1. Introduction
The world is currently facing the new pandemic of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2, the cause of coronavirus disease 2019 (COVID-19)), for which no proven specific therapies are available so far, besides a few supportive cares. Indeed, in many countries worldwide, a large number of patients are receiving off-label and compassionate use therapies based on the administration of different drugs already authorized for other diseases, including chloroquine, hydroxychloroquine, azithromycin, lopinavir-ritonavir, favipiravir, baloxavir, oseltamivir, remdesivir, ribavirin, niclosamide, interferon, corticosteroids, tocolizumab, and anti–IL-6 inhibitors, as well as convalescent plasma. The administration of the above active pharmaceutical ingredients (APIs) is based on their antiviral or anti-inflammatory properties [
1,
2]. Except for a few randomized trials, these therapies are being given without the strict control required during the evaluation of new drug candidates or repurposed drugs. However, the prescription and preparation of all these medicines for off-label use are subjected to national regulatory aspects that all healthcare professionals should know and respect. Along this line, to address the current emergency caused by COVID-19, several initiatives have been undertaken at the regulatory level to promote and facilitate clinical trials and early access to drugs, whether these are old or new molecular candidates.
In this scenario, the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) has also allowed off-label use, at the expense of the National Health Service (NHS), of some medicines already authorized for the treatment of diseases other than COVID-19. Among the compassionate use programs of medicinal products approved by AIFA, there is ruxolitinib, in particular for patients diagnosed with COVID-19 and suffering from severe/very severe lung disease [
3,
4].
In response to the promising results so far achieved with the ruxolitinib-based therapies, Novartis and Incyte are intended to set-up a clinical study aimed at testing the potential of Jakavi
® (that is, the marketed drug with the API) in patients with COVID-19 associated cytokine storm. This is a type of severe immune overreaction that can be generated from the infection and may contribute to respiratory compromise in some patients [
5].
Ruxolitinib, (3
R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-l-yI]propanenitrile (
Figure 1), is a drug already in use for the treatment of intermediate or high-risk myelofibrosis, a type of bone marrow cancer, but is also administered for the conventional or experimental treatment of other types of cancer, including polycythemia vera and lymphomas (in particular, classical Hodgkin lymphoma), as well as for a growing number of various other indications like acute and chronic graft-versus-host disease after hematopoietic stem cell transplantation; hemophagocytic lymphohistiocytosis; plaque psoriasis; and, importantly, COVID-19.
In this context, considering that commercial tablets of ruxolitinib (Jakavi®) cost the same (at least in Italy) irrespective of the dosage strength (5 mg, 10 mg, 15 mg, or 20 mg), and considering that ruxolitinib dosing in patients varies based on the indication and on tolerance, it would be very useful and cost-effective to extemporaneously prepare personalized doses of the drug, addressing the individual therapeutic needs of a specific patient.
Thus, starting from commercial tablets of Jakavi® containing 20 mg of ruxolitinib, a customized pharmaceutical dosage form (namely, capsules) was compounded containing 5 mg of ruxolitinib (the lowest dose usable in the clinic). Indeed, this strategy would allow an optimal cost-saving tailoring of drug dosage in every patient depending on the disease type and potential adverse drug reactions (e.g., cytopenia) that frequently require dose reductions. Implementation of this strategy is contingent upon analytical methods for the accurate quantitative determination of this API.
In the last two decades, a series of important studies dealing with the dosage of ruxolitinib (among other tyrosine kinase inhibitors) in biological samples for pharmacokinetics and therapeutic drug monitoring evaluations were published [
6,
7,
8,
9,
10]. In all these studies, highly performing procedures of sample treatment and quantitative analysis were developed and described in detail. In these investigations, the quantification of ruxolitinib was performed by relying upon reversed-phase (RP) liquid chromatography analysis with different detection systems (including fluorescence, mass spectrometry). In all of the cases, assay validation procedures were performed according to international recommendations.
In contrast to the valuable bioanalytical investigations involving ruxolitinib, only few RP-HPLC methods have been developed so far for the quantitative analysis of ruxolitinib in marketed tablets [
11,
12]. However, these methods suffer from some major concerns, including the following: (i) the rather low retention time of the API, which can imply a reduced selectivity of the chromatographic system; (ii) the use of eluent components (such as tetrahydrofuran (THF) and orthophosphoric acid) scarcely compatible to completely incompatible with mass-spectrometry (MS) detectors; and (iii) the completely neglected the impact of excipients on the overall chromatographic performance.
