Abstract
Pesticides and veterinary pharmaceuticals are used for effective crop production and prevention of livestock diseases; these chemicals are released into the environment via various pathways. Although the chemicals are typically present in trace amounts post-release, they could disturb aquatic ecosystems and public health through resistance development toward drugs or diseases, e.g., reproductive disorders. Thus, the residues of pesticides and veterinary pharmaceuticals in the environment must be managed and monitored. To that end, we developed a simultaneous analysis method for 41 target chemicals in environmental water samples using ultra-high-performance liquid chromatography (UHPLC)–quadrupole-orbitrap high-resolution mass spectrometry (HRMS) coupled with an on-line solid-phase extraction system. Calibration curves for determining linearity were constructed for 10–750 ng∙L−1, and the coefficient of determination for each chemical exceeded 0.99. The method’s detection and quantitation limits were 0.32–1.72 ng∙L−1 and 1.02–5.47 ng∙L−1, respectively. The on-line solid-phase extraction system exhibited excellent method reproducibility and reduced experimental error. As the proposed method is applicable to the monitoring of pesticides and veterinary pharmaceuticals in surface water and groundwater samples acquired near agricultural areas, it allows for the management of chemicals released into the environment.
1. Introduction
Pesticides and veterinary pharmaceuticals are commonly used in the agriculture sector (crop production and livestock breeding) to promote productivity and to prevent and treat diseases [1,2,3,4,5,6,7,8]. Many studies reported that pesticides and veterinary pharmaceuticals are globally found in environmental water [9,10,11,12]. Because their release rates are higher than their degradation rates, the chemicals from these sources are persistent in the environment [8,13,14,15]. Pesticides and veterinary pharmaceuticals can be released into the surface water and groundwater through various pathways (e.g., runoff from agricultural lands, direct discharge of effluents from wastewater treatment plants, and direct discharge of animal wastewater from livestock farms) [8,16,17,18,19].
The presence of pesticide and veterinary pharmaceutical residues in the environment is of concern because these residues adversely affect ecosystems and human health [19,20,21,22,23]. For example, some organochlorine pesticides are known to cause side effects by mimicking or antagonizing human hormones. In addition, long-term, low-dose exposure is associated with adverse impacts on human health such as immuno-suppression, hormone disruption, and reproductive abnormalities [1,24,25]. Moreover, antibiotics in surface water or groundwater can promote the emergence of antibiotic-resistant bacteria in spite of their low concentrations [8,14,18,25].
Therefore, monitoring the pesticide and veterinary pharmaceutical residues in environmental water of the agricultural watershed is necessary because surface water and groundwater are commonly used for drinking and irrigation. According to a U.S. Food and Drug Administration (U.S. FDA) report, the sales of veterinary antibiotics were approximately 28.8 million pounds in 2009, which is estimated to be four times higher than those of human antibiotics (approximately 7.3 million pounds in 2009) [26,27]. Many studies focused on the monitoring of pesticides and veterinary pharmaceuticals in environmental water, samples obtained from surface water, and wastewater treatment plants located near urban watersheds [9,10,11,12]. The monitoring of pesticides and veterinary pharmaceuticals in agricultural watersheds was not extensively investigated [28,29,30].
Recently, use of high-performance liquid chromatography coupled with high-resolution mass spectrometry (HRMS) was widely explored for the analysis of trace residues, such as pharmaceuticals, pesticides, metabolites, and hormones, in the environment [31,32,33,34,35]. Quadrupole-orbitrap (q-orbitrap) HRMS is advantageous because many chemicals can be simultaneously analyzed. In addition, q-orbitrap HRMS can determine preselected ion transitions corresponding to specific compounds and provide full scan data [33,34,35,36,37,38]. Moreover, q-orbitrap HRMS is known to provide accurate mass measurements that facilitate selectivity for the detection of analytes even at low concentrations in complex matrices such as environmental water [38].
Moreover, residual chemicals such as pharmaceuticals, pesticides, metabolites, and hormones exist in trace amounts in the environment; thus, their extraction and concentration are required for analyses [39,40]. Furthermore, compared to conventional methods such as off-line solid-phase extraction (SPE), an on-line SPE system has many advantages including cost and labor efficiency; therefore, it was recently applied in combination with liquid chromatography–mass spectrometry (LC–MS) to analyze trace residue chemicals in environmental water [41,42,43]. However, only a few studies applied the on-line SPE method for the analysis of trace residue chemicals in environmental water samples from agricultural watersheds [44,45]. In particular, to the best of our knowledge, a simultaneous analysis of pesticides and veterinary pharmaceuticals using an on-line SPE system combined with LC-q-orbitrap HRMS was not previously conducted.
This study aimed to develop an analytical method for the simultaneous determination of pesticides and veterinary pharmaceuticals belonging to different therapeutic classes in environmental water samples using ultra-high-performance liquid chromatography (UHPLC)–q-orbitrap HRMS combined with an on-line SPE system. The proposed method was validated based on its linearity, precision, and accuracy. Moreover, the validated method was successfully applied to analyze the concentrations of multiclass target chemicals in environmental water samples from agricultural watersheds.
2. Materials and Methods
2.1. Chemicals
Forty-one pesticides and veterinary pharmaceuticals were chosen for identification in environmental water samples, and 12 stable isotope-labeled chemicals were selected in this study as internal standards (ISTDs). The target chemicals comprised eight pesticides and 33 veterinary pharmaceuticals. The pesticides included fungicides, insecticides, and herbicides, while the veterinary pharmaceuticals were classified as antiprotozoal, anthelmintic, sedative, anti-inflammatory, antibiotic, synthetic progesterone, and digestion-stimulating agents. The classes of the target chemicals are listed in Table 1. The ISTDs used for the quantification of the target chemical were selected from compounds that were structurally like the target chemical or had a retention time similar to that of the target chemical as per the UHPLC–MS analysis (Table 2).

