1. Introduction
Tetracyclines belong to the broad-spectrum antibiotics that are active against both gram-positive and gram-negative bacteria. They are commonly used in veterinary medicine for the prevention and treatment of several infectious diseases or as feed additives to promote growth in farm animals [
1]. However, the indiscriminate use of tetracyclines in animal feed has raised substantive concerns with regards to the quality of food, posing a risk to human health. Potential harmful consequences include allergic reactions, liver damage, teeth yellowing to gastrointestinal disturbances, as well as the increase of pathogens resistance to antimicrobial agents [
2,
3].
Many sample preparation strategies have been developed for the isolation and clean-up of tetracycline residues in milk throughout the years, such as solid phase extraction (SPE) with various types of sorbents [
4,
5], solid phase microextraction (SPME) [
6], hollow-fiber liquid phase microextraction (HF-LPME) [
7] and ultrasound assisted dispersive extraction [
8].
Modern sample preparation techniques focus on the reduction of organic solvent consumption in less time. These include QuEChERS (acronym name for quick, easy, cheap, effective, rugged and safe) methodology, which was introduced in 2003 by Anastassiades et al. [
9] Target analytes are extracted by acetonitrile, followed by an included liquid-liquid partition after the addition of salts and a dispersive solid-phase extraction (D-SPE) clean-up step. This process was firstly developed for the extraction of pesticides from different matrices, such as soil [
10], sugarcane juice [
11], honey [
12], wheat grains, flour and bran [
13], but also for the extraction of drugs and phenols from soil [
14,
15,
16] and veterinary drug residues from foods of animal origin [
17,
18,
19].
Although a number of methods [
20,
21] can be found in the literature employing mass spectrometric detection for confirmation purposes, this instrumentation is not always available in analytical laboratories. The aim of this study was to develop a simple and quick HPLC-DAD method for the determination of four tetracyclines (oxytetracycline, tetracycline, chlorotetracycline and doxycycline) in milk using QuEChERS dispersive extraction. The method was validated according to the European Union Decision 2002/657/EC with regards to selectivity, linearity, accuracy, precision, sensitivity and ruggedness by applying the Youden approach [
22].
2. Materials and Methods
2.1. Reagents and Materials
Methanol and acetonitrile of HPLC grade were supplied by Fischer Scientific (Loughborough, UK). Oxalic acid (C2H2O4 100%) and ethylenediamine tetraacetic acid disodium salt hydrate (Na2EDTA) were purchased from Merck (Darmstadt, Germany). Ultra-pure water was used throughout the study, provided by a Milli-Q® purification system (Merck, Darmstadt, Germany). Oxytetracycline (OTC), Tetracycline (TC), Chlorotetracycline (CTC) and Doxycycline (DC) were purchased from Sigma-Aldrich (Steinheim, Germany). QuEChERS for fatty samples were purchased from Agilent Technologies (Santa Clara, CA, USA) and consist of 150 mg magnesium sulfate, 50 mg primary and secondary amines and 50 mg C18EC. Syringe (nylon 66) filters (13 mm diameter, 0.2 μm membrane) were purchased from Supelco (Bellefonte, PA, USA) and were used for sample filtration prior to HPLC analysis. An Orbit 100C4 (5 μm, 250 × 4.0 mm) analytical column from MZ-Analysentechnik (Mainz, Germany) was used for the chromatographic separation. Milk (skimmed, semi-skimmed and full fat) was purchased from the local markets and kept at +4 °C.
2.2. Instrumentation
Mobile phase was delivered to the analytical column by a Shimadzu LC-9ADVP pump (Shimadzu, Kyoto, Japan) equipped with a Shimadzu SCL-10ALVP System Controller. The solvent lines were mixed in an FCV-9AL mixer. For the degassing of the mobile phase, helium sparging was performed in the solvent reservoirs by a DGU-10B degassing unit. Samples were injected by means of a Shimadzu SIL-9A auto-sampler. Analytes were monitored using an SPD-M6A photodiode array detector, with data acquisition software CLASS-M10A by Shimadzu.
