Screening of Contaminants of Emerging Concern in Microalgae Food Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Microalgae
2.3. Sample Preparation
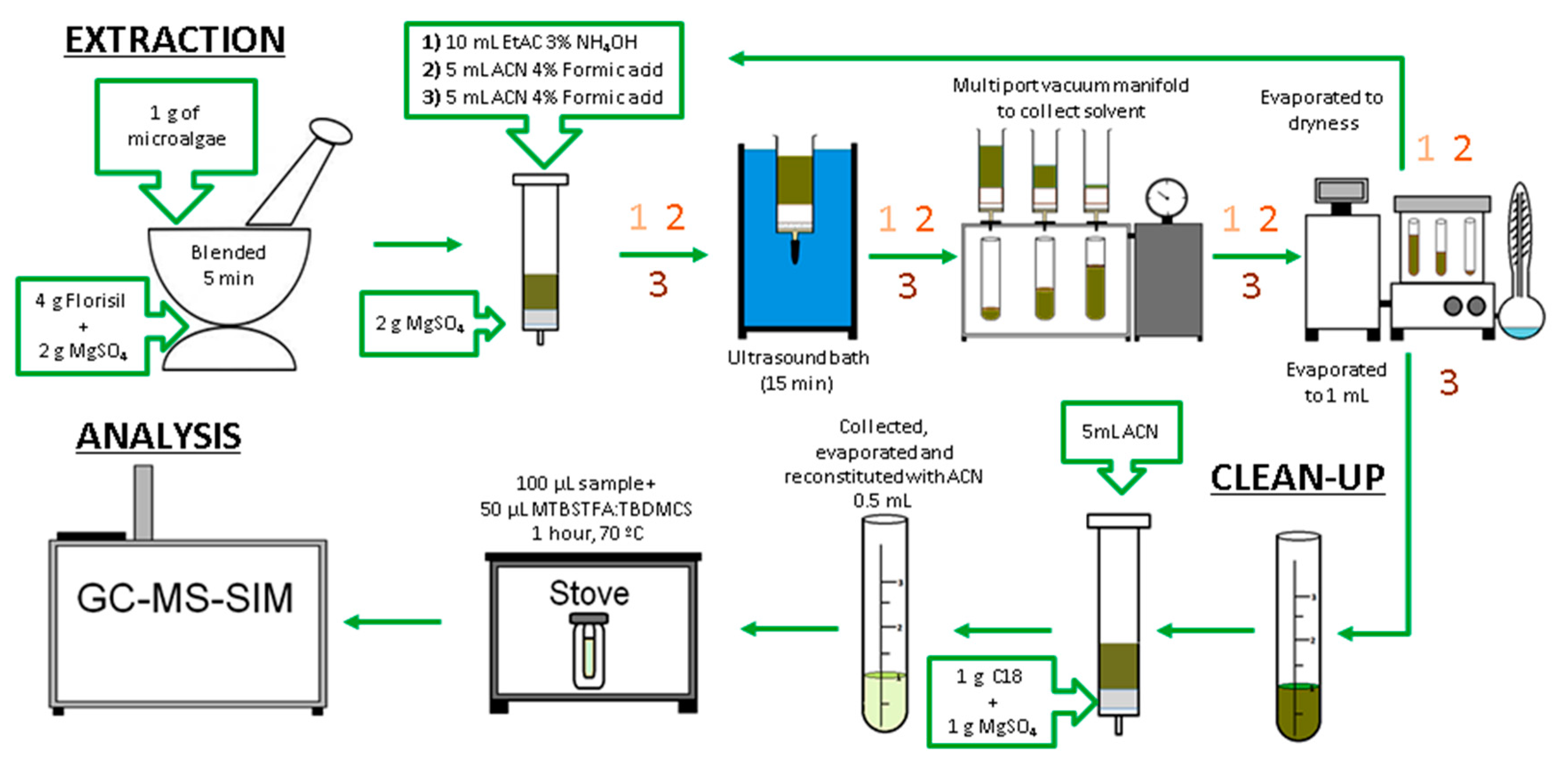
2.3.1. Method Extraction Selection
2.3.2. Clean-Up
2.3.3. Derivatization
2.4. Chromatographic Analysis
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
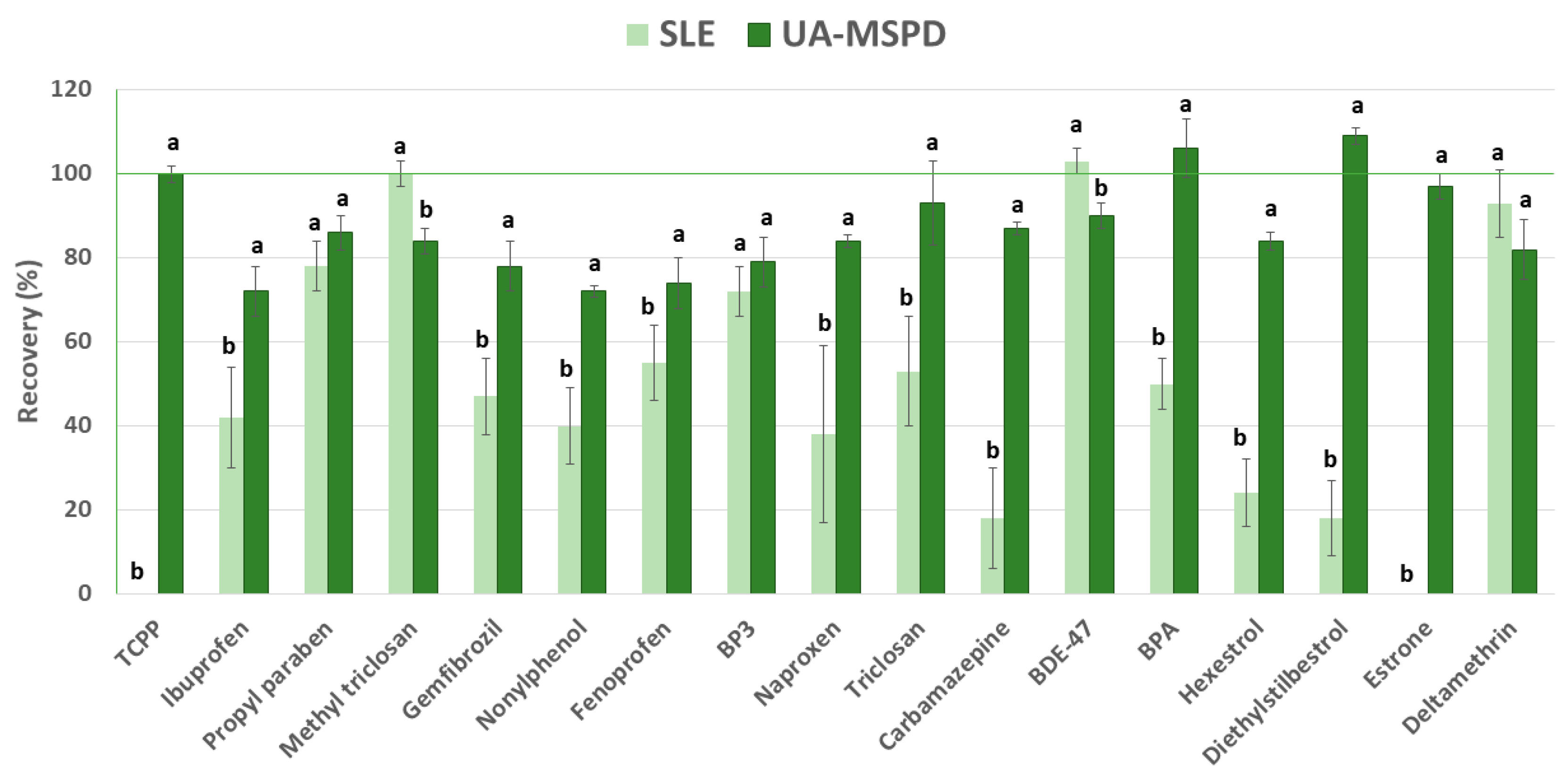
3.1. Method Selection
3.2. Method Validation
3.3. Food Supplements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, J.E. Human Population: The Next Half Century. Science 2003, 302, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.M.; Zubeck, H.M. Evaluation of Microalgae for use as Nutraceuticals and Nutritional Supplements. J. Nutr. Food Sci. 2012, 2, 2. [Google Scholar] [CrossRef]
- Wu, L.-C.; Ho, J.A.-A.; Shieh, M.-C.; Lu, I.-W. Antioxidant and antiproliferative activities of spirulina and Chlorella water extracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef] [PubMed]
- Gábor, V. Microalgae as the source of natural products. Orv. Hetil. 2018, 159, 703–708. [Google Scholar]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytherapy Res. 2003, 17, 665–670. [Google Scholar] [CrossRef]
- Hasegawa, T.; Tanaka, K.; Ueno, K.; Ueno, S.; Okuda, M.; Yoshikai, Y.; Nomoto, K. Augmentation of the resistance against Escherichia coli by oral administration of a hot water extract of Chlorella vulgaris in rats. Int. J. Immunopharmacol. 1989, 11, 971–976. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lim, Y.; Kim, Y.J.; Kim, J.Y.; Kwon, O. A dietary cholesterol challenge study to assess Chlorella supplementation in maintaining healthy lipid levels in adults: A double-blinded, randomized, placebo-controlled study. Nutr. J. 2016, 15, 54. [Google Scholar] [CrossRef]
