1. Introduction
Generally, thallium can be found as thallium(I) and thallium(III) inorganic species, and the latter is considered thousand times more toxic [
1]. Because of its adverse effects on the biosphere and its accumulation in the environment, thallium has been identified as a metal of great toxicological interest. The main sources of thallium pollution are cement production, fossil fuel combustion, as well as the high-tech industries for semiconductor and electronics production [
2]. As thallium is toxic even at very low concentrations in environmental and biological systems, analytical techniques presenting high sensitivity and desirable selectivity are required.
The actual tendency in trace metal determination involves atomic spectrometry (AS) techniques, such as flame atomic absorption spectrometry (FAAS), electrothermal atomic absorption spectrometry (ETAAS), hydride generation atomic absorption spectrometry (HG-AAS), as well as inductively coupled plasma atomic emission spectrometry (ICP–AES). Flame atomic absorption spectrometry is widely used for routine analysis, presenting inherent advantages (low cost, operational facilities) and desirable characteristics (selectivity, low interferences). However, direct determinations are limited by lack of sensitivity in case of metal quantification, as metals are present in various matrices at very low concentrations. This limitation can be overcome by using preconcentration/separation techniques either in batch or in automatic mode. Batch methods suffer from high time and reagent/sample consumption, as well as the risk of analyte loss or contamination [
3]. On the other hand, the automation of analytical procedures overcomes the above limitations. In this context, flow-injection (FI) on-line column preconcentration is a valuable tool for the enhancement of AAS sensitivity and selectivity [
4].
Out of the different approaches suggested, on-line solid-phase extraction (on-line SPE) is one of the most convenient techniques which can be applied by incorporating a microcolumn packed with the appropriate sorbent material or a commercially available SPE cartridge, within the flow injection system [
5,
6,
7,
8]. Attention should be given to the column geometry and sorbent characteristics (functional groups, particle size, and breakthrough capacity). In other words, the selected sorbent should fulfill a variety of criteria in order to accomplish the preconcentration/separation [
9].
A limited number of methods have been reported adapting on-line SPE with AAS to determine thallium using various stationary phases. Quinolin-8-ol on controlled pore glass beads [
10], oxine immobilized on coated alumina [
11], and thallium(III) ion-imprinted polymer particles [
12] were used in FI–FAAS systems for thallium determination, while in the case of FI–ETAAS, titanium dioxide particles [
13], multiwalled carbon nanotubes (MWCNs) [
14], and dibenzo-18-crown-6 (DB18C6) immobilized on surfactant-coated alumina [
15] have been adopted.
In our previous works, preconcentration columns packed with poly-tetrafluoroethylene (PTFE) turnings were constructed and used for on-line Cu(I), Co(II), Pb(II), Cd(II), Hg(II), Cr(VI), As(III), Ag(I), Ni(II), and Zn(II) determination, by either FAAS or ETAAS, using various combinations of samples that had satisfactory analytical performance characteristics like sensitivity, precision, and enrichment factor. The proposed column is low-cost, hand-made, and manufactured in the laboratory, thereby providing a large effective surface, low backpressure, high sample loading flow rates, and strong retention of hydrophobic complexes. Moreover, the excellent chemical resistance and the physical properties of the PTFE material result in a practically unlimited lifetime of the column, without the need of regeneration or repacking, thereby remaining stable during the preconcentration and elution steps [
16,
17,
18].
In this work, a FI on-line column preconcentration system for thallium determination was developed. Poly-tetrafluoroethylene (PTFE) turnings were used as the sorbent material for the retention of thallium complexes. To the best of our knowledge, PTFE turnings were used for the first time in an on-line preconcentration system for the determination of thallium. Thallium(Ι) was oxidized to thallium(III) by adding a small amount of saturated bromine solution in the presence of nitric acid, prior complexation with sodium diethyldithiocarbamate (DDTC). The on-line formed complex was retained onto the PTFE turnings and subsequently eluted with methyl isobutyl ketone (MIBK) before quantification. The proposed method was optimized and applied for the analysis of natural waters and urine samples.