For the filtration of the aquatic solutions, a glass vacuum-filtration apparatus from Alltech Associates (Deerfield, IL, USA) using Cellulose Nitrate 0.2 μm membrane filters from WHATMAN (Maidstone, UK) was used. A Glass-col Small Vortexer (Terre Haute, IN, USA) was employed for the pretreatment of milk samples. Centrifugation was carried out using a Hermle centrifuge, model Z-230 (Gosheim, Germany). A nine-port Reacti-VapTM, model 18,780 by PIERCE (Rockford, IL, USA) was used for the evaporation under nitrogen stream.
2.3. Chromatographic Conditions
An Orbit 100C4 (5 μm, 250 × 4.0 mm) analytical column, at ambient temperature, was used for the separation. The analytes were monitored at 355 nm. The elution system consisted of 0.01 M C2H2O4–10−4 M Na2EDTA/ACN, delivered at a flow rate 0.9 mL/minute, according to the gradient program: 0 min 82:18 v/v, 20 min 60:40 v/v. Dwell volume of the system used was 1.6 mL. Inlet pressure was between 215 and 230 bar. The injection volume was 100 μL.
2.4. Preparation of Standards
Stock solutions of OTC, TC, CTC and DC at 100 ng/μL were dissolved in methanol and stored refrigerated at +4 °C. They were found to be stable for at least one month. Working methanolic standards were prepared by the appropriate dilution at a range of 1–20 ng/μL.
2.5. Sample Preparation
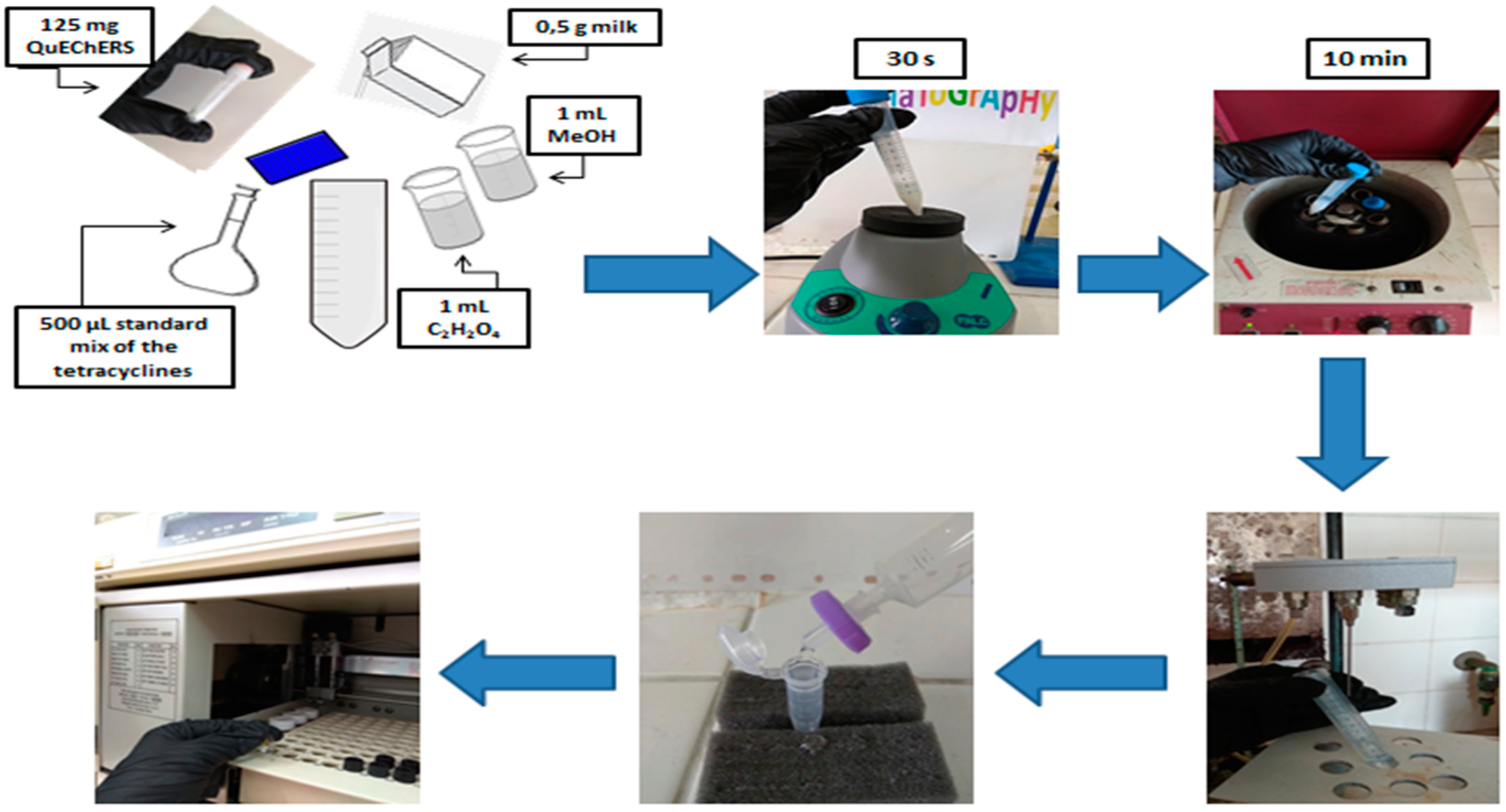
The QuEChERS method employed in this study was based on previous method developed in the Laboratory of Analytical Chemistry in the Chemistry Department of the Aristotle University of Thessaloniki in 2015 [
23]. The protocol used is as follows: an aliquot of 125 mg of QuEChERS material (25 mg of PSA, 25 mg of C18EC and 75 mg of magnesium sulfate) was placed in a falcon tube with 1 mL methanol, 1 mL ACN and 0.5 g of sample, which was spiked with 500 μL of a standard solution containing the target analytes. Then, the sample was vortexed for 30 s and centrifuged at 3500 rpm for 10 min. The supernatant was evaporated to dryness in water bath at 40 °C under a light stream of nitrogen, and the dry residue was dissolved in 500 μL in ultrapure water and was filtered prior to the injection into the HPLC system.
In the present study, the dispersive approach followed above, was optimized in terms of the type of extraction solvents, as shown in
Table 1. According to the optimized protocol: an aliquot of 125 mg of QuEChERS material was placed in a falcon tube with 1 mL methanol, 1 mL C
2H
2O
