Abstract
Virgin olive oil is unique among plant oils for its high levels of oleic acid, and the presence of a wide range of minor components, which are responsible for both its health-promoting properties and characteristic aroma, and only produced when olives are crushed during the industrial process used for oil production. The genetic variability of the major volatile compounds comprising the oil aroma was studied in a representative sample of olive cultivars from the World Olive Germplasm Collection (IFAPA, Cordoba, Spain), by means of the headspace solid-phase microextraction/gas chromatography–mass spectrometry–flame ionization detection (HS-SPME/GC-MS-FID). The analytical data demonstrated that a high variability is found for the content of volatile compounds in olive species, and that most of the volatile compounds found in the oils were synthesized by the enzymes included in the so-called lipoxygenase pathway. Multivariate analysis allowed the identification of cultivars that are particularly interesting, in terms of volatile composition and presumed organoleptic quality, which can be used both to identify old olive cultivars that give rise to oils with a high organoleptic quality, and in parent selection for olive breeding programs.
1. Introduction
Virgin olive oil (VOO) is an essential component of the traditional Mediterranean diet, and attracts the interest of the scientific community for its health-promoting properties [1,2,3,4,5,6]. VOO is unique for its high levels of monounsaturated fatty acids and the presence of a wide range of minor components that are largely responsible for both their health-promoting properties and their organoleptic characteristics. Regarding the organoleptic characteristics, some of these minor components are volatile and consequently responsible for the aroma of VOO, which is characterized by a distinctive balance of green and fruity attributes.
Olías et al. [7] established that most of VOO volatiles are synthesized when enzymes and substrates join during the olive fruit milling in the oil extraction process, and that the enzymatic activities within the lipoxygenase (LOX) pathway are involved in this synthesis. Aldehydes and alcohols of six straight-chain carbons (C6), as well as their derived esters, are the major compounds in VOO aroma, either qualitatively or quantitatively [8,9]. Linoleic (LA) and linolenic (LnA) acids are the main substrates for this synthesis. LOX enzymes catalyze the production of 13-hydroperoxide derivatives in the first step of this pathway, which are later cleaved heterolytically by the hydroperoxide lyase (HPL) enzyme, forming C6 aldehydes [7,10,11]. C6 aldehydes are then reduced by alcohol dehydrogenase (ADH) enzymes to C6 alcohols [7,12], and finally converted into the corresponding esters by alcohol acyltransferase (AAT) enzymes [7,13]. The relevance of the five straight-chain carbons (C5) compounds in VOO aroma has also been suggested [9]. They seem to be produced via a parallel branch of the LOX pathway, as demonstrated in soybeans [14,15].
The synthesis of VOO volatile compounds seems to depend primarily on the availability of fatty-acid substrates to be catabolized through the LOX pathway during the VOO extraction process. In addition, LOX activity also proved to be an important limiting factor for the production of these volatile compounds, and this limitation is seemingly characteristic of each olive cultivar [16,17].
Along with its possible health benefits, its excellent organoleptic properties may well explain the continued demand for VOO. As mentioned above, volatile compounds are responsible for the aroma of this product, which is composed, when obtained from sound fruits, of a mixture of green and fruity odor notes, spiced with some other positive notes that make VOO a unique, edible oil. Odor perception and pleasantness are determined by the functional groups in the volatile compound [18,19]. To understand the relationship between volatile compounds and odor attributes in VOO, Aparicio and Morales [20] developed a statistical sensory wheel through a compilation of the data obtained from trained VOO sensory panels across Europe. As a result, odor notes with similar description and volatile compounds with a given sensory perception were grouped together into sectors. Among the most relevant to VOO aroma are green or ripe fruit odor notes. Moreover, concentration and odor threshold determine the sensory contribution of each volatile compound in the oil. The ratio between the concentration of the volatile compound and its odor threshold is the odor activity value (OAV). Those volatile compounds with OAV below one do not theoretically contribute to the aroma of VOO.
The volatile fraction of VOO is also responsible for the off-flavors present in some oils. This is of absolute importance because VOO classification into commercial categories requires a sensory analysis. This classification is officially ruled [21] and carried out by trained test panels, which assess the green and fruity odor notes typical in the aroma of virgin olive oil, when extracted from healthy, fresh olive fruits, as well as aroma defects in the oil. When VOO was obtained from infested olive fruits or those that were incorrectly processed or stored, the volatile fraction of VOO is altered and may include off-flavor compounds responsible for the sensory defects in the oils. They are mostly aliphatic carbonyls and alcohols or acids [22,23], which are produced by chemical oxidation, or by the activity of microbial enzymes present in non-sound fruits.
One of the key characteristics of the olive species is its wide genetic patrimony. The collection and conservation of olive genetic resources has received much attention. Thus, the World Olive Germplasm Collection (WOGC, Cordoba, Spain) is internationally recognized due to its large number of cultivars, and the high degree of evaluation and identification by molecular markers and agronomical traits [24]. This high genetic diversity of the olive germplasm collection could be very useful to recuperate old cultivars which produce oils with outstanding aromas, or for the selection of parents in breeding programs, in order to find new cultivars with improved olive oil aroma. In this sense, olive breeding programs are lately addressing the selection of cultivars, based on the sensory and nutritional qualities of VOO [25,26], in addition to the traditional agronomic traits [27]. For this purpose, the aim of this work was to evaluate the volatile composition and presumed aroma properties of the oils from a representative sample of cultivars from WOGC.
