/MgSO4) delivered a recovery of >93.9% during the multi-residue pesticide analysis. We believe that the characteristics of ENVI-Carb are attributable for the highest recovery for Clean-up V. The two-dimensional layered graphite structure endows ENVI-Carb with a large surface area, makes it weakly adsorptive, and does not require the use an analyte to diffuse into pores [8,23]. For this reason, ENVI-Carb does not require a washing step, such as the dichloromethane washing step used for silica cartridge, which reduces the sample-preparation time [23]. In principle, ENVI-Carb interacts in a dispersive manner with non-polar molecules, such as aromatic sorbates, on its surface due to the presence of hybridized π-electrons [24,25,26]. Hence, the ENVI-Carb sorbent effectively removes trace compounds such as polyphenols and pigments [8,26]. Zhang et al. [26] reported that ENVI-Carb effectively reduces the color of sewage sludge and exhibits low matrix effects. Therefore, we conclude that ENVI-Carb is an effective sorbent for the analysis of multi-residue pesticides in environmental samples.
3.3. Method Validation
The clean-up procedure was validated under the optimized combined-sorbent conditions. The values of several validation parameters, namely, linearity, accuracy, precision, LOD, and LOQ, were determined in order to validate QuEChERS extraction using the Clean-up V method. Linear calibration curves were constructed by the matrix-matched standard calibration method using concentrations of 5, 10, 20, 50, and 100 μg L−1 in blank extracts of agricultural water. As summarized in Table 2, satisfactory coefficients of determination (R2 = 0.9991–0.9999) were obtained for the 55 pesticides. The LOD values of individual pesticides were within 0.02–3.0 μg L−1, which were calculated as having a signal to noise ratios of 3:1 from the chromatograms.

Table 2.
Method validation data of selected 55 pesticides in water using Clean-up V (7.5 mg ENVI-Carb, 25 mg PSA, and 150 mg MgSO4) with LC-MS/MS. Range of pesticides concentration between 0.1 to 100 (μg L−1).
