Development of an Automated Method for Selected Aromas of Red Wines from Cold-Hardy Grapes Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples, Internal Standard, SPME
2.2. GC-MS-Olfactometry System
3. Results
3.1. SPME Optimization
3.1.1. SPME Coating Selection
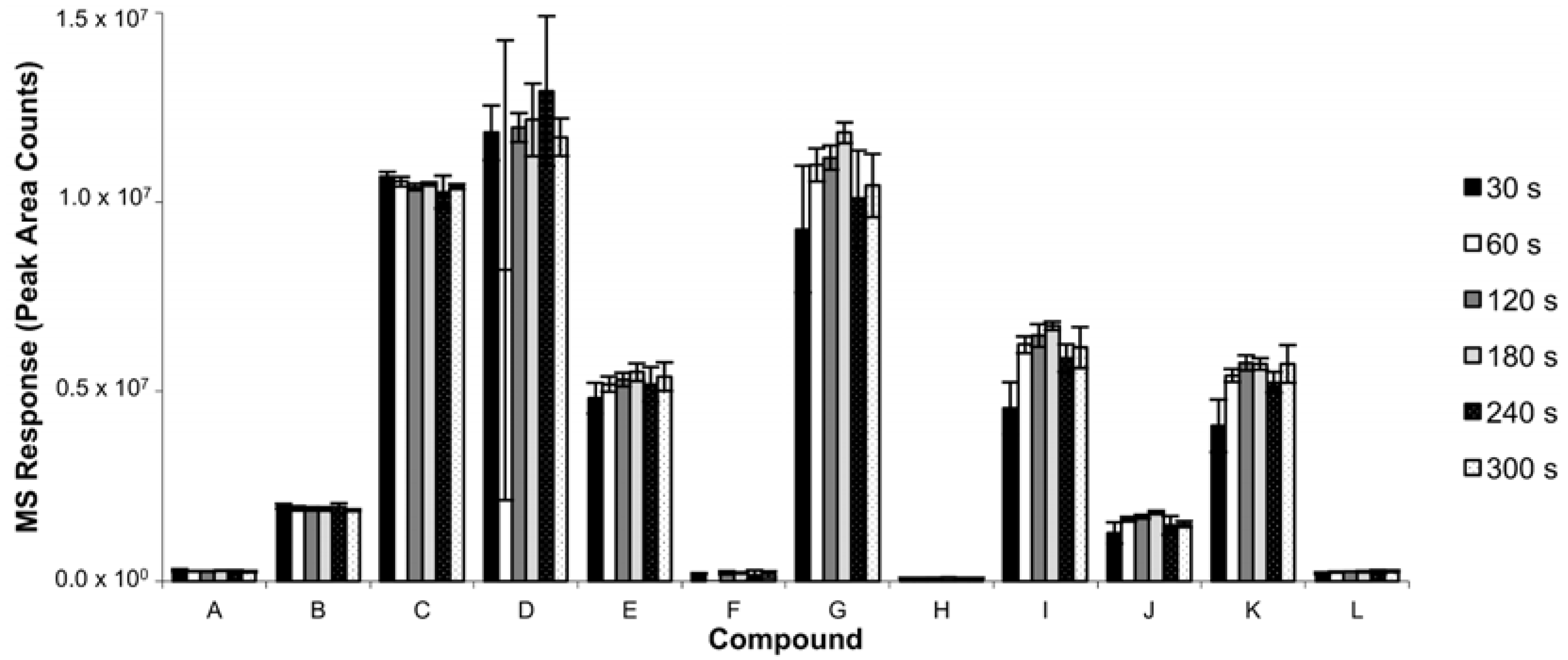
3.1.2. Extraction Time
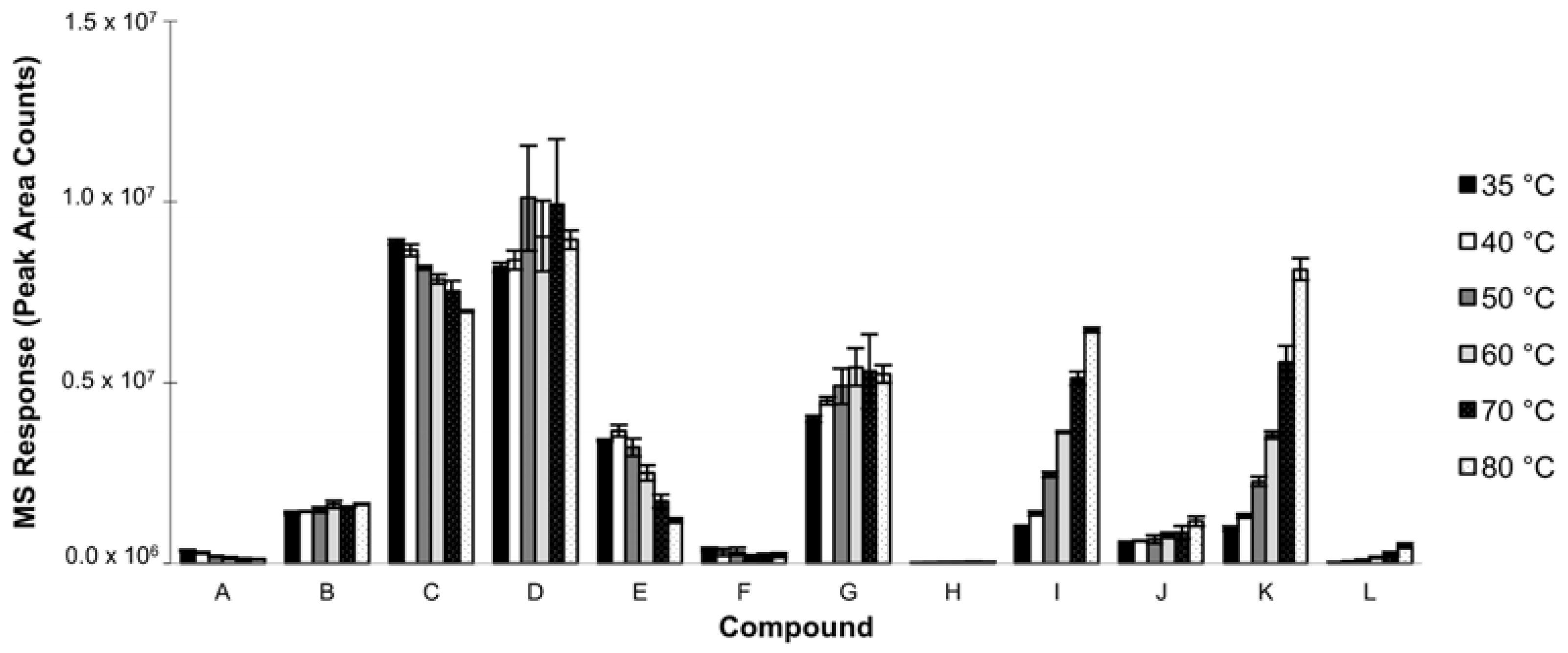
3.1.3. Extraction Temperature
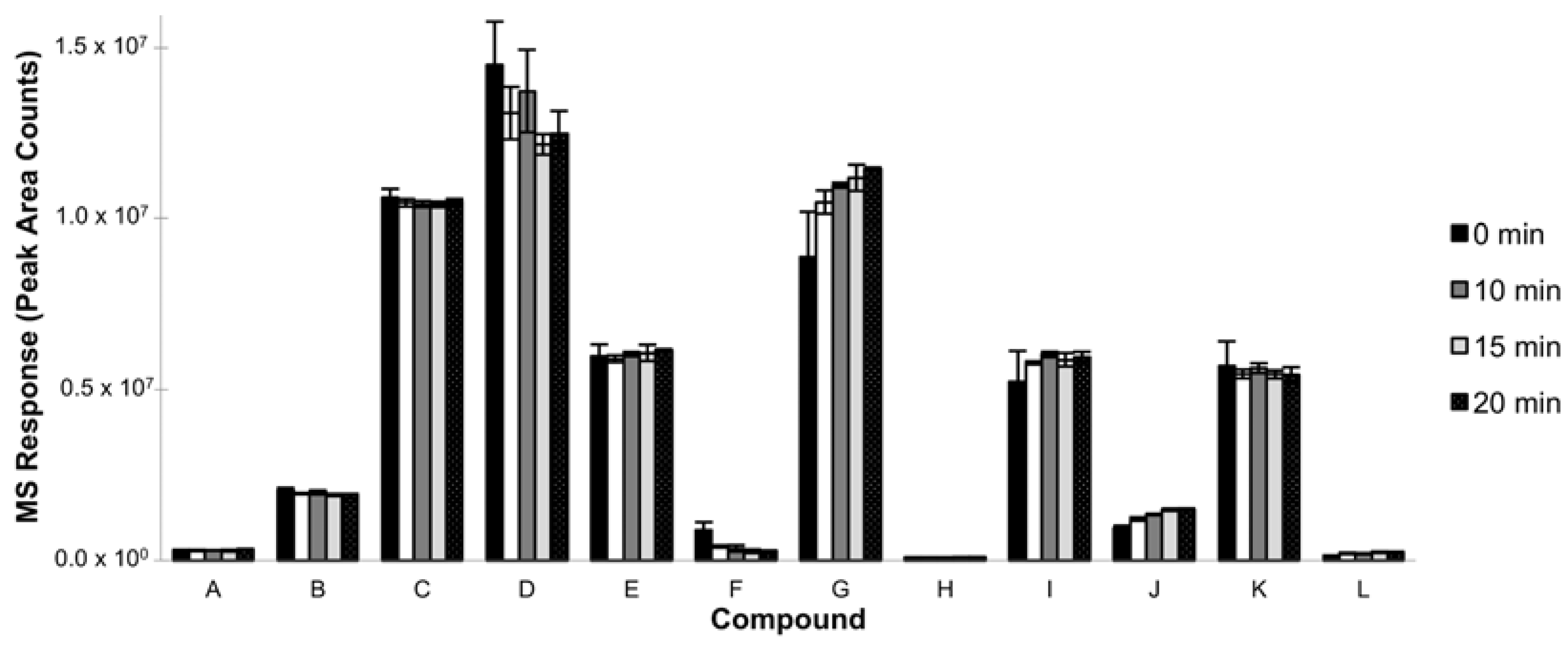
3.1.4. Incubation Time
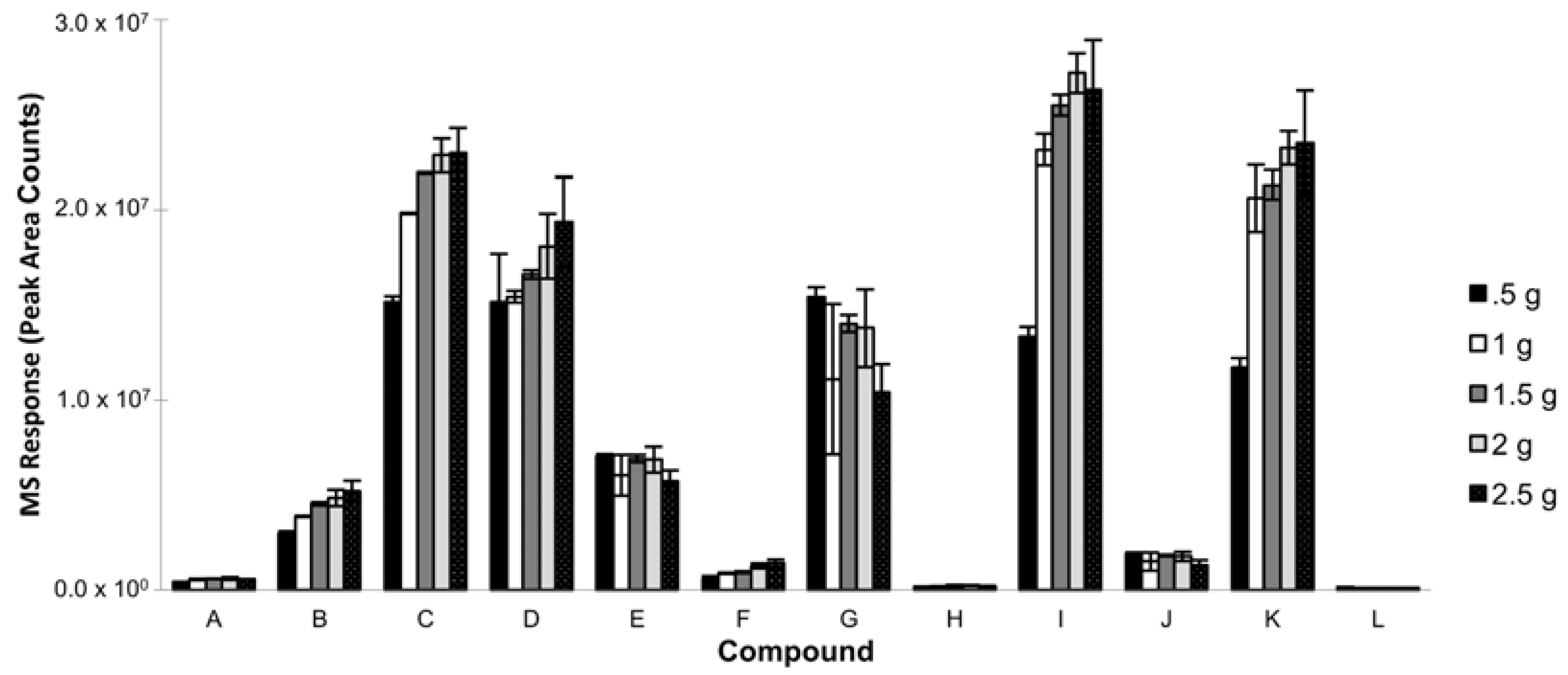
3.1.5. Sample Volume
3.1.6. Desorption Time
3.1.7. Salt Addition
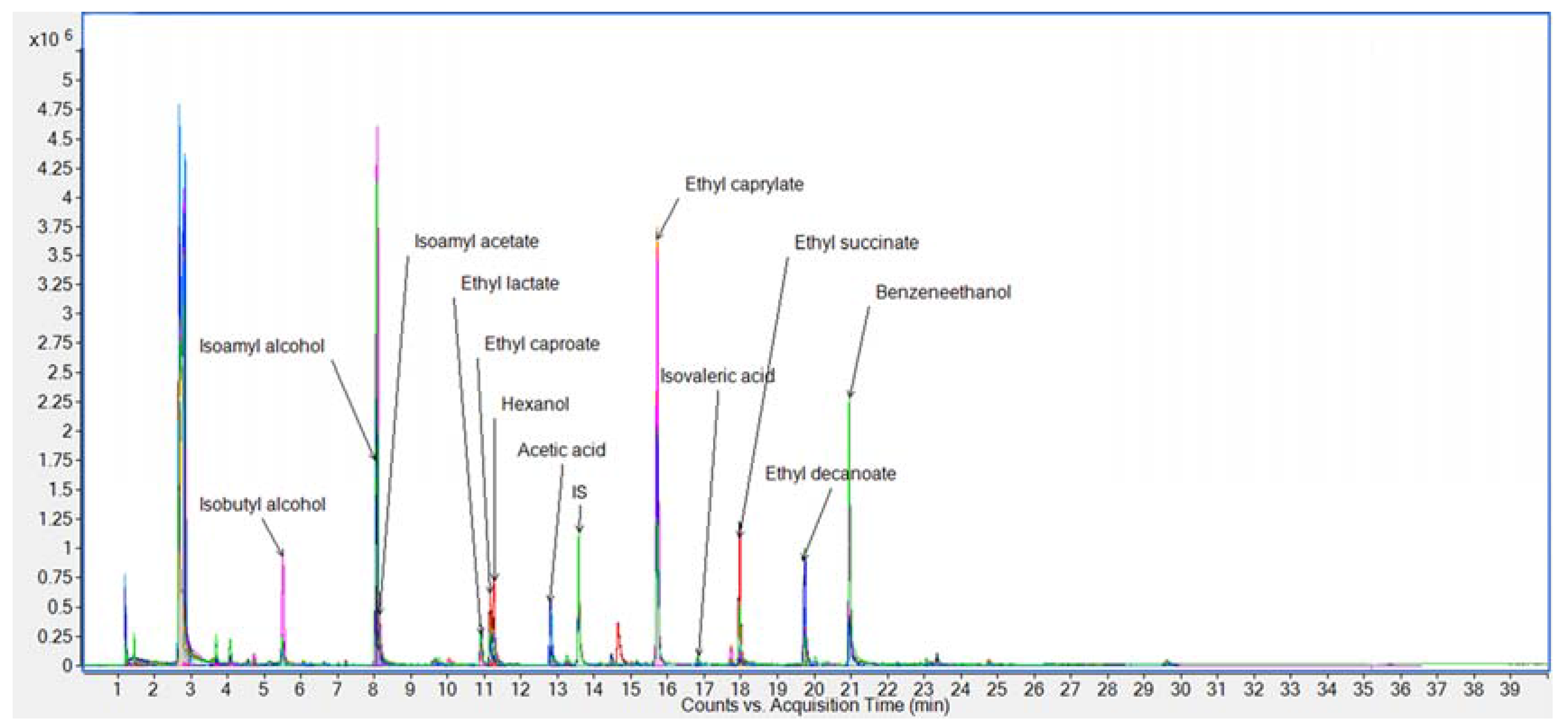
3.2. Analysis of Wine Samples
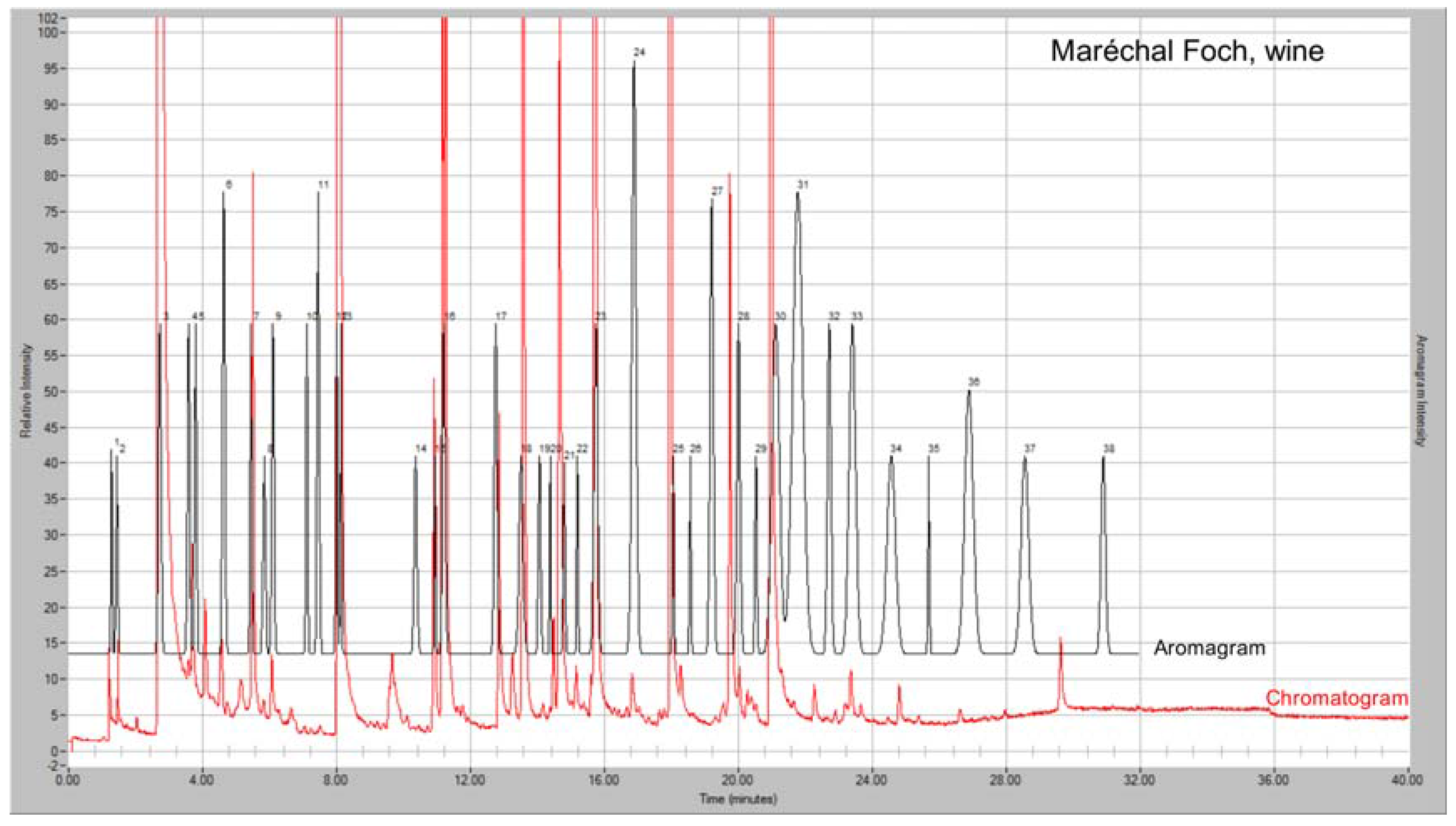
GC-MS-Olfactometry