2. Materials and Methods
2.1. Plant Material
Sixty-one olive (Olea europaea L.) cultivars from the WOGC (IFAPA Alameda del Obispo in Cordoba) were studied (Table 1). Two trees per accession were grown at the experimental orchards of IFAPA Alameda del Obispo center using drip irrigation and standard culture practices. Fruits were picked by hand on two consecutive years, when they reached the turning stage, to better compare the cultivars.

Table 1.
Cultivars selected from the World Olive Germplasm Collection (WOGC, Cordoba, Spain).
2.2. Olive Oil Extraction
Olive oil was extracted from olive fruits (2–3 kg) using an Abencor system (Comercial Abengoa, S.A., Seville, Spain) that mimics, at laboratory scale, the industrial process of VOO extraction [28]. Fruits were milled using a stainless steel hammer mill operating at 3000 rpm and with a 5 mm sieve, malaxation performed for 30 min at 28 °C, and centrifugation of the paste carried out in a basket centrifuge at 3500 rpm for 1 min. The oils were decanted, filtered through paper, and stored under nitrogen at −20 °C until analysis.
2.3. Analysis of Volatile Compounds
Analysis of volatile compounds was carried out according to Pérez et al. [29]. Olive oil samples (0.5 g) were prepared in 10-mL vials and placed in a vial heater at 40 °C for a 10 min equilibration time. Volatile compounds from the headspace were adsorbed onto a headspace solid-phase microextraction (SPME) fiber divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) 50/30 µm (Supelco Co., Bellefonte, PA, USA). Sampling time was carried out at 40 °C for 50 min. Desorption of volatile compounds was carried out directly in the GC injector. Volatile compounds were identified on a 7820A/GC-5975/MSD system (Agilent Technologies, Santa Clara, CA, USA), including a DB-Wax capillary column (60 m × 0.25 mm i.d., film thickness, 0.25 µm: J&W Scientific, Folsom, CA, USA) under the following conditions: Injection port operated in splitless mode at 250 °C; He as the carrier gas at a flow rate of 1 mL/min; column held for 6 min at 40 °C, then programmed at 2 °C min−1 to 168 °C; the mass detector was operated in the electronic impact mode at 70 eV; source temperature set at 230 °C; and the mass spectra scanned at 2.86 scans/s in the m/z 40–550 amu range. Compounds were identified by mass spectrum, comparing to the Wiley/NBS and NIST libraries, retention index in agreement with literature, and co-elution with chemical standard when available. Quantification was performed in triplicate on a HP-6890 GC apparatus (Agilent Technologies, Santa Clara, CA, USA), which was equipped with a similar column and operated under the following operating conditions in order to obtain the same retention times for volatile compounds such as those obtained with the 7820A/GC-5975/MSD system: N2 as the carrier gas at 17 psi; injector and detector at 250 °C; oven held for 6 min at 40 °C and then programmed at 2 °C min−1 to 168 °C. For the quantification of the different compounds, calibration curves obtained by adding known amounts of the different compounds to re-deodorized, high-oleic sunflower oil were used. Limits of quantification and concentration ranges were established according to typical contents in virgin olive oil volatile fractions. Linear regression curves were found for all the compounds, with regression coefficients higher than 0.99. At the beginning, and regularly during the sample analyses, a blank containing no oil and a mixture of volatile standards were run as controls.
2.4. Statistical Analysis
The data were analyzed using STATISTICA (Statsoft Inc., Tulsa, OK, USA). Factor Analysis and Principal Component Analysis (PCA) were used to assess the associations among the volatile compounds from the WOGC cultivar sample.