Moreover, the LOQ levels (0.1–9.9 μg L−1) were obtained, with the 55 pesticides exhibiting satisfactory recovery and precision. The recovery of the 55 pesticides ranged between 20.5 and 121.7% and was determined at three fortification levels (10, 50, and 100 μg L−1). However, among the tested pesticides, the MRLs are not currently set for agricultural water in Korea. Thus, we applied the uniform MRL (0.01 mg kg−1 or less) to all tested pesticides. The low recovery of pyrazolate (29.1%) and thidiazuron (59.2%) indicates that their levels in agricultural water can only be qualitatively determined, rather than quantitatively. The RSDs for repeatability and reproducibility for all 55 pesticides were found to be <14.8% and 15.8%, respectively. It was also possible to separate the 55 target components to satisfactory levels.
3.4. Matrix Effects
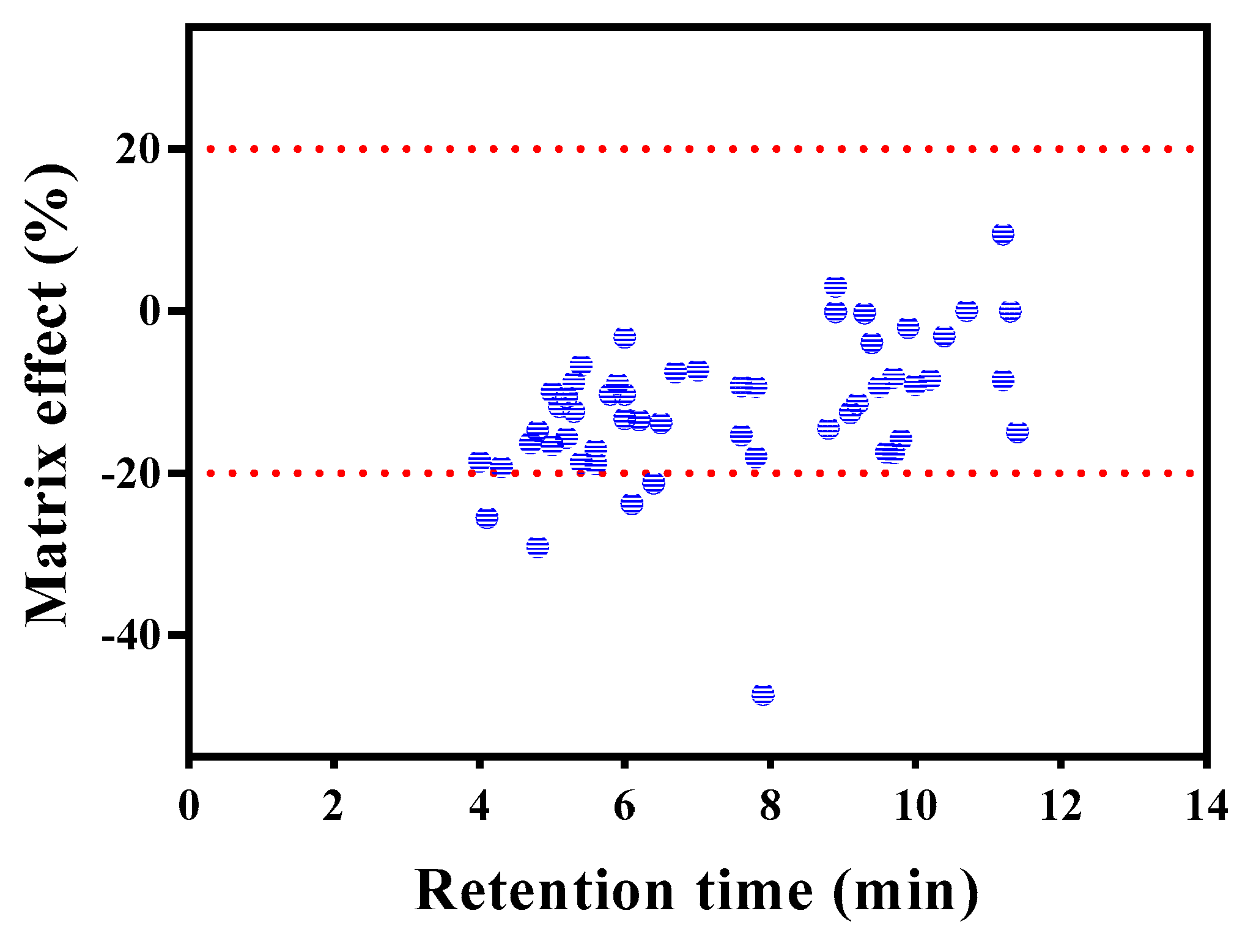
Matrix effects (MEs) are one of the main aspects that must be addressed when evaluating a multi-residue method for pesticide analysis. The slope of a calibration curve is considerably influenced by matrix components that are not removed during clean-up [27]. Minimizing MEs improves chromatographic selectivity, and dilution is an effective approach for this [18,19]. In this study, MEs (%ME) were determined on the basis of ion suppression and/or enhancement [19]. Reagent and matrix standards for each concentration (10, 50, and 100 μg L−1) were analyzed using Clean-up V. As shown in Figure 4, all compounds showed slight ion suppression, with MEs ranging from −47.3% to 9.5% (average: –11.9%), with pyrazolate exhibiting the highest ME (−47.3%). Other pesticides that showed MEs < 20% include fenbuconazole (−21.2%), fenhexamid (−23.8%), forchlorfenuron (−29.1%), and thidiazuron (−25.5%). The recoveries of these five pesticides were mostly less than 70%, except one or two fortification levels. Ion suppression and/or enhancement through MEs depend on several factors, including sample preparation procedure, sample matrix, and physicochemical properties of the pesticide [28,29]. A few alternative techniques have been suggested to compensate for MEs. Among them, external matrix-matched calibration using blank samples spiked with standard solutions is commonly employed because of its low cost and lack of complexity [29,30].
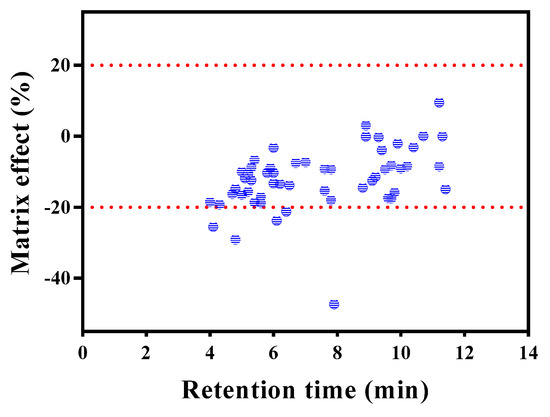
Figure 4.