Table 1.
Analytical data of high-resolution mass spectrometry (HRMS) for target chemicals in this study.

Table 2.
Analytical data of HRMS for the internal standards in this study.
Ampicillin-d5, tiamulin-d10, clanobutin, and ormethoprim were obtained from Toronto Research Chemicals Inc. (North York, ON, Canada). Triclocarban-d4, and virginiamycin were purchased from CDN isotopes (Gwangju, Korea) and LKT Laboratories Inc. (St. Louis, MO, USA), respectively, while the 38 pesticides and veterinary pharmaceuticals (altrenogest, ampicillin, azaperone, azoxystrobin, boscalid, carbendazime, ceftiofur, chlortetracycline, clopidol, colchicine, decoquinate, dexamethaxone, dimethoate, doxycycline, fenbendazole, flumequine, flunixin, imiprothrin, lincomycin, marbofloxacin, meloxicam, mepanipyrim, methabenzthiazuron, methoxyfenozide, moxidectin, narasin, orbifloxacin, oxytetracycline, penicillin-G, sulfachloropyridazine, sulfadiazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfaquinoxaline, sulfathiazole, tiamulin, and trimethoprim) and 11 ISTDs (carbendazime-d3, fenbendazole-d3, meloxicam-d 3, ofloxacin-d3, sulfadiamethoxine-d6, sulfadiazine-13C6, sulfamethazine-13C6, terbythylazine-(ethyl-d5), and trimethoprim-d9) were purchased from Sigma-Aldrich (St. Louis, MO, USA), except for lincomycin-d3 and sulfamethoxazole-d4, which were obtained from LGC standards (Teddington, UK).
LC–MS grade organic solvents (methanol and acetonitrile (ACN)) and distilled water (DW) used during LC–HRMS operation were obtained from Fisher Scientific (Fairlawn, NJ, USA) and Merck Millipore (Billerica, MA, USA), respectively. Citric acid and trisodium citrate dehydrate for the preparation of 1.5 M citrate buffer solution (pH 6.5) and ethylenediaminetetraacetic acid disodium salt dehydrate for the preparation of the 5% EDTA solution were bought from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Sampling and Sample Preparation
All groundwater and river water samples were collected using polypropylene bottles at a point in the midstream basin of the Cheongmi River from an intensive livestock farming area in Gyeonggi-do in March 2019. The collected samples were stored at <4 °C in darkness to prevent changes in the sample; the samples were extracted within 24 h. The water samples were centrifuged at 4 °C and 24,600× g for 5 min to eliminate suspended matter, and the supernatant was filtered through a 0.2-µm polyvinylidene fluoride syringe filter. The pH of the water samples was considered as an important factor during sample preparation. To prevent the degradation of target chemicals during the experiment, we adjusted the pH to 6.5 using 1.5 M citrate buffer (0.5 mL per 50 mL sample), followed by the addition of 0.5 mL of a 5% EDTA solution to the 50 mL samples. For the ISTD method, the ISTD solutions (25 µL, 500 ng∙L−1) were added to 50-mL samples. Moreover, DW as a blank was subjected to pretreatment using the same procedure as that for the environmental water samples. The prepared samples were stored in 10-mL vials; they were extracted and analyzed using UHPLC–q-orbitrap HRMS coupled with an on-line SPE system within 7 days.
2.3. UHPLC–q-Orbitrap HRMS Instrumentation and Conditions
Compared to traditional methods such as off-line SPE, an on-line SPE system has many advantages, including cost and labor efficiency. However, the pretreatment process, including sample extraction and concentration, is challenging to optimize for satisfactory accuracy and sensitivity because the target compounds have different physicochemical properties. In addition, several factors affect SPE performance, including the type of SPE cartridge, sample pH, sample column, and the solvent used for elution, among others [25,42,43,46,47,48,49,50].
The operating parameters used in the method described by Kim et al. [7] were used for the on-line SPE and UHPLC–q-orbitrap HRMS, with optimization. We used the same type of column because our samples also had a pH of 6.5; however, we combined two Hypersil columns to extract larger amounts of the target chemicals. To determine the appropriate column for separating the target chemicals in this study, the performance of the XBridge C18 column (50 mm × 2.1 mm inner diameter (i.d.), 2.5 µm, Waters, Milford, MA, USA) used by Kim et al. [7] was compared with the CORTECS C18 column in a preliminary experiment. In this preliminary experiment, samples were analyzed by injecting standard compounds at a concentration of 500 ng∙L−1 in DW, and the retention times, peak shapes, and peak intensities were compared. The CORTECS C18 column produced cleaner baselines and peak shapes than the XBridge C18 column; hence, we concluded that the CORTECS C18 column was suitable for further studies. The detailed analysis conditions for selected target chemicals are described in Table 3.

Table 3.
Analysis condition for selected target chemicals. UHPLC—ultra-high-performance liquid chromatography; SPE—solid-phase extraction; DW—distilled water.