3. Results and Discussion
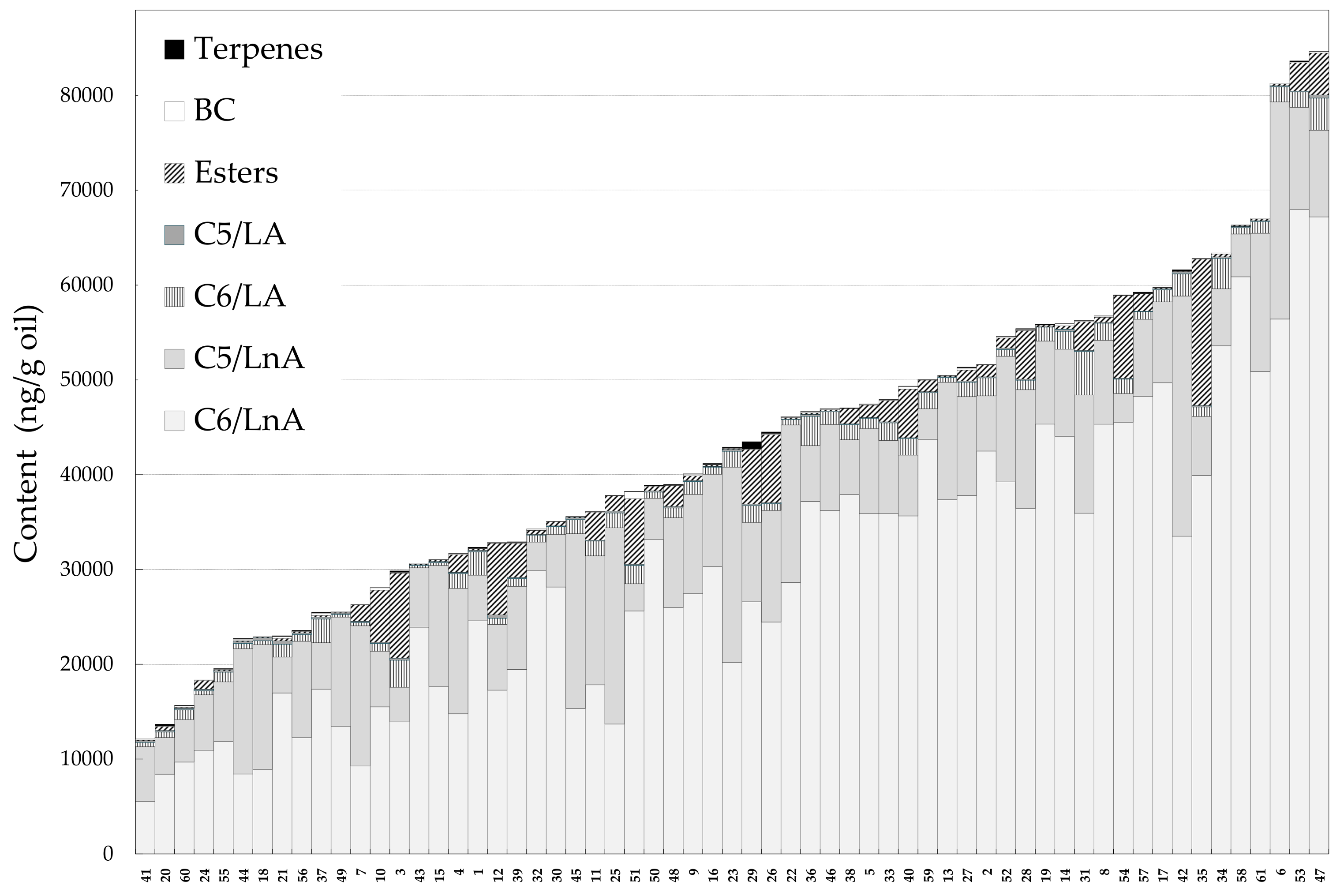
The extensive genetic patrimony of the olive tree is currently used in different olive breeding programs, but only recently includes the sensory properties of VOO as main traits, in addition to the agronomic traits [25,27,30]. Regarding these sensory properties, the natural variation of aroma compounds in VOO was studied using the WOGC olive cultivar collection. For this purpose, around 15% of this germplasm collection was considered (Table 1) [24]. Only healthy fruits were picked at the turning stage, and mild operating conditions were employed for the oil extraction process, to minimize the production of compounds that cause sensory defects in the oils. This subset of the olive germplasm collection showed a high degree of variability, in terms of the content of volatile compounds (Figure 1 and Table 2). As mentioned, most of the volatile compounds found in the oils were synthetized by the enzymes included in the so-called LOX pathway [7], and can be grouped according to the length of the chain, the fatty acid substrate (C6/LnA, C6/LA, C5/LnA, C5/LA), and the origin of esters (LOX esters). Moreover, compounds derived from amino acid metabolism, which has a branched-chain chemical structure (BC), and a terpene also contributed quantitatively to the volatile fraction of VOO. C6 compounds derived from linolenic acid (C6/LnA) represented, on average, about 70% of total volatile fraction in the oils (Figure 1). The C6/LnA compounds varied from 5.53 to 67.94 µg/g oil, a variability higher than that found for oils produced from a collection of 39 olive cultivars, cultivated under the same edaphoclimatic conditions (2.52–18.11 µg/g oil) [31]. The C6/LnA aldehyde group was the most abundant, of which (E)-hex-2-enal was the main compound (91% of total C6/LnA compounds on average, Figure 1). The mean content of (E)-hex-2-enal in the oils was 27.19 µg/g oil, ranging from 4.70 to 65.03 µg/g oil. The large amount, and the relatively low odor threshold [32], of this C6/LnA compound make it one of the main contributors to the aromas of the oils, which seems to be typical of oils from the olive species [29,31].
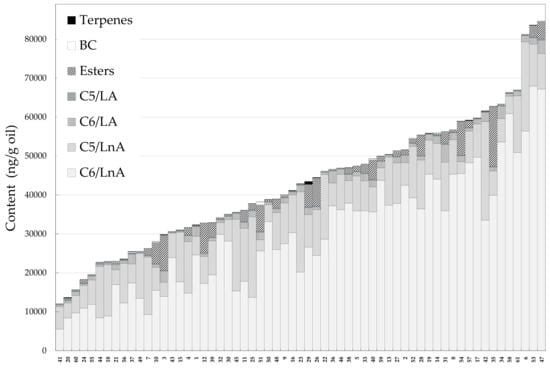
Figure 1.
Content (ng/g oil) of the main groups of volatile compounds in the oils from the WOGC cultivar subset. Numbers correspond to the WOGC cultivars displayed in Table 1.

Table 2.
Content of the volatile compounds (ng/g oil) in the oils from the WOGC cultivar subset and percentaje of cultivars with odor activity values (OAV) higher than 1 for each volatile compound.
C5/LnA compounds showed contents close to those of the C6/LnA compounds. Quantitatively, the pentene dimers are the major compounds in this group. They are thought to be produced in the same branch of the LOX pathway as the rest of the C5 compounds [33]. The pentene dimers represented 82% of the C5/LnA content on average (Figure 1), but seem to have a quite low or negligible sensory contribution to the VOO aroma, according to the estimated odor thresholds for these compounds [29]. The rest of the C5/LnA compounds seem to contribute to VOO aroma according to their OAVs (Table 2). Pent-1-en-3-one and the pent-2-en-1-ols are especially remarkable. All the cultivars showed to have contents of pent-1-en-3-one above its odor threshold (0.73 ng/g oil). The odor of this compound is considered unpleasant [9], while the aroma of pent-2-en-1-ol is pleasant, green-fruity. Most of the olive cultivars (90%) presented (E)-pent-2-en-1-ol contents above its odor threshold [29] (Table 2). On the contrary, (Z)-pent-2-en-1-ol contents suggest that this compound is of little relevance to VOO aroma, as it was present below its threshold concentration in the oils.
