Retention Behaviour of Alkylated and Non-Alkylated Polycyclic Aromatic Hydrocarbons on Different Types of Stationary Phases in Gas Chromatography
Abstract
1. Introduction
2. Materials and Methods
3. Results and discussion
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keith, L.H. The Source of US EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out153_en.pdf (accessed on 28 January 2019).
- Available online: http://apps.who.int/iris/bitstream/handle/10665/43258/WHO_TRS_930_eng.pdf?sequence=1 (accessed on 28 January 2019).
- Ding, Y.S.; Trommel, J.S.; Yan, X.Z.J.; Ashley, D.; Watson, C.H. Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from domestic cigarettes. Environ. Sci. Technol. 2005, 39, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Dagnac, T.; Llompart, M. Determination of priority and other hazardous substances in football fields of synthetic turf by gas chromatography-mass spectrometry: A health and environmental concern. Chemosphere 2018, 195, 201–211. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; Law, R.J. Developments in the use of chromatographic techniques in marine laboratories for the determination of halogenated contaminants and polycyclic aromatic hydrocarbons. J. Chromatogr. A 2003, 1000, 223–251. [Google Scholar] [CrossRef]
- Poster, D.L.; Schantz, M.M.; Sander, L.C.; Wise, S.A. Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: A critical review of gas chromatographic (GC) methods. Anal. Bioanal. Chem. 2006, 386, 859–881. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.A.; Sander, L.C.; Schantz, M.M. Analytical Methods for Determination of Polycyclic Aromatic Hydrocarbons (PAHs)—A Historical Perspective on the 16 US EPA Priority Pollutant PAHs. Polycycl. Aromat. Compd. 2015, 35, 187–247. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/sites/production/files/2015-10/documents/method_610_1984.pdf (accessed on 28 January 2019).
- Gomez-Ruiz, J.A.; Wenzl, T. Evaluation of gas chromatography columns for the analysis of the 15+1 EU-priority polycyclic aromatic hydrocarbons (PAHs). Anal. Bioanal. Chem. 2009, 393, 1697–1707. [Google Scholar] [CrossRef]
- Nalin, F.; Sander, L.C.; Wilson, W.B.; Wise, S.A. Gas chromatographic retention behavior of polycyclic aromatic hydrocarbons (PAHs) and alkyl-substituted PAHs on two stationary phases of different selectivity. Anal. Bioanal. Chem. 2018, 410, 1123–1137. [Google Scholar] [CrossRef]
- Wilson, W.B.; Sander, L.C.; Ona-Ruales, J.O.; Moessner, S.G.; Sidisky, L.M.; Lee, M.L.; Wise, S.A. Retention behavior of alkyl-substituted polycyclic aromatic sulfur heterocycle isomers in gas chromatography on stationary phases of different selectivity. J. Chromatogr. A 2017, 1484, 73–84. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W. Immobilized ionic liquids as high-selectivity/high-temperature/high-stability gas chromatography stationary phases. Anal. Chem. 2005, 77, 6453–6462. [Google Scholar] [CrossRef]
- Poole, C.F.; Poole, S.K. Ionic liquid stationary phases for gas chromatography. J. Sep. Sci. 2011, 34, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Ruiz-Angel, M.J.; Huguet, S. Nonmolecular solvents in separation methods: Dual nature of room temperature ionic liquids. Anal. Chem. 2005, 77, 4071–4080. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; Blok, D.; Ballesteros-Gomez, A. Assessment of ionic liquid stationary phases for the determination of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers. J. Chromatogr. A 2014, 1348, 158–163. [Google Scholar] [CrossRef]
- Ros, M.; Escobar-Arnanz, J.; Sanz, M.L.; Ramos, L. Evaluation of ionic liquid gas chromatography stationary phases for the separation of polychlorinated biphenyls. J. Chromatogr. A 2018, 1559, 156–163. [Google Scholar] [CrossRef]
- Antle, P.M.; Zeigler, C.D.; Wilton, N.M.; Robbat, A. A more accurate analysis of alkylated PAH and PASH and its implications in environmental forensics. Int. J. Environ. Anal. Chem. 2014, 94, 332–347. [Google Scholar] [CrossRef]
- Stout, S.A.; Emsbo-Mattingly, S.D.; Douglas, G.S.; Uhler, A.D.; McCarthy, K.J. Beyond 16 Priority Pollutant PAHs: A Review of PACs used in Environmental Forensic Chemistry. Polycycl. Aromat. Compd. 2015, 35, 285–315. [Google Scholar] [CrossRef]
- Lam, M.M.; Bulow, R.; Engwall, M.; Giesy, J.P.; Larsson, M. Methylated PACs Are More Potent Than Their Parent Compounds: A Study of Aryl Hydrocarbon Receptor-Mediated Activity, Degradability, and Mixture Interactions in the H4IIE-luc Assay. Environ. Toxicol. Chem. 2018, 37, 1409–1419. [Google Scholar] [CrossRef]
- Brack, W.; Schirmer, K.; Erdinger, L.; Hollert, H. Effect-directed analysis of mutagens and ethoxyresorufin-O-deethylase inducers in aquatic sediments. Environ. Toxicol. Chem. 2005, 24, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Kaisarevic, S.; Luebcke-von Varel, U.; Orcic, D.; Streck, G.; Schulze, T.; Pogrmic, K.; Teodorovic, I.; Brack, W.; Kovacevic, R. Effect-directed analysis of contaminated sediment from the wastewater canal in Pancevo industrial area, Serbia. Chemosphere 2009, 77, 907–913. [Google Scholar] [CrossRef]
- Meyer, W.; Seiler, T.-B.; Christ, A.; Redelstein, R.; Puettmann, W.; Hollert, H.; Achten, C. Mutagenicity, dioxin-like activity and bioaccumulation of alkylated picene and chrysene derivatives in a German lignite. Sci. Total Environ. 2014, 497, 634–641. [Google Scholar] [CrossRef]
- Xiao, H.; Krauss, M.; Floehr, T.; Yan, Y.; Bahlmann, A.; Eichbaum, K.; Brinkmann, M.; Zhang, X.; Yuan, X.; Brack, W. Effect-Directed Analysis of Aryl Hydrocarbon Receptor Agonists in Sediments from the Three Gorges Reservoir, China. Environ. Sci. Technol. 2016, 50, 11319–11328. [Google Scholar] [CrossRef] [PubMed]
- Arp, H.P.H.; Azzolina, N.A.; Cornelissen, G.; Hawthorne, S.B. Predicting Pore Water EPA-34 PAH Concentrations and Toxicity in Pyrogenic-Impacted Sediments Using Pyrene Content. Environ. Sci. Technol. 2011, 45, 5139–5146. [Google Scholar] [CrossRef] [PubMed]
- Richter-Brockmann, S.; Achten, C. Analysis and toxicity of 59 PAH in petrogenic and pyrogenic environmental samples including dibenzopyrenes, 7H-benzo c fluorene, 5-methylchrysene and 1-methylpyrene. Chemosphere 2018, 200, 495–503. [Google Scholar] [CrossRef]
- Available online: https://clu-in.org/conf/tio/porewater1/resources/EPA-ESB-Procedures-PAH-mixtures.pdf (accessed on 28 January 2019).