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. CRC Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A. Wine aroma substances from gas chromatographic analysis. In Modern Methods of Plant Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin, Germany, 1988; Volume 6, pp. 29–66. [Google Scholar] [CrossRef]
- Sauvageot, F.; Vivier, P. Effects of malolactic fermentation on sensory properties of four burgundy wines. Am. J. Enol. Vitic. 1997, 48, 187–192. [Google Scholar]
- De Revel, G.; Bertrand, A. Dicarbonyl compounds and their reduction products in wine. Identification of wine aldehydes. In Trends in Flavour Research; Maarse, H., van der Heij, D.G., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994; Volume 35, p. 353. ISBN 0444815872. [Google Scholar]
- Allen, M. What level of methoxypyrazines is desired in red wines? The flavour perspective of the classic red wines of Bordeaux. Aust. Grapegrow. Winemak. 1995, 381, 7–9. [Google Scholar]
- Davis, C.R.; Wibowo, D.; Eschenbruch, R.; Lee, T.H.; Fleet, G.H. Practical implications of malolactic fermentation: A review. Am. J. Enol. Vitic. 1985, 36, 290–301. [Google Scholar]
- Rapp, A.; Güntert, M.; Almy, J. Identification and significance of several sulfur-containing compounds in wine. Am. J. Enol. Vitic. 1985, 36, 219–221. [Google Scholar]
- Montedoro, G.; Bertuccioli, M. The flavour of wines, vermouth and fortified wines. In Food flavours, Part B, The Flavour of Beverages; Morton, I.D., MacLeod, A.J., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1986; p. 171. ISBN 9780444425997. [Google Scholar]