Fruity odor notes are positive attributes of the oil aroma. Volatile esters are the main compounds responsible for these odor notes, especially LOX esters. Their content in the olive collection subset was 1844 ng/g oil on average, and is present in a range of 14–15,369 ng/g oil, much higher than those values found in a progeny of the cross of cultivars Picual and Arbequina [29]. Only a few accessions (3%) had (E)-hex-2-en-1-yl acetate contents that contribute to their aroma (OAV > 1, Table 2). However, (Z)-hex-3-en-1-yl acetate contents suggest this compound to be an important contributor to VOO aroma in more than half of the olive collection subset (Table 2).
The levels of the branched-chain (BC) compounds in the different accessions of the olive collection subset are also important for the aroma of the oils, despite their low concentrations in the oils (average 106 ng/g oil, range of 20–740 ng/g oil). The 2- and 3-methyl-butanal contents suggest that these BC aldehydes contribute decisively to the VOO aroma, since they have OAV values higher than one in all the cultivars. This aroma significance for VOO is especially notable because they are located in the ‘ripe fruit’ sector of the statistical sensory wheel [20]. However, 2-methyl-butan-1-ol, which is responsible for the fusty off-flavor of the oil [34], was found below its odor threshold in all the cultivars assessed (Table 2), so it should not contribute to the aroma of the oils. Finally, only 2% of the cultivars of the olive collection subset had an OAV above one for limonene (Table 2), which suggests that this terpene is not an important contributor to VOO.
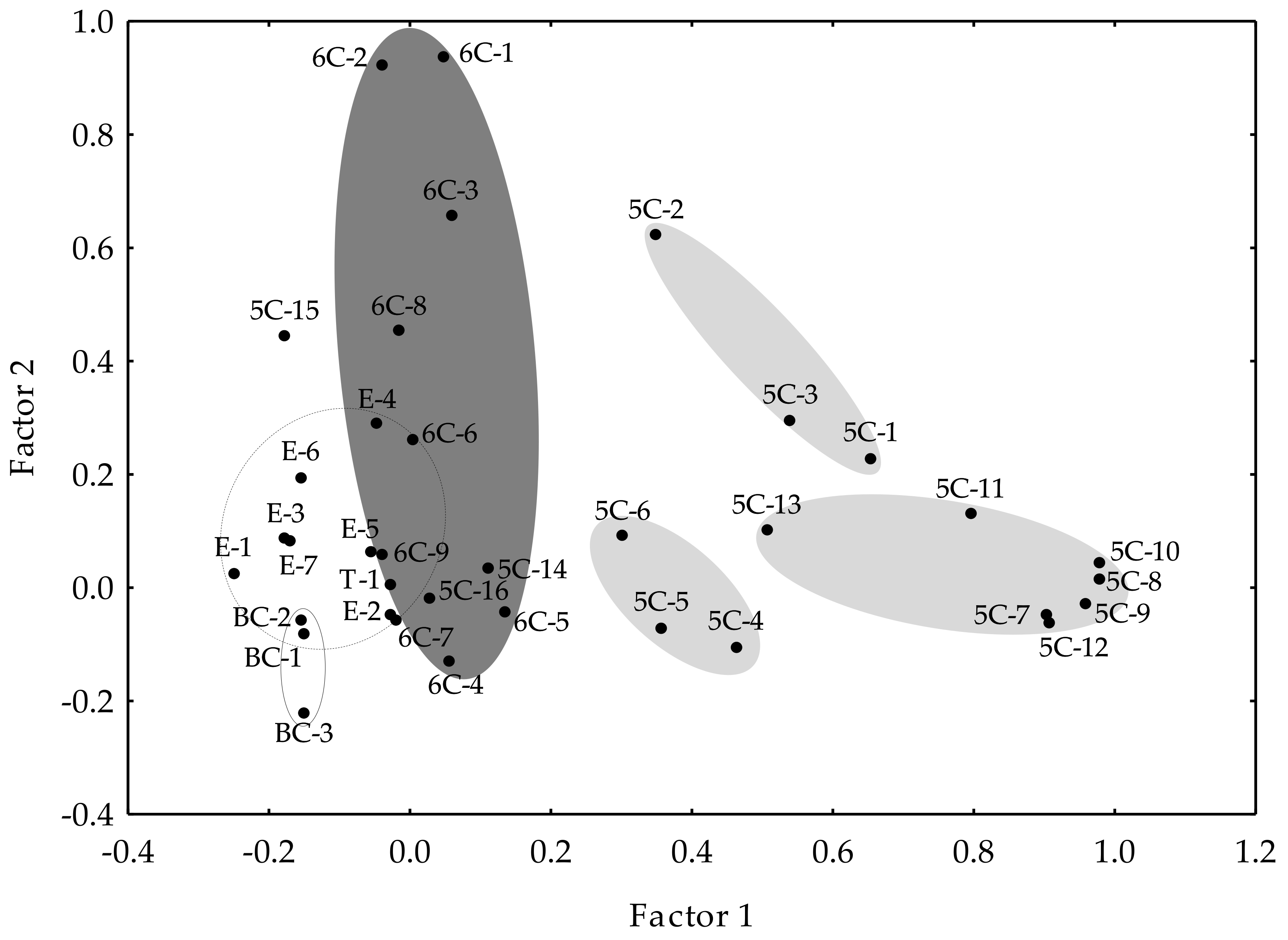
Factor analysis allowed the correlations within the different volatile compounds in the olive oils to be explained. Figure 2 displays the factor analysis, considering only those factors with eigenvalues higher than one and using the normalized Varimax method. Those factors explained 77.97% of the total