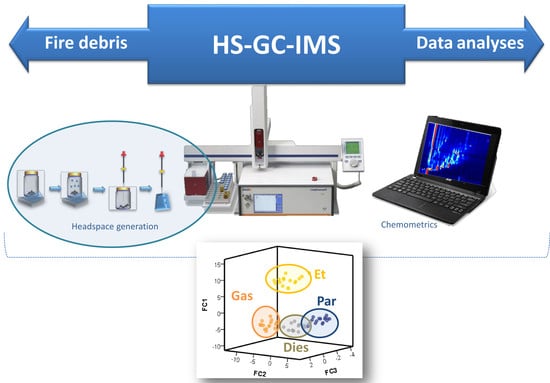
Application of Headspace Gas Chromatography-Ion Mobility Spectrometry for the Determination of Ignitable Liquids from Fire Debris
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. HS-GC-IMS Analysis Acquisition
2.3. Data Analysis
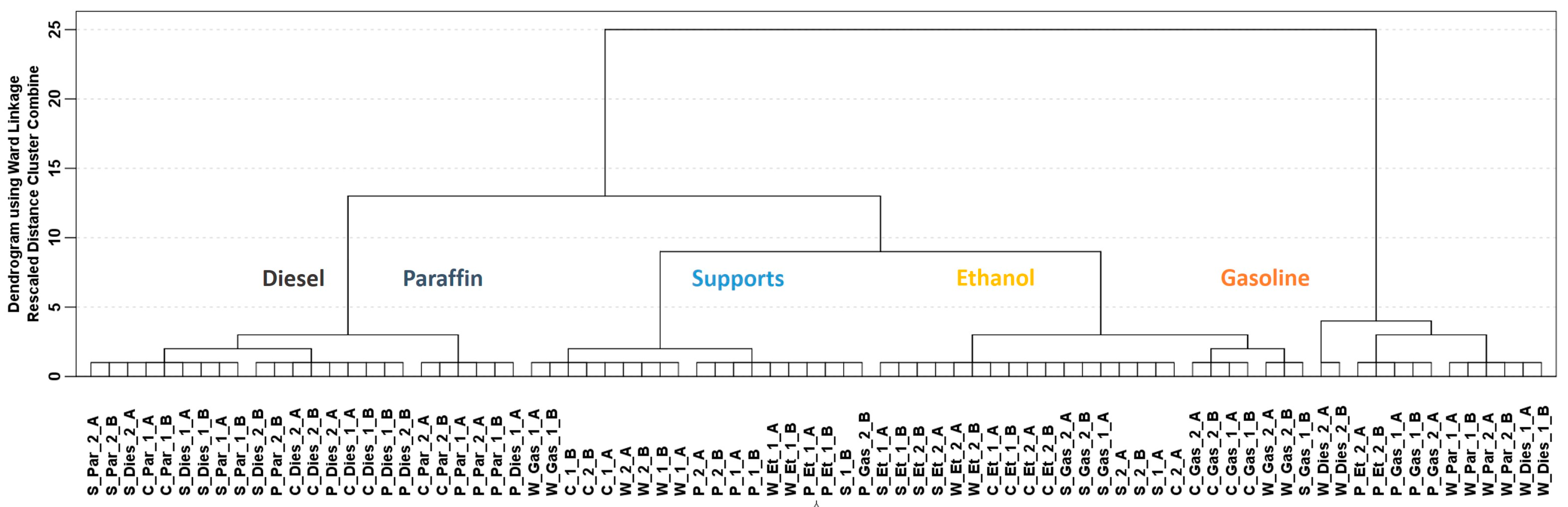
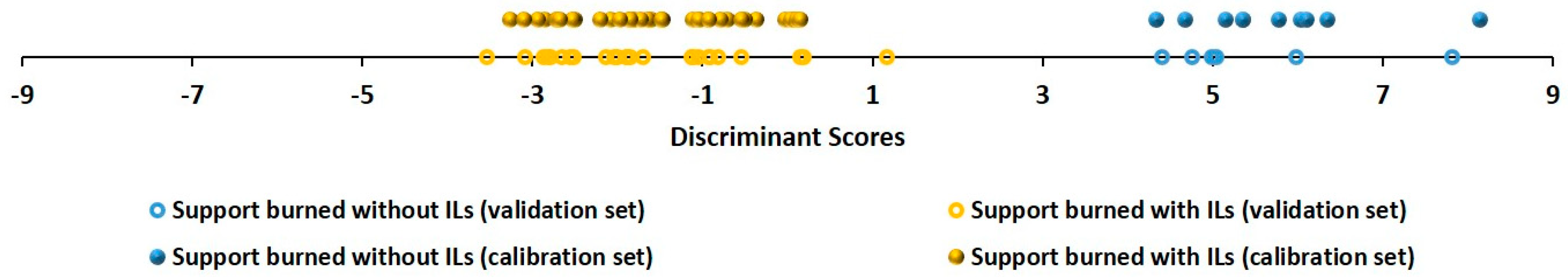
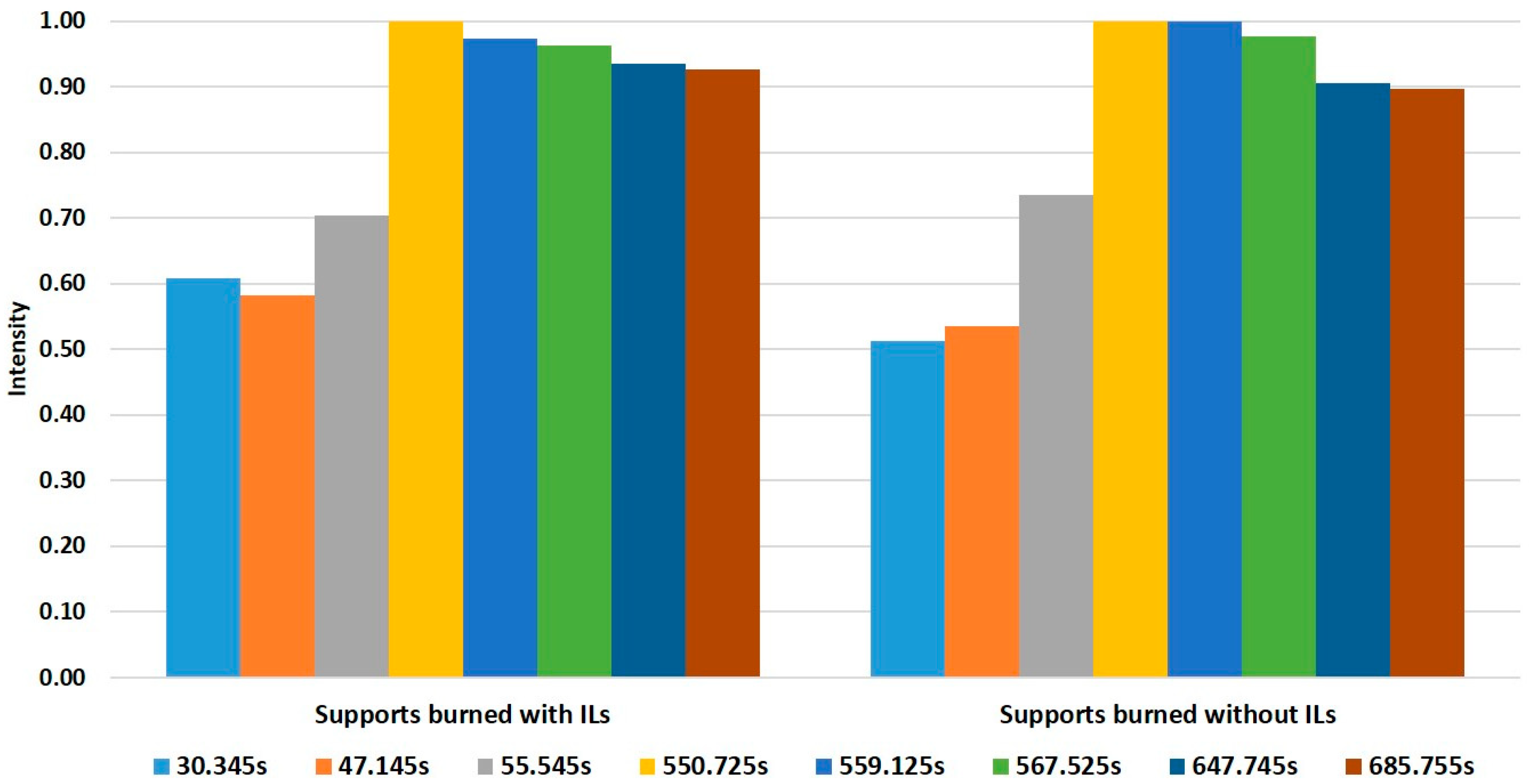
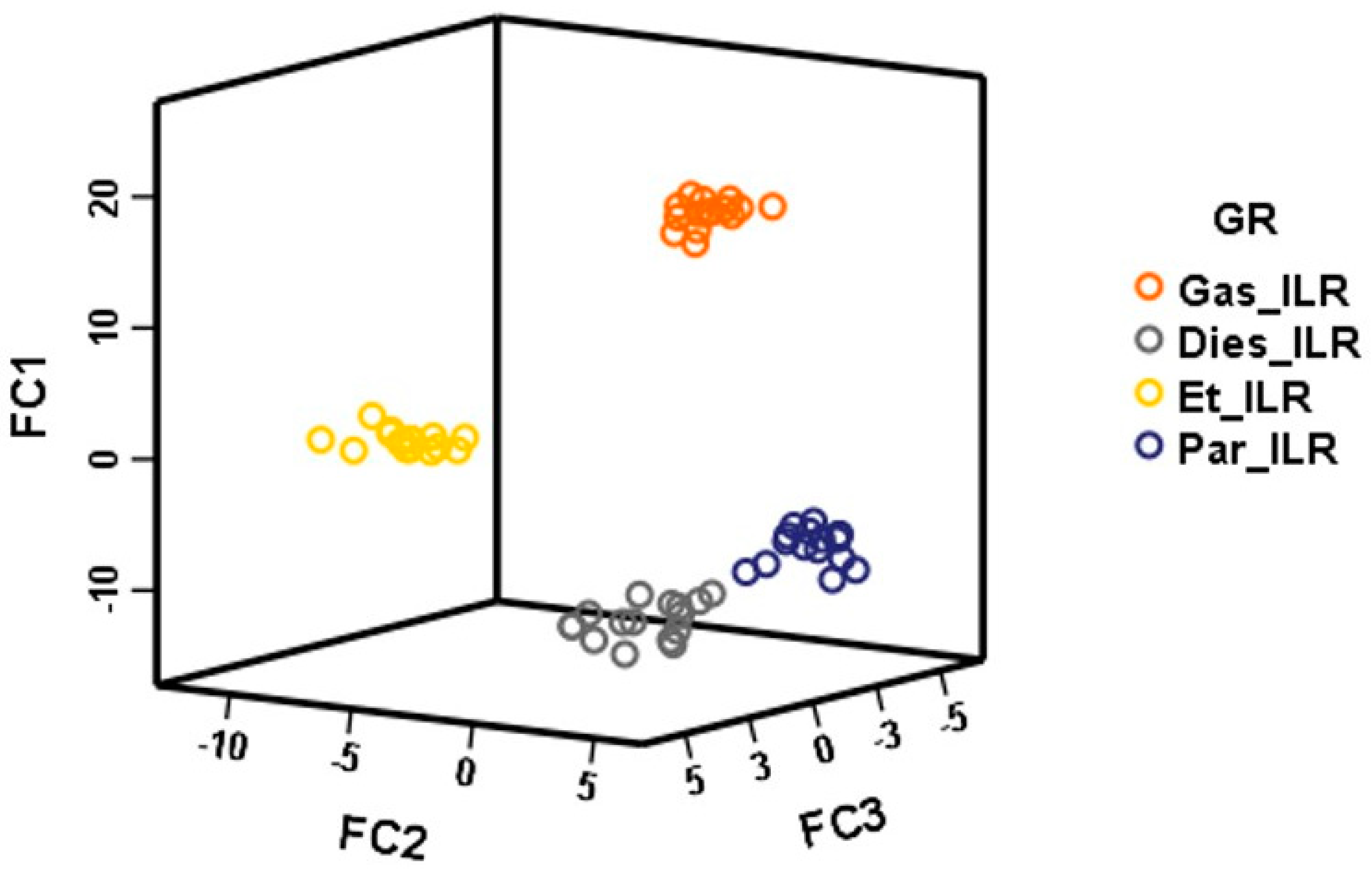
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dennis, D.-M.K.; Williams, M.R.; Sigman, M.E. Investigative probabilistic inferences of smokeless powder manufacturers utilizing a bayesian network. Forensic Chem. 2017, 3, 41–51. [Google Scholar] [CrossRef]
- Falatova, B.; Ferreiro-González, M.; Martín-Alberca, C.; Kačíková, D.; Galla, Š.; Palma, M.G.; Barroso, C. Effects of fire suppression agents and weathering in the analysis of fire debris by HS-MS eNose. Sensors 2018, 18, 1933. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alberca, C.; Carrascosa, H.; San Román, I.; Bartolomé, L.; García-Ruiz, C. Acid alteration of several ignitable liquids of potential use in arsons. Sci. Justice 2018, 58, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alberca, C.; García-Ruiz, C.; Delémont, O. Study of chemical modifications in acidified ignitable liquids analysed by GC-MS. Sci. Justice 2015, 55, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Kindell, J.H.; Williams, M.R.; Sigman, M.E. Biodegradation of representative ignitable liquid components on soil. Forensic Chem. 2017, 6, 19–27. [Google Scholar] [CrossRef]
