Monolithic Silica Capillary Columns with Improved Retention and Selectivity for Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
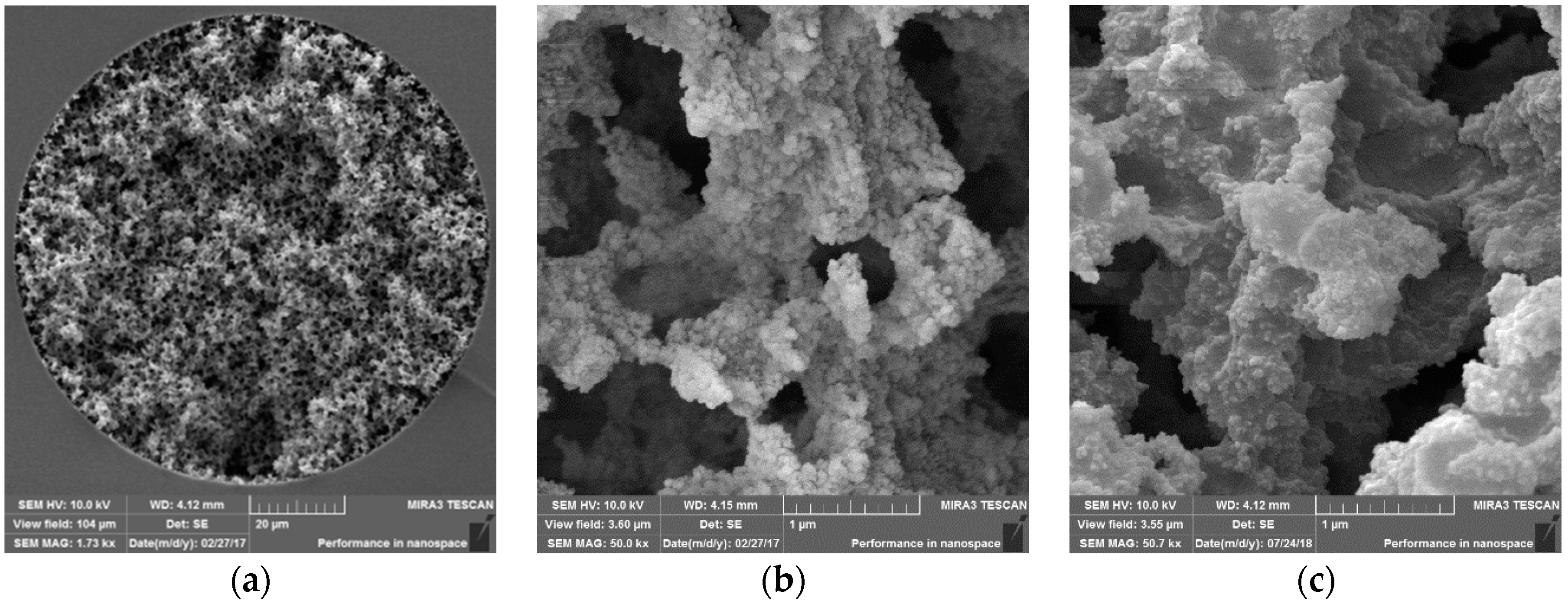
2.3. Preparation of Monolithic Capillary Columns
2.4. Preparation of Mobile Phases and Sample Solutions
3. Results and Discussion
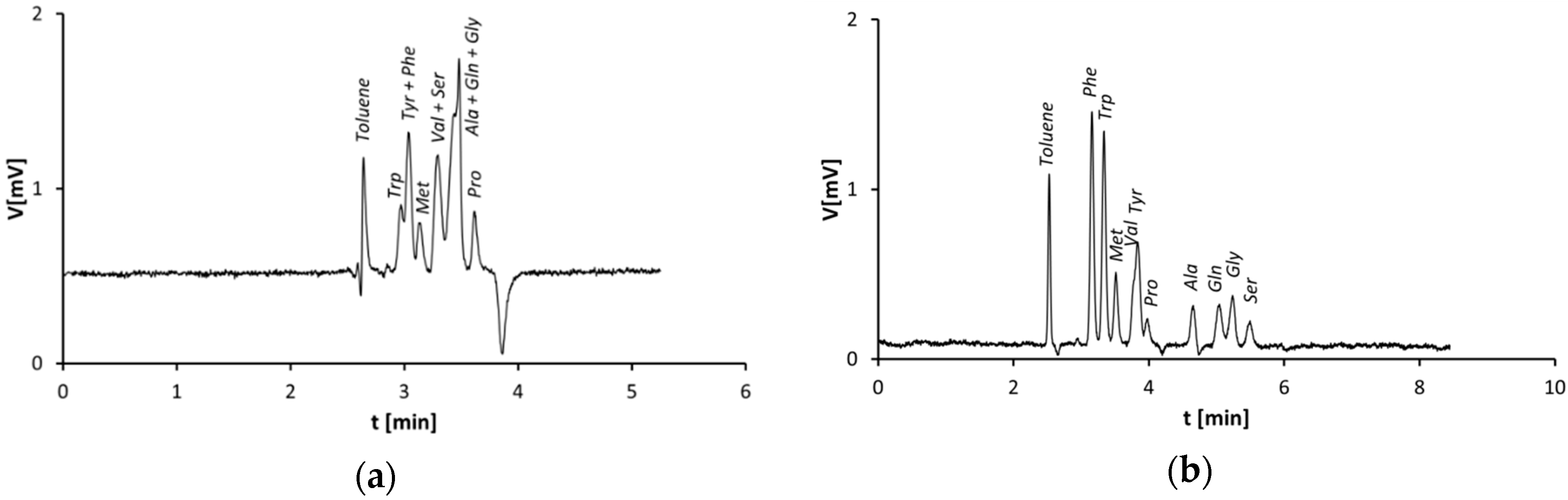
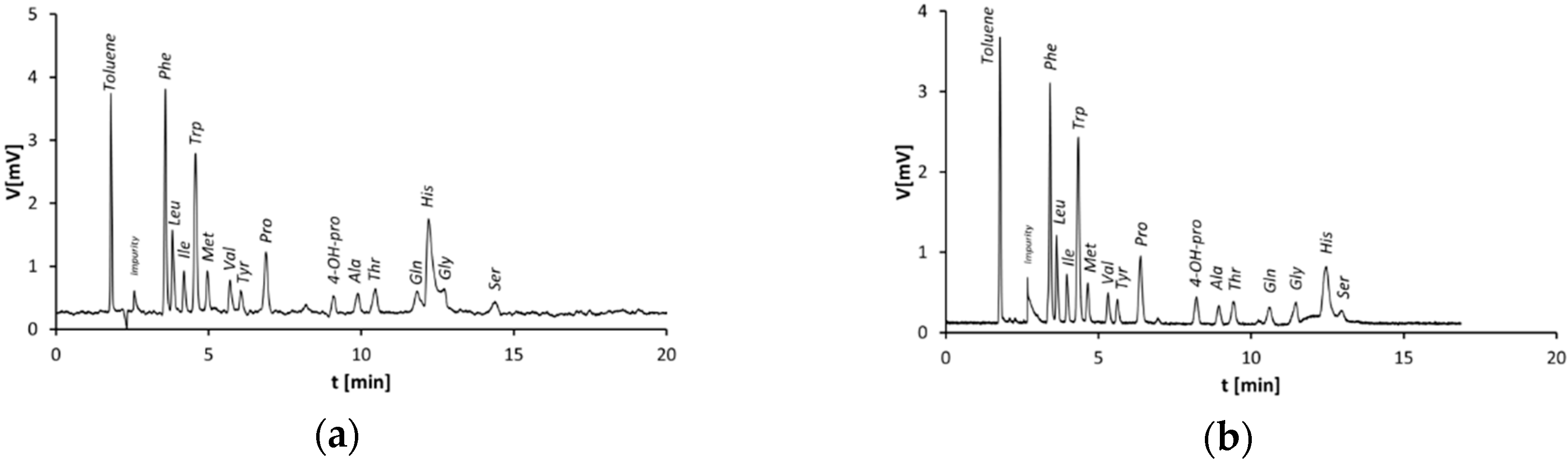
3.1. Separation of Amino Acids on Monolithic Silica Capillary Columns—Plain Silica vs. Zwitterionic Stationary Phase
3.2. Capillary Columns Modified by the Stepwise Modification Procedure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, Y.; Xu, C.; Kuroki, H.; Liao, Y.; Tsunoda, M. Recent trends in analytical methods for the determination of amino acids in biological samples. J. Pharm. Biomed. Anal. 2018, 147, 35–49. [Google Scholar] [CrossRef]
- Gar, C.; Rottenkolber, M.; Prehn, C.; Adamski, J.; Seissler, J.; Lechner, A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit. Rev. Clin. Lab. Sci. 2018, 55, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Begou, O.; Gika, H.G.; Wilson, I.D.; Theodoridis, G. Hyphenated MS-based targeted approaches in Metabolomics. Analyst 2017, 142, 3079–3100. [Google Scholar] [CrossRef] [PubMed]
- Manig, F.; Kuhne, K.; von Neubeck, C.; Schwarzenbolz, U.; Yue, Z.; Kessler, B.M.; Pietzsch, J.; Kunz-Schughart, L.A. The why and how of amino acid analytics in cancer diagnostics and therapy. J. Biotechnol. 2017, 242, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Wahl, O.; Holzgrabe, U. Amino acid analysis for pharmacopoeial purposes. Talanta 2016, 154, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Baghdady, Y.Z.; Schug, K.A. Review of in situ derivatization techniques for enhanced bioanalysis using liquid chromatography with mass spectrometry. J. Sep. Sci. 2016, 39, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Marrubini, G.; Appelblad, P.; Maietta, M.; Papetti, A. Hydrophilic interaction chromatography in food matrices analysis: An updated review. Food Chem. 2018, 257, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Šesták, J.; Moravcová, D.; Kahle, V. Instrument platforms for nano liquid chromatography. J. Chromatogr. A 2015, 1421, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Nazario, C.E.D.; Silva, M.R.; Franco, M.S.; Lanças, F.M. Evolution in miniaturized