variance. The first factor explained 20.95% of the variance, while the second factor explained 12.66%. As shown, most of the C6 compounds spread along Factor 2 (dark gray ellipse), while most of the C5 compounds distributed along Factor 1. Three different groupings may be distinguished for the latter (Figure 2, light gray ellipses), which correspond to the C5/LnA alcohols, carbonyls, and pentene dimers, respectively. The group of C5/LnA alcohols is the closest to the C6 compounds, which would indicate a greater metabolic proximity and would support the hypothesis that C5/LnA alcohols would be the first products formed from the homolytic branch of the LOX pathway, which involves the activity of a LOX protein forming an alkoxy radical from a polyunsaturated fatty acid [33], parallel to the pentene dimers formation but in different ways, and finally oxidized enzymatically to C5/LnA carbonyls. Moreover, it was found previously that the pentene dimer content is not related to the contents of the C5/LA carbonyls (5C-14, 5C-15) or alcohol (5C-16), suggesting that pentene dimers are only synthesized from LnA [35]. Accordingly, those compounds are located in Figure 2 far from the group of pentene dimers.
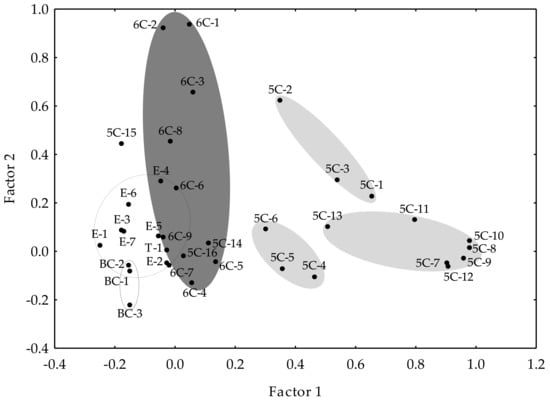
Figure 2.
Factor analysis. Position of the volatile compounds in the oils from the WOGC cultivar subset on the first two factors using the normalized Varimax method.
On the other hand, the esters grouped together, overlapping the space of the C6 compounds, where the main precursors of esters are located [(hexan-1-ol (6C-9) and (Z)-hex-3-enol (6C-7)]. This partial disconnection from the LOX pathway mainstream could be due to the limitation of alcohol production during the oil extraction process, mainly due to the inactivation of ADH activity during VOO production, as previously demonstrated [36].
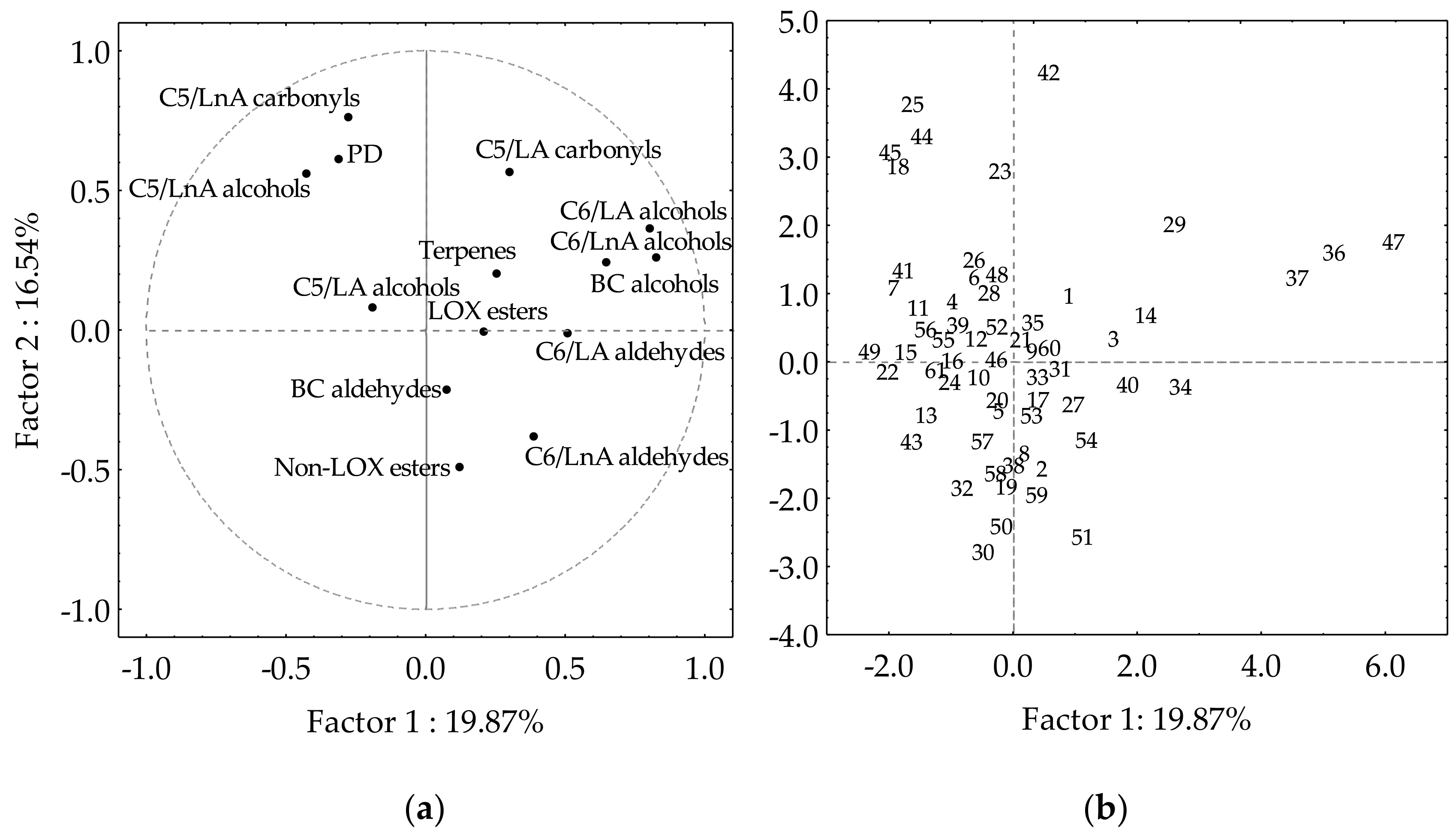