- Skoczynska, E.; Leonards, P.; de Boer, J. Identification and quantification of methylated PAHs in sediment by two-dimensional gas chromatography/mass spectrometry. Anal. Methods 2013, 5, 213–218. [Google Scholar] [CrossRef]
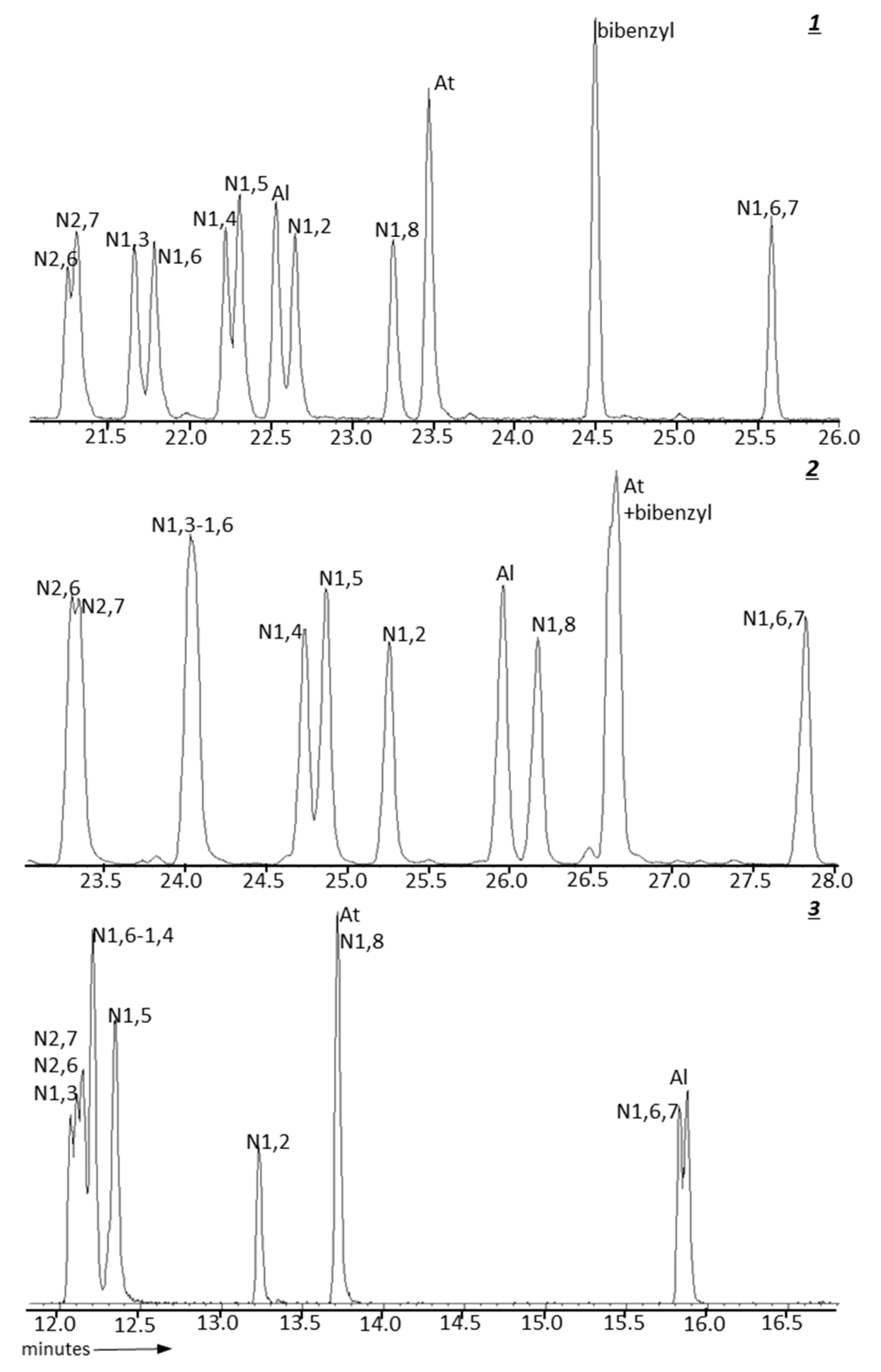
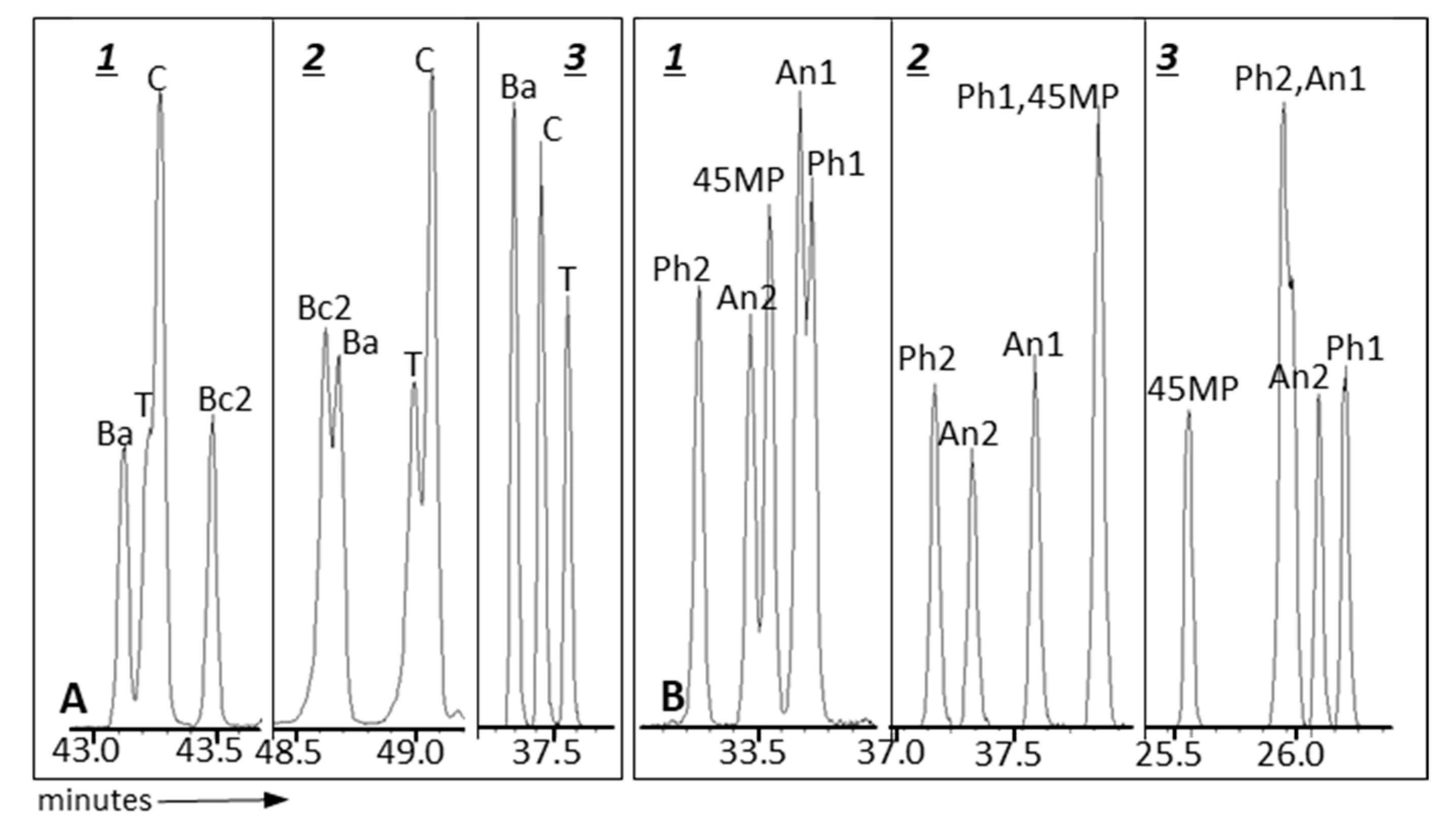
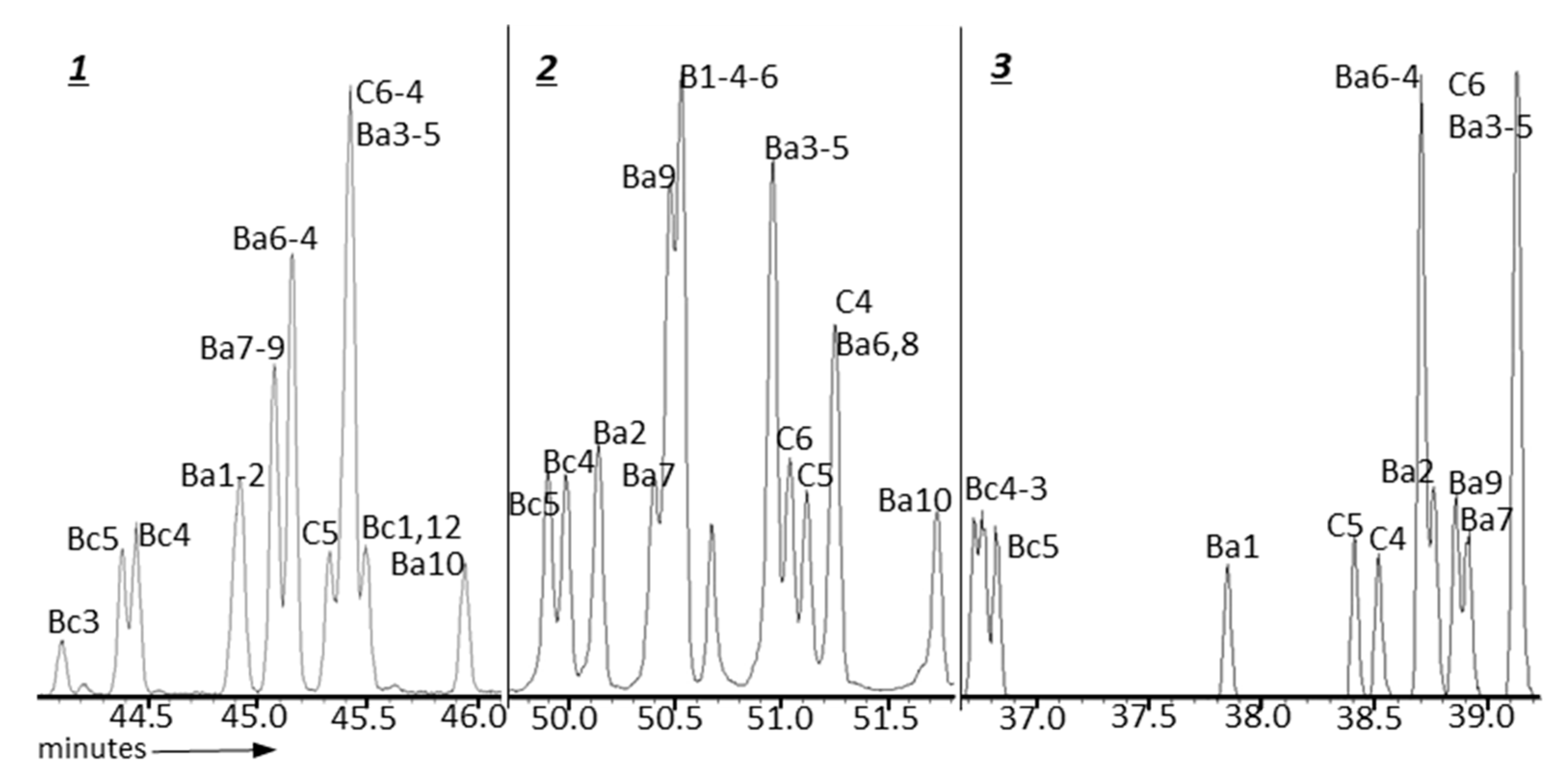
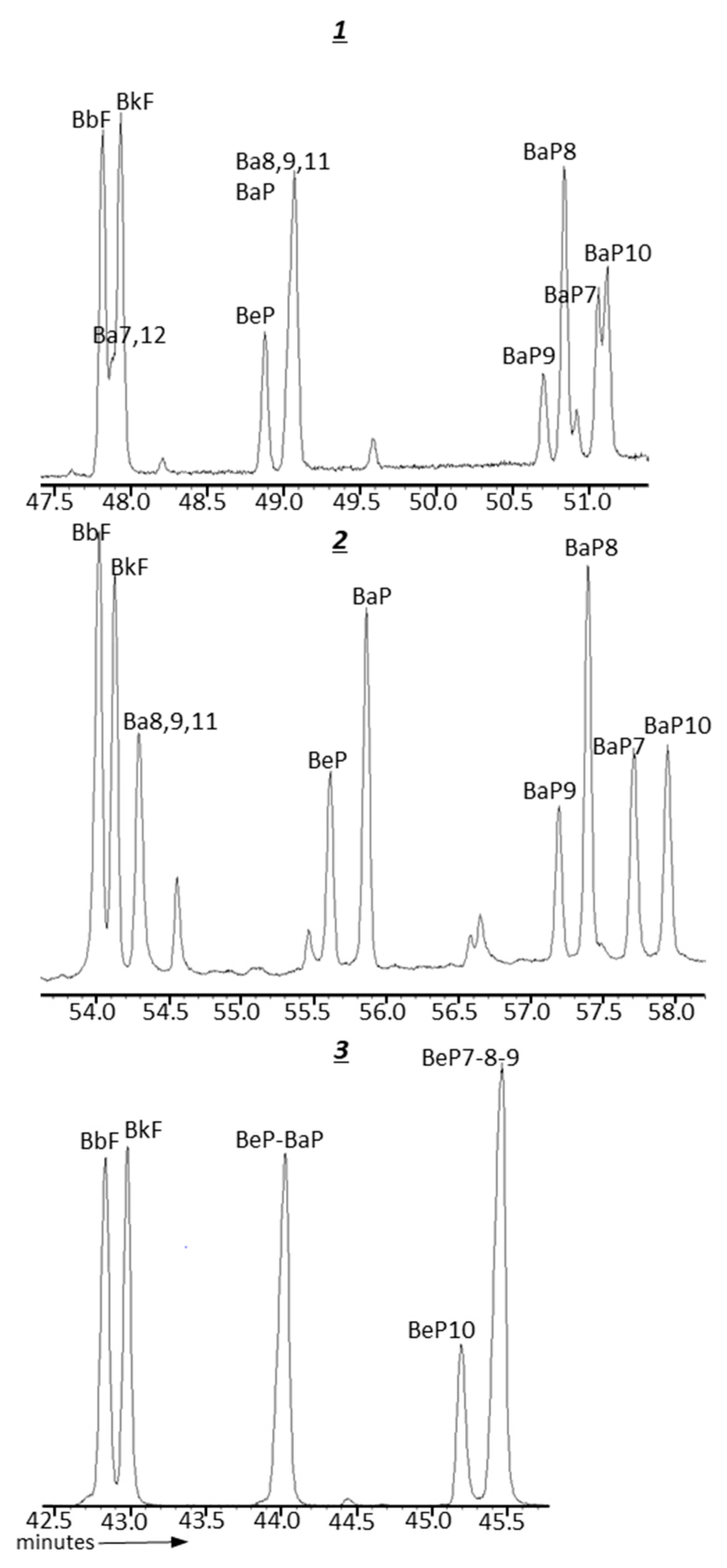
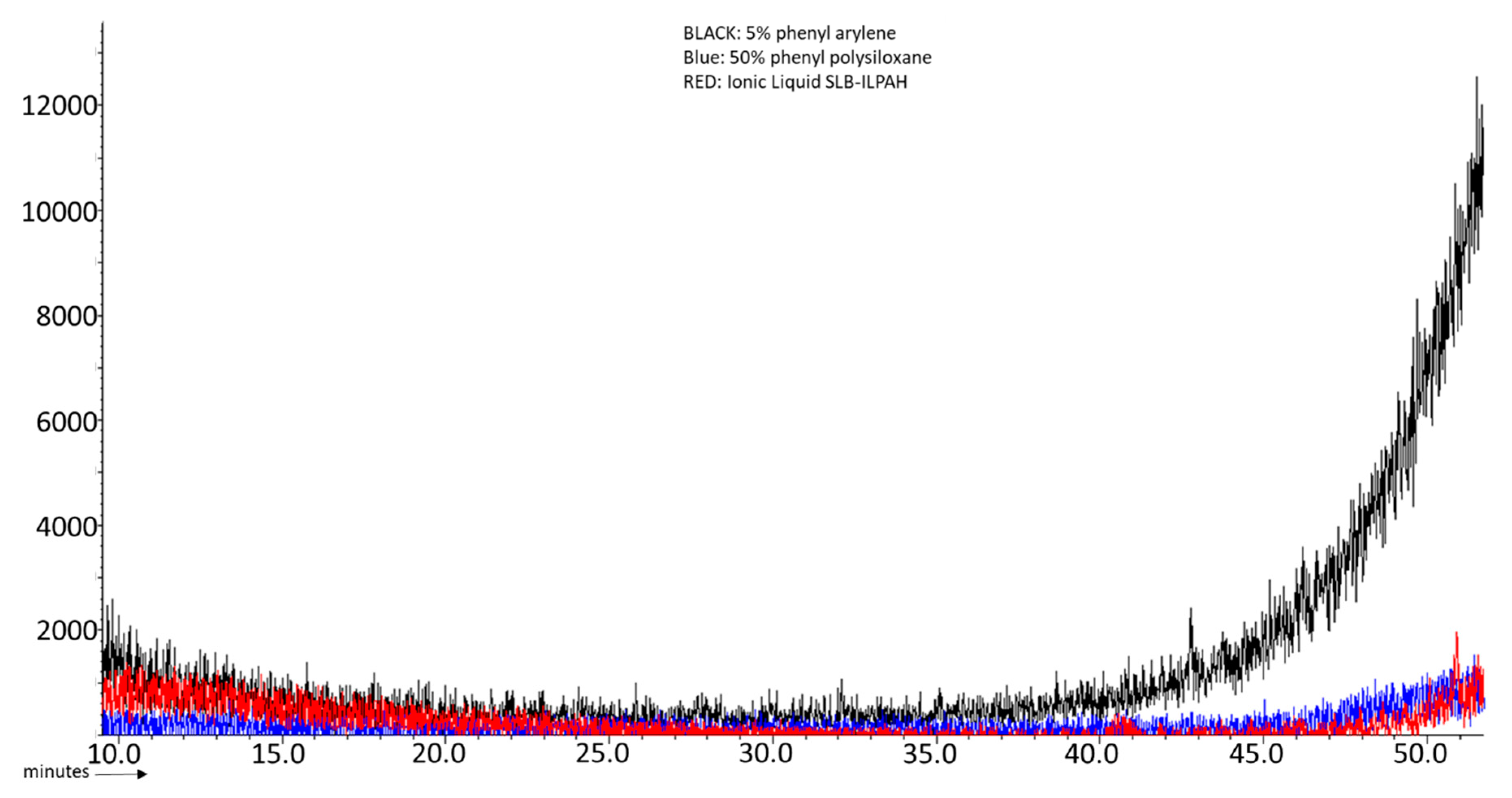
| GC Column | Stationary Phase | Dimensions | Max. temp. (Isotherm/Programmed) °C |
|---|---|---|---|
| VDB-5ms | Phenyl Arylene polymer, virtually equivalent to 5%-phenyl-methylpolysiloxane | 30 m × 0.25 mm ID × 0.25 µm | 300/320 °C |
| SLB PAHms (Supelco) | Denoted as 50% phenyl dimethylpolysiloxane | 30 m × 0.25 mm ID × 0.25 µm | 350/360 °C |
| SLB®-ILPAH (Supelco) | Non-bonded, 1,12-Di(tripropylphosphonium) dodecane bis(trifluoromethanesulfonyl)imide | 20 m × 0.18 mm ID × 0.05 µm | 300/300 °C |
| Code | DB-5ms | RT | RRT | Code | SLB PAHms | RT | RRT | Code | SLB-ILPAH | RT | RRT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Naphthalene | 13.20 | 0.353 | N | Naphthalene | 16.12 | 0.379 | N | Naphthalene | 5.86 | 0.188 |
| N2 | 2-Methylnaphthalene | 17.65 | 0.472 | N2 | 2-Methylnaphthalene | 20.03 | 0.471 | N2 | 2-Methylnaphthalene | 9.13 | 0.292 |
| N1 | 1-Methylnaphthalene | 18.19 | 0.486 | N1 | 1-Methylnaphthalene | 20.82 | 0.489 | N1 | 1-Methylnaphthalene | 9.33 | 0.299 |
| N2,6 | 2,6-Dimethylnaphthalene | 21.25 | 0.568 | N2,6 | 2,6-Dimethylnaphthalene | 23.31 | 0.548 | N2,7 | 2,7-Dimethylnaphthalene | 12.07 | 0.386 |
| N2,7 | 2,7-Dimethylnaphthalene | 21.31 | 0.570 | N2,7 | 2,7-Dimethylnaphthalene | 23.35 | 0.549 | N2,6 | 2,6-Dimethylnaphthalene | 12.11 | 0.388 |