4 0.01M and 0.5 g skimmed milk (stored at +4 °C in the fridge), which was spiked with 500 μL of a standard solution containing OTC, TC, CTC and DC. High recovery rates were obtained with an exception of OTC, which shows lower recovery, attributed to its polarity, which leads to analyte losses during various steps. Then, the sample was vortexed for 30 s. Subsequently centrifugation was applied at 3500 rpm for 10 min. The supernatant was evaporated to dryness in water bath at 40 °C under a light stream of nitrogen, and the dry residue was dissolved in 500 μL in ultrapure water and was filtered prior to the HPLC analysis. The analytical procedure is shown in
Figure 1.
2.6. Method Validation
Validation of the developed method was carried out according to the European Union Decision 2002/657/EC [
22] using spiked samples, since no validated reference material was available. Selectivity, linearity, precision (within-day repeatability and between-day precision), accuracy, decision limit (CCα), detection capability (CCβ) and ruggedness were studied.
Linearity was examined using working standards at concentration levels between 1 and 20 ng/μL. In milk, linearity was studied using spiked samples covering the range between 50 μg/kg and 1000 μg/kg and calibration curves were calculated. The Limit of Detection (LOD) was calculated from the calibration curve, according to the formula:
and the Limit of Quantification (LOQ), according to the formula:
where S = signal and N = noise.
The selectivity of this method was expressed as lack of interference of endogenous compounds examined by the analysis of blank samples of milk. Precision and accuracy were calculated for OTC, TC, CTC and DC by analyzing spiked samples of milk at the concentration levels of 100 μg/kg, 150 μg/kg and 200 μg/kg. Within-day repeatability was examined by five measurements at the above concentration levels. Between-day precision was studied applying the same procedure in a period of three days. The recovery was calculated using the formula of the percentage of the ratio of the analyte mass that was found in the spiked sample, to the spiked mass. Calculation of the decision limit (CCα) was done by the MRL concentration (100 μg/kg) of the target analytes, as determined by the Regulation 37/2010 of the European Commission [
24], plus 1.64 times the SD of duplicate measurements of 20 samples at MRL, while calculation of the detection capability (CCβ) was based on CCα plus 1.64 times the SD of duplicate measurements of 20 samples spiked at levels of CCα.