In this paper, a new direct RP-HPLC method enabling the quantitative analysis of ruxolitinib in commercially available tablets and galenic capsules containing 20 and 5 mg of API, respectively, is described. For the present study, the highly economic and widely diffused UV/vis detector was used. All the weaknesses of the previous studies made by other authors have been conveniently overcome by the method proposed herein, which is based on the use of a simple eluent system and was mostly validated in accordance to the International Council on Harmonisation (ICH) guidelines, and applied for the accurate analysis of commercially available 20 mg containing API tablets and 5 mg containing galenically prepared capsules.
Furthermore, the application of the so-called “inverted chirality column approach (ICCA)” [
13,
14], performed with the Whelk-O1 chiral stationary phases (CSPs) under RP conditions, allowed us to demonstrate that the optical configuration at the asymmetric carbon atom in ruxolitinib is retained upon compounding the customized pharmaceutical dosage. To the best of our knowledge, this is the first paper describing the enantioselective liquid chromatography (LC) analysis of ruxolitinib.
The two chromatographic methods proposed in the present manuscript, along with the easy-to-perform extraction procedure of the API from the two formulations, can enter the frame of the quality control pipeline, assisting the production of ruxolitinib containing drugs for medical use.
2. Materials and Methods
2.1. Chemicals and Reagents
HPLC-grade ethanol (EtOH), methanol (MeOH), acetonitrile (ACN), and formic acid (FA) were purchased from VWR (Milan, Italy). Water for HPLC analysis was purified with a Milli-Q Plus 185 system from Millipore (Milford, MA, USA). The mobile phase components were degassed by sonication for 20 min before use. Highly pure (99.8%) ruxolitinib phosphate (from Axon Medchem BV, Groningen, The Netherlands) was used as standard during analytical validation.
Jakavi® (ruxolitinib) tablets labelled to contain 20 mg of ruxolitinib (as phosphate salt) were obtained from Novartis (Basel, Switzerland), while capsules containing 5 mg of API were provided by Hospital Pharmacy—A.O. Perugia—Ospedale S. Maria della Misericordia (Perugia, Italy).
2.2. Instrumentation and Chromatographic Conditions
Both the achiral and the chiral HPLC analyses were performed on a Shimadzu (Kyoto, Japan) LC-20A Prominence, equipped with a CBM-20A communication bus module, two LC-20 AD dual piston pumps, an SPD-M20A photodiode array detector, and a Rheodyne 7725i injector (Cotati, CA, USA) with a 20 μL stainless steel loop.
A Robusta C18 column (SepaChrom, Rho, Italy) 250 × 4.6 mm i.d., 5 μm, 100 Å pore size, was used as the analytical column for the RP achiral chromatography analyses.
For the enantioselective (chiral) HPLC analyses, the columns (
R,
R)-Whelk-O1 (CSP 1;
Figure S1, Supplementary Materials) and (
S,
S)-Whelk-O1 (CSP 2,
Figure S1, Supplementary Materials) (both 250 × 4.6 mm ID, 5 µm, 100 Å pore size; containing the 1-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene chiral selector (SO) motif; from Regis Technologies Inc. (Morton Grove, IL, USA)) were used.
For both the achiral and the chiral analyses, the employed column was conditioned at a 1.0 mL/min flow rate for at least 30 min before use, with the selected mobile phase. Column temperature was controlled through a Grace (Sedriano, Italy) heater/chiller (Model 7956R) thermostat. The injection volume was set at 20 µL, and the chromatographic analyses were followed at 227 nm. This wavelength of detection was selected by visual inspection of the UV/vis spectrum of ruxolitinib recorded with the Photodiode Array (PDA) detector during the chromatographic run with the optimized eluent system.
A mixture of water/ACN (70:30, v/v) was identified as the best mobile phase for the purpose of the study. For the achiral analysis, 0.1% v/v FA in both eluent components was added. The flow rate was set at 1.0 mL/min, while the column temperature was 25 °C.