- Rapp, A.; Versini, G. Methylanthranilate (“foxy taint”) concentrations of hybrid and Vitis vinifera wines. Vitis 1996, 35, 215–216. [Google Scholar]
- Margalit, Y. Concepts in Wine Chemistry, 3rd ed.; The Wine Appreciation Guild: San Francisco, CA, USA, 2012; p. 203. ISBN 1-935879-94-4. [Google Scholar]
- Fuller, G.H.; Steltenkamp, R.; Tisserand, G.A. The gas chromatograph with human sensor: Perfumer model. Ann. N. Y. Acad. Sci. 1964, 116, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengrove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 2. Chemical and sensory analysis. Am. J. Enol. Viticult. 2014, 65, 25–42. [Google Scholar] [CrossRef]
- Panighel, A.; Flamini, R. Solid phase extraction and solid phase microextraction in grape and wine volatile compounds analysis. Sample Prep. 2015, 2, 55–65. [Google Scholar] [CrossRef]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Chemical Industry Press: Beijing, China, 2009; pp. 90–103. ISBN 978-7-122-04701-4. [Google Scholar]
- Zhang, S.; Koziel, J.A.; Cai, L.; Hoff, S.J.; Heathcote, K.Y.; Chen, L.; Jacobson, L.D.; Akdeniz, N.; Hetchler, B.P.; Parker, D.B.; et al. Odor and odorous chemical emissions from animal buildings: Part 5. Simultaneous chemical and sensory analysis with gas chromatography-mass spectrometry-olfactometry. Trans. ASABE 2015, 58, 1349–1359. [Google Scholar] [CrossRef]
- LRI & Odour Database. Available online: http://www.webcitation.org/6rDRATLKY (accessed on 14 June 2017).
- Flavornet and Human Odor Space. Available online: http://www.webcitation.org/6qr4xTprN (accessed on 30 May 2017).