| N1,3 | 1,3-Dimethylnaphthalene | 21.66 | 0.579 | N1,3 | 1,3-Dimethylnaphthalene | 24.04 | 0.565 | N1,3 | 1,3-Dimethylnaphthalene | 12.14 | 0.389 |
| N1,6 | 1,6-Dimethylnaphthalene | 21.78 | 0.583 | N1,6 | 1,6-Dimethylnaphthalene | 24.05 | 0.565 | N1,6 | 1,6-Dimethylnaphthalene | 12.21 | 0.391 |
| N1,4 | 1,4-Dimethylnaphthalene | 22.22 | 0.594 | N1,4 | 1,4-Dimethylnaphthalene | 24.73 | 0.581 | N1,4 | 1,4-Dimethylnaphthalene | 12.21 | 0.391 |
| N1,5 | 1,5-Dimethylnaphthalene | 22.31 | 0.597 | N1,5 | 1,5-Dimethylnaphthalene | 24.87 | 0.584 | N1,5 | 1,5-Dimethylnaphthalene | 12.34 | 0.395 |
| Al | Acenaphthylene | 22.53 | 0.603 | N1,2 | 1,2-Dimethylnaphthalene | 25.26 | 0.593 | N1,2 | 1,2-Dimethylnaphthalene | 13.23 | 0.424 |
| N1,2 | 1,2-Dimethylnaphthalene | 22.65 | 0.606 | Al | Acenaphthylene | 25.96 | 0.610 | N1,8 | 1,8-Dimethylnaphthalene | 13.72 | 0.439 |
| N1,8 | 1,8-Dimethylnaphthalene | 23.25 | 0.622 | N1,8 | 1,8-Dimethylnaphthalene | 26.17 | 0.615 | At | Acenaphthene | 13.72 | 0.439 |
| At | Acenaphthene | 23.48 | 0.628 | At | Acenaphthene | 26.66 | 0.626 | N1,6,7 | 1,6,7-Trimethylnaphthalane | 15.83 | 0.507 |
| N1,6,7 | 1,6,7-Trimethylnaphthalane | 25.59 | 0.684 | N1,6,7 | 1,6,7-Trimethylnaphthalane | 27.82 | 0.654 | Al | Acenaphthylene | 15.88 | 0.508 |
| Fl | Fluorene | 26.13 | 0.699 | Fl | Fluorene | 29.38 | 0.690 | Fl | Fluorene | 17.29 | 0.554 |
| Ph | Phenanthrene | 30.75 | 0.822 | Ph | Phenanthrene | 34.88 | 0.819 | Ph | Phenanthrene | 23.84 | 0.763 |
| A | Anthracene | 30.99 | 0.829 | A | Anthracene | 35.10 | 0.825 | A | Anthracene | 23.98 | 0.768 |
| Ph2 | 2-Methylphenanthrene | 33.25 | 0.889 | Ph2 | 2-Methylphenanthrene | 37.22 | 0.874 | 45MP | 4,5-Methylenephenanthrene | 25.49 | 0.816 |