A Q ExactiveTM Plus Hybrid Quadrupol-OrbitrapTM mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) fitted to the UHPLC system was used to detect the pesticides and veterinary pharmaceuticals in water. The mass spectrometer employed a heated electrospray ionization source (HESI-II) in positive mode with the following conditions: ion spray voltage, capillary temperature, flow rate of sheath gas, sweep gas, auxiliary gas, and auxiliary temperature of 3.5 kV, 250 °C, 45 arb, 2 arb, 10 arb, and 400 °C, respectively. The resolving power, automatic gain control (AGC), target, and maximum injection time (IT) were set at 17,500 full width at half maximum (FWHM. m/z 200), 5 × 105, and 100 ms, respectively. The precursor ions were fragmented in the high-energy collisional dissociation (HCD) collision cell with a normalized collision energy (NCE) of 30.
Xcalibur 4.0 (Thermo Fisher Scientific, San Joes, CA, USA) software was used for instrument control and data acquisition. Positive ionization mode was selected to qualify and quantify the pesticides and veterinary pharmaceuticals. The positive ions of the target chemicals were all in the [M + H]+ form, except for virginiamycin which was in the [M + Na]+ form (Table 1). To confirm the identity of each analyte, the m/z values of the monitored adduct (mass error < 5 ppm), two fragment ions, and the relevant retention time were verified (Table 1).
2.4. Method Validation
To validate the method developed in this study, a calibration curve was constructed for each chemical using the ISTD method; the method detection limit (MDL), method quantitation limit (MQL), precision, and relative accuracy were determined for each target chemical. Standard solutions used to construct the calibration curves were prepared in DW and pretreated in the same manner as the environmental water samples. Solutions at seven concentrations in the 10–750 ng∙L−1 range containing 250 ng∙L−1 of the ISTD mixture were used to construct the calibration curves, and the coefficients of determination (R2) were evaluated. The values of MDL and MQL for each chemical were obtained by multiplying the standard deviation, which was obtained by analyzing the 10 ng∙L−1 standard solution seven times, by 3.14 and 10, respectively [51,52,53]. The recovery and relative standard deviation (RSD, %) for determining the precision data were derived by analyzing seven replicate DW samples spiked with a