Heterogeneous Catalytic Ozonation of p-Chlorobenzoic Acid in Aqueous Solution by FeMnOOH and PET
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Modification of TMFx Hydrophilicity
2.3. Adsorption Experiments
2.4. Ozonation Procedures
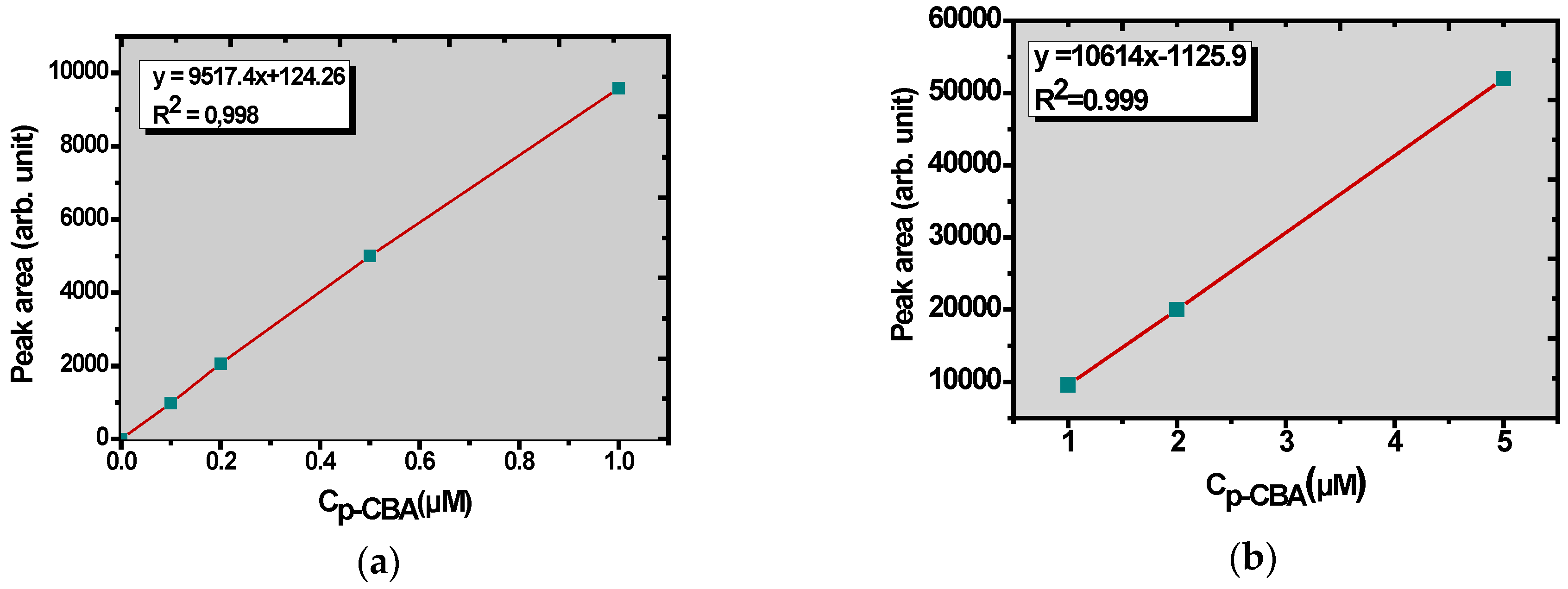
2.5. Analytical Methods
3. Results
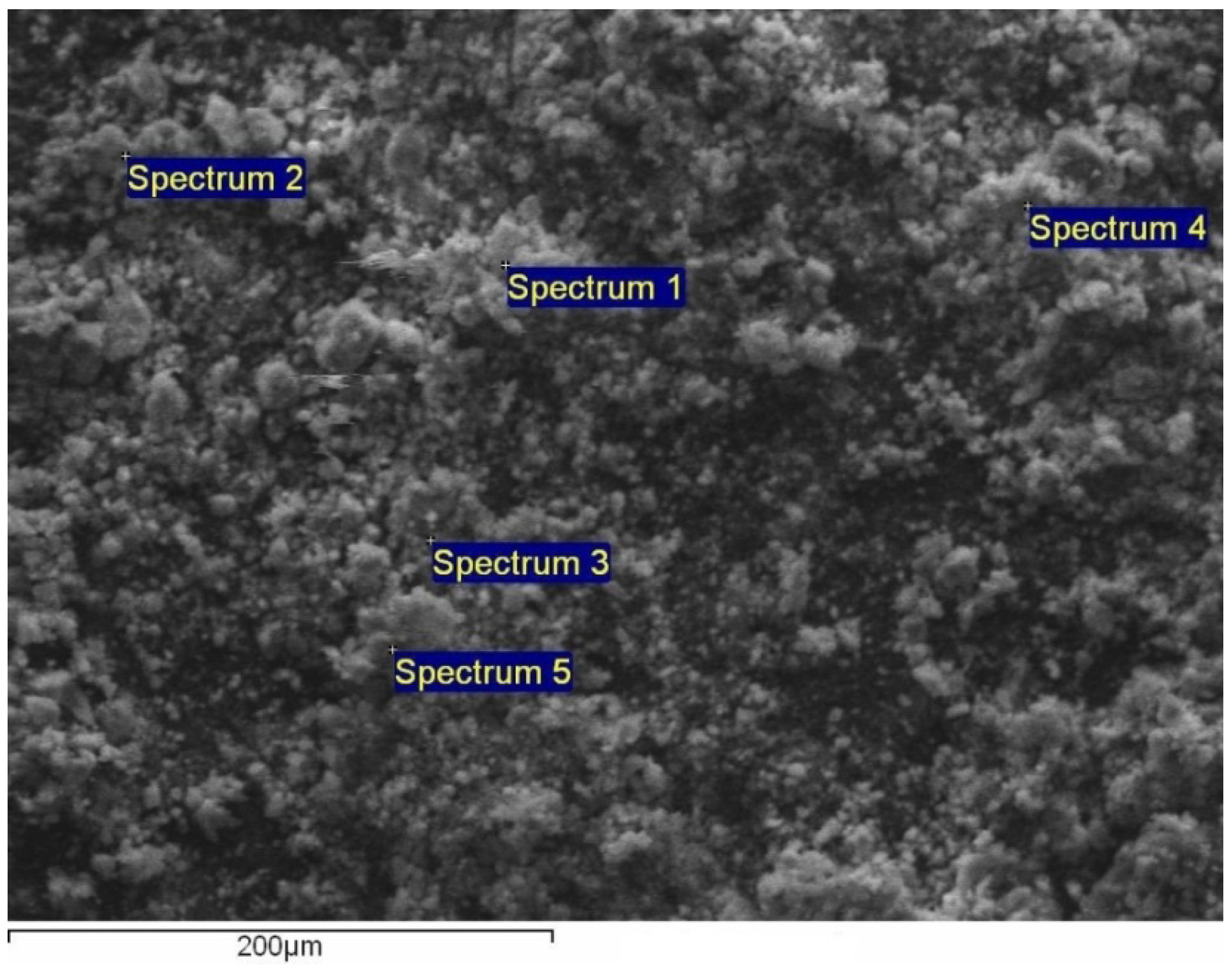
3.1. Characterization of the Hydrophobically Modified TMFx
3.2. Adsorption of p-CBA by the Examined Catalysts
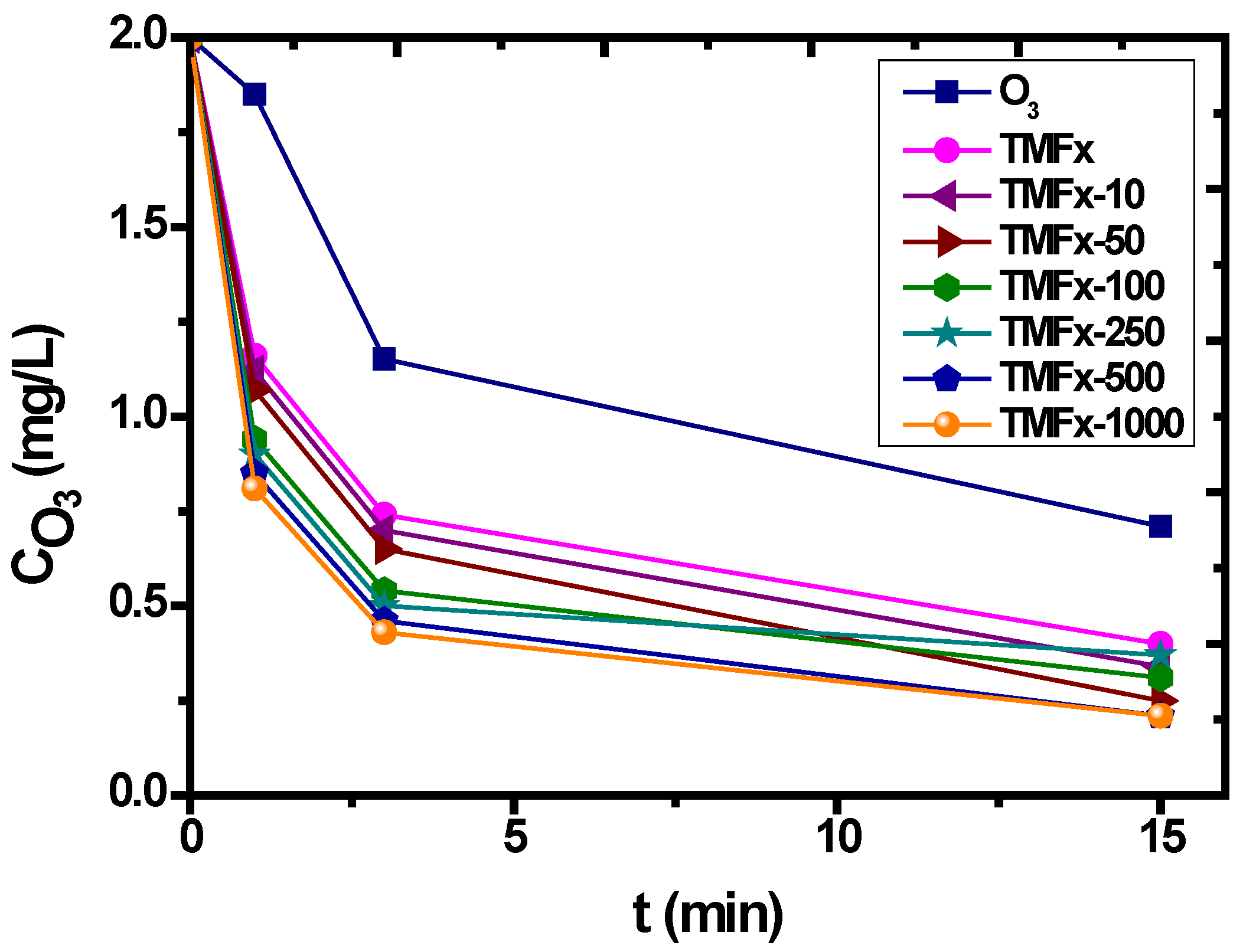
3.3. Catalytic Ozonation withTMFx
3.4. Catalytic Ozonation with PET
- The p-CBA can cover greater percentage of active PET sites, enhancing its degradation rate of this organic compound.
- Simultaneously, by increasing the PET dosage, the free active sites are proportionally increased, resulting in enhanced ozone decomposition and lower OH· production, even without the presence of p-CBA.
4. Discussion
- Chemisorption of ozone onto the catalyst surface and further reaction with the free (non-sorbed) organic molecules.
- Chemisorption of the organic molecules on the surface of the catalyst and reaction with aqueous (dissolved) or gaseous ozone.