The PCA approach explains the associations between the different classes of volatile compounds in the olive collection subset, and allows us to distinguish those olive cultivars that are especially rich in some compounds (Figure 3). The first factor explains 19.87% of the variance, whereas the second factor explains 16.54%. Similar values were found when evaluating the content of the main volatile compounds in the oils of the Core-36 nuclear collection from the WOGC [35], and the progeny of the cross of cultivars Picual and Arbequina [29]. Cultivars producing oils with high contents of C6/LnA aldehydes are situated in the fourth quadrant of the plot, while those producing oils with high contents of C5/LnA are located opposite in the second quadrant. This distribution allows the selection in the fourth quadrant of those cultivars producing oils characterized by high contents of C6/LnA aldehydes, LOX esters, as well as BC aldehydes, which might act synergistically to provide green fruity odor notes. Most of the compounds included in those volatile classes are situated in sectors ‘green fruit’ and ‘ripe fruit’ of the statistical sensory wheel [20]. Therefore, it is possible to select cultivars from the germplasm collection subset whose oils have a prevailing green aroma, such as those cultivars located in the bisector of the first quadrant.
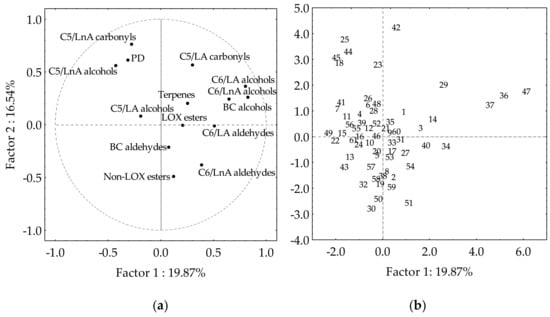
Figure 3.
Main groups of volatile compounds in the oils from the WOGC cultivar subset. (a) Vector distribution of the volatile groups. (b) Distribution of the cultivars.
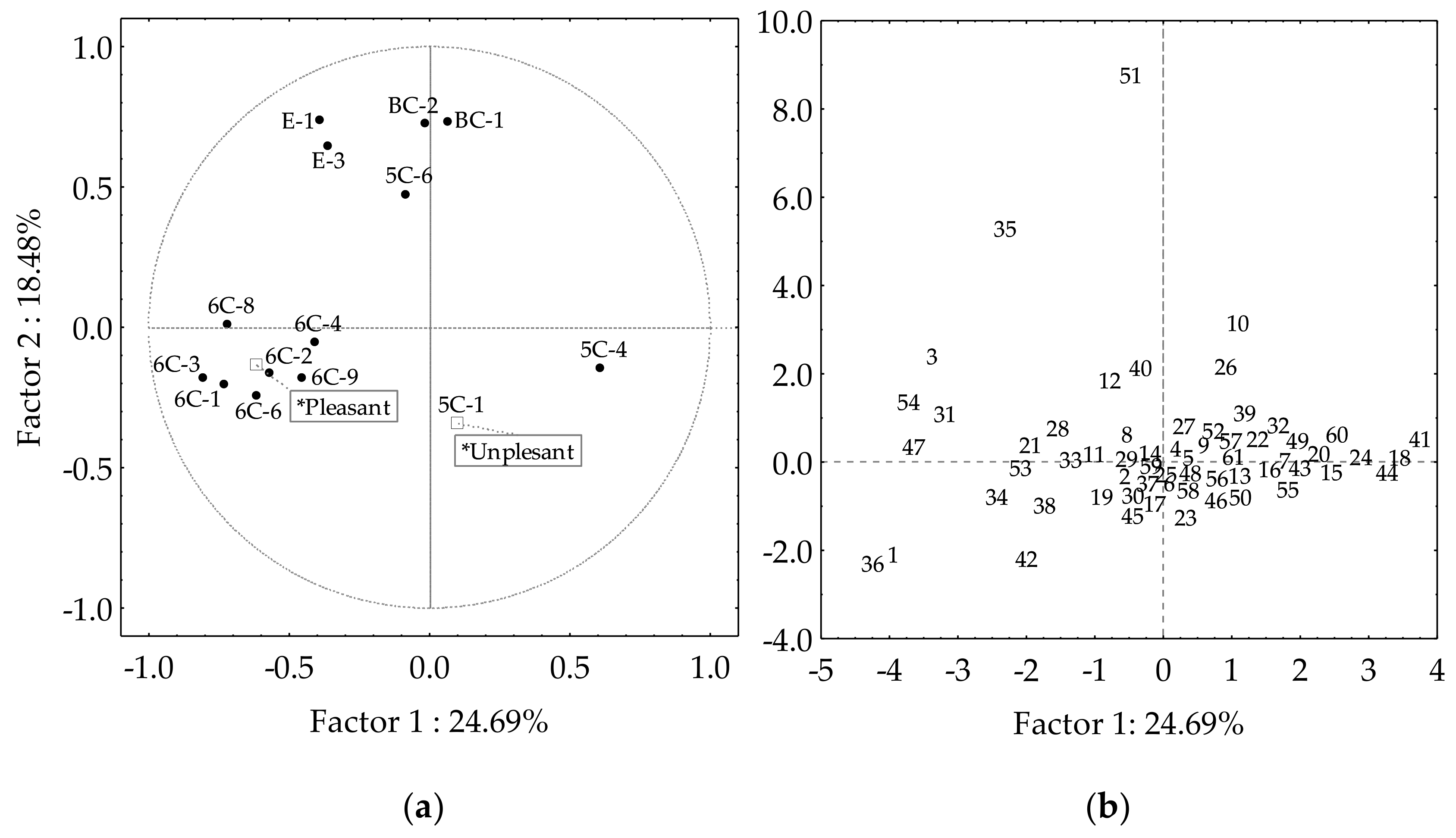
Table 2 shows those volatile compounds which present contents in the cultivar oils that make them major contributors to the oil aroma (OAV > 1). However, other volatile compounds contribute to the oil aroma of only a certain number of cultivars. A new PCA was carried out considering as variables those volatiles with OAV higher than one in more than 5% of the cultivars, thus truly contributing to the aroma of the oil (Figure 4). Most of them are considered