mixed target chemical solution (10 ng∙L−1, 50 ng∙L−1, and 500 ng∙L−1). The relative recovery for determining the accuracy data was achieved by analyzing the groundwater and river water samples in triplicate containing the target chemicals (50 ng∙L−1 and 500 ng∙L−1); all samples also contained 250 ng∙L−1 of the mixed ISTD solution. The value of the accuracy was calculated using Equation (1) and expressed as relative recovery (%).
where CM and CC are the measured preside and veterinary pharmaceutical concentrations of the spiked and un-spiked samples, respectively, and CS is the expected concentration of the spiked sample.
3. Results and Discussion
3.1. Method Validation
3.1.1. Linearity
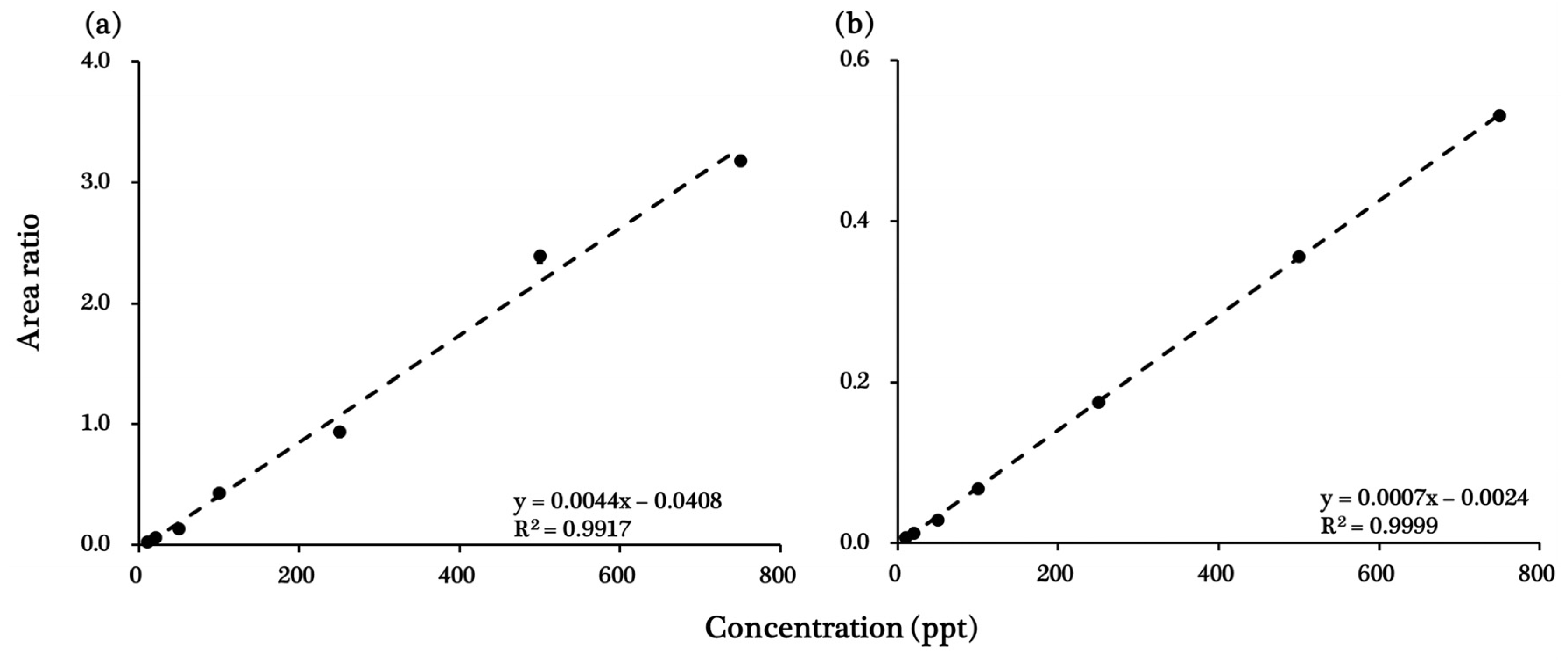
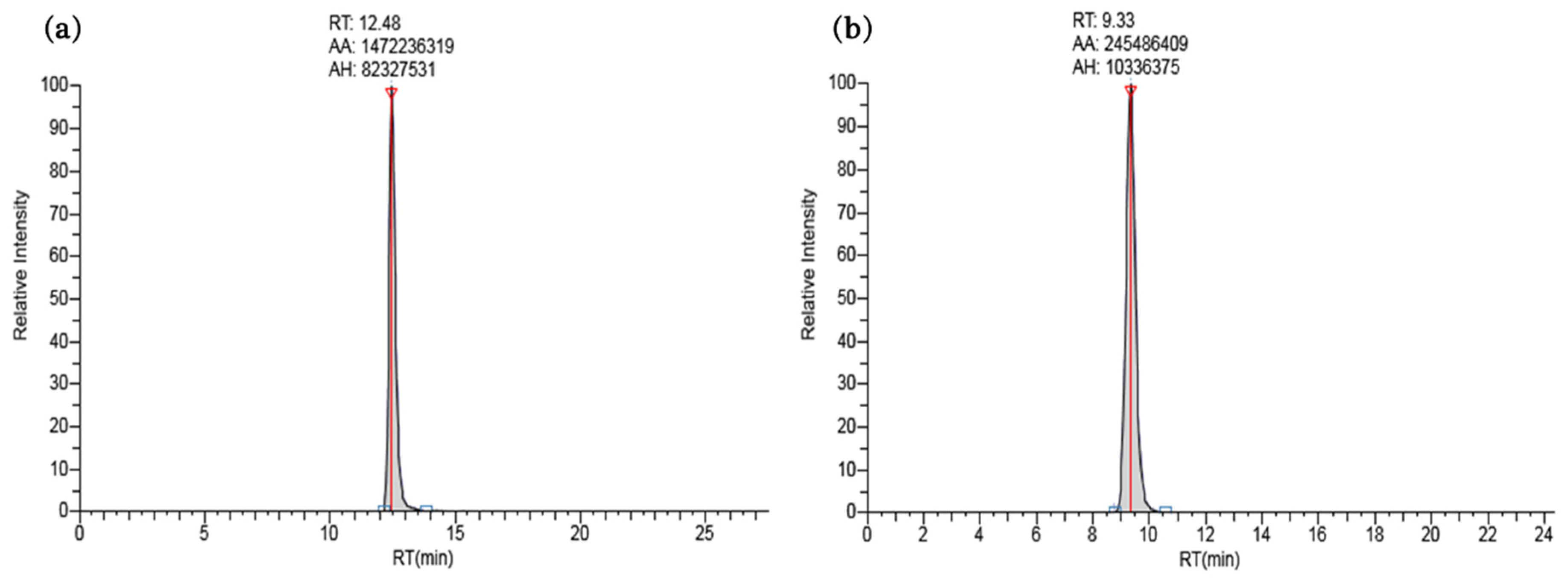
The ISTD method was used to create a calibration curve for each chemical to minimize potential errors that may occur during analysis. Each of the 12 stable isotope-labeled chemicals was used at a concentration of 250 ng∙L−1. The ISTD list of target chemicals is summarized in Table 2 according to retention time and chemical structure. The calibration curve for each chemical was constructed by plotting the area ratios of the ISTD and target chemical peaks against the concentration of the target chemical (10–750 ng∙L−1), and R2 was determined as a measure of linearity. R2 values ranged from 0.9917 (tiamulin) to 0.9999 (clanobutin). Details of R2 values, calibration curves, and peak-analysis results for each chemical are listed in Table 4 and displayed in Figure 1 and Figure 2, and Figures S1–S3 (Supplementary Materials).

Table 4.
Method validation data of the developed method with on-line SPE for target chemicals. MDL—method detection limit; MQL—method quantitation limit.
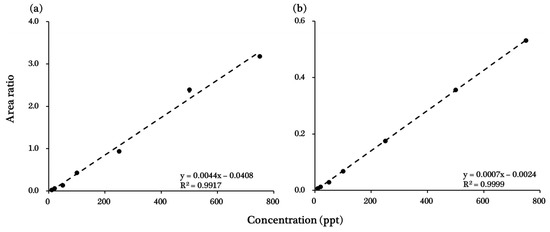
Figure 1.
Calibration curves for (a) tiamulin and (b) clanobutin. These calibration curves are representative of the 41 target pesticides and veterinary pharmaceuticals; calibration curves for the remaining 39 target chemicals are available in the Supplementary Materials (Figure S3).
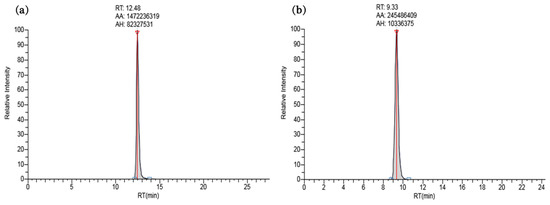
Figure 2.
UHPLC traces of standard solutions (750 ng∙L−1) of (a) altrenogest, and (b) ampicillin. These chromatograms are representative of the 41 target pesticides and veterinary pharmaceuticals; traces for the remaining 39 chemicals are available in the Supplementary Materials (Figures S1 and S2).