- Chemisorption of both organic molecule and ozone onto the catalyst surface and further reaction between them.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Nawrocki, J. Catalytic ozonation in water: Controversies and questions. Discussion paper. Appl. Catal. B Environ. 2013, 142–143, 465–471. [Google Scholar] [CrossRef]
- Andreozzi, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Martins, R.C.; Quinta-Ferreira, R.M. Catalytic ozonation of phenolic acids over a Mn-Ce-O catalyst. Appl. Catal. B Environ. 2009, 90, 268–277. [Google Scholar] [CrossRef]
- Ma, J.; Sui, M.; Zhang, T.; Guan, C. Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene. Water Res. 2005, 39, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Gonzalo, M.S.; García-Calvo, E. Catalytic ozonation of naproxen and carbamazepine on titanium dioxide. Appl. Catal. B Environ. 2008, 84, 48–57. [Google Scholar] [CrossRef]
- Tong, S.P.; Liu, W.P.; Leng, W.H.; Zhang, Q.Q. Characteristics of MnO2 catalytic ozonation of sulfosalicylic acid and propionic acid in water. Chemosphere 2003, 50, 1359–1364. [Google Scholar] [CrossRef]
- Amutha, R.; Sillanpää, M.; Lee, G.J.; Lin, J.C.; Yang, C.K.; Wu, J.J. Catalytic ozonation of 2-ethoxy ethyl acetate using mesoporous nickel oxalates. Catal. Commun. 2014, 43, 88–92. [Google Scholar] [CrossRef]
- Sui, M.; Sheng, L.; Lu, K.; Tian, F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal. B Environ. 2010, 96, 94–100. [Google Scholar] [CrossRef]
- He, K.; Dong, Y.M.; Li, Z.; Yin, L.; Zhang, A.M.; Zheng, Y.C. Catalytic ozonation of phenol in water with natural brucite and magnesia. J. Hazard. Mater. 2008, 159, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Perez, C.A.; Soltan, J.; Robertson, J. Kinetics of catalytic ozonation of atrazine in the presence of activated carbon. Sep. Purif. Technol. 2011, 79, 8–14. [Google Scholar] [CrossRef]
- Valdés, H.; Murillo, F.A.; Manoli, J.A.; Zaror, C.A. Heterogeneous catalytic ozonation of benzothiazole aqueous solution promoted by volcanic sand. J. Hazard. Mater. 2008, 153, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; He, K.; Zhao, B.; Yin, Y.; Yin, L.; Zhang, A. Catalytic ozonation of azo dye active brilliant red X-3B in water with natural mineral brucite. Catal. Commun. 