After each analysis, the achiral C18 column was washed with a mixture of water/ACN/methanol (50:25:25, v/v/v) for 10 min at a flow rate of 1.5 mL/min. With this procedure, the possible occurrence of carry-over is completely abolished. For the same reason, the chiral columns were washed with EtOH for 15 min at a flow rate of 1.0 mL/min after each analysis.
In order to appraise the column performance, uracil from Sigma-Aldrich (Milano, Italy) was used as the unretained marker in all the achiral analyses, while the performance of the chiral analyses was appraised using the system peak at 2.5 min.
2.3. Method Validation
The developed HPLC method was validated mostly according to ICH guidelines [
15,
16,
17]. A “research validation” study was actually performed. The following parameters were assessed: specificity, linearity, limits of detection (LOD) and quantitation (LOQ), trueness and precision, and robustness and ruggedness. The accuracy profile was established as well.
The method was validated using the simplest and cheapest UV/visible detector. The specificity of the HPLC method was assessed for potential interferences in the drug formulations at the retention time of ruxolitinib (at around 8.1 min). With this aim, each excipient contained in ruxolitinib commercial tablets (colloidal anhydrous silica, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, type A sodium carboxymethyl starch, povidone) was treated with the selected eluent system to get a 1.0 mg/mL concentration. The resulting suspensions were filtered through 0.45 µm nylon syringe filters to eliminate the insoluble particles, and then analyzed via RP-HPLC according to the method reported in the Instrumentation and chromatographic conditions section.
One milligram of each excipient was separately weighted and then a homogeneous mixture of the entire pool was obtained in a mortar. In order to perform the quantitative analysis of ruxolitinib in the two drug formulations, a seven-point calibration curve was used. The curve was built up with solutions prepared by diluting the standard stock solution (0.2 mg/mL) with the eluent system to obtain concentrations corresponding to 0.024, 0.04, 0.06, 0.10 (actually, 0.096), 0.11, 0.12, and 0.144 mg/mL of ruxolitinib. A 0.12 mg/mL target test concentration was chosen. In order to better simulate the matrix, one milligram of the excipients’ mixture was added to each calibration solution. Before analysis, the six resulting suspensions were sonicated for 5 min and then filtered through a 0.45 µm nylon syringe filter to eliminate the insoluble particles of excipients. Three independent replicates were performed at each concentration level.
For the LOD estimation, a signal-to-noise (s/n) ratio of 3:1 was considered. Accordingly, LOD was considered as the ruxolitinib concentration, producing a three-times higher area value than the system noise. Instead, the LOQ value was assessed considering the minimum concentration in which the precision (measured as coefficient of variation, CV) was lower than 5.0%. Both LOD and LOQ were determined using filtered API solutions prepared in the eluent systems added with the surrogate matrix.
Trueness and precision (repeatability and intermediate precision) were assessed carrying out three independent determinations on three different days at three concentration levels within the calibration range (40, 100, and 120% of the selected test concentration). These solutions were prepared using the surrogate matrix described previously. The three resulting suspensions were sonicated for 5 min to disintegrate the solid particles and then filtered through a 0.45 µm nylon syringe filter to eliminate the insoluble excipient. As a part of the precision evaluation, the robustness and ruggedness of the method were also investigated. As far as the robustness is concerned, two parameters were deliberately varied: the eluent flow rate and the column temperature. In order to evaluate the effect of subtle variations of the mobile phase velocity on the peak area value produced by the analysis of a 0.12 mg/mL ruxolitinib solution, three flow rate levels were compared: 0.9, 1.0, and 1.1 mL/min. Instead, the effect played by minimal changes of column temperature was appraised at 24, 25, and 26 °C. In order to investigate the ruggedness of the method, the difference in the analytical outcome from analyst to analyst was evaluated at three concentration levels of ruxolitinib (0.04, 0.10 (actually, 0.096), and 0.12 mg/mL). Nine analyses were performed interchangeably by two analysts over three consecutive days. These two last parameters were evaluated by injecting ruxolitinib phosphate standard solutions.
Analyzing the solutions of standard ruxolitinib phosphate with and without excipients at the test concentrations revealed that co-formulants do not affect the peak area value (>99% of similarity).