- Rodríguez-García, I.; Guil-Guerrero, J.L. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008, 108, 1023–1026. [Google Scholar] [CrossRef]
- Danxiang, H.; Yonghong, B.; Zhengyu, H. Industrial production of microalgal cell-mass and secondary products—Species of high potential:Nostoc. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 304–311. [Google Scholar]
- Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013. [Google Scholar]
- Farrar, W.V. Tecuitlatl; A glimpse of Aztec food technology. Nature 1966, 211, 341–342. [Google Scholar] [CrossRef]
- Lee, J.; Park, A.; Kim, M.J.; Lim, H.J.; Rha, Y.A.; Kang, H.G. Spirulina extract enhanced a protective effect in type 1 diabetes by anti-apoptosis and anti-ROS production. Nutrients 2017, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Szulinska, M.; Gibas-Dorna, M.; Miller-Kasprzak, E.; Suliburska, J.; Miczke, A.; Walczak-Gałezewska, M.; Stelmach-Mardas, M.; Walkowiak, J.; Bogdanski, P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2473–2481. [Google Scholar] [PubMed]
- Hernández-Corona, A.; Nieves, I.; Meckes, M.; Chamorro, G.; Barron, B.L. Antiviral activity of Spirulina maxima against herpes simplex virus type 2. Antivir. Res. 2002, 56, 279–285. [Google Scholar] [CrossRef]
- Girardin-Andréani, C. Spiruline: Système sanguin, système immunitaire et cancer*. Phytothérapie 2005, 3, 158–161. [Google Scholar] [CrossRef]
- Chamorro, G.; Salazar, M.A.; Araújo, K.G.D.L.; Dos Santos, C.P.; Ceballos, G.A.; Castillo, L.H.F. Update on the pharmacology of Spirulina (Arthrospira), an unconventional food. Arch Lat. Nutr. 2002, 52, 232–240. [Google Scholar]