| An2 | 2-Methylanthracene | 33.47 | 0.895 | An2 | 2-Methylanthracene | 37.38 | 0.878 | Ph2 | 2-Methylphenanthrene | 25.89 | 0.829 |
| 45MP | 4,5-Methylenephenanthrene | 33.55 | 0.897 | An1 | 1-Methylanthracene | 37.64 | 0.884 | An1 | 1-Methylanthracene | 25.93 | 0.830 |
| An1 | 1-Methylanthracene | 33.68 | 0.901 | Ph1 | 1-Methylphenanthrene | 37.90 | 0.890 | An2 | 2-Methylanthracene | 26.03 | 0.833 |
| Ph1 | 1-Methylphenanthrene | 33.73 | 0.902 | 45MP | 4,5-Methylenephenanthrene | 37.92 | 0.891 | Ph1 | 1-Methylphenanthrene | 26.15 | 0.837 |
| An9 | 9-Methylanthracene | 34.40 | 0.920 | An9 | 9-Methylanthracene | 38.84 | 0.912 | An9 | 9-Methylanthracene | 26.58 | 0.851 |
| Ph3,6 | 3,6-Dimethylphenanthrene | 35.36 | 0.946 | Ph3,6 | 3,6-Dimethylphenanthrene | 38.87 | 0.913 | Ph3,6 | 3,6-Dimethylphenanthrene | 27.72 | 0.888 |
| Fa | Fluoranthene | 36.39 | 0.973 | An2,3 | 2,3-Dimethylanthracene | 40.64 | 0.955 | Ph9,10 | 9,10-Dimethylanthracene | 28.90 | 0.925 |
| An2,3 | 2,3-Dimethylanthracene | 36.63 | 0.980 | Fa | Fluoranthene | 41.16 | 0.967 | An2,3 | 2,3-Dimethylanthracene | 29.10 | 0.932 |
| Py | Pyrene | 37.39 | 1.000 | An9,10 | 9,10-Dimethylanthracene | 42.36 | 0.995 | Fa | Fluoranthene | 30.42 | 0.974 |
| An9,10 | 9,10-Dimethylanthracene | 37.63 | 1.006 | Py | Pyrene | 42.57 | 1.000 | Py | Pyrene | 31.23 | 1.000 |
| Fa2 | 2-Methylfluoranthene | 38.58 | 1.032 | Fa2 | 2-Methylfluoranthene | 43.11 | 1.013 | Fl2 | 2-Methylfluoranthene | 32.29 | 1.034 |
| Py1 | 1-Methylpyrene | 40.07 | 1.072 | Py1 | 1-Methylpyrene | 45.25 | 1.063 | Bc1 | 1-Methylbenzo(c)phenanthrene | 33.37 | 1.069 |
| Bc1 | 1-Methylbenzo(c)phenanthrene | 42.36 | 1.133 | Bc1 | 1-Methylbenzo(c)phenanthrene | 47.71 | 1.121 | Py1 | 1-Methylpyrene | 33.43 | 1.070 |