2.4. Analysis of Tablets and Capsules
Eight tablets labelled to contain 20 mg of ruxolitinib (as phosphate) were blended in the mortar. An aliquot of powder was weighted and transferred into a volumetric flask, and mobile phase was added until the expected concentration (0.12 mg/mL of API) was reached. The resulting mixture was sonicated for 5 min, filtered through a 0.45 µm nylon filter, and finally analyzed according to the experimental conditions reported in the Instrumentation and chromatographic conditions section. Similarly, 25 capsules containing 5 mg of ruxolitinib were opened, the powder was transferred into a volumetric flask, and the selected mobile phase was added until the expected test concentration (0.12 mg/mL of API) was obtained. Before the HPLC analysis, the resulting mixture was sonicated for 5 min, and then filtered through a 0.45 µm nylon filter.
3. Results and Discussion
The first experimental step was to develop an RP-HPLC method able to efficiently analyze the API of interest in the two formulations under investigation. Accordingly, a limited number of isocratic analyses conditions were preliminarily screened, mostly addressing the attention to identify the experimental setting providing satisfactory system suitability parameters. The following parameters were iteratively evaluated according to an “one-variable-at-time, OVAT” approach, although the method under investigation could also be profitably optimized by applying dedicated design of experiment (DoE) procedures [
18]: water-to-ACN ratio, FA concentration, column temperature, and flow-rate. Prior to obtaining results on the real samples, system suitability was evaluated analyzing a standard solution of ruxolitinib at the test concentration. All the system suitability parameters were found to be satisfactory [
19] with the optimized experimental conditions reported in the Instrumentation and chromatographic conditions section: tailing factor, in the range 1.7–2.0 (acceptance criteria ≤ 2.0); retention factor, 2.2 (acceptance criteria ≥ 2.0); number of theoretical plates, >9000 (acceptance criteria ≥ 2000).
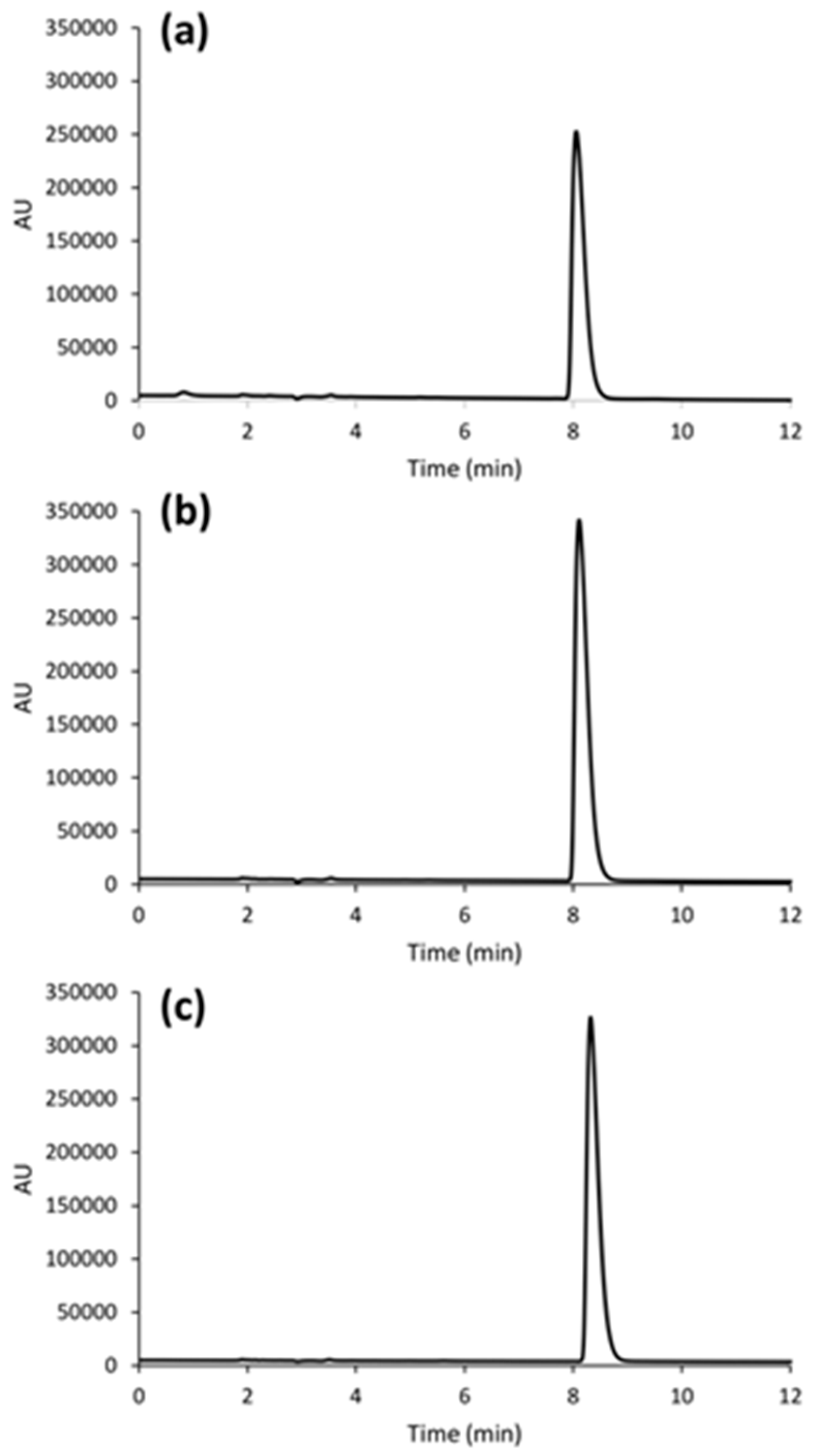
The chromatogram of a ruxolitinib phosphate standard solution is shown in
Figure 2a. With the optimized eluent conditions, a retention time of about 8.1 min was obtained for the analyte of interest. To have a good analyte retention is of prime importance to enhance the selectivity of the chromatographic system.
The chromatograms shown in
Figure 2 were obtained with the optimized conditions reported in the Instrumentation and chromatographic conditions section. The analysis of the ruxolitinib phosphate standard solution (
Figure 2a) was performed after the filtration of the surrogate matrix components, according to the procedure reported in Method validation section.