- Gao, F.; Yang, Z.H.; Li, C.; Zeng, G.M.; Ma, D.H.; Zhou, L. A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour. Technol. 2015, 179, 8–12. [Google Scholar] [CrossRef]
- Jasinska, E.J.; Goss, G.G.; Gillis, P.L.; Van Der Kraak, G.J.; Matsumoto, J.; de Souza Machado, A.A.; Giacomin, M.; Moon, T.W.; Massarsky, A.; Gagné, F.; et al. Assessment of biomarkers for contaminants of emerging concern on aquatic organisms downstream of a municipal wastewater discharge. Sci. Total Environ. 2015, 530–531, 140–153. [Google Scholar] [CrossRef]
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Marchesi, V.; Tremolada, L.; Daverio, D.; Dellavedova, P. Hyphenated high performance liquid chromatography–tandem mass spectrometry techniques for the determination of perfluorinated alkylated substances in Lombardia region in Italy, profile levels and assessment: One year of monitoring activities during 2018. Separations 2020, 7, 17. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Hurtado, C.; Domínguez, C.; Pérez-Babace, L.; Canameras, N.; Comas, J.; Bayona, J.M. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J. Hazard. Mater. 2016, 305, 139–148. [Google Scholar] [CrossRef]
- Wang, F.; Guo, X.Y.; Zhang, D.N.; Wu, Y.; Wu, T.; Chen, Z.G. Ultrasound-assisted extraction and purification of taurine from the red algae Porphyra yezoensis. Ultrason. Sonochemistry 2015, 24, 36–42. [Google Scholar] [CrossRef] [PubMed]