desirable for VOO aroma, except for hexan-1-ol (6C-9), pent-1-en-3-one (5C-1), and pent-1-en-3-ol (5C-4). The latter give unpleasant sensations [9,20,34]. Factors 1 and 2 explain 43% of the data variation, quite similar to what was found for the Core-36 nuclear collection from the WOGC [35], and the progeny of the cross of cultivars Picual and Arbequina [29]. When using as variables those volatile compounds with OAV higher than one in more than 5% of the cultivars, the cultivars are distributed mainly along the Factor 1 axis (Figure 4b). When using the sums of the OAV of the compounds as supplementary variables in the PCA, which according to the literature are desirable (Pleasant) or undesirable (Unpleasant) to the oil aroma, the area in which the cultivars had desirable odor notes (Figure 4b, third quadrant) is clearly differentiated from that in which those cultivars characterized by having undesirable odor notes are located (Figure 4b, fourth quadrant).
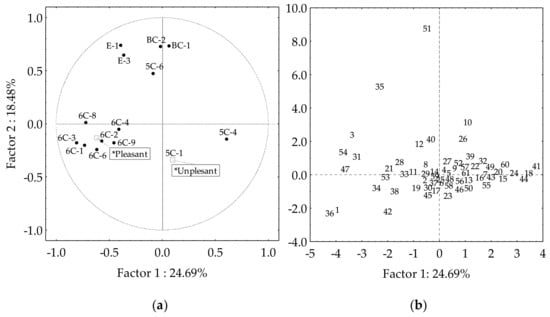
Figure 4.
Selected volatile compound contributors to the aroma (OAV > 1) of the oils from the WOGC cultivar subset. (a) Vector distribution of the selected volatiles. (b) Distribution of the cultivars.
4. Conclusions
Olive species present a high variability for the VOO volatile compounds and, likewise, of the aroma quality. This natural variation of the aroma quality, and the high genetic diversity of the cultivar germplasm collection, suggest that it is possible both to pinpoint old olive cultivars that give rise to oils with a high organoleptic quality, and to select parents useful for breeding programs in order to develop olive cultivars with an enriched oil aroma. Multivariate analysis seems be a useful tool for this purpose.
Acknowledgments
Funding for this research came from the OLEAGEN project of Genoma España and the project AGL2011-24442 from the Programa Nacional de Recursos y Tecnologías Agroalimentarias, both financed by the Spanish Government.