- Ferreira, A.C.S.; de Pinho, P.G. Analytical method for determination of some aroma compounds on white wines by solid phase microextraction and gas chromatography. J. Food Sci. 2003, 68, 2817–2820. [Google Scholar] [CrossRef]
- Howard, K.L.; Mike, J.H.; Riesen, R. Validation of a solid-phase microextraction method for headspace analysis of wine aroma compounds. Am. J. Enol. Vitic. 2005, 56, 37–45. [Google Scholar]
- Hall, B.J.; Brodbelt, J.S. Determination of barbiturates by solid-phase microextraction (SPME) and ion trap gas chromatography-mass spectrometry. J. Chromatogr. A 1997, 777, 275–282. [Google Scholar] [CrossRef]
- Slegers, A.; Angers, P.; Ouellet, E.; Truchon, T.; Pedneault, K. Volatile compounds from grape skin, juice, and wine from five interspecific hybrid grape cultivars grown in Quebec (Canada) for wine production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, K.; Martine, D.; Angers, P. Flavor of cold-hardy grapes: Impact of berry maturity and environmental conditions. J. Agric. Food Chem. 2013, 61, 10418–10438. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Liu, Y.; Yue, T.X.; Zhang, Z.W. Comparison between aroma compounds in wines from four Vitis vinifera grape varieties grown in different shoot positions. Food Sci. Technol. Camp. 2015, 35, 237–246. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science Principles and Applications, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008; p. 662. ISBN 978-0-12-373646-8. [Google Scholar]
- Gunst, R.F. Response surface methodology: Process and product optimization using designed experiments. Technometrics 1996, 38, 284–286. [Google Scholar] [CrossRef]





| RT (min) | Compound | 50/30 µm DVB/CAR/PDMS | 100 µm PDMS | 7 µm PDMS | 85 µm PA | 65 µm PDMS/DVB | 70 µm CW/DVB | 85 µm CAR/PDMS |
|---|---|---|---|---|---|---|---|---|
| 4.77 | Ethyl isobutyrate | 100 | 58 | 0 | 13 | 75 | 29 | 104 |
| 5.53 | Isobutyl alcohol | 100 | 42 | 1 | 95 | 81 | 97 | 83 |
| 8.10 | Isoamyl alcohol | 100 | 35 | 0 | 73 | 75 | 66 | 92 |
| 11.00 | Ethyl lactate | 100 | 17 | 0 | 71 | 60 | 79 | 132 |
| 11.75 | Ethyl caproate | 100 | 24 | 0 | 7 | 53 | 17 | 109 |
| 12.92 | Acetic acid | 100 | 5 | 3 | 124 | 38 | 242 | 158 |
| 16.38 | Ethyl caprylate | 100 | 67 | 1 | 21 | 98 | 45 | 64 |
| 18.44 | Vitispirane | 100 | 61 | 0 | 16 | 90 | 38 | 99 |
| 18.34 | Diethyl succinate | 100 | 52 | 0 | 45 | 105 | 56 | 63 |
| 20.42 | Ethyl decanoate | 100 | 114 | 12 | 56 | 121 | 72 | 53 |
| 21.13 | Benzenethanol | 100 | 23 | 0 | 74 | 86 | 67 | 82 |
| 24.03 | Ethyl myristate | 100 | 94 | 32 | 59 | 92 | 66 | 41 |
| 30.37 | Ethyl palmitate | 100 | 187 | 132 | 135 | 194 | 155 | 17 |
| # | RT (min) | LRI A | Stationary Phase 1–10 | Compound | MW | B Foch | B St. Croix | B Frontenac | B Foch/Frontenac | C Vincent | D Frontenac | D Foch |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.58 | 949 | 2 | 2,3-Butanedione | 86 | 1.11 | 5.18 | 0.85 | 0.32 | 0.94 | 1.39 | 1.05 |
| 963 | 2 | |||||||||||
| 955 | 4 | |||||||||||
| 558 | 5 | |||||||||||
| 619 | 8 | |||||||||||
| 575 | 8 | |||||||||||
| 976 | 9 | |||||||||||
| 2 | 3.7 | 880 | 2 | Acetal | 118 | 1.24 | 0.96 | 8.57 | 23.1 | 6.75 | 4.9 | 8.08 |
| 710 | 8 | |||||||||||
| 3 | 4.08 | 774 | 3 | Ethyl propanoate | 102 | 1.73 | 1.05 | 7.26 | 19.6 | 3.42 | 6.85 | 5.38 |
| 4 | 4.72 | 956 | 2 | Ethyl isobutyrate | 116 | 5.94 | 1.67 | 3.99 | 3.54 | 6.36 | 2.3 | 1 |