The ruggedness of the method was assessed according to the Youden’s approach [
25]. For this purpose, eight different experiments are carried out with seven changes of the operating parameters (variables). The following parameters were altered: milk mass, QuEChERS mass, centrifugation time, vortex time, type and volume of organic solvents and evaporation temperature. Milk samples were spiked at 100 μg/kg and recovery of target analytes was estimated. Standard deviation was calculated according to the equation:
when SDi is significantly larger than the standard deviation of the method carried out under intermediate precision conditions, it can be concluded that all factors collectively have an effect on the result, even if every single factor does not show a significant influence, and that the method is not sufficiently robust against the modifications that are chosen. The investigated factors are reported in
Table 2, while their levels of variation are reported in
Table 3.
3. Results and Discussion
3.1. Chromatography
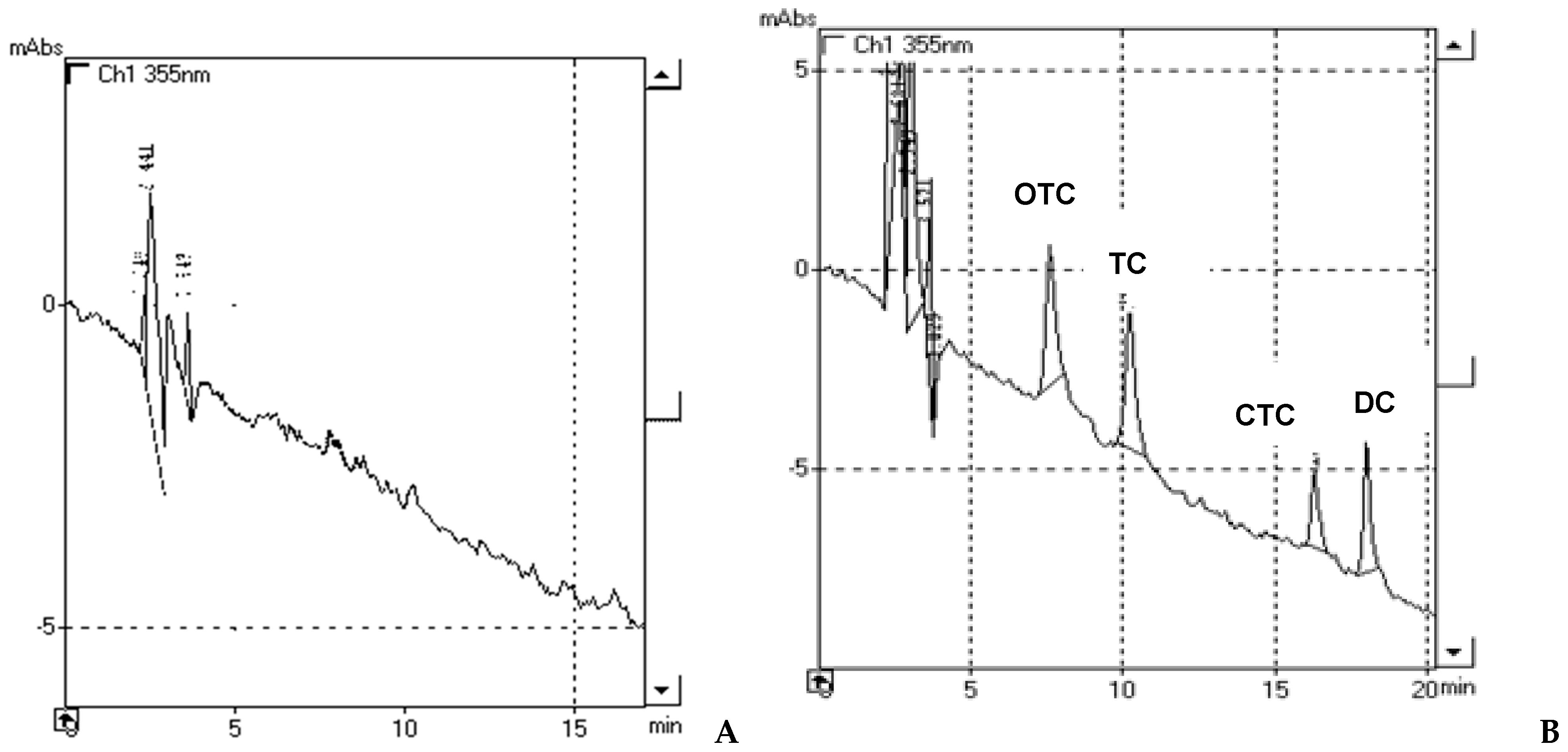
Quantitative analysis was performed on a HPLC system with a diode array detector (355 nm). The separation of OTC, TC, CTC and DC was achieved within 18 min. Retention time for the examined compounds were 6.150 min for OTC, 8.826 min for TC, 16.178 min for CTC and 18.070 min for DC.