2007, 8, 1599–1603. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Nie, J.; Ma, L.; Huang, Y.; Li, L.; Liu, Y.; Guo, Z. Pilot-scale study on catalytic ozonation of bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst. Sci. Rep. 2018, 8, 7555. [Google Scholar] [CrossRef] [PubMed]
- Niedan, V.; Scholer, H.F. Natural Formation of Chlorobenzoic Acids (CBA) and Distinction between PCB-Degraded CBA. Science 1997, 35, 1233–1241. [Google Scholar] [CrossRef]
- Han, W.; Zhang, P.; Zhu, W.; Yin, J.; Li, L. Photocatalysis of p-chlorobenzoic acid in aqueous solution under irradiation of 254 nm and 185 nm UV light. Water Res. 2004, 38, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.S.; von Gunten, U. Hydroxyl Radical/Ozone Ratios during Ozonation Processes. I. The Rct Concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Pi, Y.; Schumacher, J.; Jekel, M. The use of para-chlorobenzoic acid (pCBA) as an ozone/hydroxyl radical probe compound. Ozone Sci. Eng. 2005, 27, 431–436. [Google Scholar] [CrossRef]
- Centurião, A.P.S.L.; Baldissarelli, V.Z.; Scaratti, G.; de Amorim, M.S.; Moreira, R.F.P.M. Enhanced ozonation degradation of petroleum refinery wastewater in the presence of oxide nanocatalysts. Environ. Technol. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Lu, X.; Liu, J.; Zhu, L.; Ma, Z.; Wu, Y. Catalytic ozonation of sulfosalicylic acid over manganese oxide supported on mesoporous ceria. Chemosphere 2016, 144, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, W.; Yin, X.; Liu, Y. The role of Mn-doping for catalytic ozonation of phenol using Mn/γ-Al2O3nanocatalyst: Performance and mechanism. J. Environ. Chem. Eng. 2016, 4, 3415–3425. [Google Scholar] [CrossRef]
- Kokkinos, E.; Simeonidis, K.; Pinakidou, F.; Katsikini, M.; Mitrakas, M. Optimization of tetravalent manganese feroxyhyte’s negative charge density: A high-performing mercury adsorbent from drinking water. Sci. Total Environ. 2017, 574, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, S.K.; Sklari, S.D.; Zamboulis, D.; Zaspalis, V.T.; Zouboulis, A.I. Development of bubble-less ozonation and membrane filtration process for the treatment of contaminated water. J. Membr. Sci. 2015, 492, 40–47. [Google Scholar] [CrossRef]