3.1.2. MDLs and MQLs
A solution of mixed target chemicals (10 ng∙L−1) in DW was analyzed seven times to determine MDL and MQL values. The highest MDL value was observed for moxidectin (1.72 ng∙L−1), while chlorotetracycline exhibited the lowest (0.32 ng∙L−1), with MQL values between 1.02 and 5.47 ng∙L−1 (Table 3). International organizations like the European Union (EU) regulate the residual allowable concentration in surface water for 33 types of pesticides [54]. They also set the residual allowable concentration in drinking water for all pesticides at 0.1 µg∙L−1 [54]. Comparing the residual allowable concentration with MDL values obtained in this study, the MDL value was found to be lower than the residual criteria. This suggests that the MDL and MQL values in this study are sufficiently low for environmental monitoring.
The literature MDL values were obtained via LC–MS/MS coupled with an on-line SPE system equivalent to that used in this study. Most of the values in the current study were found to be several times lower than the values reported in the literature [20,47,54,55,56]. Panditi et al. [47] developed a method for the analysis of 31 antibiotics (sulfonamide and macrolide) in several environmental water samples (groundwater, river water, and reclaimed water). Eight of these 31 chemicals (lincomycin, sulfachloropyridazine, sulfadiazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfathiazole, and trimethoprim) reported overlap with this study, and the MDLs were found to be 2.52–14.82 times higher than those in our study. The MDL value of 5.4 ng∙L−1 for sulfachloropyridazine reported by Panditi et al. [47] differs the most from that in our study (0.36). Based on these results, we conclude that the method developed in this study has satisfactorily low MDL and MQL values for the analysis of target chemicals in environmental water samples.
3.1.3. Precision and Accuracy
The values of recovery and RSD for each chemical were determined in order to obtain a measure of precision. The recovery and RSD values were calculated using the averages and standard deviations of the analysis results for samples spiked with DW with 10, 50, and 500 ng∙L−1 of a mixed solution of 41 pesticides and veterinary pharmaceuticals; all recovery and RSD values were found to be over 90% and lower than 15%, respectively (Table 5).

Table 5.
Precisions including recovery and relative standard deviation (RSD) of the developed method with on-line SPE for target chemicals.
Environmental water samples (groundwater and river water) containing 50 and 500 ng∙L−1 of the target chemicals were analyzed three times, while the relative recoveries and coefficients of variation (CVs, %) determined the accuracy of the developed method. The relative recoveries and CVs of the 41 pesticides and veterinary pharmaceuticals were 70–120% and 0.172–10.83%, respectively. The data for each chemical are listed in Table 6.

Table 6.
Accuracy including relative recovery and coefficient of variation (CV) data of the developed method with on-line SPE for target agrochemicals.
Even though the ISTD method using stable isotope-labeled chemicals was applied to correct the matrix effect, ionization was enhanced or suppressed during the LC–MS/MS analyses of the target chemicals [57]. This shows that extraction and ionization performances are affected by the complexity of the matrix. According to widely applied guidelines for pesticide residue analysis, mean recoveries must be within the range of 70–120%, with RSD values ≤20% for all analytes [58]. The result of method validation satisfied the criteria of the guidelines [58]. Therefore, the method developed in this study has excellent precision and accuracy for the analysis of residual pesticides and veterinary pharmaceuticals.
3.1.4. Method Application
The concentrations of the target chemicals in environmental water samples were analyzed using the developed method. Six chemicals (boscalid, ceftiofur, dexamethasone, methabenzthiazuron, sulfadiazine, and virginiamycin) were not detected in groundwater and river water, while 19 and 31 target chemicals were identified in groundwater and river water, respectively. In addition, eight of the 19 chemicals in the groundwater samples, and 12 of the 31 chemicals in the river water samples were found to be below their MQL values, which means that these chemicals were present in these samples but were not quantifiable. Furthermore, five chemicals (altrenogest, decoquinate, doxycycline, flunixin, and trimethoprim) were found at levels above their MQLs in all samples, with decoquinate (71.46 ng∙L−1 in groundwater) and doxycycline (42.98 ng∙L−1 in river water) detected at the highest levels in the environmental water samples. In contrast, trimethoprim and altrenogest were analyzed at levels of 10.33 ng∙L−1 and 7.84 ng∙L−1 in groundwater and river water, respectively, which were the lowest concentrations found in this study (Table 7).

Table 7.
Analysis results using the developed method with on-line SPE for target agrochemicals in environmental water samples (groundwater and surface water). ND—not detected.
Among the 41 target chemicals, sulfathiazole was identified only in the river water samples, and was present at the highest concentration (103.07 ng∙L−1), while decoquinate was found to be present at the highest concentration (71.46 ng∙L−1) in groundwater. While this chemical was also detected in river water, it was 3.11 times lower than that in groundwater.
Most of the 31 veterinary pharmaceuticals selected in this study were identified in river water or groundwater. In contrast, the pesticides were detected at very low concentrations, or not at all. Because large numbers of livestock are bred in the sampling area, the use of veterinary pharmaceuticals is high; therefore, various veterinary pharmaceuticals were expected to be detected in river water samples.
4. Conclusions
In this study, we developed a method for the simultaneous analysis of 41 pesticides and veterinary pharmaceuticals in environmental water samples by using on-line SPE combined with UHPLC–MS/MS. The developed method was verified by measuring the linearities, MDLs, MQLs, and relative recoveries in water samples containing mixtures of the pesticides and veterinary chemicals. The developed method was successfully applied for determining the concentration of the target chemicals in groundwater and river water samples collected from near the agricultural watersheds. Thirty-five of the 41 chemicals were detected in concentrations that ranged from 0.35 to 103.07 ng∙L−1 from groundwater and river water samples.
