Evaluation of the Performance of Cryogen-Free Thermal Modulation-Based Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC×GC-TOFMS) for the Qualitative Analysis of a Complex Bitumen Sample
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
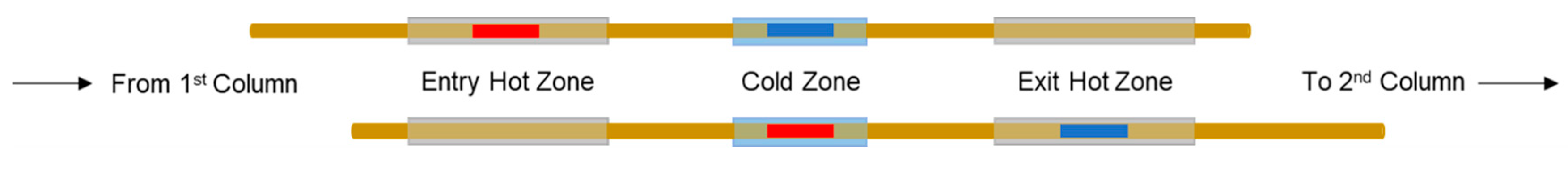
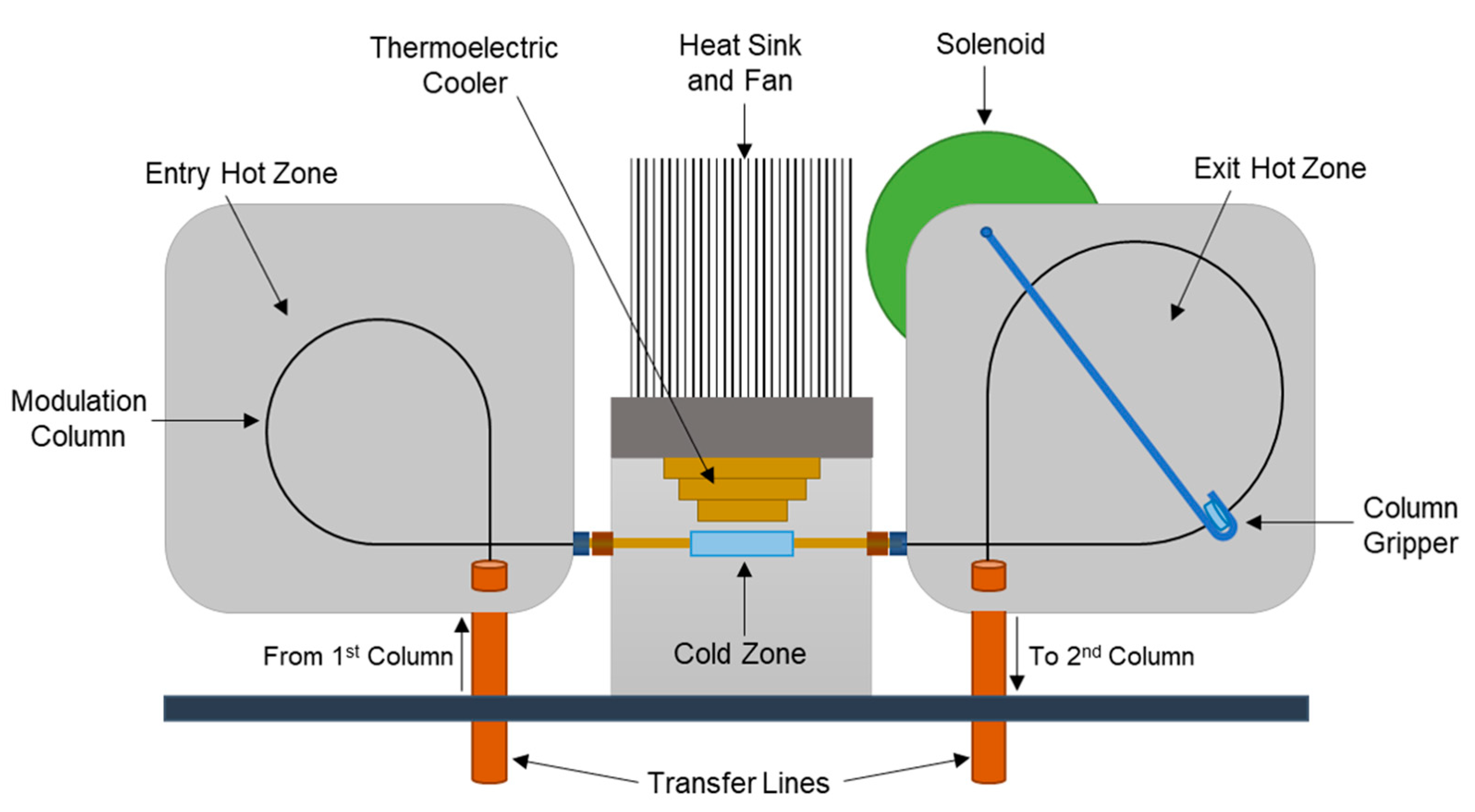
2.2. Solid-State Modulation
2.3. Analysis Conditions
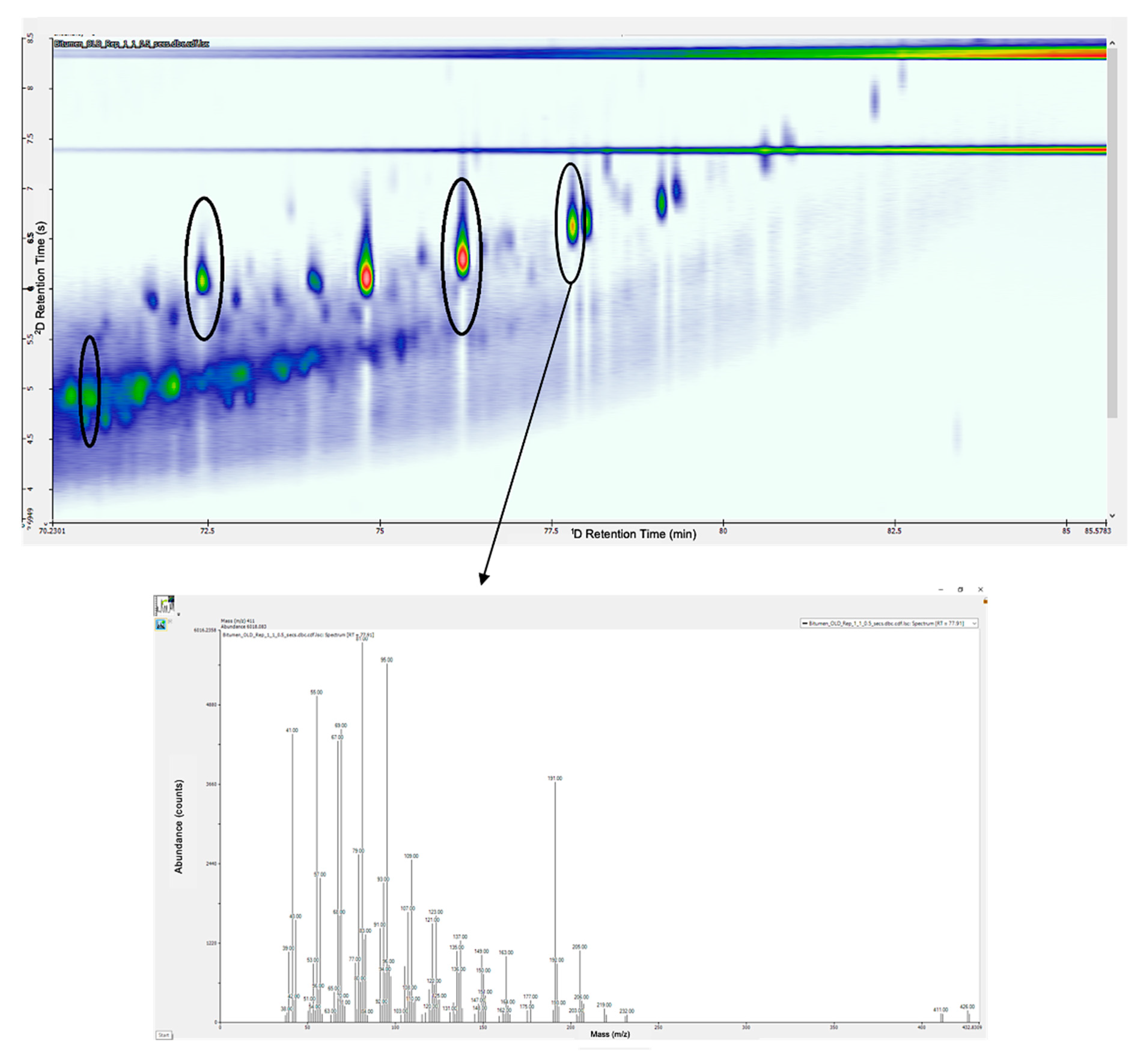
3. Results and Discussion