Matrix effects of 55 pesticides obtained by Clean-up V (contained ENVI-Carb) procedure.
4. Conclusions
We developed a clean-up procedure that uses a combined sorbent for the LC-MS/MS determination of multi-residue pesticides in agricultural water. The GCB sorbent is commonly used to effectively remove pigments, such as chlorophyll, in an agricultural water sample; however, GCB exhibits low recovery for multi-residue pesticides. As an alternative, we selected ENVI-Carb and showed that it positively influenced the clean-up process for extracts from agricultural water samples. The combined use of PSA and ENVI-Carb facilitated good linearity, precision, and accuracy during the multi-residue pesticide analysis. This approach is suitable for quantitative analysis and for detection of multi-residue pesticides in agricultural water.
Author Contributions
Conceptualization, T.G.N.; methodology, T.G.N. and N.-E.S.; validation, N.-E.S. and Y.S.J.; formal analysis, J.Y.C. and D.-H.S.; investigation, T.-G.L.; writing—original draft preparation, Y.S.J. and T.G.N.; writing—review and editing, T.G.N.; supervision, T.G.N.; funding acquisition, H.-K.C. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Main Research Program (E0187200-03) of the Korea Food Research Institute funded by the Ministry of Science and ICT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caldas, S.S.; Bolzan, C.M.; Cerqueira, M.B.; Tomasini, D.; Furlong, E.B.; Fagundes, C.; Primel, E.G. Evaluation of a modified QuEChERS extraction of multiple classes of pesticides from a rice paddy soil by LC-APCI-MS/MS. J. Agric. Food Chem. 2011, 59, 11918–11926. [Google Scholar] [CrossRef] [PubMed]
- Hrynko, I.; Łozowicka, B.; Kaczyński, P. Comprehensive analysis of insecticides in melliferous weeds and agricultural crops using a modified QuEChERS/LC-MS/MS protocol and of their potential risk to honey bees (Apis mellifera L.). Sci. Total Environ. 2019, 657, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jennings, A. Worldwide regulations of standard values of pesticides for human health risk control: A review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Arias, J.L.; Rombaldi, C.; Caldas, S.S.; Primel, E.G. Alternative sorbents for the dispersive solid-phase extraction step in quick, easy, cheap, effective, rugged and safe method for extraction of pesticides from rice paddy soils with determination by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1360, 66–75. [Google Scholar] [CrossRef]
- Abdel Ghani, S.B.; Hanafi, A.H. QuEChERS method combined with GC‒MS for pesticide residues determination in water. J. Anal. Chem. 2016, 71, 508–512. [Google Scholar] [CrossRef]
- Tolosa, I.; Douy, B.; Carvalho, F.P. Comparison of the performance of graphitized carbon black and poly(styrene–divinylbenzene) cartridges for the determination of pesticides and industrial phosphates in environmental waters. J. Chromatogr. A 1999, 864, 121–136. [Google Scholar] [CrossRef]
- Shimelis, O.; Yang, Y.; Stenerson, K.; Kaneko, T.; Ye, M. Evaluation of a solid-phase extraction dual-layer carbon/primary secondary amine for clean-up of fatty acid matrix components from food extracts in multiresidue pesticide analysis. J. Chromatogr. A 2007, 1165, 18–25. [Google Scholar] [CrossRef]
- Rutkowska, E.; Łozowicka, B.; Kaczyński, P.M. Modification of multiresidue QuEChERS protocol to minimize matrix effect and improve recoveries for determination of pesticide residues in dried herbs followed by GC-MS/MS. Food Anal. Meth. 2018, 11, 709–724. [Google Scholar] [CrossRef]
- Lawal, A.; Wong, R.C.S.; Tan, G.H.; Abdulra’uf, L.B.; Alsharif, A.M.A. Recent modifications and validation of QuEChERS-dSPE coupled to LC-MS and GC-MS instruments for determination of pesticide/agrochemical residues in fruits and vegetables: Review. J. Chromatogr. Sci. 2018, 56, 656–669. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Checchini, L.; Carlo, R.M.D.; Orlandini, S.; Rivoira, L.; Bubba, M.D. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef]
- Kim, L.; Lee, D.; Cho, H.-K.; Choi, S.-D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063. [Google Scholar] [CrossRef]