Pesticides and veterinary pharmaceuticals have adverse effects on health and the environment, including antibiotic resistance of the ecosystem, thereby necessitating monitoring. The on-line SPE method developed in this study reduces the consumption of organic solvents, time, cost, and labor required during the extraction experiment while delivering reliable results. HRMS might not be used as widely as LC–MS because of the high initial installation cost. However, as shown in this study, when using HRMS, low contaminant concentrations in environmental water can be accurately analyzed, which is very useful for the evaluation of the contamination levels in environmental water. Analytical methods using UHPLC–q-orbitrap HRMS combined with the on-line SPE system were previously developed for the detection of contaminants in environmental water samples. Although various therapeutic classes of chemicals coexist in environmental water, analytical methods were developed to separately analyze the different classes of chemicals such as pesticides and pharmaceuticals. In contrast, the developed method in this study analyzes various therapeutic classes of chemicals. Moreover, the developed method has the advantage of simultaneously analyzing multiple classes of chemicals in environmental water. This study shows novelty in that the developed method with a very fast system using on-line SPE can be applied with high performance for pesticide and veterinary pharmaceutical monitoring of environmental water in agricultural watersheds, as well as other environmental water sources such as urban watersheds. Because of this widescale applicability, the developed method will facilitate the management of chemical use and control the amounts released into the environment. The developed method could not be adequately applied to the identification of some pharmaceuticals such as aminoglycoside and macrolide antibiotics. Therefore, a method with on-line SPE for the analysis of amoxicillin, aminoglycoside, and macrolide antibiotics needs to be developed in the near future.
Supplementary Materials
The following are available online at https://www.mdpi.com/2297-8739/7/1/14/s1: Figure S1: HPLC traces of standard solutions (750 ng∙L−1) for the target chemicals, Figure S2: HPLC traces of standard solutions (250 ng∙L−1) of the ISTDs, Figure S3. Calibration curve for the selected target chemicals.
Author Contributions
Conceptualization C.K. and E.G.C.; formal analysis H.-J.L.; writing—original draft preparation, H.-J.L.; investigation, H.-D.R. and E.G.C.; supervision, E.G.C.; project administration, E.G.C., D.S., and J.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the National Institute of Environment Research (NIER), funded by the Ministry of Environment (MOE) of the Republic of Korea [grant number 1900-1946-303-210].
Acknowledgments
The authors thank all the members of the Livestock Excreta Management Research Team including Do Young Lim, Deok-Woo Kim, Un-il Baek, and Seon-Jeong Kim.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdisk Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Yang, G.-G.; Liu, S.; Zhao, J.-L.; Chen, F.; Zhang, R.-Q.; Peng, F.-Q.; Zhang, Q.-Q. Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2012, 1244, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Śkrbicć, B.; Živančev, J.; Ferrando-Climent, L.; Barceló, D. Determinarion of 81 pharmaceutical drugs by high performance liquid chromatography coupled to mass spectrometry with hybrid triple quadrupole-linear ion trap in different types of water in Serbia. Sci. Total Environ. 2014, 468–469, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cornejo, J.; Nehring, R.; Osteen, C.; Wechsler, S.; Martin, A.; Vialou, A. Pesticide Use in U.S. Agriculture: 21 Selected Crops, 1960–2008; EIB-124; U.S. Department of Agriculture: Washington, DC, USA, 2014.
- Hong, Y.; Sharma, V.K.; Chiang, P.-C.; Kim, H. Fast-target analysis and hourly variation of 60 pharmaceuticals in wastewater using UPLC-high resolution mass spectrometry. Arch. Environ. Contam. Toxicol. 2015, 69, 525–534. [Google Scholar] [CrossRef]
- Speight, J.G. Environmental Organic Chemistry for Enginners, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 153–201. [Google Scholar]
- Kim, C.; Ryu, H.-D.; Chung, E.G.; Kim, Y. Determination of 18 veterinary antibiotics in environmental water using high-performance liquid chromatography-q-orbitrap combined with on-line solid-phase extraction. J. Chromatogr. B 2018, 1084, 158–165. [Google Scholar] [CrossRef]
- Licul-kucera, V.; Ladányi, M.; Hizsnyik, G.; Záray, G.; Mihucz, V.G. A filtration optimized on-line SPE-HPLC-MS/MS method for determination of three macrolide antibiotics dissolved and bound to suspended solids in surface water. Microchem. J. 2019, 148, 480–492. [Google Scholar] [CrossRef]
- Dougherty, J.A.; Swarzenski, P.W.; Dinicola, R.S.; Reinhard, M. Occurrence of herbicides and pharmaceutical and personal care products in surface water and groundwater around Liberty bay, Puget Sound, ashington. J. Environ. Qual. 2010, 39, 1173–1180. [Google Scholar] [CrossRef]
- Al Aukidly, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barceló, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef]
- Nebot, C.; Falcon, R.; Boyd, K.G.; Gibb, S.W. Introduction of human pharmaceuticals from wastewater treatment plants into the aquatic environment: A rural perspective. Environ. Sci. Pollut. Res. 2015, 22, 10559–10568. [Google Scholar] [CrossRef]