- De Godos, I.; Vargas, V.A.; Blanco, S.; González, M.C.G.; Soto, R.; García-Encina, P.A.; Becares, E.; Muñoz, R. A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour. Technol. 2010, 101, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; García-González, M.C.; González-Fernández, C. Performance comparison of two photobioreactors configurations (open and closed to the atmosphere) treating anaerobically degraded swine slurry. Bioresour. Technol. 2010, 101, 5144–5149. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.B.; Costanza-Robinson, M.S.; Spatafora, G.A. Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biomass Bioenergy 2011, 35, 40–49. [Google Scholar] [CrossRef]
- Ungsethaphand, T. Production of Spirulina platensis using dry chicken manure supplemented with urea and sodium bicarbonate. Maejo Int. J. Sci. Technol. 2009, 3, 379–387. [Google Scholar]
- Albero, B.; Sánchez-Brunete, C.; Miguel, E.; Aznar, R.; Tadeo, J.L. Rapid determination of natural and synthetic hormones in biosolids and poultry manure by isotope dilution GC-MS/MS. J. Sep. Sci. 2014, 37, 811–819. [Google Scholar] [CrossRef]
- Aznar, R.; Albero, B.; Sánchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Multiresidue analysis of insecticides and other selected environmental contaminants in poultry manure by gas chromatography/mass spectrometry. J. AOAC Int. 2014, 97, 978–986. [Google Scholar] [CrossRef]
- Directive 2002/46/EC on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Commun. 2002, L183/51.
- Flynn, A.; Hirvonen, T.; Mensink, G.B.M.; Ocké, M.C.; Serra-Majem, L.; Stos, K.; Szponar, L.; Tetens, I.; Turrini, A.; Fletcher, R.; et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr. Res. 2009, 53. [Google Scholar] [CrossRef]
- Commision Regulation (EU) No. 37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. 2009, L15/1.
- SANTE/11813/2017 Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 1 May 2020).