- Staehelld, J.; Hoigne, J. Decomposition of Ozone in Water in the Presence of Organic Solutes Acting as Promoters and Inhibitors of Radical Chain Reactions. Environ. Sci. Technol. 1985, 19, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Clesceri, S.L.; Greenberg, E.A.; Trussell, R.R. Inorganic Nonmetals. In Standard Methods for Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989; pp. 162–165. ISBN 0-87553-161-X. [Google Scholar]
- Kosmulski, M. Surface Charging and Points Zero Charge; CRC: Boca Raton, FL, USA, 2009; Volume 145, ISBN 978-1-4200-51889-9. [Google Scholar]
- Beltrán, F.J.; Rivas, F.J.; Montero-De-Espinosa, R. A TiO2/Al2O3 catalyst to improve the ozonation of oxalic acid in water. Appl. Catal. B Environ. 2004, 47, 101–109. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Activated carbon catalytic ozonation of oxamic and oxalic acids. Appl. Catal. B Environ. 2008, 79, 237–243. [Google Scholar] [CrossRef]

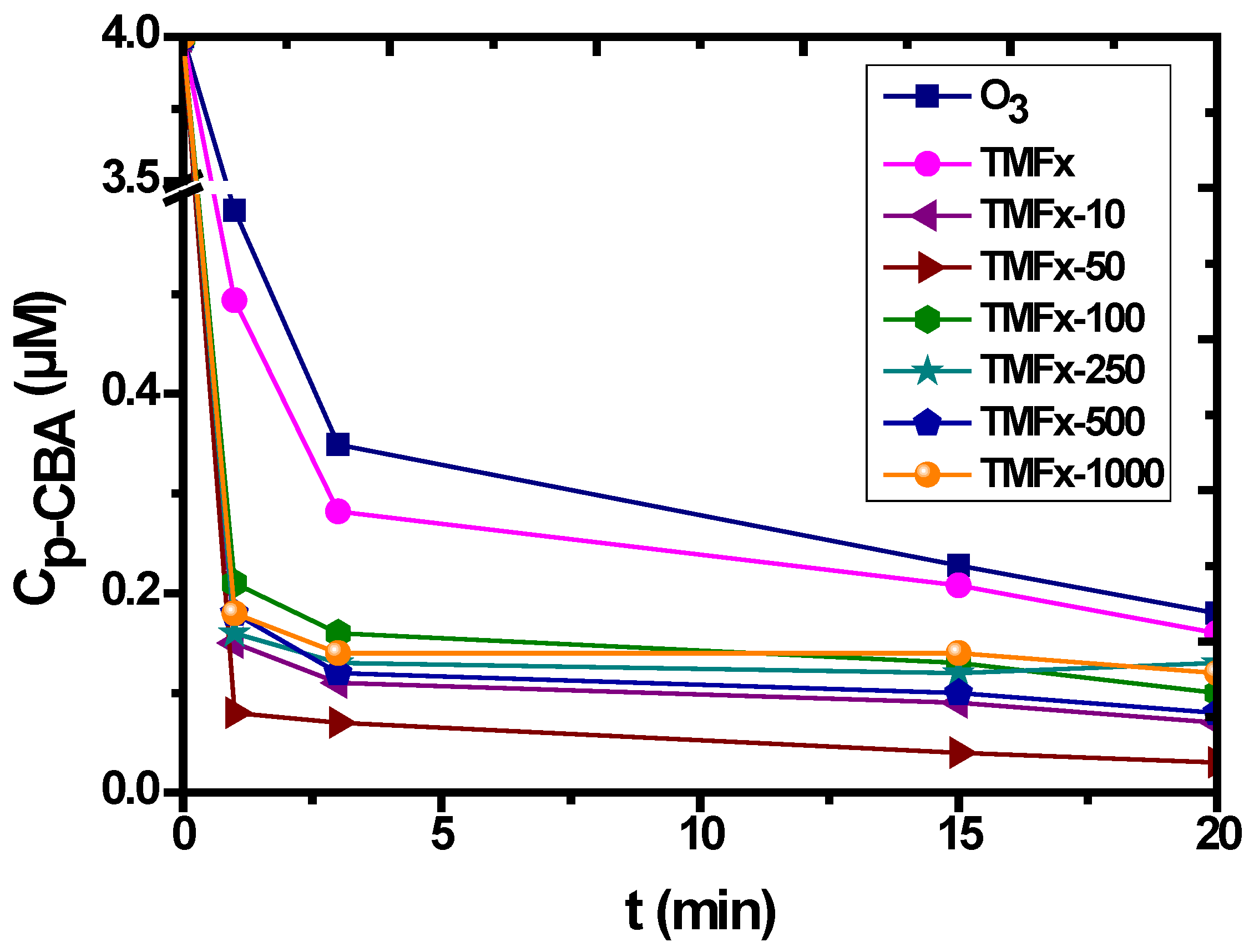
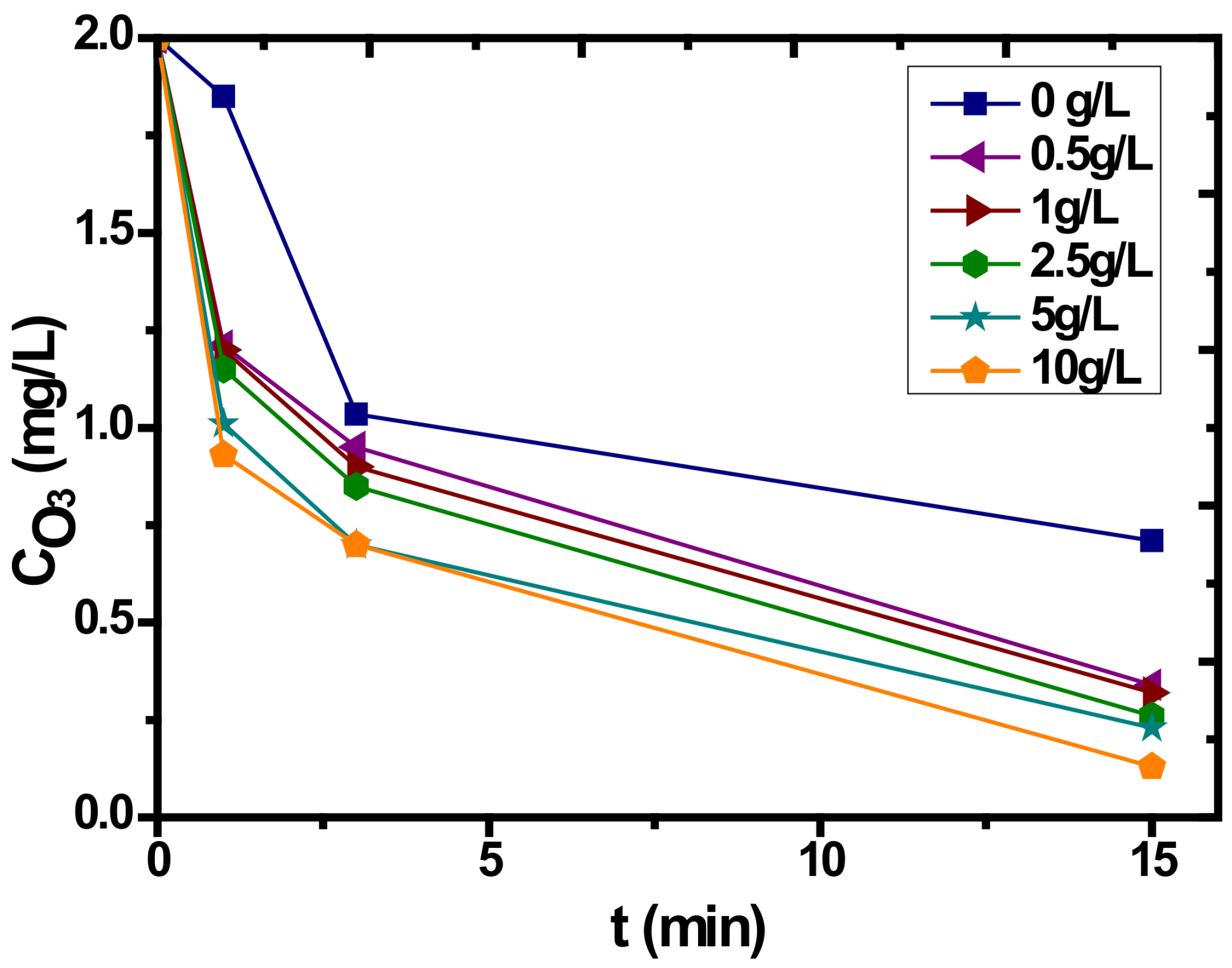
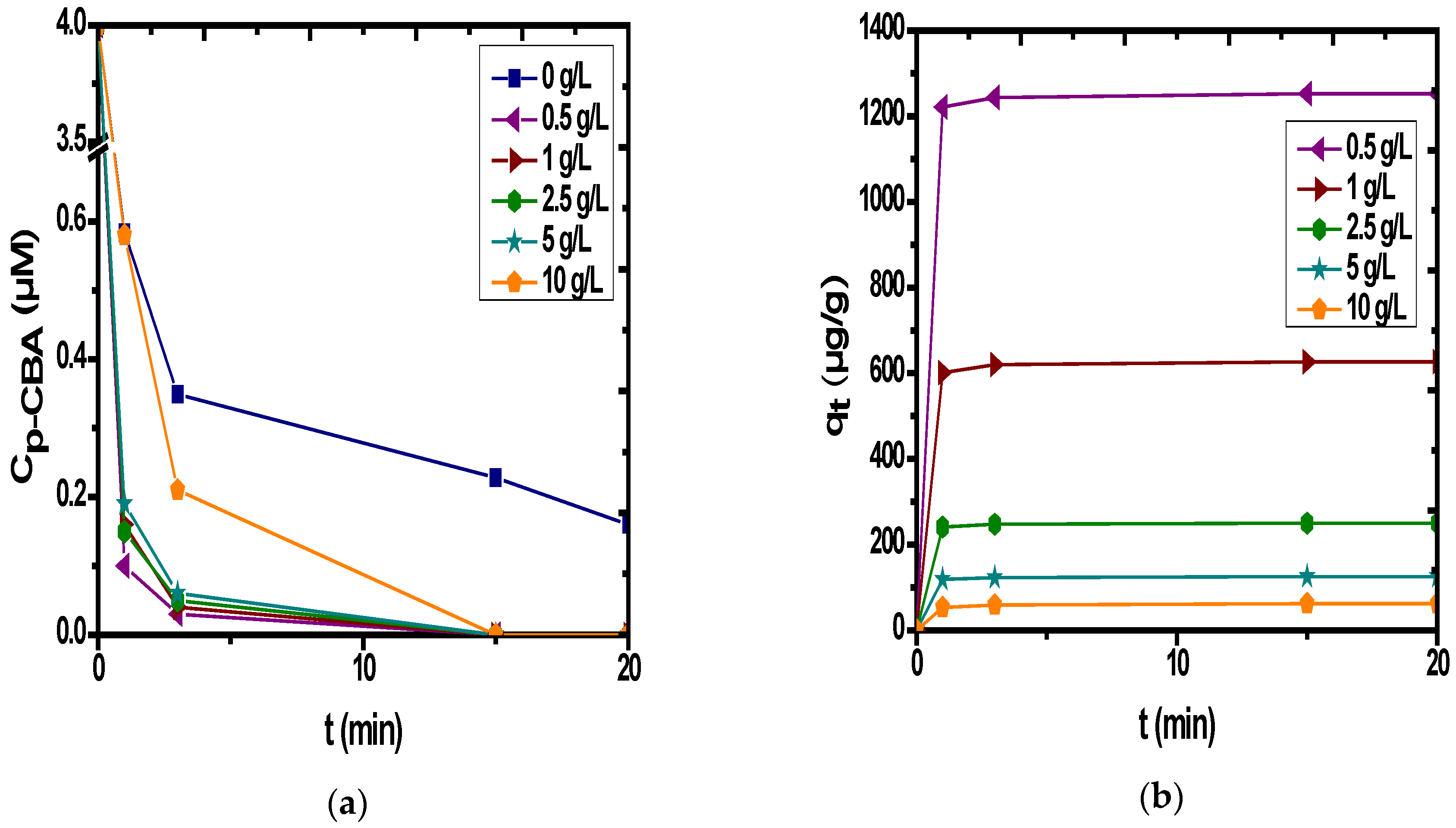