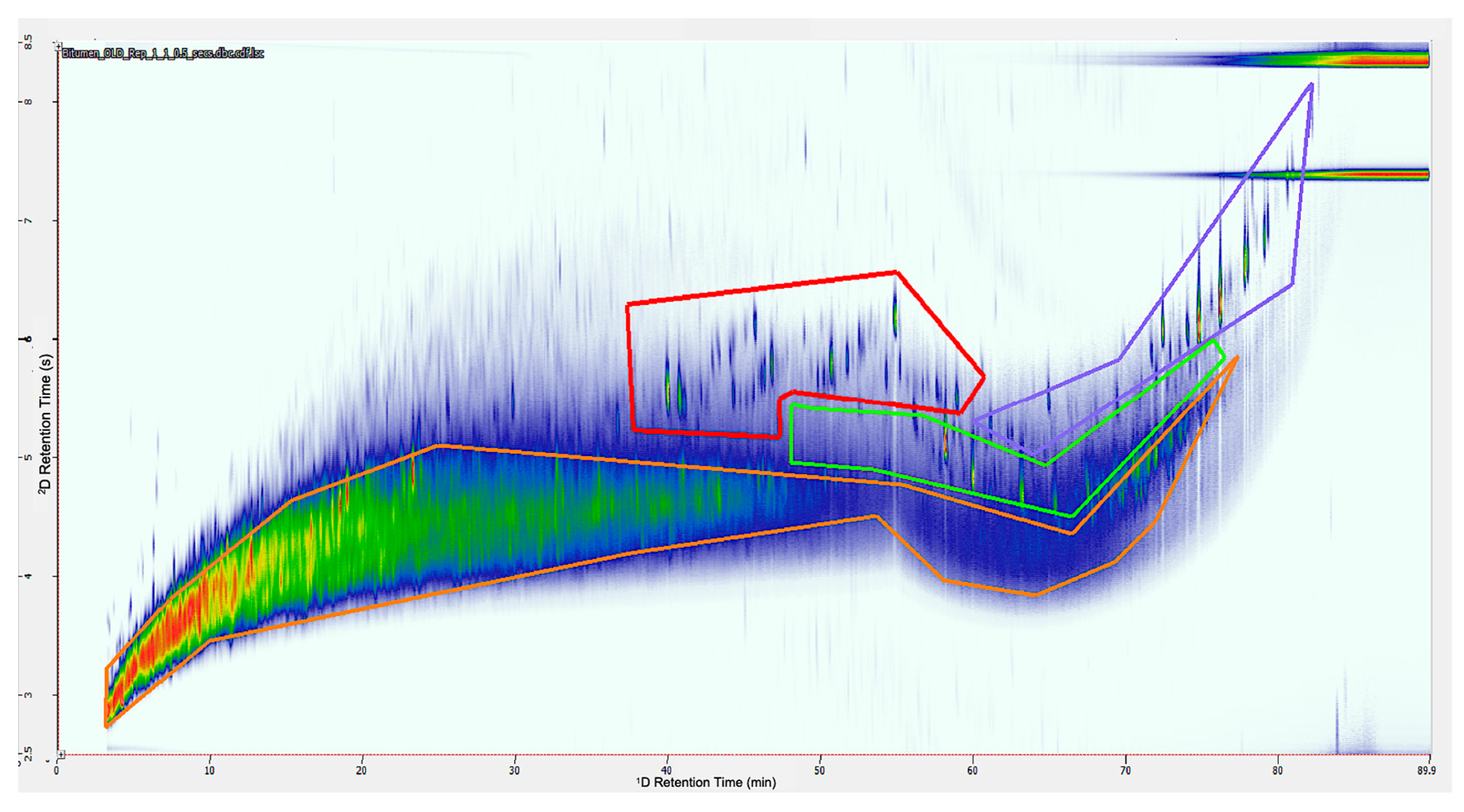
3.1. Group-Type Identification
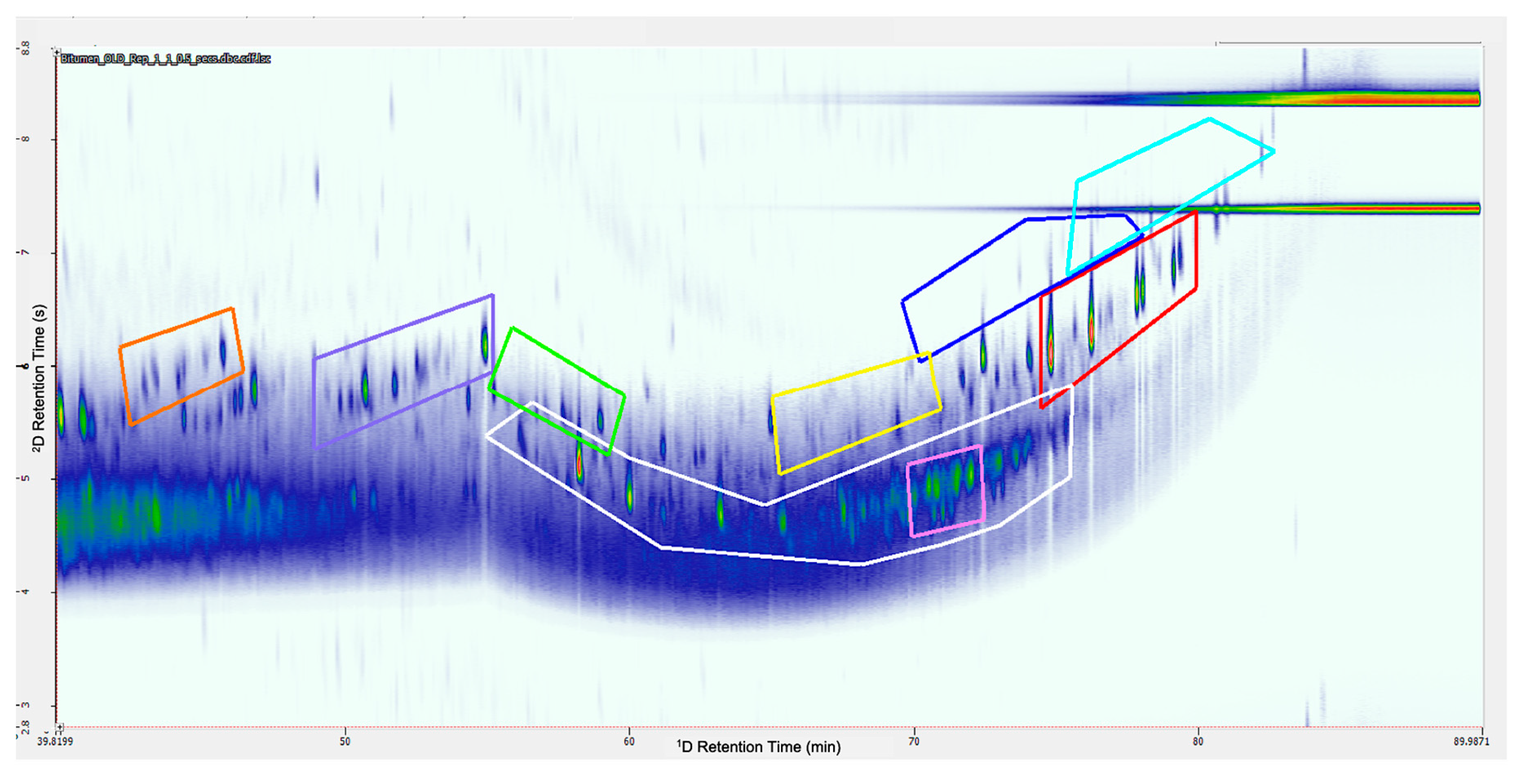
3.2. Biomarker Analysis
3.3. Biomarker Identification
3.4. Evaluation of Modulator Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afework, B.; Hanania, J.; Jenden, J.; Stenhouse, K.; Donev, J. Energy Education-Bitumen, University of Calgary. 2018. Available online: https://energyeducation.ca/encyclopedia/Bitumen (accessed on 30 June 2019).