column liquid chromatography instrumentation and applications: An overview. J. Chromatogr. A 2015, 1421, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Novotny, M.V. Development of capillary liquid chromatography: A personal perspective. J. Chromatogr. A 2017, 1523, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Piehowski, P.D.; Shi, T.; Smith, R.D.; Qian, W.J. Advances in microscale separations towards nanoproteomics applications. J. Chromatogr. A 2017, 1523, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Fanali, S. An overview to nano-scale analytical techniques: Nano-liquid chromatography and capillary electrochromatography. Electrophoresis 2017, 38, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Jia, M.; Liu, R.; Liu, Q.; Xiao, H.; Li, J.; Xue, Y.; Wang, Y.; Yan, C. Recent advances in microscale separation. Electrophoresis 2018, 39, 8–33. [Google Scholar] [CrossRef] [PubMed]
- Svec, F.; Lv, Y.Q. Advances and recent trends in the field of monolithic columns for chromatography. Anal. Chem. 2015, 87, 250–273. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Tanaka, N. Recent progress in monolithic silica columns for high-speed and high-selectivity separations. Annu. Rev. Anal. Chem. 2016, 9, 317–342. [Google Scholar] [CrossRef] [PubMed]
- Eeltink, S.; Wouters, S.; Dores-Sousa, J.L.; Svec, F. Advances in organic polymer-based monolithic column technology for high-resolution liquid chromatography-mass spectrometry profiling of antibodies, intact proteins, oligonucleotides, and peptides. J. Chromatogr. A 2017, 1498, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Rathnasekara, R.; Khadka, S.; Jonnada, M.; El Rassi, Z. Polar and nonpolar organic polymer-based monolithic columns for capillary electrochromatography and high-performance liquid chromatography. Electrophoresis 2017, 38, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Dinga, X.; Yanga, J.; Dong, Y. Advancements in the preparation of high-performance liquid chromatographic organic polymer monoliths for the separation of small-molecule drugs. J. Pharm. Anal. 2018, 8, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Soga, N. Phase separation in silica sol-gel system containing polyacrylic acid I. Gel formation behavior and effect of solvent composition. J. Non-Cryst. Solids 1992, 139, 1–13. [Google Scholar] [CrossRef]