- Ferreiro-Gonzalez, M.; Ayuso, J.; Alvarez, J.A.; Palma, M.; Barroso, C.G. Application of an HS-MS for the detection of ignitable liquids from fire debris. Talanta 2015, 142, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro-Gonzalez, M.; Barbero, G.F.; Palma, M.; Ayuso, J.; Alvarez, J.A.; Barroso, C.G. Determination of ignitable liquids in fire debris: Direct analysis by electronic nose. Sensors 2016, 16, 695. [Google Scholar] [CrossRef] [PubMed]
- Green, M.K.; Kuk, R.J.; Wagner, J.R. Collection and analysis of fire debris evidence to detect methamphetamine, pseudoephedrine, and ignitable liquids in fire scenes at suspected clandestine laboratories. Forensic Chem. 2017, 4, 82–88. [Google Scholar] [CrossRef]
- Choi, S.; Yoh, J.J. Fire debris analysis for forensic fire investigation using laser induced breakdown spectroscopy. Spectrochim. Acta Part B Atomic Spectrosc. 2017, 134, 75–80. [Google Scholar] [CrossRef]
- Martín-Alberca, C.; Ortega-Ojeda, F.E.; García-Ruiz, C. Analytical tools for the analysis of fire debris. A review: 2008–2015. Anal. Chim. Acta 2016, 928, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sturaro, A.; Vianello, A.; Denti, P.; Rella, R. Fire debris analysis and scene reconstruction. Sci. Justice 2013, 53, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, É.; Lentini, J.J. ASTM standards for fire debris analysis: A review. Forensic Sci. Int. 2003, 132, 63–67. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Practice for Separation of Ignitable Liquid Residues from Fire Debris Samples by Passive Headspace Concentration with Activated Charcoal; ASTM E1412 (2012); ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- American Society for Testing and Materials (ASTM). Standard