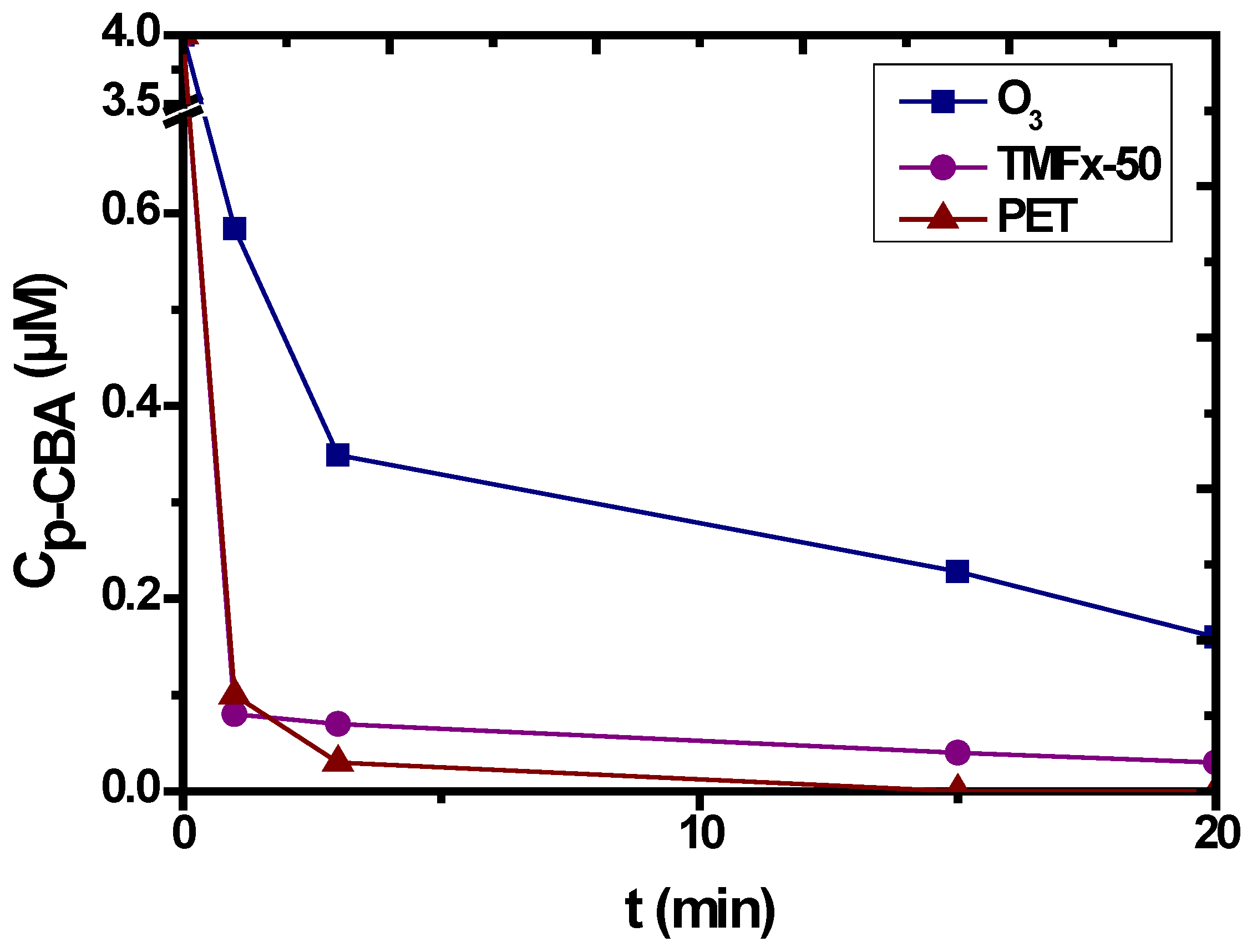
| Characteristics | Fe/Mnratio (wt %) | IEP | PZC | Surface Charge (mmol OH−/g) | Surface Charge (mmol H+/g) | SBET (m2/g) | Pore Volume (mL/g) | Pore Diameter (Å) |
|---|---|---|---|---|---|---|---|---|
| TMFx | 41/12.7 | 7.5 | 3.4 | 3.24 | 0.13 | 85 | 0.12 | 29 |
| Characteristics | Fe/Mn (wt %) | Si (wt %) | PZC | Surface Charge (mmol OH−/g) | Surface Charge (mmol H+/g) | SBET (m2/g) | Pore Volume (mL/g) |
|---|---|---|---|---|---|---|---|
| TMFx | 41/12.7 | 0 | 3.4 | 3.24 | 0.13 | 85 | 0.12 |
| TMFx-10 | 41/12.7 | 0 | 3.05 | 3.32 | 0.10 | 60.4 | 0.45 |
| TMFx-50 | 41/12.7 | <0.5 | 3.05 | 3.36 | 0.10 | 63.5 | 0.43 |
| TMFx-100 | 41/12.7 | <0.5 | 3.05 | 3.37 | 0.16 | 62.7 | 0.46 |
| TMFx-250 | 41/12.7 | <0.5 | 3.05 | 3.40 | 0.27 | 67.6 | 0.52 |
| TMFx-500 | 41/12.7 | 0.5 | 3.05 | 3.44 | 0.27 | 63.3 | 0.41 |
| TMFx-1000 | 41/12.7 | 1.5 | 3.05 | 3.37 | 0.27 | 60.0 | 0.47 |
| Parameter | TMFx | TMFx-10 | TMFx-50 | TMFx-100 | TMFx-250 | TMFx-500 | TMFx-1000 |
|---|---|---|---|---|---|---|---|
| q (μg p-CBA/g TMFx) | 125 | 125 | 250 | 167 | 160 | 167 | 136 |