- Minakuchi, H.; Nakanishi, K.; Soga, N.; Ishizuka, N.; Tanaka, N. Octadecylsilylated porous silica rods as separation media for reversed-phase liquid chromatography. Anal. Chem. 1996, 68, 3498–3501. [Google Scholar] [CrossRef] [PubMed]
- Núñez, O.; Nakanishi, K.; Tanaka, N. Preparation of monolithic silica columns for high-performance liquid chromatography. J. Chromatogr. A 2008, 1191, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Liu, Z.; Wang, H.; Lin, H.; Dong, J.; Zou, H. Recent development of hybrid organic-silica monolithic columns in CEC and capillary LC. Electrophoresis 2015, 36, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Viklund, C.; Sjögren, A.; Irgum, K.; Nes, I. Chromatographic interactions between proteins and sulfoalkylbetaine-based zwitterionic copolymers in fully aqueous low-salt buffers. Anal. Chem. 2001, 73, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Smith, N.W.; Ferguson, P.D.; Taylor, M.R. Hydrophilic interaction chromatography using methacrylate-based monolithic capillary column for the separation of polar analytes. Anal. Chem. 2007, 79, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Škeříková, V.; Jandera, P.; Kubíčková, R.; Pospíšilová, M. Preparation and characterization of polymethacrylate monolithic capillary columns with dual hydrophilic interaction reversed-phase retention mechanism for polar compounds. J. Sep. Sci. 2009, 32, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, J.; Karas, M.; Jiang, W.; Hendriks, R.; Andrecht, S. Enhanced glyco-profiling by specific glycopeptide enrichment and complementary monolithic nano-LC (ZIC-HILIC/RP18e)/ESI-MS analysis. J. Sep. Sci. 2010, 33, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, D.; Planeta, J.; Kahle, V.; Roth, M. Zwitterionic silica-based monolithic capillary columns for isocratic and gradient hydrophilic interaction liquid chromatography. J. Chromatogr. A 2012, 1270, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ou, J.; Zhang, Z.; Dong, J.; Wu, M.; Zou, H. Facile preparation of zwitterionic organic-silica hybrid monolithic capillary column with an improved “one-pot” approach for hydrophilic-interaction liquid chromatography (HILIC). Anal. Chem. 2012, 84, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, D.; Planeta, J.; King, A.W.T.; Wiedmer, S.K. Immobilization of