2. Materials and Methods
2.1. Instrumentation
A Perkin-Elmer Model 5100 PC flame atomic absorption spectrometer (Perkin-Elmer, Norwalk, CT, USA) with deuterium background corrector was furnished with a thallium electrodeless discharge lamp (EDL) operated at 7 W. The wavelength was set to 276.8 nm, and the monochromator spectral bandpass (slit) at 0.7 nm. A standard air-acetylene burner with a flow spoiler into the spray chamber was operated with oxidizing flame at 10.0 L min−1 air flow rate and 1.0 L min−1 acetylene flow rate. Under the above conditions, the nebulizer aspiration rate was 5.5 mL min−1. Peak height was used for signal evaluation throughout the study.
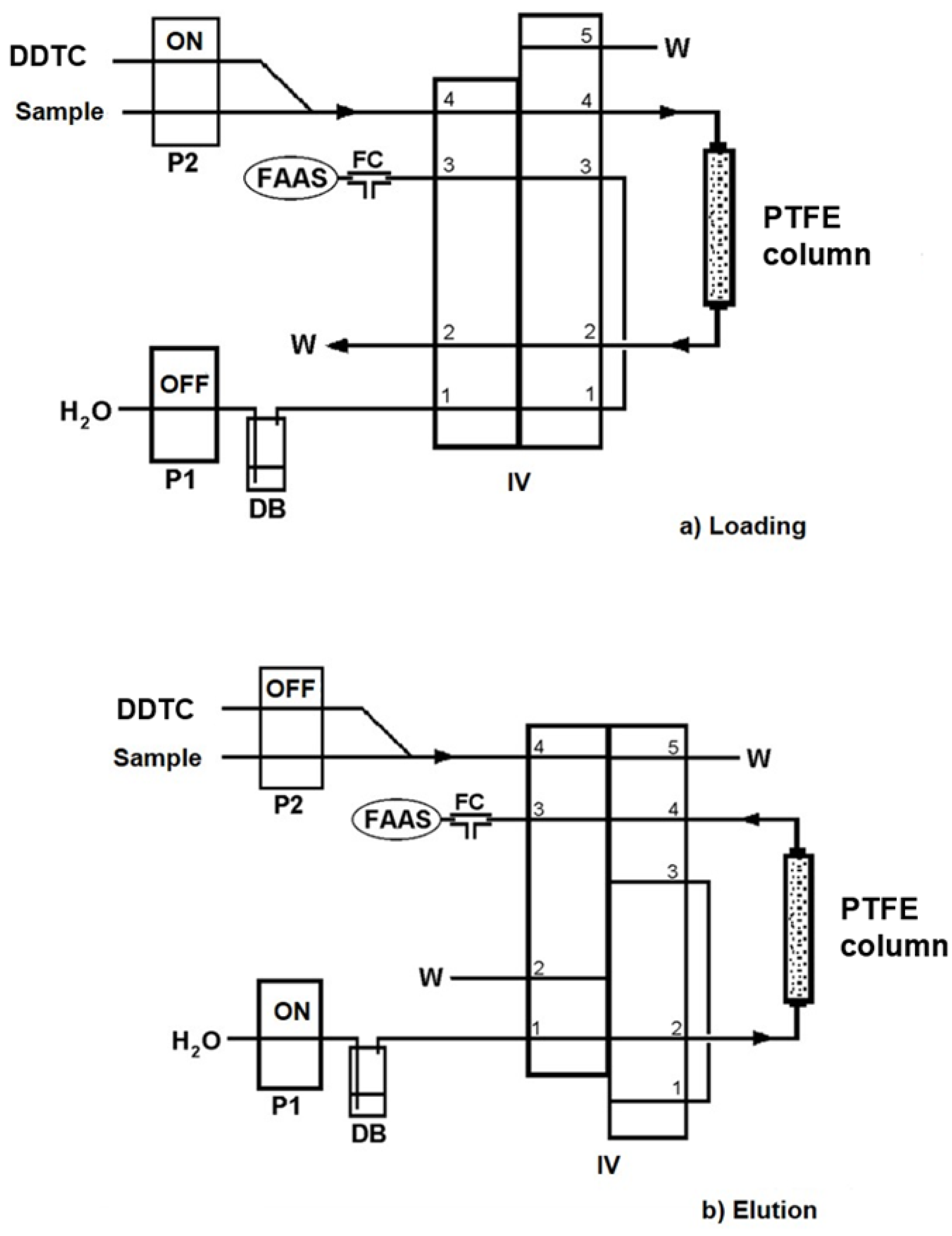
A Perkin-Elmer (Norwalk, CT, USA) Model FIAS-400 flow-injection analysis system (
Figure 1) was coupled to the nebulizer system of the FAAS spectrometer operated in preconcentration mode. The FIAS-400 manifold consisted of two peristaltic pumps, P1 and P2, equipped with Tygon peristaltic tubing, a five-port two-position injection valve (IV), and a packed column with PTFE turnings for the on-line preconcentration of the target analyte. The FAAS and FIAS-400 flow systems were controlled by a personal computer running the AA Lab Benchtop version 7.2 software. A “T” type mixing device before the inlet of the nebulizer was adopted as a flow compensation (FC) unit between the elution flow rate (2.6 mL min
−1) and the nebulizer aspiration rate (5.5 mL min
−1). A displacement bottle, DB, (Tecator, Hoganas, Sweden) was used for the delivery of methyl isobutyl ketone (MIBK), as this organic solvent is not compatible with Tygon peristaltic tubing.