- Guibal, R.; Lissalde, S.; Brizard, Y.; Guibaud, G. Semi-continuous pharmaceutical and human tracer monitoring by POCIS sampling at the watershed-scale in an agricultural rural headwater river. J. Hazard. Mater. 2018, 360, 106–114. [Google Scholar] [CrossRef]
- Darwano, H.; Duy, S.V.; Sauvé, S. A new protocol for the analysis of pharmaceuticals, pesticides and hormones in sediments and suspended particulate matter from rivers and municipal wastewaters, Arch. Environ. Contam. Toxicol. 2014, 66, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Mokh, S.; Khatib, M.E.; Koubar, M.; Daher, Z.; Iskandarani, M.A. Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: A case study natural water sources in Lebanon. Sci. Total Environ. 2017, 609, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Ribeiro, C.; Tiritan, M.E.; Pereira, M.F.R.; Silva, A.M.T. Monitoring of the 17 EU watch list contaminants of emerging concern in the Ave and the Sousa Rivers. Sci. Total Environ. 2019, 649, 1083–1095. [Google Scholar] [CrossRef]
- Dolliver, H.; Gupta, S.; Noll, S. Antibiotic degradation during manure composting. J. Environ. Qual. 2008, 37, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-R.; Owens, G.; Kwon, S.-I.; So, K.-H.; Lee, D.-B.; Ok, Y.S. Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air Soil Pollut. 2011, 214, 163–174. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.-H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef]
- Mainero Rocca, L.; Gentili, A.; Pérez-Fernández, V.; Tomai, P. Veterinary drugs residues: A review of the latest analytical research on sample preparation and LC-MS based methods. Food Addit. Contam. A 2017, 34, 766–784. [Google Scholar] [CrossRef]
- Pozo, O.J.; Guerrero, C.; Sancho, J.V.; Ibáñez, M.; Pitarch, E.; Hogendoorn, E.; Hernández, F. Efficient approach for the reliable quantification and confirmation of antibiotics in water using on-line solid-phase extraction liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2006, 1103, 83–93. [Google Scholar] [CrossRef]
- Ntzani, E.; Chondrogiorge, M.; Ntritsos, G.; Evangelou, E.; Tzoulaki, I. Literature review on epidemiological studies linking exposure to pesticides and health effect. EFSA Supporting Publ. 2013, 10, 497E. [Google Scholar] [CrossRef]
- Wolecki, D.; Carban, M.; Pazdro, K.; Mulkiewicz, E.; Stepnowski, P.; Kumirska, J. Simultaneous determination of non-steroidal anti-inflammatory drugs and natural estrogens in the mussel Mytilus edulis trossulus. Talanta 2019, 200, 316–323. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Karry, V.V.S.R. Experimental design in pesticide extraction methods: A review. Food Chem. 2019, 289, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Yoon, Y.S.; Kim, H.S.; Jeon, S.J.; Cole, E.; Lee, J.; Kho, Y.; Cho, Y.H. Distribution of fipronil in humans, and adverse health outcomes of in utero fipronil sulfone exposure in newborns. Int. J. Hyg. Envir. Heal. 2019, 222, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Pessah, I.N.; Lein, P.J.; Seegal, R.F.; Sagiv, S.K. Neurotoxicity of polychlorinated biphenyls and relat5ed organohalogens. Acta Neuropathol. 2019, 138, 363–387. [Google Scholar] [CrossRef] [PubMed]
- Seifrtová, M.; Nováková, L.; Lino, C.; Pena, A.; Solich, P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal. Chim. Acta 2009, 649, 158–179. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; US Food and Drug Administration: Silver Spring, MD, USA, 2010.
- US Environmental Protection Agency. Literature Review of Contaminants in Livestock and Poultry Manure and Implications for Water Quality; US Environmental Protection Agency: Washington, DC, USA, 2013; EPA 820-R-13-002.
- Jaffrézic, A.; Jardé, E.; Soulier, A.; Carrera, L.; Marengue, E.; Cailleau, A.; Le Bot, B. Veterinary pharmaceutical contamination in mixed land use watersheds: From agricultural headwater to water monitoring watershed. Sci. Total Environ. 2017, 609, 992–1000. [Google Scholar] [CrossRef]
- Charuaud, L.; Jardé, E.; Jaffrézic, A.; Liotaud, M.; Goyat, Q.; Mercier, F.; Le Bot, B. Veterinary pharmaceutical residues in water resources and tap water in an intensive husbandry area in France. Sci. Total Environ. 2019, 66, 605–615. [Google Scholar] [CrossRef]
- Gómez Pérez, M.L.; Romero-González, R.; Plaza-Bolaños, P.; Génin, E.; Vidal, J.L.M.; Frenich, A.G. Wide-scope analysis of pesticide and veterinary drug residues in meatmatrices by high resolution MS: Detection and identification using exactive-orbitrap. J. Mass Spectrom. 2014, 49, 27–36. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Bletsou, A.A.; Koulis, G.A.; Thomaidis, N.S. Qualitative multiresidue screening method for 143 veterinary drugs and pharmaceuticals in milk and fish tissue using liquid chromatography quadrupole-time-of flight mass spectrometry. J. Agric. Food Chem. 2015, 63, 4493–4508. [Google Scholar] [CrossRef]
- Masiá, A.; Saurez-Varela, M.M.; Gonzalez-Llopis, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.; Lu, S.; Yan, P.; Sui, Q. Recent advances in pharmaceuticals and personal care products in the surface water and sediments in China. Front Environ. Sci. Eng. 2016, 10, 2–12. [Google Scholar] [CrossRef]
- Kong, C.; Wang, Y.; Huang, Y.; Yu, H. Multiclass screening of >200 pharmaceutical and other residues in aquatic foods by ultrahigh-performance liquid chromatography-quadrupole-Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 5545–5553. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Hollender, J.; López de Alda, M.; Müller, C.; Schulze, T.; Schymanski, E.; Slobodnik, J.; Krauss, M. High-resolution mass spectrometry to complement monitoring and track emerging chemicals and pollution trends in European water resources. Environ. Sci. Eur. 2019, 31, 62–67. [Google Scholar] [CrossRef]