3.1. Method Validation
The developed analytical method was validated according to ICH guidelines [
15,
16,
17], aiming at performing a reliable “research validation” designed on purpose for this specific method application.
Method specificity was evaluated by injecting the individual solution of each excipient present in the formulation prepared as described in the Method validation section. None of the peaks corresponding to excipients were detected at the retention time of ruxolitinib phosphate. The chromatograms related to the excipients’ analysis are reported as
Supplementary Materials (Figure S2).
In order to establish the range of the developed chromatographic method, a rather extended concentration interval was investigated, spanning from 5% (0.006 mg/mL) to about 140% (0.17 mg/mL) of the selected test concentration (0.12 mg/mL) (
Table S1). For all ten tested concentrations, the coefficient of variation (CV%) values were calculated on the peak area values and then, based on the obtained results, a more restricted concentration range (that is, the linearity range made up with seven points in the 0.024–0.144 mg/mL interval; 20–120% of the selected test concentration) was selected to build up the mathematical model for the quantitative analyses.
Either considering all the 10 concentration levels or 7 of them, regression lines with R
2 equal to 0.99 were obtained (this was also the case for the R
2 value computed with the leave-one-our procedure—R
2xv). However, for all seven concentration levels, CV% ≤ 2.0 was computed, while higher values turned out for the more external concentrations (0.006 mg/mL, 0.16 mg/mL, and 0.17 mg/mL). The CV%-concentration plot is shown in
Figure S3.
By using the least squares method, the seven-point calibration curve, y = 50,907,123 (±1,256,761) + 148,530 (±119,637), was built by plotting the peak area values versus the corresponding analyte concentration.
Solutions of ruxolitinib phosphate with concentrations lower than 0.004 mg/mL were then analysed, which allowed us to establish an LOQ equal to 0.002 mg/mL (CV% of 5.2).
In order to appraise the LOD, the signal-to-noise (S/N) value has been firstly determined. Accordingly, a solution of the sole eluent system was injected and twenty “base-line” peaks were integrated. As a result, considering a 3× S/N value, a 7 ng/mL LOD was estimated. LOD and LOQ values can be considered satisfactory, as they are suitable for the proposed method application. In order to evaluate the accuracy and precision of the developed method, three concentration levels at 40% (0.045 mg/mL), 100% (0.12 mg/mL), and 120% (0.144 mg/mL) of the selected test concentration were analysed, and the results are summarized in
Table 1.