The proposed column was constructed by using a glass syringe tube with an appropriate length of 54 mm and an inner diameter of 4.0 mm. PTFE turnings were mechanically produced, using a lathe, and an amount of 850 mg with a mean width of 0.1 mm was firmly packed into the column. No frits or glass wool were necessary at either end of the column to block the PTFE turnings. The image of the PTFE turnings using an optical microscope was shown previously [
16]. The turnings were thoroughly washed by ethanol followed by 1 M HNO
3 and finally with de-ionized water. The column was initially flushed with de-ionized water and subsequently with MIBK, without the need of pre-conditioning or stabilizing procedures. The experimental study showed that the performance of the column was stable, repeatable, and unaffected by continuous use for at least 600 sorption/elution cycles.
2.2. Reagents and Samples
All reagents were of analytical reagent grade available from Merck (Darmstadt, Germany), while doubly de-ionized water was used throughout the study. Working standard solutions of thallium(I) were prepared daily by appropriate stepwise dilution of a 1000 mg L−1 thallium(I) stock standard solution in 0.5 mol L−1 HNO3 (Titrisol, Merck) to the required microgram per liter levels. The acidity of the standards was adjusted with dilute HNO3. The chelating reagent, 0.05% m/v sodium diethyl dithiocarbamate (DDTC) (Merck), was prepared daily by dissolving the appropriate amount in de-ionized water. Methyl isobutyl ketone (MIBK) was used to elute the retained complexes after saturation with de-ionized water. A saturated solution of bromine (Fluka, Buchs, Switzerland) was prepared in de-ionized water in order to oxidize thallium(I) into thallium(III). Suprapure concentrated acids (65% m/m HNO3, 40% m/m HF and 70% m/m HClO4) were used for sample digestion and adjustment of sample acidity.
Two certified reference materials were investigated: NIST 1643e, containing trace elements in water, and SRM 2704 Buffalo River Sediment (National Institute of Standard and Technology, NIST, Gaithersburg, MD, USA). The proposed method was applied for the determination of thallium in aqueous samples: tap water (from Thessaloniki) and river water (Axios river, Northern Greece). All samples were filtered through 0.45 μm membrane filters, acidified to 0.01 mol L−1 HNO3, stored at 4 °C in acid-cleaned polyethylene bottles, and then used to determine the “dissolved metal” fraction. A urine sample (600 mL) provided by the researchers themselves on a voluntary basis, was also analyzed after a wet-digestion procedure.
The sediment sample (SRM 2704) was digested using a mixture of HNO3–HClO4–HF, while, for the urine sample, concentrated HNO3 was used. Approximately 0.3 g of river sediment was precisely weighed into sealed Teflon crucibles and wetted by a mixture of HNO3–HClO4–HF in a volume ratio of 3:2:1. The digestion procedure was carried out at 130–140 °C into a stainless-steel pressurized bomb according to the manufacturer’s recommendations. After cooling the system, the digests were properly diluted in doubly de-ionized water, and the resulting solutions were used for the analysis.