| 746 | 8 | |||||||||||
| 5 | 5.49 | 1110 | 2 | Isobutyl alcohol | 74 | 14.6 | 23.8 | 21.9 | 27.6 | 86.1 | 21.4 | 28.7 |
| 1054 | 2 | |||||||||||
| 1083 | 4 | |||||||||||
| 1093 | 4 | |||||||||||
| 609 | 6 | |||||||||||
| 616 | 8 | |||||||||||
| 6 | 6.07 | 863 | 3 | Ethyl butanoate | 116 | 1.55 | 3.43 | 3.5 | 2.2 | 2.63 | 1.05 | 3.59 |
| 788 | 5 | |||||||||||
| 7 | 6.66 | 1138 | 4 | n-Butanol | 74 | 0.9 | 1.85 | 1.44 | 4.07 | 1.76 | 4.32 | 1.68 |
| 1149 | 4 | |||||||||||
| 634 | 5 | |||||||||||
| 653 | 6 | |||||||||||
| 8 | 7.22 | 1060 | 2 | Ethyl isovalerate | 130 | 4.29 | 0.41 | 2 | 1.76 | 0.9 | 0.38 | 0.29 |
| 856 | 6 | |||||||||||
| 840 | 8 | |||||||||||
| 9 | 8.05 | 1184 | 2 | Isoamyl alcohol | 88 | 132 | 355 | 275 | 477 | 475 | 221 | 377 |
| 719 | 8 | |||||||||||
| 10 | 8.12 | 1110 | 2 | Isoamyl acetate | 130 | 32.2 | 3.89 | 3.87 | 4.02 | 3.89 | 3.22 | 0 |
| 860 | 8 | |||||||||||
| 11 | 10.03 | 1250 | 2 | Styrene | 104 | nd | 16.9 | 3.22 | 0.05 | 0.03 | nd | 0.68 |
| 12 | 10.91 | 1312 | 2 | Ethyl lactate | 118 | 13.7 | 34.1 | 39.9 | 34.3 | 30 | 67.1 | 19.8 |
| 1341 | 4 | |||||||||||
| 803 | 8 | |||||||||||
| 13 | 11.15 | 1230 | 2 | Ethyl caproate | 144 | 26.4 | 71 | 40.4 | 33 | 34.7 | 21.5 | 64.3 |
| 1060 | 3 | |||||||||||
| 1232 | 4 | |||||||||||
| 1238 | 4 | |||||||||||
| 985 | 5 | |||||||||||
| 981 | 5 | |||||||||||
| 996 | 6 | |||||||||||
| 996 | 6 | |||||||||||
| 14 | 11.27 | 1316 | 2 | n-Hexanol | 102 | 8.64 | 0 | 0 | 19.6 | 12.6 | 7.82 | 51.7 |
| 1330 | 2 | |||||||||||
| 1332 | 2 | |||||||||||
| 1352 | 4 | |||||||||||
| 847 | 5 | |||||||||||
| 848 | 5 | |||||||||||
| 848 | 5 | |||||||||||
| 862 | 6 | |||||||||||
| 1354 | 9 | |||||||||||
| 15 | 12.81 | 1400 | 2 | Acetic acid | 60 | 51.4 | 6.61 | 58.2 | 10.6 | 11.6 | 13.2 | 18.7 |
| 1401 | 2 | |||||||||||
| 791 | 3 | |||||||||||
| 1435 | 4 | |||||||||||
| 1442 | 4 | |||||||||||
| 1459 | 4 | |||||||||||
| 621 | 5 | |||||||||||
| 723 | 8 | |||||||||||
| 16 | 13.25 | 1437 | 2 | Furfural | 96 | nd | nd | nd | 10.2 | nd | nd | nd |
| 1438 | 2 | |||||||||||
| 1450 | 2 | |||||||||||
| 1449 | 2 | |||||||||||
| 1447 | 4 | |||||||||||
| 1456 | 4 | |||||||||||
| 1466 | 4 | |||||||||||
| 1465 | 4 | |||||||||||
| 802 | 5 | |||||||||||
| 829 | 5 | |||||||||||
| 800 | 5 | |||||||||||
| 800 | 5 | |||||||||||
| 836 | 6 | |||||||||||
| 868 | 6 | |||||||||||
| 830 | 6 | |||||||||||
| 815 | 8 | |||||||||||
| 17 | 13.57 | 3-Nonanone (IS) | 142 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 18 | 14.37 | 1375 | 1 | Methyl octanoate | 158 | 0.39 | 1.01 | 0.32 | 0.23 | 0.35 | 0.18 | 0.33 |
| 1392 | 4 | |||||||||||
| 1109 | 10 | |||||||||||
| 19 | 14.48 | 1692 | 2 | 1,3-Butanediol | 90 | 1.89 | 4.03 | 11 | 3.38 | 1.48 | 8.15 | 4.43 |
| 941 | 8 | |||||||||||
| 20 | 14.81 | 1518 | 1 | Benzaldehyde | 106 | 0.18 | 0.93 | 0.95 | 1.14 | 0.73 | 0.12 | 55.2 |
| 1516 | 1 | |||||||||||
| 1509 | 2 | |||||||||||
| 1454 | 2 | |||||||||||
| 1520 | 2 | |||||||||||
| 1482 | 2 | |||||||||||
| 1502 | 2 | |||||||||||
| 1086 | 3 | |||||||||||
| 1515 | 4 | |||||||||||
| 1496 | 4 | |||||||||||
| 1513 | 4 | |||||||||||
| 1538 | 4 | |||||||||||
| 1530 | 4 | |||||||||||
| 1522 | 4 | |||||||||||
| 1516 | 4 | |||||||||||
| 926 | 5 | |||||||||||
| 926 | 5 | |||||||||||
| 926 | 5 | |||||||||||
| 924 | 6 | |||||||||||
| 960 | 6 | |||||||||||
| 962 | 6 | |||||||||||