Author Contributions
C.S. and A.G.P. conceived and designed the experiments; A.S.-O., A.G.P., and C.S. performed the experiments; A.G.P. and C.S. analyzed the data; A.B. contributed materials; C.S. collected data, performed statistical analysis, and wrote the first draft of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Bernardini, E. Extra virgin olive oil’s polyphenols: Biological activities. Curr. Pharm. Des. 2011, 17, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Covas, M.I.; Muñoz-Aguayo, D.; Khymenets, O.; de La Torre, R.; Saez, G.; del Carmen Tormos, M.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of VOO polyphenols within the frame of the Mediterranean diet: A randomized trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, F.; Ruano, J.; Pérez-Martínez, P.; López-Segura, F.; López-Miranda, J. The influence of olive oil on human health: Not a question of fat alone. Mol. Nutr. Food Res. 2007, 51, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Olias, J.M.; Perez, A.G.; Rios, J.J.; Sanz, C. Aroma of virgin olive oil: Biogenesis of the green odor notes. J. Agric. Food Chem. 1993, 41, 2368–2373. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio, R.; Ríos, J.J. Dynamic headspace gas chromatographic method for determining volatiles in virgin olive oil. J. Chromatogr. A 1994, 68, 455–462. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odour notes: Their relationships with the volatile compound from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Salas, J.; Williams, M.; Harwood, J.L.; Sanchez, J. Lipoxygenase activity in olive (Olea europaea L.) fruit. J. Am. Oil Chem. Soc. 1999, 76, 1163–1169. [Google Scholar] [CrossRef]
- Salas, J.; Sanchez, J. Hydroperoxide lyase from olive (Olea europaea L.) fruits. Plant Sci. 1999, 143, 19–26. [Google Scholar] [CrossRef]
- Salas, J.J.; Sanchez, J. Alcohol dehydrogenases from olive (Olea europaea) fruit. Phytochemistry 1998, 48, 35–40. [Google Scholar] [CrossRef]
- Salas, J.J. Characterization of alcohol acyltransferase from olive fruit. J. Agric. Food Chem. 2004, 52, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W.; Grove, M.J.; Salch, Y.P. Enzymic pathway to ethyl vinyl ketone and 2-pentenal in soybean preparations. J. Agric. Food Chem. 1996, 44, 882–886. [Google Scholar] [CrossRef]
- Fisher, A.J.; Grimes, H.D.; Fall, R. The biochemical origin of pentenol emissions from wounded leaves. Phytochemistry 2003, 62, 159–163. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Pérez, A.G.; Sanz, C. Cultivar differences on nonesterified polyunsaturated fatty acid as a limiting factor for biogenesis of virgin olive oil aroma. J. Agric. Food Chem. 2007, 55, 7869–7873. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortiz, A.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Synthesis of volatile compounds of virgin olive oil is limited by the lipoxygenase activity load during the oil extraction process. J. Agric. Food Chem. 2012, 60, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Bedoukian, P.Z. The seven primary hexenols and their olfactory characteristics. J. Agric. Food Chem. 1971, 19, 1111–1114. [Google Scholar] [CrossRef]
- Hatanaka, A.; Kajiwara, T.; Horino, H.; Inokuchi, K.I. Odor-structure relationships in n-hexanols and n-hexenales. Z. Naturforsch. 1992, 47, 183–189. [Google Scholar]
- Aparicio, R.; Morales, M.T. Sensory wheels: A statistical technique for comparing QDA panels Application to virgin olive oil. J. Sci. Food Agric. 1995, 67, 247–257. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation 640/2008/EC. Off. J. Eur. Union 2008, L178, 11–16. [Google Scholar]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Belaj, A.; Dominguez-Garcia, M.C.; Atienza, S.G.; Martin-Urdiroz, N.; de la Rosa, R.; Satovic, Z.; Martin, A.; Kilian, A.; Trujillo, I.; Valpuesta, C.; et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- León, L.; Beltrán, G.; Aguilera, M.P.; Rallo, L.; Barranco, D.; de la Rosa, R. Oil composition of advanced selections from an olive breeding program. Eur. J. Lipid Sci. Technol. 2011, 113, 870–875. [Google Scholar] [CrossRef]
- Rjiba, I.; Gazzah, N.; Dabbou, S.; Hammami, M. Evaluation of virgin olive oil minor compounds in progenies of controlled crosses. J. Food Biochem. 2011, 35, 1413–1423. [Google Scholar] [CrossRef]
- Lavee, S. Aims, methods and advances in breeding of new olive (Olea europaea L.) cultivars. Acta Hortic. 1989, 286, 23–40. [Google Scholar] [CrossRef]
- Martinez, J.M.; Munoz, E.; Alba, J.; Lanzon, A. Report about the use of the ‘Abencor’ analyser. Grasas Aceites 1975, 26, 379–385. [Google Scholar]
- Perez, A.G.; de la Rosa, R.; Pascual, M.; Sanchez-Ortiz, A.; Romero-Segura, C.; Leon, L.; Sanz, C. Assessment of volatile compound profiles and the deduced sensory significance of virgin olive oils from the progeny of Picual × Arbequina cultivars. J. Chromatogr. A 2016, 1428, 305–315. [Google Scholar] [CrossRef] [PubMed]
- El Riachy, M.; Priego-Capote, F.; Rallo, L.; Luque de Castro, M.D.; León, L. Phenolic profile of virgin olive oil from advanced breeding selections. Span. J. Agric. Res. 2012, 10, 443–453. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile composition. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Reiners, J.; Grosch, W. Odorants of virgin olive oils with different flavor profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
- Angerosa, F.; Camera, L.; d’Alessandro, N.; Mellerio, G. Characterization of seven new hydrocarbon compounds present in the aroma of virgin olive oils. J. Agric. Food Chem. 1998, 46, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; Lanza, B.; Marsilio, V. Biogenesis of “fusty” defect in virgin olive oils. Grasas Aceites 1996, 47, 142–150. [Google Scholar] [CrossRef]
- García-Vico, L.; Belaj, A.; Sanchez-Ortiz, A.; Martínez-Rivas, J.M.; Pérez, A.G.; Sanz, C. Volatile compound profiling by HS-SPME/GC-MS-FID of a core olive cultivar collection as a tool for aroma improvement of virgin olive oil. Molecules 2017, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ortiz, A.; Romero-Segura, C.; Gazda, V.E.; Graham, I.A.; Sanz, C.; Perez, A.G. Factors limiting the synthesis of virgin olive oil volatile esters. J. Agric. Food Chem. 2012, 60, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).