Practice for Separation and Concentration of Ignitable Liquid Residues from Fire Debris Samples by Passive Headspace Concentration with Solid Phase Microextraction (SPME); ASTM E2154 (2008); ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- American Society for Testing and Materials (ASTM). Standard Test Method for Ignitable Liquid Residues in Extracts from Fire Debris Samples by Gas Chromatography-Mass Spectrometry; ASTM E1618 (2014); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Hupp, A.M.; Marshall, L.J.; Campbell, D.I.; Smith, R.W.; McGuffin, V.L. Chemometric analysis of diesel fuel for forensic and environmental applications. Anal. Chim. Acta 2008, 606, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Sigman, M.E.; Lewis, J.; Pitan, K.M. Combined target factor analysis and bayesian soft-classification of interference-contaminated samples: Forensic fire debris analysis. Forensic Sci. Int. 2012, 222, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Borusiewicz, R.; Zadora, G.; Zieba-Palus, J. Application of headspace analysis with passive adsorption for forensic purposes in the automated thermal desorption-gas chromatography-mass spectrometry system. Chromatographia 2004, 60, 133–142. [Google Scholar] [CrossRef]
- Lopatka, M.; Sigman, M.E.; Sjerps, M.J.; Williams, M.R.; Vivó-Truyols, G. Class-conditional feature modeling for ignitable liquid classification with substantial substrate contribution in fire debris analysis. Forensic Sci. Int. 2015, 252, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.E.; Williams, M.R. Assessing evidentiary value in fire debris analysis by chemometric and likelihood ratio approaches. Forensic Sci. Int. 2016, 264, 113–121. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, J.; Sissons, N.; Robinson, S. Fire debris analysis by raman spectroscopy and chemometrics. J. Anal. Appl. Pyrolysis 2011, 91, 210–218. [Google Scholar] [CrossRef]
- Li, F.; Xie, Z.; Schmidt, H.; Sielemann, S.; Baumbach, J.I. Ion mobility spectrometer for online monitoring of trace compounds. Spectrochim. Acta Part B Atomic Spectrosc. 2002, 57, 1563–1574. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; del MarDobao-Prieto, M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC-IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Delgado, R.; Mercader-Trejo, F.; Sielemann, S.; de Bruyn, W.; Arce, L.; Valcárcel, M. Direct classification of olive oils by using two types of ion mobility spectrometers. Anal. Chim. Acta 2011, 696, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Vautz, W.; Baumbach, J.I.; Jung, J. Continuous monitoring of the fermentation of beer by ion mobility spectrometry. Int. J. Ion Mobil. Spectrom. 2004, 7, 3–5. [Google Scholar]
- Camara, M.; Gharbi, N.; Cocco, E.; Guignard, C.; Behr, M.; Evers, D.; Orlewski, P. Fast screening for presence of muddy/earthy odorants in wine and in wine must using a hyphenated gas chromatography-differential ion mobility spectrometry (GC-DMS). Int. J. Ion Mobil. Spectrom. 2011, 14, 39–47. [Google Scholar] [CrossRef]
- Márquez-Sillero, I.; Cárdenas, S.; Valcárcel, M. Direct determination of 2,4,6-tricholoroanisole in wines by single-drop ionic liquid microextraction coupled with multicapillary column separation and ion mobility spectrometry detection. J. Chromatogr. A 2011, 1218, 7574–7580. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; Wiesenhofer, H.; Allers, M.; Zimmermann, S.; Güntner, A.T.; Pineau, N.J.; Lederer, W.; Agapiou, A.; Mayhew, C.A.; Ruzsanyi, V. Monitoring of selected skin- and breath-borne volatile organic compounds emitted from the human body using gas chromatography ion mobility spectrometry (GC-IMS). J. Chromatogr. B 2018, 1076, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Rapid detection of hydrogen sulfide produced by pathogenic bacteria in focused growth media using SHS-MCC-GC-IMS. Microchem. J. 2018, 140, 232–240. [Google Scholar] [CrossRef]
- Armenta, S.; de la Guardia, M.; Alcalà, M.; Blanco, M.; Perez-Alfonso, C.; Galipienso, N. Ion mobility spectrometry evaluation of cocaine occupational exposure in forensic laboratories. Talanta 2014, 130, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Keller, A.; Tutsch-Bauer, E.; Monticelli, F. Application of ion mobility spectrometry in cases of forensic interest. Forensic Sci. Int. 2006, 161, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; McCall, H.; Yeager, B.; Bell, S. Characterization and validation of ion mobility spectrometry in methamphetamine clandestine laboratory remediation. Talanta 2012, 100, 196–206. [Google Scholar] [CrossRef] [PubMed]
| Classification Results a,c | |||||||
|---|---|---|---|---|---|---|---|
| GR | Predicted Group Membership | Total | |||||
| Gas_ILR | Dies_ILR | Et_ILR | Par_ILR | ||||
| Original | Count | Gas_ILR | 16 | 0 | 0 | 0 | 16 |
| Dies_ILR | 0 | 15 | 0 | 1 | 16 | ||
| Et_ILR | 0 | 0 | 16 | 0 | 16 | ||
| Par_ILR | 0 | 0 | 0 | 16 | 16 | ||
| % | Gas_ILR | 100.0 | 0 | 0 | 0 | 100.0 | |