2.3. FI–SPE–FAAS Analytical Procedure
A schematic diagram of the on-line flow-injection column preconcentration (on-line FI–SPE) system for thallium determination by FAAS is presented in
Figure 1. The operational sequences of the FI–SPE–FAAS method are presented in
Table 1 and run through 3 steps. In step 1 (
Figure 1a), the injection valve (IV) was in the “Load” position, and pump 2 (P2) was activated for the propulsion of the mixture of sample and chelating reagent into the column at a flow rate of 12.4 mL min
−1 for a preconcentration time of 60 s (see
Table 1). The sample was mixed on-line with DDTC, and the formed complex was absorbed quantitatively onto the hydrophobic surface of the PTFE turnings. In step 2 (
Figure 1b), the injection valve (IV) switched to the “Elute” position, and pump 1 (P1) was activated for the delivery of MIBK through the column at a flow rate of 2.6 mL min
−1 for 30 s in order to effectively elute the preconcentrated complex and introduce it into the FAAS nebulizer for measurement and quantification. MIBK flowed in the reverse direction compared with the sample and chelating reagent, in order to minimize the dispersion of the eluted analyte. During step 2, P2 remained inactive to avoid additional consumption of the sample solution. Five replicate measurements were made throughout the study.
3. Results and Discussion
Chemical and flow parameters affecting the sensitivity of the proposed method were thoroughly studied on the basis of the univariate methodology, so that the optimum analytical conditions could be attained. A standard aqueous solution of thallium(I) at 100.0 μg L−1 concentration in the presence of 0.3% v/v bromine solution was used for all optimization studies, with a preconcentration time of 60 s.
3.1. Selection of Chelating Reagent
Dithiocarbamates comprise an important class of chelating reagents for heavy metals in trace analysis. Diethyldithiocarbamate (DDC) and pyrrolidinedithiocarbamate (PDC) are two of its derivatives commonly used in routine analysis [
19]. As free bases, dithiocarbamates have a good water solubility, while when forming a complex with a metal cation, their solubility becomes low. Dithiocarbamate salts, like ammonium pyrrolidinedithiocarbamate (APDC) and sodium diethyl dithiocarbamate (DDTC), provide a stable form of the dithiocarbamate structure and are commercially available at low cost. A remarkable feature of metal complexes of dithiocarbamates is their hydrophobic nature, resulting in their efficient sorption on non-polar surfaces of various sorbent materials.
In this work, DDTC and APDC aqueous solutions at a concentration of 0.05% (m/v) were studied. The obtained results showed that DDTC presented better sensitivity, as the absorbance was approximately 10% higher than that obtained when using APDC. Thus, DDTC was adopted for further experiments. At higher concentrations of DDTC, the absorbance remained practically stable, whereas, in the absence of DDTC, no detectable amount of target analyte was measured.
3.2. Effect of Sample Acidity and Bromine Concentration
It is well known that thallium(I) is distinctly more stable than thallium(III) in aqueous solutions, while the latter is rapidly reverted to thallium(I) [
20]. To avoid reduction, an oxidant such as aqueous bromine in acidic solution is necessary [
21]. Thallium (I) was oxidized to thallium(III) with dilute HNO
3 and a small amount of bromine solution, resulting in the formation of a stable [TlBr
4]
− anionic bromo complex. From preliminary experiments, it was proved that thallium(III) in the form of [TlBr
4]
− anionic bromo complex was efficiently retained in the presence of DDTC onto the PTFE turnings, while the retention of thallium(I) was very poor (<10%).
One critical parameter for the formation of Tl(III)-DDTC complex and its retention onto the sorbent surface is sample acidity. Herein, sample acidity was studied using variable concentrations of HNO
3 ranging from 0.0001 to 0.5 mol L
−1. The recorded absorbance reached a plateau level in the range of 0.01–0.001 mol L
−1 HNO
3. A concentration of 0.01 mol L
−1 HNO
3 was finally selected for further experiments, as this is the most frequently recommended by standard methods for sample preservation during the analysis of trace elements [
19].
The bromine concentration was studied in the range up to 0.5% v/v. In the absence of bromine, a very low absorbance was recorded, while a sharp increase was observed by adding small amounts of bromine up to 0.3% v/v, proving that the bromine solution is necessary for maintaining thallium in its highest oxidizing state, thallium(III). For higher concentrations, the absorbance remained stable. Finally, a concentration of 0.3% v/v was finally adopted for further experiments.