- Kiefer, K.; Müller, A.; Singer, H.; Hollender, J. New relevant pesticide transformation products in groundwater detected using target and suspect screening for agricultural and urban micropollutants with LC-HRMS. Water Res. 2019, 165, 114972–114984. [Google Scholar] [CrossRef] [PubMed]
- Turnipseed, S.B.; Storey, J.M.; Wu, I.L.; Andersen, W.C.; Madson, M.R. Extended liquid chromatography high resolution mass spectrometry screening method for veterinary drug, pesticide and human pharmaceutical residues in aquaculture fish. Food Addict. Contam. A 2019, 36, 1501–1514. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Rizzetti, T.M.; Kemmerich, M.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization of a QuEChERS based method by means of central composite design for pesticide multiresidue determination in orange juice by UHPLC-MS/MS. Food Chem. 2016, 196, 25–33. [Google Scholar] [CrossRef]
- Postigo, C.; López de Alda, M.J.; Barceló, D.; Ginebreda, A.; Garrido, T. Analysis of occurrence of selected medium to highly polar pesticides in groundwater of Catalonia (NE Spain): An approach based on on-line solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry detection. J. Hydrol. 2010, 383, 83–92. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Determination of 19 sulfonamides in environmental water samples by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry (SPE-LC-MS/MS). Talanta 2010, 81, 355–366. [Google Scholar] [CrossRef]
- López-Serna, R.; Pérez, S.; Ginebreda, A.; Petrović, M.; Barceló, D. Fully automated determination of 74 pharmaceuticals in environmental and waste waters by online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta 2010, 83, 410–424. [Google Scholar] [CrossRef]
- Pocurull, E.; Aguilar, C.; Borrull, F.; Marcé, R.M. On-line coupling of solid-phase extraction to gas chromatography with mass spectrometric detection to determine pesticides in water. J. Chromatogr. A 1998, 818, 85–93. [Google Scholar] [CrossRef]
- Chen, Z.; Megharaj, M.; Naidu, R. On-line solid phase extraction of pesticide residues in natural water, coupled with liquid chromatography and UV detection, using various sorbents. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 1779–1790. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Q.; Qu, G.-B.; Song, S.-J.; Sun, J.-T.; Shi, J.-B.; Jiang, G.-B. Simultaneous determination of five estrogens and four androgens in water samples by online solid-phase extraction coupled with high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1281, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Panditi, V.R.; Batchu, S.R.; Gardinali, P.R. Online solid-phase extraction-liquid chromatography-electrospray-tandem mass spectrometry determination of multiple classes of antibiotics in environmental and treated waters. Anal. Bioanal. Chem. 2013, 405, 5953–5964. [Google Scholar] [CrossRef] [PubMed]
- Anumal, T.; Snyder, S.A. Rapid analysis of trace organic compounds in water by automated online solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. Talanta 2015, 132, 77–86. [Google Scholar] [CrossRef]
- Axel, M.; Ewelina, K.; Jenny-Maria, B.; Leif, K. An online SPE LC-MS/MS method for the analysisof antibiotics in environmental water. Environ. Sci. Pollut. Res. 2017, 24, 8692–8699. [Google Scholar] [CrossRef]
- Reemtsma, T.; Alder, L.; Ursula, B. A multimethod for the determination of 150 pesticide metabolites in surface water and groundwater using direct injection liquid chromatography-mass spectrometry. J. Chromatogr. A 2013, 1271, 95–104. [Google Scholar] [CrossRef]
- Ripp, J. Analytical Detection Limit Guidance: Laboratory Guide for Determining Method Detection Limits; PUBL-TS-059-96; Wsiconsin Department of Natural Resources: Madison, WI, USA, 1996. [Google Scholar]
- US Environmental Protection Agency. Guidelines Establishing Test Procedures for the Analyses of Pollutants, Appendix B to Part 136-definition and Procedure for the Determination of Method Detection Limit rev. 2; U.S. Code of Federal Regulations: Washington, DC, USA, 2016.
- Ministry of Environment Republic of Korea. MoE Notification 2016-65, Standard Method for the Examination of Water Pollution; ES 04001.b; Ministry of Environment Republic of Korea: Sejong, Korea, 2016.
- Gulkowska, A.; Buerge, I.J.; Poiger, T. Online solid phase extraction LC-MS/MS method for the analysis of succinate dehydrogenase inhibitor fungicides and its applicability to surface water samples. Anal. Bioanal. Chem. 2014, 406, 6419–6427. [Google Scholar] [CrossRef]
- Chitescu, C.L.; Kaklamanos, G.; Nicolau, A.I.; Stolker, A.A.M.L. High sensitive multiresidue analysis of pharmaceuticals and antifungals in surface water using U-HPLC-Q-Exactive Orbitrap HRMS. Application to the Danube river basin on the Romanian Territory. Sci. Total Environ. 2015, 532, 501–511. [Google Scholar] [CrossRef]
- Puig, P.; Alexander Tempeles, F.W.; Somsen, G.W.; de Jong, G.J.; Borrull, F.; Aguilar, C.; Calull, M. Use of large-volume sample stacking in on-line solid-phase extraction-capillary electrophoresis for improved sensitivity. Electrophoresis 2008, 29, 1339–1346. [Google Scholar] [CrossRef]
- Annesley, T.M. Ion suppression in mass spectrometry. Clin. Chem. 2003, 49, 1041–1044. [Google Scholar] [CrossRef]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; SANTE/11813/2017; European Commission: Brussels, Belgium, 2017. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).