| Ba | Benz(a)anthracene | 43.12 | 1.153 | Bc2 | 2-Methylbenzo(c)phenanthrene | 48.63 | 1.142 | Bc2 | 2-Methylbenzo(c)phenanthrene | 35.98 | 1.152 |
| T | Triphenylene | 43.22 | 1.156 | Ba | Benz(a)anthracene | 48.68 | 1.144 | Bc1,12 | 1,12-Dimethylbenzo(c)phenanthrene | 36.46 | 1.168 |
| C | Chrysene | 43.27 | 1.157 | T | Triphenylene | 49.00 | 1.151 | Bc4 | 4-Methylbenzo(c)phenanthrene | 36.72 | 1.176 |
| Bc2 | 2-Methylbenzo(c)phenanthrene | 43.49 | 1.163 | C | Chrysene | 49.07 | 1.153 | Bc3 | 3-Methylbenzo(c)phenanthrene | 36.77 | 1.178 |
| 23BA | 2,3-Benzanthracene | 43.72 | 1.169 | Bc3 | 3-Methylbenzo(c)phenanthrene | 49.42 | 1.161 | Bc5 | 5-Methylbenzo(c)phenanthrene | 36.82 | 1.179 |
| Bc3 | 3-Methylbenzo(c)phenanthrene | 44.11 | 1.180 | 23BA | 2,3-Benzanthracene | 49.51 | 1.163 | Ba | Benz(a)anthracene | 37.29 | 1.194 |
| Bc5 | 5-Methylbenzo(c)phenanthrene | 44.39 | 1.187 | Bc5 | 5-Methylbenzo(c)phenanthrene | 49.90 | 1.172 | C | Chrysene | 37.43 | 1.199 |
| Bc4 | 4-Methylbenzo(c)phenanthrene | 44.45 | 1.189 | Bc4 | 4-Methylbenzo(c)phenanthrene | 49.98 | 1.174 | T | Triphenylene | 37.56 | 1.203 |
| Ba2 | 2-Methylbenz(a)anthracene | 44.92 | 1.201 | Ba2 | 2-Methylbenz(a)anthracene | 50.13 | 1.178 | 23Ba | 2,3-Benzanthracene | 37.85 | 1.212 |
| Ba1 | 1-Methylbenz(a)anthracene | 44.92 | 1.201 | Ba7 | 7-Methylbenz(a)anthracene | 50.39 | 1.184 | Ba1 | 1-Methylbenz(a)anthracene | 37.85 | 1.212 |
| Ba7 | 7-Methylbenz(a)anthracene | 45.08 | 1.206 | Ba9 | 9-Methylbenz(a)anthracene | 50.47 | 1.186 | C5 | 5-Methylchrysene | 38.41 | 1.230 |
| Ba9 | 9-Methylbenz(a)anthracene | 45.08 | 1.206 | Ba1 | 1-Methylbenz(a)anthracene | 50.52 | 1.187 | C4 | 4-Methylchrysene | 38.51 | 1.234 |