| Parameter (g/L) | 0.5 | 1 | 2.5 | 5 | 10 |
|---|---|---|---|---|---|
| q (μg p-CBA/g PET) | 28 | 16 | 7 | 7 | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psaltou, S.; Stylianou, S.; Mitrakas, M.; Zouboulis, A. Heterogeneous Catalytic Ozonation of p-Chlorobenzoic Acid in Aqueous Solution by FeMnOOH and PET. Separations 2018, 5, 42. https://doi.org/10.3390/separations5030042
Psaltou S, Stylianou S, Mitrakas M, Zouboulis A. Heterogeneous Catalytic Ozonation of p-Chlorobenzoic Acid in Aqueous Solution by FeMnOOH and PET. Separations. 2018; 5(3):42. https://doi.org/10.3390/separations5030042
Chicago/Turabian StylePsaltou, Savvina, Stylianos Stylianou, Manasis Mitrakas, and Anastasios Zouboulis. 2018. "Heterogeneous Catalytic Ozonation of p-Chlorobenzoic Acid in Aqueous Solution by FeMnOOH and PET" Separations 5, no. 3: 42. https://doi.org/10.3390/separations5030042
APA StylePsaltou, S., Stylianou, S., Mitrakas, M., & Zouboulis, A. (2018). Heterogeneous Catalytic Ozonation of p-Chlorobenzoic Acid in Aqueous Solution by FeMnOOH and PET. Separations, 5(3), 42. https://doi.org/10.3390/separations5030042