- Aznar, R.; Albero, B.; Sánchez-Brunete, C.; Miguel, E.; Martín-Girela, I.; Tadeo, J.L. Simultaneous determination of multiclass emerging contaminants in aquatic plants by ultrasound-assisted matrix solid-phase dispersion and GC-MS. Environ. Sci. Pollut. Res. 2017, 24, 7911–7920. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Springer: Berlin/Heidelberg, Germany, 1975; pp. 29–60. [Google Scholar]
- Rodríguez-López, M. Influence of the inoculum and the medium on the growth of Chlorella pyrenoidosa. Nature 1964, 203, 666–667. [Google Scholar] [CrossRef]
- Aznar, R.; Sánchez-Brunete, C.; Albero, B.; Rodríguez, J.A.; Tadeo, J.L. Occurrence and analysis of selected pharmaceutical compounds in soil from Spanish agricultural fields. Environ. Sci. Pollut. Res. 2014, 21, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Albero, B.; Sánchez-Brunete, C.; Miguel, E.; Pérez, R.A.; Tadeo, J.L. Analysis of natural-occurring and synthetic sexual hormones in sludge-amended soils by matrix solid-phase dispersion and isotope dilution gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1283, 39–45. [Google Scholar] [CrossRef]
- Gil García, M.D.; Galera, M.M.; Uclés, S.; Lozano, A.; Fernández-Alba, A.R. Ultrasound-assisted extraction based on QuEChERS of pesticide residues in honeybees and determination by LC-MS/MS and GC-MS/MS. Anal. Bioanal. Chem. 2018, 410, 5195–5210. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.P.; Chang, J.S. Mild cell disruption methods for bio-functional proteins recovery from microalgae—Recent developments and future perspectives. Algal Res. 2018, 31, 506–516. [Google Scholar] [CrossRef]
- Lv, M.; Tang, X.; Zhao, Y.; Li, J.; Zhang, B.; Li, L.; Jiang, Y.; Zhao, Y. The toxicity, bioaccumulation and debromination of BDE-47 and BDE-209 in Chlorella sp. under multiple exposure modes. Sci. Total Environ. 2020, 723, 138086. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Li, J.; Yuan, M.; Zhang, J.; Xu, F.; Xu, H.; Zheng, X.; Wang, L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Arashiro, L.; Santos, L.H.M.L.M.; Insa, S.; Rodríguez-Mozaz, S.; Barceló, D.; Ferrer, I.; Garfí, M. Fate of priority pharmaceuticals and their main metabolites and transformation products in microalgae-based wastewater treatment systems. J. Hazard. Mater. 2020, 390, 121771. [Google Scholar] [CrossRef]
- Muñoz, R.; Alvarez, M.T.; Muñoz, A.; Terrazas, E.; Guieysse, B.; Mattiasson, B. Sequential removal of heavy metals ions and organic pollutants using an algal-bacterial consortium. Chemosphere 2006, 63, 903–911. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef] [PubMed]

| Name | Use | tR | SIM Parameters | ||
|---|---|---|---|---|---|
