Non-Targeted Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) Fingerprints for the Chemometric Characterization and Classification of Turmeric and Curry Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. UHPLC-HRMS Instrumentation
2.3. Samples and Sample Treatment
2.4. Data Analysis
3. Results and Discussion
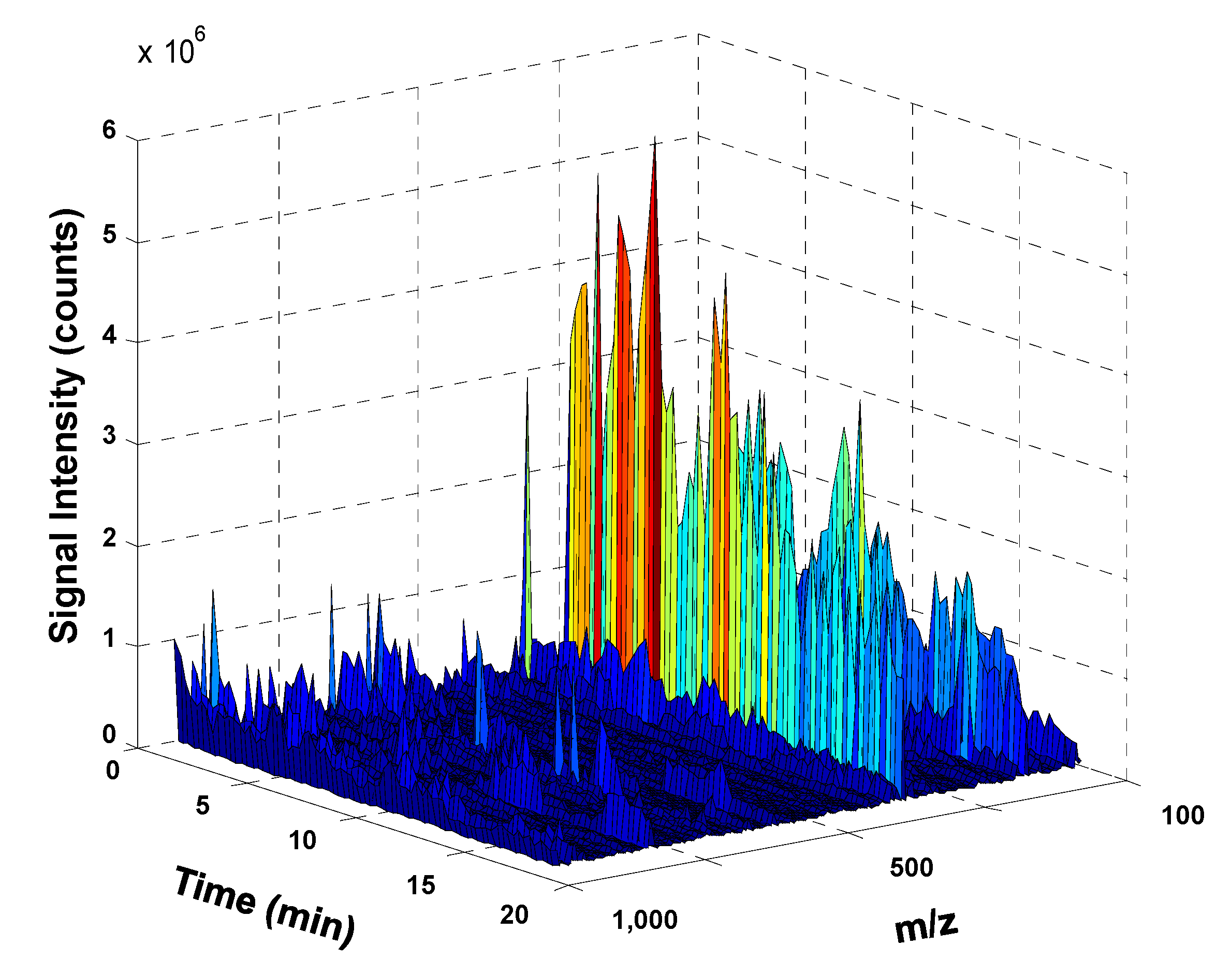
3.1. Non-Targeted UHPLC-HRMS Fingerprinting
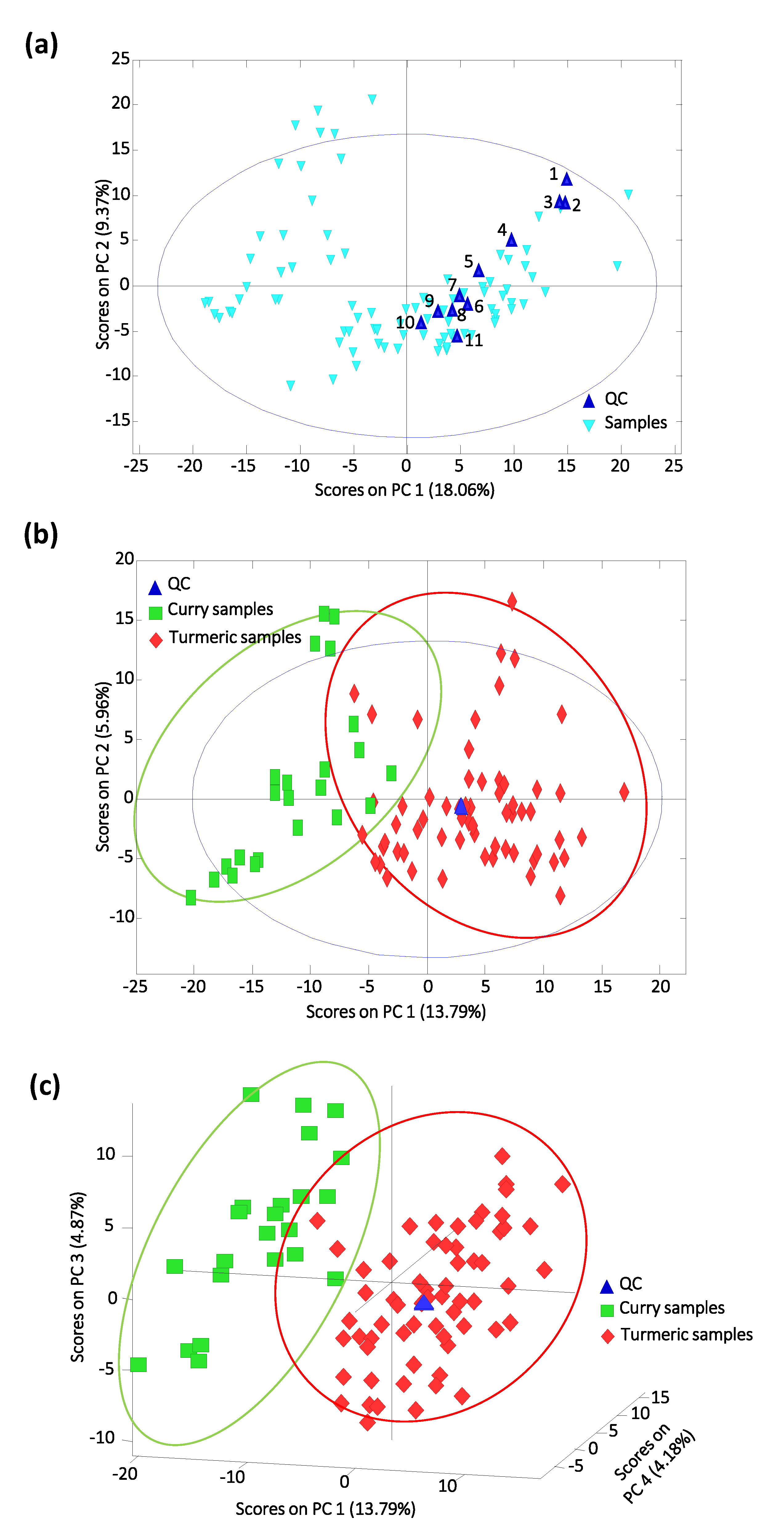
3.2. PCA Study
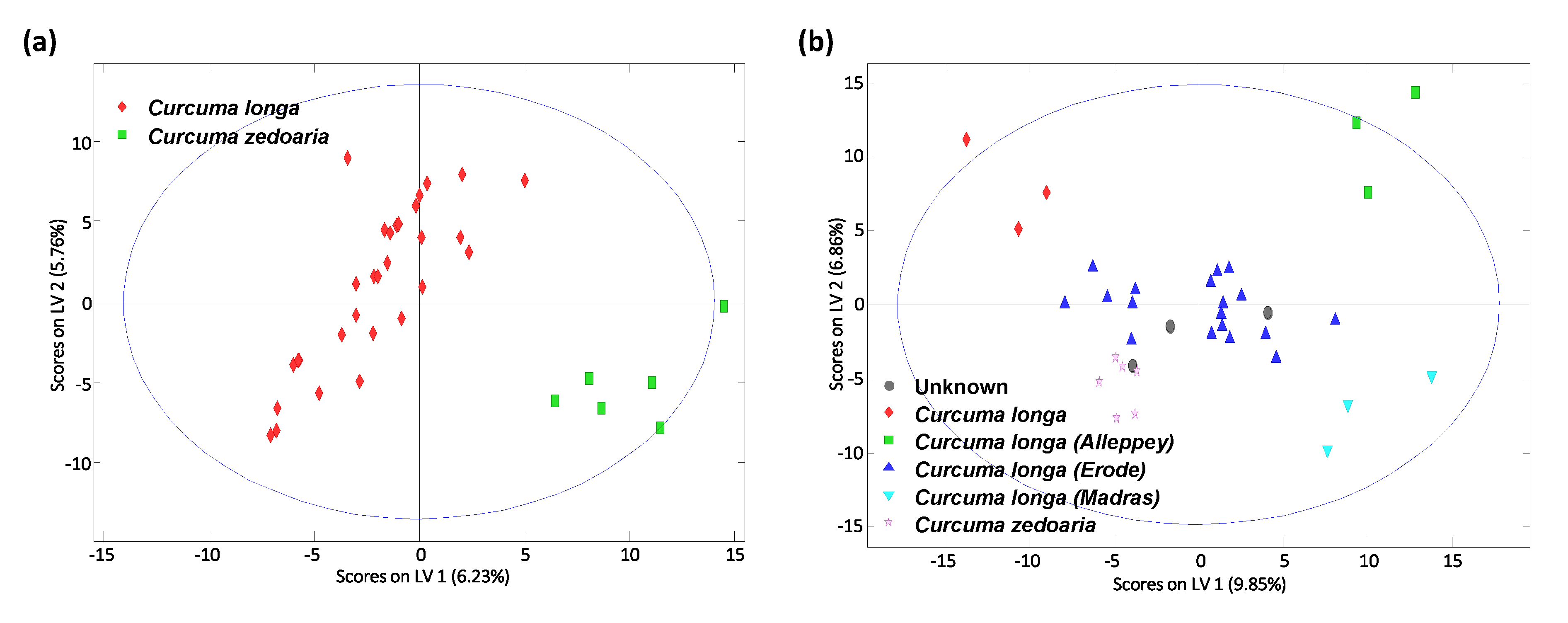
3.3. Sample Classification by PLS-DA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Carolina Alves, R.; Perosa Fernandes, R.; Fonseca-Santos, B.; Damiani Victorelli, F.; Chorilli, M. A critical review of the properties and analytical methods for the determination of curcumin in biological and pharmaceutical matrices. Crit. Rev. Anal. Chem. 2019, 49, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.R.; Parnell, L.D.; Ordovas, J.M.; Lai, C.Q. Curcumin and aging. BioFactors 2013, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin nanoformulations for colorectal cancer: A review. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Clifton, P. Curcumin, cardiometabolic health and dementia. Int. J. Environ. Res. Public Health 2018, 15, 2093. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, Y.; Li, H. Bin Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin: An effective or deceptive dietary factor? Challenges for functional food scientists. J. Agric. Food Chem. 2018, 66, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A critical review of analytical methods for determination of curcuminoids in turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Bouzabata, A. Application of chemometrics in quality control of Turmeric (Curcuma longa) based on Ultra-violet, Fourier