| Ba6 | 6-Methylbenz(a)anthracene | 45.16 | 1.208 | Ba6 | 4-Methylbenz(a)anthracene | 50.52 | 1.187 | Ba6 | 6-Methylbenz(a)anthracene | 38.70 | 1.240 |
| Ba4 | 4-Methylbenz(a)anthracene | 45.16 | 1.208 | Ba4 | 6-Methylbenz(a)anthracene | 50.52 | 1.187 | Ba4 | 4-Methylbenz(a)anthracene | 38.70 | 1.240 |
| C5 | 5-Methylchrysene | 45.33 | 1.212 | Ba3 | 3-Methylbenz(a)anthracene | 50.96 | 1.197 | Ba2 | 2-Methylbenz(a)anthracene | 38.76 | 1.242 |
| C6 | 6-Methylchrysene | 45.42 | 1.215 | Ba5 | 5-Methylbenz(a)anthracene | 50.96 | 1.197 | Ba9 | 9-Methylbenz(a)anthracene | 38.85 | 1.244 |
| Ba3 | 3-Methylbenz(a)anthracene | 45.42 | 1.215 | C6 | 6-Methylchrysene | 51.03 | 1.199 | Ba7 | 7-Methylbenz(a)anthracene | 38.91 | 1.246 |
| C4 | 4-Methylchrysene | 45.42 | 1.215 | C5 | 5-Methylchrysene | 51.12 | 1.201 | C6 | 6-Methylchrysene | 39.13 | 1.253 |
| Ba5 | 5-Methylbenz(a)anthracene | 45.42 | 1.215 | C4 | 4-Methylchrysene | 51.25 | 1.204 | Ba3 | 3-Methylbenz(a)anthracene | 39.13 | 1.253 |
| Bc1,12 | 1,12-Dimethylbenzo(c)phenanthrene | 45.49 | 1.217 | Ba6,8 | 6,8-Dimethylbenz(a)anthracene | 51.26 | 1.204 | Ba5 | 5-Methylbenz(a)anthracene | 39.13 | 1.253 |
| Ba10 | 10-Methylbenz(a)anthracene | 45.95 | 1.229 | Ba10 | 10-Methylbenz(a)anthracene | 51.73 | 1.215 | Ba10 | 10-Methylbenz(a)anthracene | 39.45 | 1.264 |
| Ba6,8 | 6,8-Dimethylbenz(a)anthracene | 46.74 | 1.250 | Bc1,12 | 1,12-Dimethylbenzo(c)phenanthrene | 51.91 | 1.219 | Ba6,8 | 6,8-Dimethylbenz(a)anthracene | 39.61 | 1.269 |
| Ba3,9 | 3,9-Dimethylbenz(a)anthracene | 46.93 | 1.255 | Ba3,9 | 3,9-Dimethylbenz(a)anthracene | 52.17 | 1.226 | Ba7,12 | 7,12-Dimethylbenz(a)anthracene | 39.71 | 1.272 |