| Dies_ILR | 0 | 93.8 | 0 | 6.3 | 100.0 | ||
| Et_ILR | 0 | 0 | 100.0 | 0 | 100.0 | ||
| Par_ILR | 0 | 0 | 0 | 100.0 | 100.0 | ||
| Cross-validated b | Count | Gas_ILR | 16 | 0 | 0 | 0 | 16 |
| Dies_ILR | 0 | 15 | 0 | 1 | 16 | ||
| Et_ILR | 0 | 0 | 16 | 0 | 16 | ||
| Par_ILR | 0 | 0 | 0 | 16 | 16 | ||
| % | Gas_ILR | 100.0 | 0 | 0 | 0 | 100.0 | |
| Dies_ILR | 0 | 93.8 | 0 | 6.3 | 100.0 | ||
| Et_ILR | 0 | 0 | 100.0 | 0 | 100.0 | ||
| Par_ILR | 0 | 0 | 0 | 100.0 | 100.0 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Barroso, C.G. Application of Headspace Gas Chromatography-Ion Mobility Spectrometry for the Determination of Ignitable Liquids from Fire Debris. Separations 2018, 5, 41. https://doi.org/10.3390/separations5030041
Aliaño-González MJ, Ferreiro-González M, Barbero GF, Palma M, Barroso CG. Application of Headspace Gas Chromatography-Ion Mobility Spectrometry for the Determination of Ignitable Liquids from Fire Debris. Separations. 2018; 5(3):41. https://doi.org/10.3390/separations5030041
Chicago/Turabian StyleAliaño-González, María José, Marta Ferreiro-González, Gerardo F. Barbero, Miguel Palma, and Carmelo G. Barroso. 2018. "Application of Headspace Gas Chromatography-Ion Mobility Spectrometry for the Determination of Ignitable Liquids from Fire Debris" Separations 5, no. 3: 41. https://doi.org/10.3390/separations5030041
APA StyleAliaño-González, M. J., Ferreiro-González, M., Barbero, G. F., Palma, M., & Barroso, C. G. (2018). Application of Headspace Gas Chromatography-Ion Mobility Spectrometry for the Determination of Ignitable Liquids from Fire Debris. Separations, 5(3), 41. https://doi.org/10.3390/separations5030041