transform-infrared and 1H NMR spectroscopy. Food Chem. 2017, 237, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Saleem, M.; Atta, B.M.; Khan, S.S.; Hammad, G. Determination of curcuminoid content in turmeric using fluorescence spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lestari, H.P.; Martono, S.; Wulandari, R.; Rohman, A. Simultaneous analysis of Curcumin and demethoxycurcumin in Curcuma xanthorriza using FTIR spectroscopy and chemometrics. Int. Food Res. J. 2017, 24, 2097–2101. [Google Scholar]
- Chao, K.; Dhakal, S.; Schmidt, W.F.; Qin, J.; Kim, M.; Peng, Y.; Huang, Q. Raman and IR spectroscopic modality for authentication of turmeric powder. Food Chem. 2020, 320, 126567. [Google Scholar] [CrossRef] [PubMed]
- Windarsih, A.; Rohman, A.; Swasono, R.T. Application of 1H-NMR based metabolite fingerprinting and chemometrics for authentication of Curcuma longa adulterated with C. heyneana. J. Appl. Res. Med. Aromat. Plants 2019, 13, 100203. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Quan, F.; Chen, P.; Qian, J.; Zhou, L.; Pu, Q. Sensitive analysis of curcuminoids via micellar electrokinetic chromatography with laser-induced native fluorescence detection and mixed micelles-induced fluorescence synergism. J. Chromatogr. A 2018, 1564, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, R.; Yang, F.; Xiao, W.; Chen, C.; Xia, Z. Determination of three curcuminoids in Curcuma longa by microemulsion electrokinetic chromatography with protective effects on the analytes. Anal. Methods 2014, 6, 2566–2571. [Google Scholar] [CrossRef]
- Anubala, S.; Sekar, R.; Nagaiah, K. Determination of Curcuminoids and Their Degradation Products in Turmeric (Curcuma longa) Rhizome Herbal Products by Non-aqueous Capillary Electrophoresis with Photodiode Array Detection. Food Anal. Methods 2016, 9, 2567–2578. [Google Scholar] [CrossRef]