| 957 | 6 | |||||||||||
| 961 | 6 | |||||||||||
| 944 | 7 | |||||||||||
| 938 | 7 | |||||||||||
| 947 | 8 | |||||||||||
| 947 | 8 | |||||||||||
| 1540 | 9 | |||||||||||
| 21 | 15.02 | 1501 | 2 | Isobutyric acid | 88 | 2.45 | 0.28 | 1.38 | 0.78 | 2.46 | 0.45 | 0.32 |
| 935 | 3 | |||||||||||
| 22 | 15.15 | 2,3-Butanediol | 90 | 1.41 | 1.56 | 2.31 | 2.03 | 1.5 | 2.62 | 2.35 | ||
| 23 | 15.72 | 1258 | 3 | Ethyl caprylate | 172 | 164 | 398 | 209 | 116 | 187 | 181 | 205 |
| 1429 | 4 | |||||||||||
| 1466 | 4 | |||||||||||
| 1196 | 6 | |||||||||||
| 1193 | 6 | |||||||||||
| 1195 | 6 | |||||||||||
| 24 | 16.83 | 1631 | 2 | Isovaleric acid | 102 | 8.97 | 2.58 | 7.79 | 5.1 | 3.73 | 3.54 | 3.55 |
| 834 | 6 | |||||||||||
| 25 | 17.66 | 1276 | 7 | Vitispirane | 192 | 0 | 3.02 | 0.31 | 4.5 | 15.7 | 3.39 | 0 |
| 26 | 17.79 | 1278 | 5 | Ethyl nonanoate | 186 | 0.83 | nd | 1.68 | 0 | 0 | 0 | 0.78 |
| 27 | 17.99 | 1642 | 2 | Diethyl succinate | 174 | 3.62 | 11.5 | 18.9 | 43.8 | 12.9 | 5.77 | 122 |
| 1153 | 8 | |||||||||||
| 28 | 19.74 | 1390 | 6 | Ethyl decanoate | 200 | 54.9 | 99.5 | 83.9 | 29.8 | 33.3 | 98.5 | 34.4 |
| 1391 | 6 | |||||||||||
| 1394 | 6 | |||||||||||
| 29 | 20.27 | 1788 | 2 | Phenethyl acetate | 164 | 0.95 | 1.56 | 1.26 | 1.41 | 0.95 | 1.47 | 1.2 |
| 1785 | 2 | |||||||||||
| 1233 | 8 | |||||||||||
| 30 | 20.98 | 1903 | 4 | Benzeneethanol | 122 | 41.3 | 146 | 56 | 232 | 111 | 53.7 | 134 |
| 31 | 23.06 | 2007 | 2 | Octanoic acid | 144 | 1.39 | 1.07 | 6.63 | 0.09 | 1.99 | 2.22 | 0.7 |
| 2007 | 2 | |||||||||||
| 2100 | 2 | |||||||||||
| 2013 | 2 | |||||||||||
| 2075 | 4 | |||||||||||
| 1183 | 6 | |||||||||||
| 1256 | 7 | |||||||||||
| 32 | 23.36 | 1595 | 6 | Ethyl laurate | 228 | 3.3 | 2.63 | 6.73 | 1.62 | 1.28 | 8.24 | 2.72 |
| 33 | 24.76 | 4-Ethylphenol | 122 | nd | 7.49 | 2.32 | nd | nd | 3.4 | 2.33 | ||
| 34 | 24.81 | 8-Pentadecanone | 226 | 1.06 | 7.49 | 2.31 | 2.53 | 2.33 | 3.4 | 2.32 | ||
| 35 | 26.57 | Glycerol | 92 | nd | nd | 26.8 | nd | nd | nd | nd | ||
| 36 | 26.63 | 1793 | 6 | Ethyl myristate | 256 | 0.39 | 0.53 | 0.48 | 0.38 | 0.57 | 0.84 | 0.99 |
| 37 | 29.63 | 1993 | 6 | Ethyl palmitate | 284 | 2.35 | 2.1 | 5.75 | 1.92 | 2.81 | 4.12 | 5.14 |
| 1985 | 7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Rice, S.; Koziel, J.A.; Dharmadhikari, M. Development of an Automated Method for Selected Aromas of Red Wines from Cold-Hardy Grapes Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry. Separations 2017, 4, 24. https://doi.org/10.3390/separations4030024
Cai L, Rice S, Koziel JA, Dharmadhikari M. Development of an Automated Method for Selected Aromas of Red Wines from Cold-Hardy Grapes Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry. Separations. 2017; 4(3):24. https://doi.org/10.3390/separations4030024
Chicago/Turabian StyleCai, Lingshuang, Somchai Rice, Jacek A. Koziel, and Murlidhar Dharmadhikari. 2017. "Development of an Automated Method for Selected Aromas of Red Wines from Cold-Hardy Grapes Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry" Separations 4, no. 3: 24. https://doi.org/10.3390/separations4030024
APA StyleCai, L., Rice, S., Koziel, J. A., & Dharmadhikari, M. (2017). Development of an Automated Method for Selected Aromas of Red Wines from Cold-Hardy Grapes Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry. Separations, 4(3), 24. https://doi.org/10.3390/separations4030024