As reported in
Table 1, trueness (expressed as recovery) was between 96.3 and 106.3%. With regard to method precision, CV% values (repeatability: CV
w and intermediate precision: CV
IP) were 1.3% and 2.9% or lower, respectively. More details are reported as
Supplementary Materials (Table S2).
Both robustness and ruggedness results indicated that the analysis was not affected by experiments performed in different conditions and by two different analysts. Indeed, the peak area values experienced only marginal variations for both parameters. The method was found to be robust and rugged, as indicated by the CV% values below 1.6% and 3.1%, respectively (
Tables S3 and S4).
As one can note, all validation assays provided satisfactory results and the method can thus be considered suitable for application to ruxolitinib analysis in drug formulations.
3.2. Application of the Developed Method to Drug Formulations Analysis
The developed method was finally applied to analyze commercially available ruxolitinib containing tablets, and capsules prepared as described in the Analysis of tablets and capsules section. The chromatograms related to solutions prepared with the two formulations are shown in
Figure 2b,c, respectively. Importantly, system suitability parameters according to the acceptance criteria were also maintained for the ruxolitinib phosphate peak during the analysis of the medicinal products.
The total content of ruxolitinib in tablets was found to be 20.0 ± 0.9 mg, while that of capsules was equal to 5.0 ± 0.1 mg. Both values are in line with our expectation, thus indicating a good compounding procedure and quality of the developed RP-HPLC method.
3.3. Enantioselective Analysis
In order to evaluate the accidental racemization of ruxolitinib during drug compounding and reformulation, an enantioselective LC method was developed and applied on both drug formulations. With this aim, the two well-known Pirkle-type Whelk-O1 CSPs (
Figure S1, Supplementary Materials) operating according to a donor-acceptor mechanism were employed. The two CSPs carry totally synthetic enantiomeric chiral selectors and are specific for the analysis of compounds with aromatic moieties [
14,
20].
Whelk-O1 columns are multimodal, as analyses with all the most relevant elution regimes for liquid chromatography applications can be performed. However, these CSPs are typically used under NP conditions, while the RP mode selected for the present study is only barely exploited.
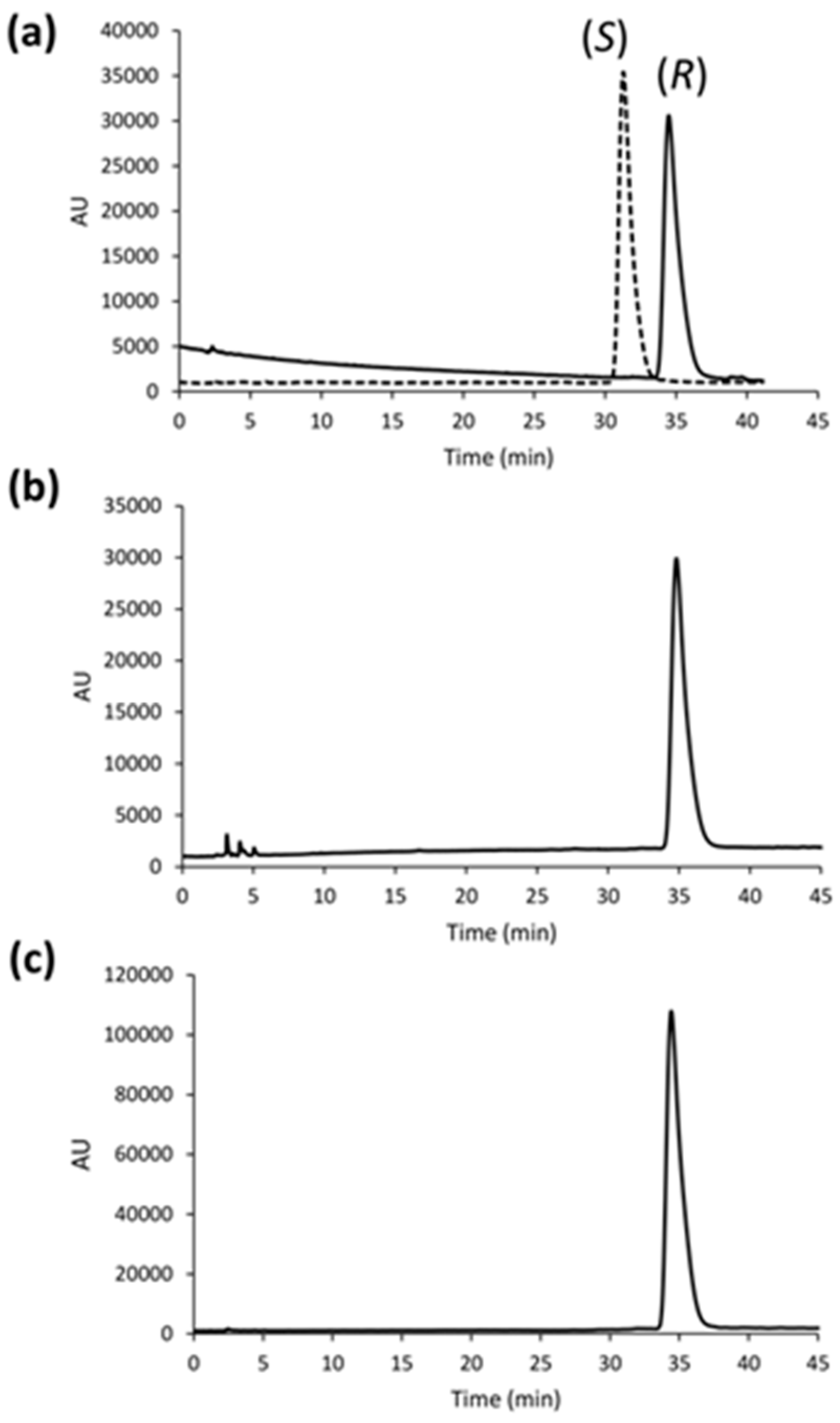
With the ICCA method [
13,
14], the use of the two CSPs with enantiomeric chiral selectors makes it possible to generate a “virtual” racemate (