3.3. Selection of Eluent
The key property that has encouraged the extensive use of MIBK as an eluent is the high sensitivity provided in FAAS. Good combustion properties, low miscibility with water, as well as hydrophobicity are characteristics that make MIBK an ideal organic solvent for efficient elution of the retained formed complex, resulting in the production of higher and sharper signals than those provided by other extractant solvents. In addition, during the elution step, the dispersion of the preconcentrated analyte is much lower. Thus, MIBK was chosen as the extractant solvent in the proposed method.
3.4. Study of Loading and Elution Flow Rates
The effect of total loading flow rate (sample + DDTC solution) on the absorbance was studied in the range of 4.8–12.4 mL min−1. In all cases, the ratio of sample flow rate to DDTC flow rate was fixed at approximately 9. An almost linear pattern of increased absorbance was observed in the above-examined range, proving that the contact time for thallium was adequate and the formation of the complex was fast. For higher sensitivity, a total loading flow rate of 12.4 mL min−1 (11.2 for sample and 1.2 mL min−1 for DDTC) was adopted.
The elution flow rate was examined in the range of 1.8–4.4 mL min−1. Maximum signals were recorded at flow rates between 2.6 and 3.6 mL min−1. For lower flow rates, the absorbance was decreased probably because of the insufficient elution of the preconcentrated complex, while for higher flow rates, the absorbance remained practically stable. Finally, a flow rate of 2.6 mL min−1 was adopted for MIBK in further experiments.
3.5. Effect of Preconcentration Time
The preconcentration time was studied in the range of 30–180 s. It was proved that the integrated absorbance of thallium increased almost linearly with the increase of time, thereby indicating that the higher preconcentration time, the higher analytical signals recorded. This fact proved that no negative effect was produced from partial leaching of the complex, even during long loading times and prolonged sample flow. Taking into account the sensitivity, time of analysis, and sample consumption, a preconcentration time of 60 s was adopted in the proposed method.
3.6. Interference Studies
The effect of potential interferences from various coexisting metals and other ions was tested on the recovery of 50.0 μg L−1 thallium, using the proposed on-line system for 60 s preconcentration time. A variation of the recovery higher than ±5% was considered as interference. The results revealed that Cu(II), Cd(II), and Hg(II) were tolerated up to 2 mg L−1, while Co(II), Fe(III), Mn(II), Ni(II), and Zn(II) were tolerated up to 5 mg L−1. In addition, alkaline and alkaline–earth metals like Na(I), K(I), Ca(II), Mg(II), and Ba(II) did not interfere at concentrations at least up to 800 mg L−1, and SO4−2, NO3−, HCO3− up to 1000 mg L−1.
4. Analytical Performance Data and Application in Certified Reference Materials and Real Samples
Under the optimized conditions described above, the analytical performance data of the proposed method are summarized in
Table 2. For a preconcentration time of 60 s, the sampling frequency was 40 h
−1. The detection limit was calculated by the 3
s criterion and was found to be 1.93 μg L
−1. The precision, expressed as relative standard deviation (RSD) at 50.0 μg L
−1 thallium(I) concentration, was 3.2%. The regression equation was A = (0.0018 ± 0.0089) + (0.0012 ± 0.0001) [Tl(I)], with a determination coefficient of 0.9959, while the linear range was between 6.4–200 μg L
−1. By direct aspiration of aqueous standard solutions into FAAS, without preconcentration, the regression equation was calculated as A = (0.0032 ± 0.0152) + (0.0114 ± 0.0009) [Tl(I)] (mg L
−1, n = 5). The enhancement factor, calculated as the slope ratio of the calibration curves with and without preconcentration procedure, was found to be 105.
The figures of merit of the proposed work and other published on-line SPE methods with FAAS [
10,
11,
12] are summarized for comparative purposes in
Table 3. The proposed method features good sensitivity and precision, with better detection limit than earlier works [
10,
11] using a shorter preconcentration time. Only one method shows a comparable detection limit but with higher preconcentration time and sample volume consumption [
12].
In order to evaluate the accuracy of the proposed method, two certified reference materials, SRM 1643e and SRM 2704, were analyzed. The student
t test revealed that no statistically significant differences were found at the 95% probability level, as all
texp values were lower than
tcrit,95% = 4.30, as shown in
Table 4. The proposed method was also applied for the determination of thallium in tap water, river water, and urine samples, and results are presented in
Table 5. The obtained recoveries varied within the range 94.0–104.0%, showing good applicability of the method in such types of samples.