- Canada’s Oil & Natural Gas Producers. Oil Sands. Available online: https://www.capp.ca/canadian-oil-and-natural-gas/oil-sands (accessed on 30 June 2019).
- Aguiar, A.; Silva Junior, A.I.; Azevedo, D.A.; Aquino Neto, F.R. Application of comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry to biomarker characterization in Brazilian oils. Fuel 2010, 89, 2760–2768. [Google Scholar] [CrossRef]
- Aguiar, A.; Aguiar, H.G.M.; Azevedo, A.; Aquino Neto, F.R. Identification of Methylhopane and Methylmoretane series in Ceara Basin Oils, Brazil, using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. Energy Fuels 2011, 25, 1060–1065. [Google Scholar] [CrossRef]
- Silva, R.S.; Aguiar, H.G.; Rangel, M.D.; Azevedo, D.A.; Awuino Neto, F.R. Comprehensive two-dimensional gas chromatography with time of flight mass spectrometry applied to biomarker analysis of oils from Colombia. Fuel 2011, 90, 2694–2699. [Google Scholar] [CrossRef]
- Forsythe, J.C.; Omerantz, A.E.; Seifert, D.J.; Wang, K.; Chen, Y.; Zuo, Y.; Nelson, R.K.; Reddy, C.M.; Schimmelmann, A.; Sauer, P.; et al. A geological model for the origin of fluid compositional gradients in a large Saudi Arabian oilfield: An investigation by two-dimensional gas chromatography (GC×GC) and asphaltene chemistry. Energy Fuels 2015, 29, 5666–5680. [Google Scholar] [CrossRef]
- J&X Technologies. Analysis of the Chemical Composition of Kerosene by GC×GC-FID (Document No. E103); J&X Technologies: Shanghai, China, 2018. [Google Scholar]
- J&X Technologies. Group Analysis of Light Cycle Oil (LCO) by Comprehensive Two-Dimensional Gas Chromatography (Document No. E101); J&X Technologies: Shanghai, China, 2018. [Google Scholar]
- J&X Technologies. Group Analysis of Diesel by Comprehensive Two-Dimensional Gas Chromatography (Document No. E102); J&X Technologies: Shanghai, China, 2018. [Google Scholar]
- Zoccali, M.; Giocastro, B.; Tranchida, P.Q.; Mondello, L. Use of a recently developed thermal modulator within the context of comprehensive two-dimensional gas chromatography combined with time-of-flight mass spectrometry: Gas flow optimization aspects. J. Sep. Sci. 2019, 42, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, C.; Zhao, R.; Zhu, C.; Yang, C.; Liu, C. Solvent extraction of bitumen from oil sands. Energy Fuels 2014, 28, 2297–2304. [Google Scholar] [CrossRef]
- Wu, J.; Dabros, T. Process for Solvent Extraction of Bitumen from Oil Sand. Energy Fuels 2012, 26, 1002–1008. [Google Scholar] [CrossRef]
- J&X Technologies. Solid State Modulator for Comprehensive Two-Dimensional Gas Chromatography (GC×GC) SSM 1800/1810 Installation and Operation; J&X Technologies: Shanghai, China, 2019. [Google Scholar]
- Luong, J.; Guan, X.; Xu, S.; Gras, R.; Shellie, R. Thermal independent modulator for comprehensive two-dimensional gas chromatography. Anal. Chem. 2016, 88, 8428–8432. [Google Scholar] [CrossRef] [PubMed]
- J&X Technologies. Principles of SSM. Available online: http://www.jnxtec.com/page111?_1=en (accessed on 4 March 2019).