3.2. Method Validation Results
3.2.1. Linearity and Sensitivity
The calibration curves of standard solution and spiked milk samples were linear with coefficient of determination values in the range from 0.9802 to 0.9999. The LOQ of the method was found at 50 μg/kg and the LOD at 15 μg/kg. The linearity was extended up to 1000 μg/kg.
Calibration curves for working standards were y = 82511x − 61,494 (R2 = 0.9986) for OTC, y = 90545x − 173,029 (R2 = 0.9999) for TC, y = 39009x − 32,087 (R2 = 0.9802) for CTC and y = 57256x − 50,800 (R2 = 0.9841) for DC. The respective calibration curves in spiked milk matrix were y = 3.3763x + 26,222 (R2 = 0.9965) for OTC, y = 6.0085x – 783.5 (R2 = 0.997) for TC, y = 4.1561x + 6468.6 (R2 = 0.9848) for CTC and y = 4.5491x + 12,389 (R2 = 0.9869) for DC.
3.2.2. Selectivity
Selectivity was investigated by the analysis of blank milk samples. No endogenous interferences were found. Typical chromatograms of blank and spiked milk samples are illustrated in
Figure 2.
3.2.3. Precision and Accuracy
The precision of the method was based on within-day repeatability and between-day reproducibility. The former was assessed by replicate (n = 5) measurements from three spiked samples of milk at 100, 150 and 200 μg/kg for the four tetracyclines. The spiked samples recoveries were calculated at three different concentrations by comparison of the peak area ratios for extracted compounds toward the values derived from calibration curves. Between-day reproducibility was determined using the same concentrations. Determination of each concentration in triplicates was performed for a period of three days. RSD values were <15.5% for all analytes. Recovery rates for each compound ranged as follows: for OTC 82.4%–101.8%, for TC 93.8%–105.9%, for CTC 83.07%–106.3% and for DC 94.7%–100.5%. Results are summarized in
Table 4.
3.2.4. Decision Limit and Detection Capability
Complying with the European Commission Regulation [
18], the CCα and CCβ were calculated after spiking 20 milk samples with OTC, TC, CTC and DC at their MRL level (100 μg/kg).
Table 5 shows the CCα and CCβ values for milk.
3.2.5. Ruggedness
A Youden test was applied to estimate ruggedness. Seven factors were selected due to their potential critical influence. These were centrifugation time, QuEChERS material quantity, evaporation temperature, milk weight, volume and type of solvents and vortex time. Blank milk samples spiked at 100 μg/kg were used for these eight experiments. A set of A-G indicates the nominal values for the seven selected factors that could potentially influence the results, if their nominal values are changed to some extent. Their alternative values are represented by the corresponding lower case letters a-g. Eight determinations are performed using a combination of the chosen factors (A-G). The letters “s-z” express the observed results as analyte amounts from each Youden experiment as shown in
Table 2. The results from eight relevant robustness experiments are reported in the same table. The extraction solvent that was used had a negative impact only for DC, which at the same time was the only one of the four tetracyclines that was influenced in a positive way from the quantity of the QuEChERS material. The vortex time had a negative impact for OTC and DC, while the milk weight had a negative influence for OTC and CTC. No negative effect was observed from the solvent volume and from the evaporation temperature as well. The only factor that had a negative impact for all the tetracyclines was the centrifugation time.