- Han, L.; Sapozhnikova, Y.; Lehotay, S.J. Streamlined sample cleanup using combined dispersive solid-phase extraction and in-vial filtration for analysis of pesticides and environmental pollutants in shrimp. Anal. Chim. Acta 2014, 827, 40–46. [Google Scholar] [CrossRef]
- Rasche, C.; Fournes, B.; Dirks, U.; Speer, K. Multi-residue pesticide analysis (gas chromatography–tandem mass spectrometry detection)—Improvement of the quick, easy, cheap, effective, rugged, and safe method for dried fruits and fat-rich cereals—Benefit and limit of a standardized apple purée calibration (screening). J. Chromatogr. A 2015, 1403, 21–31. [Google Scholar] [PubMed]
- Walorczyk, S. Application of gas chromatography/tandem quadrupolemass spectrometry to the multi-residue analysis ofpesticides in green leafy vegetables. Rapid Commun. Mass Spectrom. 2008, 22, 3791–3801. [Google Scholar] [CrossRef] [PubMed]
- Rajski, Ł.; Lozano, A.; Uclés, A.; Ferrer, C.; Fernández-Alba, A.R. Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2013, 1304, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, Y.; Zheng, J.; Zhang, Y.; Yang, L.; Liao, C.; Su, L.; Zhou, Y.; Gong, D.; Chen, L.; et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review. TrAC-Trends Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- European Commission. SANTE/12682/2019. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission Directorate-General for Health and Food Safety. (rev.0). 2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 7 July 2020).
- Song, N.-E.; Lee, J.Y.; Mansur, A.R.; Jang, H.W.; Lim, M.-C.; Lee, Y.; Yoo, M.; Nam, T.G. Determination of 60 pesticides in hen eggs using the QuEChERS procedure followed by LC-MS/MS and GC-MS/MS. Food Chem. 2019, 298, 125050. [Google Scholar] [CrossRef]
- Song, N.-E.; Seo, D.-H.; Choi, J.Y.; Yoo, M.; Koo, M.; Nam, T.G. Dispersive solid–liquid extraction coupled with LC-MS/MS for the determination of sulfonylurea herbicides in strawberries. Foods 2019, 8, 273. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Muhammad, R.; Ahad, K.; Mehboob, F. Extraction techniques for pesticide residues analysis in edible oils and role of sorbents in cleanup. Sep. Sci. Plus 2020, 3, 51–62. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 980–1010. [Google Scholar] [CrossRef]
- Jing, H.; Ge, S.; Lian-Feng, A.; Jian-Chen, L. Determination of six polyether antibiotic residues in foods of animal origin by solid phase extraction combined with liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2016, 1017, 187–194. [Google Scholar]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Powley, C.R.; George, S.W.; Ryan, T.W.; Buck, R.C. Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrixes. Anal. Chem. 2005, 77, 6353–6358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Gerecke, A.C.; Kannan, K.; Müller, C.E.; Alder, A.C. Comparison of two extraction methods for the analysis of per- and polyfluorinated chemicals in digested sewage sludge. J. Chromatogr. A 2010, 1217, 5026–5034. [Google Scholar] [CrossRef] [PubMed]
- Polgár, L.; Kmellár, B.; García-Reyes, J.F.; Fodor, P.B. Comprehensive evaluation of the clean-up step in QuEChERS procedure for the multi-residue determination of pesticides in different vegetable oils using LC-MS/MS. Anal. Methods 2012, 4, 1142–1148. [Google Scholar] [CrossRef]
- Rutkowska, E.; Łozowicka, B.; Kaczyński, P. Compensation of matrix effects in seed matrices followed by gas chromatography-tandem mass spectrometry analysis of pesticide residues. J. Chromatogr. A 2020, 1614, 460738. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.; Shin, J.Y.; Kim, M.; Kim, J.H. Development and verification for analysis of pesticides in eggs and egg products using QuEChERS and LC–MS/MS. Food Chem. 2015, 173, 1236–1242. [Google Scholar] [CrossRef]
- Kittlaus, S.; Schimanke, J.; Kempe, G.; Speer, K. Assessment of sample cleanup and matrix effects in the pesticide residue analysis of foods using postcolumn infusion in liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 8399–8410. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).