- Walters, C.C.; Wang, F.C.; Higgins, M.B.; Madincea, M.E. Universal biomarker analysis using GCxGC with dual FID and ToF-MS (EI/FI) detection. Org. Geochem. 2018, 115, 57–66. [Google Scholar] [CrossRef]
- Nardella, F.; Landi, N.; Degano, I.; Colombo, M.; Serradimigni, M.; Tozzi, C.; Ribechini, E. Chemical investigations of bitumen from Neolithic archaelogical excavations in Italy by GC/MS combined with principal component analysis. Anal. Methods 2019, 11, 1449–1459. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Volume 1 Biomarkers and Isotopes in the Environment and Human History; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Volume 2 Biomarkers and Isotopes in Petroleum Exploration and Earth History; Cambridge Univeristy Press: Cambridge, UK, 2005. [Google Scholar]
- Scarlett, A.G.; Despaigne-Diaz, A.I.; Wilde, S.A.; Grice, K. An examination by GC×GC-TOFMS of organic molecules present in highly degraded oils emerging from Caribbean terrestial seeps of Cretaceous age. Geosci. Front. 2019, 10, 5–15. [Google Scholar] [CrossRef]
- LECO Corporation. LECO’s GC×GC Utilizing a Consumable-Free Modulator and Second Column Modulation; LECO Corporation: St. Joseph, MI, USA, 2013; Available online: https://www.leco.com/component/edocman/?task=document.viewdoc&id=1161&Itemid=1161 (accessed on 30 June 2019).
- ZOEX Corporation. Introducing the NEW ZX-2 Closed Cycle Refrigeration Thermal Modulated GC×GC System. Available online: http://zoex.com/wp-content/uploads/2012/07/ZX2-Product-Specs.pdf (accessed on 30 June 2019).
- ZOEX Corporation. ZX-1 Thermal Modulation GC×GC System. Available online: http://zoex.com/wp-content/uploads/2012/07/ZX1-Product-Specs.pdf (accessed on 30 June 2019).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boswell, H.; Tarazona Carrillo, K.; Górecki, T. Evaluation of the Performance of Cryogen-Free Thermal Modulation-Based Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC×GC-TOFMS) for the Qualitative Analysis of a Complex Bitumen Sample. Separations 2020, 7, 13. https://doi.org/10.3390/separations7010013
Boswell H, Tarazona Carrillo K, Górecki T. Evaluation of the Performance of Cryogen-Free Thermal Modulation-Based Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC×GC-TOFMS) for the Qualitative Analysis of a Complex Bitumen Sample. Separations. 2020; 7(1):13. https://doi.org/10.3390/separations7010013
Chicago/Turabian StyleBoswell, Haleigh, Kieran Tarazona Carrillo, and Tadeusz Górecki. 2020. "Evaluation of the Performance of Cryogen-Free Thermal Modulation-Based Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC×GC-TOFMS) for the Qualitative Analysis of a Complex Bitumen Sample" Separations 7, no. 1: 13. https://doi.org/10.3390/separations7010013
APA StyleBoswell, H., Tarazona Carrillo, K., & Górecki, T. (2020). Evaluation of the Performance of Cryogen-Free Thermal Modulation-Based Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC×GC-TOFMS) for the Qualitative Analysis of a Complex Bitumen Sample. Separations, 7(1), 13. https://doi.org/10.3390/separations7010013