| T | Q1 | Q2 | |||
| Methyl Paraben (tBDMS) | Preservative | 11.27 | 209 | 210 | 266 |
| TCEP | Plasticizer | 11.38 | 249 | 250 | 63 |
| TCPP | Plasticizer | 11.57 | 125 | 99 | 277 |
| Ibuprofen (tBDMS) | NSAID | 11.85 | 263 | 264 | 117 |
| Propyl Paraben (tBDMS) | Preservative | 12.15 | 237 | 238 | 294 |
| Methyl Triclosan | Antifungal | 13.38 | 302 | 304 | 252 |
| Nonylphenol (tBDMS) | Surfactant | 13.38 | 334 | 277 | 278 |
| Gemfibrozil (tBDMS) | Lipid regulator | 13.45 | 243 | 179 | 307 |
| Fenoprofen (tBDMS) | NSAID | 13.68 | 299 | 197 | 206 |
| Benzophenone-3 (BP3) (tBDMS) | Sunscreen | 14.11 | 285 | 242 | 286 |
| Naproxen (tBDMS) | NSAID | 14.13 | 287 | 185 | 288 |
| Triclosan (tBDMS) | Antifungal | 14.34 | 347 | 345 | 200 |
| Mefenamic acid (tBDMS) | NSAID | 14.64 | 298 | 224 | 355 |
| Ketoprofen (tBDMS) | NSAID | 14.65 | 311 | 295 | 105 |
| Bifenthrin | Pesticide | 14.80 | 181 | 165 | 166 |
| Fenpropathrin | Pesticide | 14.87 | 125 | 181 | 265 |
| Carbamazepine (tBDMS) | Antiepileptic | 14.95 | 193 | 194 | 293 |
| BDE-47 | Flame retardant | 15.16 | 486 | 326 | 488 |
| Fenofibrate | Lipid regulator | 15.19 | 121 | 273 | 139 |
| λ-Cyhalothrin | Pesticide | 15.24 | 197 | 181 | 208 |
| Permethrin | Pesticide | 15.74 | 183 | 163 | 165 |
| BPA (tBDMS) | Plasticizer | 15.70 | 441 | 442 | 456 |
| BDE-100 | Flame retardant | 15.92 | 404 | 406 | 566 |
| Cyfluthrin | Pesticide | 16.06 | 163 | 206 | 226 |
| Hexestrol (tBDMS) | Hormone | 16.35 | 249 | 250 | 337 |
| α-Cypermethrin | Pesticide | 16.41 | 163 | 165 | 181 |
| Diethylstilbestrol (tBDMS) | Hormone | 16.50 | 496 | 497 | 498 |
| τ-Fluvalinate | Pesticide | 17.09 | 250 | 252 | 181 |
| Estrone (tBDMS) | Hormone | 17.10 | 327 | 384 | 328 |
| Esfenvalerate | Pesticide | 17.20 | 125 | 167 | 181 |
| Deltamethrin | Pesticide | 17.74 | 181 | 253 | 251 |
| Chlorella spp. | Arthrospira platensis | |||||||
|---|---|---|---|---|---|---|---|---|
| 100 ng·g−1 | 25 ng·g−1 | LOD ng·g−1 | LOQ ng·g−1 | 100 ng·g−1 | 25 ng·g−1 | LOD ng·g−1 | LOQ ng·g−1 | |
| Methyl Paraben | 70 (8) | 82(8) | 0.4 | 1.2 | 76 (5) | 72 (9) | 1.8 | 3.6 |
| TCEP | 84 (4) | 80 (9) | 1.5 | 3.2 | 74 (3) | 94 (6) | 1.5 | 3.2 |
| TCPP | 94 (9) | 81 (8) | 0.8 | 2.4 | 100 (3) | 96 (9) | 0.9 | 1.7 |
| Ibuprofen | 100 (3) | 71 (3) | 0.8 | 2.7 | 70 (6) | 72 (5) | 1.3 | 2.6 |
| Propyl Paraben | 89 (3) | 80 (6) | 0.3 | 1.0 | 86 (2) | 80 (5) | 1.5 | 2.9 |
| Methyl Triclosan | 88 (3) | 74 (7) | 1.3 | 4.0 | 87 (4) | 83 (8) | 2.3 | 4.6 |
| Gemfibrozil | 86 (6) | 81 (5) | 0.5 | 1.5 | 77 (7) | 79 (10) | 0.8 | 2.6 |
| Nonylphenol | 87 (2) | 83 (8) | 0.4 | 1.5 | 70 (2) | 77 (5) | 1.3 | 2.6 |