| BbF | Benzo(b)fluoranthene | 47.82 | 1.279 | Ba7,12 | 7,12-Dimethylbenz(a)anthracene | 53.98 | 1.268 | Ba3,9 | 3,9-Dimethylbenz(a)anthracene | 40.37 | 1.293 |
| Ba7,12 | 7,12-Dimethylbenz(a)anthracene | 47.88 | 1.281 | BbF | Benzo(b)fluoranthene | 54.02 | 1.269 | Ba8,9,11 | 8,9,11-Trimethylbenz(a)anthracene | 41.40 | 1.326 |
| BkF | Benzo(k)fluoranthene | 47.94 | 1.282 | BkF | Benzo(k)fluoranthene | 54.12 | 1.271 | BbF | Benzo(b)fluoranthene | 42.86 | 1.373 |
| BeP | Benzo(e)pyrene | 48.88 | 1.307 | Ba8,9,11 | 8,9,11-Trimethylbenz(a)anthracene | 54.30 | 1.276 | BkF | Benzo(k)fluoranthene | 43.02 | 1.378 |
| Ba8,9,11 | 8,9,11-Trimethylbenz(a)anthracene | 49.03 | 1.311 | BeP | Benzo(e)pyrene | 55.61 | 1.306 | BeP | Benzo(e)pyrene | 44.04 | 1.411 |
| BaP | Benzo(a)pyrene | 49.08 | 1.313 | BaP | Benzo(a)pyrene | 55.86 | 1.312 | BaP | Benzo(a)pyrene | 44.08 | 1.412 |
| BaP9 | 9-Methylbenzo(a)pyrene | 50.71 | 1.356 | BaP9 | 9-Methylbenzo(a)pyrene | 57.19 | 1.343 | BaP10 | 10-Methylbenzo(a)pyrene | 45.24 | 1.449 |
| BaP8 | 8-Methylbenzo(a)pyrene | 50.84 | 1.360 | BaP8 | 8-Methylbenzo(a)pyrene | 57.39 | 1.348 | BaP9 | 9-Methylbenzo(a)pyrene | 45.49 | 1.457 |
| BaP7 | 7-Methylbenzo(a)pyrene | 51.07 | 1.366 | BaP7 | 7-Methylbenzo(a)pyrene | 57.71 | 1.356 | BaP8 | 7-Methylbenzo(a)pyrene | 45.49 | 1.457 |
| BaP10 | 10-Methylbenzo(a)pyrene | 51.12 | 1.367 | BaP10 | 10-Methylbenzo(a)pyrene | 57.94 | 1.361 | BaP7 | 8-Methylbenzo(a)pyrene | 45.49 | 1.457 |
| BaP7,10 | 7,10-Dimethylbenzo(a)pyrene | 52.93 | 1.416 | BaP7,10 | 7,10-Dimethylbenzo(a)pyrene | 59.77 | 1.404 | BaP7,10 | 7,10-Dimethylbenzo(a)pyrene | 46.27 | 1.482 |
| Indeno(1,2,3-c,d)pyrene | 53.26 | 1.424 | Indeno(1,2,3-c,d)pyrene | 60.86 | 1.430 | Dibenz(a,h)anthracene | 48.68 | 1.559 | |||