- Vidal, Ò.; Castilla, X.; Aliaga-Alcalde, N.; López-Periago, A.M.; Domingo, C.; Sentellas, S.; Saurina, J. Determination of curcuminoids by liquid chromatography with diode array detection: Application to the characterization of turmeric and curry samples. Curr. Anal. Chem. 2018, 16, 95–105. [Google Scholar] [CrossRef]
- Vidal-casanella, O.; Nuñez, N.; Sentellas, S.; Oscar, N. Characterization of turmeric and curry samples by liquid chromatography with spectroscopic detection based on polyphenolic and curcuminoid contents. Separations 2020, 7, 23. [Google Scholar] [CrossRef]
- Chao, I.C.; Wang, C.M.; Li, S.P.; Lin, L.G.; Ye, W.C.; Zhang, Q.W. Simultaneous quantification of three curcuminoids and three volatile components of curcuma longa using pressurized liquid extraction and high-performance liquid chromatography. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Manap, M.Y.A.; Khatib, A.; Razis, A.F.A. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Hiserodt, R.; Hartman, T.G.; Ho, C.T.; Rosen, R.T. Characterization of powdered turmeric by liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry. J. Chromatogr. A 1996, 740, 51–63. [Google Scholar] [CrossRef]
- Singh, S.; Rajesh, B.S.S.; Sahoo, K.; Subudhi, E.; Nayak, S. Chemical composition of turmeric oil (Curcuma longa L. cv. Roma) and its antimicrobial activity against eye infecting pathogens. J. Essent. Oil Res. 2011, 23, 11–18. [Google Scholar] [CrossRef]
- Kulyal, P.; Kuchibhatla, L.N.; Uma Maheshwari, K.; Nirmal Babu, K.; Tetali, S.D.; Raghavendra, A.S. Highly sensitive HPLC method for estimation of total or individual curcuminoids in Curcuma cultivars and commercial turmeric powders. Curr. Sci. 2016, 111, 1816–1824. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Alvarenga, J.F.R.; Martinez-Huelamo, M.; Leal, L.N.; Lamuela-Raventos, R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Jia, S.; Du, Z.; Song, C.; Jin, S.; Zhang, Y.; Feng, Y.; Xiong, C.; Jiang, H. Identification and characterization of curcuminoids in turmeric using ultra-high performance liquid chromatography-quadrupole time of flight tandem mass spectrometry. J. Chromatogr. A 2017, 1521, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Jude, S.; Amalraj, A.; Kunnumakkara, A.B.; Divya, C.; Löffler, B.M.; Gopi, S. Development of validated methods and quantification of curcuminoids and curcumin metabolites and their pharmacokinetic study of oral administration of complete natural turmeric formulation (CureitTM) in human plasma via UPLC/ESI-Q-TOF-MS spectrometry. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, K.; Mujeeb, M.; Ahmad, A.; Ahmad, N.; Amir, M. Determination of curcuminoids in curcuma longa linn. by UPLC/Q-TOF-MS: An application in turmeric cultivation. J. Chromatogr. Sci. 2015, 53, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Rodríguez, L.; Ruiz-Samblás, C.; Valverde-Som, L.; Pérez-Castaño, E.; González-Casado, A. Chromatographic fingerprinting: An innovative approach for food “identitation” and food authentication—A tutorial. Anal. Chim. Acta 2016, 909, 9–23. [Google Scholar] [CrossRef]
- Campmajó, G.; Núñez, N.; Núñez, O. The role of liquid chromatography-mass spectrometry in food integrity and authenticity. In Mass Spectrometry—Future Perceptions and Applications; Kamble, G.S., Ed.; IntechOpen: London, UK, 2019; pp. 3–20. ISBN 978-953-51-7845-3. [Google Scholar]
- Eigenvector Research Incorporated. Powerful Resources for Intelligent Data Analysis. Available online: https://eigenvector.com/software/solo/ (accessed on 7 May 2020).