| Fenoprofen | 78 (9) | 71 (3) | 0.9 | 2.6 | 72 (8) | 78 (2) | 2.1 | 4.1 |
| BP3 | 97 (4) | 98 (6) | 0.8 | 2.5 | 78 (7) | 80 (4) | 0.8 | 1.7 |
| Naproxen | 82 (8) | 98 (2) | 1.0 | 3.1 | 86 (3) | 79 (5) | 0.8 | 1.8 |
| Triclosan | 89 (4) | 84 (2) | 0.3 | 1.0 | 94 (11) | 80 (7) | 2.1 | 4.0 |
| Mefenamic acid | 91 (8) | 94 (8) | 0.4 | 1.4 | 70 (9) | 70 (3) | 1.3 | 2.8 |
| Ketoprofen | 73 (2) | 72 (2) | 0.3 | 1.0 | 75 (10) | 78 (8) | 0.7 | 1.9 |
| Bifenthrin | 86 (3) | 89 (7) | 0.4 | 1.3 | 94 (5) | 82 (5) | 0.3 | 1.2 |
| Fenpropathrin | 84 (3) | 89 (7) | 1.2 | 3.6 | 90 (6) | 73 (5) | 2.1 | 3.9 |
| Carbamazepine | 90 (2) | 83 (2) | 0.5 | 1.5 | 88 (3) | 75 (9) | 2.4 | 4.9 |
| BDE-47 | 76 (8) | 84 (9) | 0.7 | 2.5 | 89 (3) | 82 (6) | 1.3 | 4.5 |
| Fenofibrate | 83 (3) | 82 (8) | 1.0 | 2.7 | 89 (5) | 83 (5) | 1.8 | 3.9 |
| λ-Cyhalothrin | 83 (11) | 80 (5) | 0.9 | 3.0 | 85 (6) | 90 (11) | 1.9 | 3.9 |
| Permethrin | 83 (2) | 83 (8) | 1.1 | 3.6 | 91 (5) | 79 (6) | 1.6 | 4.8 |
| BPA | 77 (7) | 77 (4) | 1.5 | 3.2 | 107 (10) | 105 (3) | 1.0 | 1.9 |
| BDE-100 | 86 (2) | 80 (9) | 1.1 | 3.6 | 88 (4) | 79 (9) | 1.8 | 5.4 |
| Cyfluthrin | 80 (4) | 84 (7) | 1.0 | 3.1 | 87 (5) | 82 (8) | 1.2 | 3.6 |
| Hexestrol | 90 (0) | 80 (7) | 0.5 | 1.8 | 85 (3) | 78 (6) | 1.9 | 3.7 |
| α-Cypermethrin | 81 (3) | 79 (8) | 1.1 | 3.5 | 83 (4) | 87 (10) | 1.1 | 3.7 |
| Diethylstilbestrol | 97 (6) | 101 (2) | 0.3 | 1.0 | 111 (4) | 106 (6) | 0.7 | 2.1 |
| τ-Fluvalinate | 74 (3) | 85 (8) | 0.9 | 2.8 | 82 (6) | 86 (6) | 1.2 | 2.6 |
| Estrone | 103 (11) | 97 (9) | 0.5 | 1.8 | 95 (5) | 74 (10) | 1.2 | 3.1 |
| Esfenvalerate | 82 (11) | 74 (6) | 0.6 | 2.0 | 95 (6) | 89 (2) | 1.5 | 4.8 |
| Deltamethrin | 80 (5) | 73 (11) | 1.3 | 3.3 | 83 (7) | 95 (7) | 1.4 | 3.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Girela, I.; Albero, B.; Tiwari, B.K.; Miguel, E.; Aznar, R. Screening of Contaminants of Emerging Concern in Microalgae Food Supplements. Separations 2020, 7, 28. https://doi.org/10.3390/separations7020028
Martín-Girela I, Albero B, Tiwari BK, Miguel E, Aznar R. Screening of Contaminants of Emerging Concern in Microalgae Food Supplements. Separations. 2020; 7(2):28. https://doi.org/10.3390/separations7020028
Chicago/Turabian StyleMartín-Girela, Isabel, Beatriz Albero, Brijesh K. Tiwari, Esther Miguel, and Ramón Aznar. 2020. "Screening of Contaminants of Emerging Concern in Microalgae Food Supplements" Separations 7, no. 2: 28. https://doi.org/10.3390/separations7020028
APA StyleMartín-Girela, I., Albero, B., Tiwari, B. K., Miguel, E., & Aznar, R. (2020). Screening of Contaminants of Emerging Concern in Microalgae Food Supplements. Separations, 7(2), 28. https://doi.org/10.3390/separations7020028