| Dibenz(a,h)anthracene | 53.44 | 1.429 | Dibenz(a,h)anthracene | 60.94 | 1.432 | Indeno(1,2,3-c,d)pyrene | 49.02 | 1.570 | |||
| Benzo(g,h,i)perylene | 54.26 | 1.451 | Benzo(g,h,i)perylene | 62.89 | 1.477 | Benzo(g,h,i)perylene | 50.01 | 1.602 |
| GC Columns | Phenyl Arylene | 50% Phenyl Polysiloxane | SLB-ILPAH |
|---|---|---|---|
| Overlap > 90% | 12 peaks | 11 peaks | 19 peaks |
| 90% > overlap > 50% | 7 peaks | 2 peaks | 3 peaks |
| Overlap < 50% | 4 peaks | 4 peaks | 1 peak |
| Peak shape | Good | Good | Good |
| Analysis time | Long | Long | Shorter than on the other two columns |
| Bleeding | Substantial bleeding above 260 °C | No bleeding till 300 °C | No bleeding till 300 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoczyńska, E.; de Boer, J. Retention Behaviour of Alkylated and Non-Alkylated Polycyclic Aromatic Hydrocarbons on Different Types of Stationary Phases in Gas Chromatography. Separations 2019, 6, 7. https://doi.org/10.3390/separations6010007
Skoczyńska E, de Boer J. Retention Behaviour of Alkylated and Non-Alkylated Polycyclic Aromatic Hydrocarbons on Different Types of Stationary Phases in Gas Chromatography. Separations. 2019; 6(1):7. https://doi.org/10.3390/separations6010007
Chicago/Turabian StyleSkoczyńska, Ewa, and Jacob de Boer. 2019. "Retention Behaviour of Alkylated and Non-Alkylated Polycyclic Aromatic Hydrocarbons on Different Types of Stationary Phases in Gas Chromatography" Separations 6, no. 1: 7. https://doi.org/10.3390/separations6010007
APA StyleSkoczyńska, E., & de Boer, J. (2019). Retention Behaviour of Alkylated and Non-Alkylated Polycyclic Aromatic Hydrocarbons on Different Types of Stationary Phases in Gas Chromatography. Separations, 6(1), 7. https://doi.org/10.3390/separations6010007