Figure 3a), thus allowing the evaluation of the enantiomeric composition in a given sample without the need for pure enantiomeric standards. Indeed, running the analysis under the same experimental conditions, an inversion of the enantiomeric elution order (EEO) is produced by switching the absolute configuration of the chiral selector from CSP 1 to CSP 2. As a result, we found that ruxolitinib was enantiomerically pure in the commercialized tablets (
Figure 3b), and also retained this feature in the deriving capsules (
Figure 3c). The chromatographic runs were carried out with the optimized conditions reported in the Instrumentation and chromatographic conditions section, while the analysis of the ruxolitinib phosphate in the tablet and capsule was performed after the filtration of the excipients with 0.45 µm nylon syringe filters.
The rather long run time of enantioselective analysis is only an apparent drawback because chiral columns of smaller dimension and particle sizes are commercially available, thus giving the possibility to shorten the enantiomers retention time without compromising the chromatographic performance as a whole.
4. Conclusions
Only a few RP-HPLC methods have been proposed so far for the quantitative analysis of ruxolitinib in approved marketed tablets. However, these methods suffer from some serious concerns, including the following: (i) the scarce system selectivity owing to the extremely low retention time of the API, (ii) the use of eluent components to not allow a facile coupling of the chromatographic system to MS-based detectors, and (iii) the completely neglected effect exerted by the excipients’ pool on the overall chromatographic performance. All these weaknesses have been addressed in the present contribution. Indeed, all the system suitability parameters were within the acceptance criteria, which allowed the method to be validated mostly according to the ICH guidelines.
The good statistical quality of the developed HPLC method allowed us to demonstrate its suitability for the quantitative determination of ruxolitinib in two drug formulations: commercially available 20 mg API tablets, and not commercially available galenic formulations (5 mg API capsules).
The proposed method was found to be simple, rapid, sensitive, precise, cost-effective, and accurate, and will be applied to evaluate the forthcoming stress-test as well as stability testing required by regulatory agencies before the release of clinical batches as part of the clinical trials.
Furthermore, an original enantioselective RP-HPLC-UV method based on the use of the Whelk-O1 CSPs and the ICCA method was developed and applied to demonstrate that the enantiomeric purity of ruxolitinib in commercial tablets is retained in the galenic capsules after compounding and reformulation.
In conclusion, the two chromatographic methods proposed herein, coupled to the easy-to-perform extraction procedure of the API from the two formulations, can be conveniently used in the quality control pipeline, assisting the production of ruxolitinib containing drugs.