3.2.6. Application to Real Samples
The method was developed and optimized using skimmed milk, however in order to prove its applicability, it was further applied to semi-skimmed and full fat milk as well. It was proved to have no interference in all types of milk. Twelve samples (four of each category) were analyzed, and found to be negative with regards to the four tetracyclines.
4. Conclusions
The method developed and validated in this study is a simple, validated assay for the simultaneous determination of oxytetracycline, tetracycline, chlorotetracycline and doxycycline in milk by HPLC-DAD using QuEChERS dispersive extraction. It was validated according to European Union Decision 2002/657/EC in terms of selectivity, linearity, accuracy, precision, sensitivity and ruggedness. RSD values were <15.5% and recoveries achieved were in the range between 83.07% and 106.3%.
The performance characteristics of the method are comparable with those obtained by other methods, including those in which dispersive solid-phase microextraction [
6] and ultrasound-assisted dispersive extraction [
8] were applied for the determination of tetracycline residues in milk samples.
To the best of our knowledge, QuEChERS dispersive extraction methodology followed by HPLC-DAD analysis was applied for the first time for the determination of tetracyclines in milk.
The method is quick, easy, economic, and environmentally friendly, with simple instrumentation easily found in any laboratory.
Author Contributions
Methodology, E.M. and V.F.S.; Project administration, V.F.S.; Supervision, V.F.S. and I.N.P.; Validation, E.M.; Writing—original draft, E.M.; Writing—review, V.F.S. and I.N.P.; Editing and proof reading, V.F.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsai, W.-H.; Huang, T.-C.; Huang, J.-J.; Hsue, Y.-H.; Chuang, H.-Y. Dispersive solid-phase microextraction method for sample extraction in the analysis of four tetracyclines in water and milk samples by high-performance liquid chromatography with diode array detection. J. Chromatogr. A 2009, 1216, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Önal, A. Overview on liquid chromatographic analysis of tetracycline residues in food matrices. Food Chem. 2011, 127, 197–203. [Google Scholar] [CrossRef]
- Rodriguez, J.-A.; Espinosa, J.; Aguilar-Artega, K.; Ibarra, L.-S.; Miranda, J.-M. Determination of tetracyclines in milk samples by magnetic solid phase extraction flow injection analysis. Microchim. Acta 2010, 171, 407–413. [Google Scholar] [CrossRef]
- Spisso, B.-F.; Oliveira-Jesus, A.-L.; Gonçalves de Araújo Júnior, M.-A.; Alves-Monteiro, M. Validation of a high-performance liquid chromatographic method with fluorescence detection for the simultaneous determination of tetracyclines residues in bovine milk. Anal. Chim. Acta 2007, 581, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Q.; Yang, C.-X.; Yan, X.-P. Zeolite imidazolate framework-8 as sorbent for on-line solid-phase extraction coupled with high-performance liquid chromatography for the determination of tetracyclines in water and milk samples. J. Chromatogr. A 2013, 1304, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Meth. 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Tajabadi, F.; Chambarian, M.; Yamini, Y.; Yazdanfar, N. Combination of hollow fiber liquid phase microextraction followed by HPLC-DAD and multivariate curve resolution to determine antibacterial residues in foods of animal origin. Talanta 2016, 160, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.; Armeni, M.; Moschou, I.; Samanidou, V. Ultrasound-assisted dispersive extraction for the high pressure liquid chromatographic determination of tetracyclines residues in milk with diode array detection. Food Chem. 2014, 150, 328–334. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.-J.; Stajnbaher, D.; Schenck, F.-J. Fast and easy multi-residue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. JAOAC Int. 2003, 86, 412–431. [Google Scholar]
- Caldas, S.-S.; Bolzan, C.-M.; Cerqueira, M.-B.; Tomasini, D.; Furlong, E.-B.; Fagundes, C.; Primel, E.-G. Evaluation of a modified QuEChERS extraction of multiple classes of pesticides from a rice paddy soil by LC-APCI-MS/MS. J. Agric. Food Chem. 2011, 59, 11918–11926. [Google Scholar] [CrossRef]