a phosphonium ionic liquid on a silica monolith for hydrophilic interaction chromatography. J. Chromatogr. A 2018, 1552, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, L.; Pyszka, M.; Okońska, M.; Niedźwiecki, M.; Bączek, T. Bioanalysis of underivatized amino acids in non-invasive exhaled breath condensate samples using liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2018, 1542, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Schiesel, S.; Lämmerhofer, M.; Lindner, W. Multitarget quantitative metabolic profiling of hydrophilic metabolites in fermentation broths of β-lactam antibiotics production by HILIC-ESI-MS/MS. Anal. Bioanal. Chem. 2010, 396, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
| Monolith | Total Porosity εT | kuracil | Nuracil |
|---|---|---|---|
| Silica | 0.945 | 0.04 | 180,200 |
| 1st modification | 0.916 | 0.13 | 176,020 |
| 2nd modification | 0.836 | 0.21 | 169,170 |
| 3rd modification | 0.739 | 0.31 | 155,720 |
| 4th modification | 0.669 | 0.45 | 146,870 |
| Amino Acid | k5 * | k25 ** |
|---|---|---|
| Phenylalanine | 0.92 | 0.99 |
| Leucine | 1.04 | 1.12 |
| Isoleucine | 1.23 | 1.33 |
| Tryptophan | 1.44 | 1.54 |
| Methionine | 1.61 | 1.76 |
| Valine | 1.98 | 2.17 |
| Tyrosine | 2.13 | 2.37 |
| Proline | 2.56 | 2.83 |
| 4-Hydroxy proline | 3.59 | 4.06 |
| Alanine | 4.01 | 4.51 |
| Threonine | 4.30 | 4.82 |
| Glutamine | 4.97 | 5.58 |
| Histidine | 6.03 | 5.79 |
| Glycine | 5.46 | 6.07 |
| Serine | 6.29 | 7.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moravcová, D.; Planeta, J. Monolithic Silica Capillary Columns with Improved Retention and Selectivity for Amino Acids. Separations 2018, 5, 48. https://doi.org/10.3390/separations5040048
Moravcová D, Planeta J. Monolithic Silica Capillary Columns with Improved Retention and Selectivity for Amino Acids. Separations. 2018; 5(4):48. https://doi.org/10.3390/separations5040048
Chicago/Turabian StyleMoravcová, Dana, and Josef Planeta. 2018. "Monolithic Silica Capillary Columns with Improved Retention and Selectivity for Amino Acids" Separations 5, no. 4: 48. https://doi.org/10.3390/separations5040048
APA StyleMoravcová, D., & Planeta, J. (2018). Monolithic Silica Capillary Columns with Improved Retention and Selectivity for Amino Acids. Separations, 5(4), 48. https://doi.org/10.3390/separations5040048