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]



| Time (min) | Acetonitrile (%) | Elution Mode |
|---|---|---|
| 0 | 10 | Initial conditions |
| 1 | 10 | Isocratic step |
| 20 | 95 | Linear gradient |
| 23 | 95 | Isocratic step |
| 24 | 10 | Linear gradient |
| 30 | 10 | Isocratic step for column re-equilibration |
| Sample | Commercial Brand | Number of Samples | Number of Extracts | Compositional Characteristics |
|---|---|---|---|---|
| Turmeric | Hacendado | 5 | 15 | Curcuma longa (Erode) |
| MG | 1 | 3 | Curcuma longa (Alleppey) | |
| Burriac | 1 | 3 | Curcuma longa | |
| Carmencita | 1 | 3 | Curcuma longa (Erode) | |
| Ducros | 1 | 3 | Curcuma longa | |
| Artemis Bio | 1 | 3 | Curcuma longa | |
| Natco | 1 | 3 | Curcuma longa | |
| Pelotari | 1 | 3 | Unknown | |
| Dani | 2 | 6 | Curcuma zedoaria | |
| Especies | 1 | 3 | Curcuma longa (Alleppey) | |
| Ocena | 1 | 3 | Curcuma longa (Madras) | |
| Tata Sampann | 1 | 3 | Unknown | |
| Herbalist | 1 | 3 | Curcuma longa (Madras) | |
| Street market | 1 | 3 | Curcuma longa (Madras) | |
| Biospirit | 1 | 3 | Curcuma longa | |
| NAAI | 1 | 3 | Unknown | |
| Curry | Hacendado | 2 | 6 | White pepper, coriander, ginger, cardamom, clove, cinnamon, anise, mustard |
| Carrefour | 1 | 3 | Pepper, coriander, ginger, cumin, fenugreek, laurel, fennel, mustard | |
| Species Kania | 1 | 3 | Pepper, coriander, cumin, fenugreek, parsley, chili, garlic, fennel | |
| Condis | 1 | 3 | Pepper, coriander, fennel, cumin, cayenne, garlic, anise | |
| Burriac | 1 | 3 | White pepper, coriander, ginger, cardamom, clove, cinnamon, anise, mace | |
| Eroski | 1 | 3 | Coriander, cardamom, ginger, fenugreek, anise, garlic, clove, mustard | |
| Ducros | 1 | 3 | Pepper, coriander, cumin, ginger, laurel, anise, garlic, clove, cinnamon, mace | |
| Street market | 1 | 3 | Unknown |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez, N.; Vidal-Casanella, O.; Sentellas, S.; Saurina, J.; Núñez, O. Non-Targeted Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) Fingerprints for the Chemometric Characterization and Classification of Turmeric and Curry Samples. Separations 2020, 7, 32. https://doi.org/10.3390/separations7020032
Núñez N, Vidal-Casanella O, Sentellas S, Saurina J, Núñez O. Non-Targeted Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) Fingerprints for the Chemometric Characterization and Classification of Turmeric and Curry Samples. Separations. 2020; 7(2):32. https://doi.org/10.3390/separations7020032
Chicago/Turabian StyleNúñez, Nerea, Oscar Vidal-Casanella, Sonia Sentellas, Javier Saurina, and Oscar Núñez. 2020. "Non-Targeted Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) Fingerprints for the Chemometric Characterization and Classification of Turmeric and Curry Samples" Separations 7, no. 2: 32. https://doi.org/10.3390/separations7020032
APA StyleNúñez, N., Vidal-Casanella, O., Sentellas, S., Saurina, J., & Núñez, O. (2020). Non-Targeted Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) Fingerprints for the Chemometric Characterization and Classification of Turmeric and Curry Samples. Separations, 7(2), 32. https://doi.org/10.3390/separations7020032