- Sampaio, M.-R.-F.; Tomasini, D.; Cardoso, L.-V.; Caldas, S.-S.; Primel, E.-G. Determination of pesticides residues in sugarcane honey by QuEChERS and liquid chromatography. J. Braz. Chem. Soc. 2012, 23, 197–205. [Google Scholar] [CrossRef]
- Tomasini, D.; Sampaio, M.-R.-F.; Caldas, S.-S.; Buffon, J.-G.; Duarte, F.-A.; Primel, E.-G. Simultaneous determination of pesticides and 5-hydroxymethylfurfural in honey by the modified QuEChERS method and liquid chromatography coupled to tandem mass spectrometry. Talanta 2012, 99, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, D.-I.; Prestes, O.-D.; Adaime, M.-B.; Zanella, R. Development of a fast multiresidue method for the determination of pesticides in dry samples (wheat, grains, flour and bran) using QuEChERS based method and GC-MS. Food Chem. 2011, 125, 1436–1442. [Google Scholar] [CrossRef]
- Padilla-Sánchez, J.-A.; Plaza-Bolaños, P.; Romero-González, R.; Garrido-Frenich, A.; Martínez-Vidal, J.-L. Application af a quick, easy, cheap, effective, rugged and safe-based method for the simultaneous extraction of chlorophenols, alkylphenols, nitrophenols and cresols in agricultural soils, analyzed by using gas chromatography-triple quadrupole-mass spectrometry/mass spectrometry. J. Chromatogr. A 2010, 1217, 5724–5731. [Google Scholar] [PubMed]
- Salvia, M.-V.; Vulliet, E.; Wiest, L.; Baudot, R.; Cren-Olivé, C. Development of a multi-residue method using acetonitrile-based extraction followed by liquid chromatography-tandem mass spectrometry for the analysis of steroids and veterinary and human drugs at trace levels in soil. J. Chromatogr. A 2012, 1245, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Bragança, I.; Plácido, A.; Paíga, P.; Domingues, V.-F.; Delerue-Matos, C. QuEChERS: A new sample preparation approach for the determination of ibuprofen and its metabolites in soils. Sci. Total Environ. 2012, 433, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Pérez, J.-F.; Arroyo-Manzanares, N.; Havlíkova, L.; Gámiz-Gracia, L.; Solich, P.; García-Campaña, A.-M. Method optimization and validation for the determination of eight sulfonamides in chicken muscle and eggs by modified QuEChERS and liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 2016, 124, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Fan, C.L.; Cao, Y.F.; Wang, H.J.; Peng, X.; Wang, Z.B.; Chang, Q.Y.; Hu, X.Y.; Pang, G.F. Multi-residue screening of multi-class veterinary drugs in milk powder by liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Anal. Methods 2014, 6, 8337–8349. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Ren, J.; Guo, B. Development of a method for the analysis of multiclass antibiotic residues in milk using QuEChERS and liquid chromatography-tandem mass spectrometry. Foodborne Pathog. Dis. 2015, 12, 693–703. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Anizan, S.; Tien, M.M.; Savoy, M.C.; Bion, C. Determination of five tetracyclines and their epimers by LC-MS/MS based on a liquid-liquid extraction with low temperature partitioning. Food Add. Contamin. A 2018, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, B.; König, J.; Lesueur, C. Development and Validation of a Multi-class UHPLC-MS/MS Method for Determination of Antibiotic Residues in Dairy Products. Food Anal. Meth. 2018, 11, 1417–1434. [Google Scholar] [CrossRef]
- Eur-Lex. European commission decision 2002/657/EC. Off. J. Eur. Commun. 2002, 221, 8–36. [Google Scholar]
- Tsartsali, N.; Samanidou, V.-F. Sample preparation of eggs from laying hens using QuEChERS dispersive extraction for the simultaneous determination of melamine and cyromazine residues by HPLC-DAD. Anal. Chem. Ins. 2015, 10, 53–58. [Google Scholar] [CrossRef]
- Eur-Lex. European commission regulation 37/2010/EC. Off. J. Eur. Commun. 2010, 53, 1–72. [Google Scholar]
- Karageorgou, E.; Samanidou, V. Youden test application in robustness assays during method validation. J. Chromatogr. A 2014, 1353, 131–139. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).