Abstract
Since the introduction in 2014 of fabric phase sorptive extraction (FPSE) as a sample preparation technique, it has attracted the attention of many scientists working in the field of separation science. This novel sorbent extraction technique has successfully utilized the benefits of sol–gel derived hybrid sorbents and a plethora of fabric substrates, resulting in a highly efficient, sensitive and green sample pretreatment methodology. The proposed procedure is an easy and efficient pathway to extract target analytes from different matrices providing inherent advantages such as high sample loading capacity and short pretreatment time. The present review mainly focuses on the background and sol–gel chemistry for the preparation of new fabric sorbents as well as on the applications of FPSE for extracting target analytes, from the time that it was first introduced. New modes of FPSE including stir FPSE, stir-bar FPSE, dynamic FPSE, and automated on-line FPSE are also highlighted and commented upon in detail. FPSE has been effectively applied for the determination of various organic and inorganic analytes in different types of environmental and biological samples in high throughput analytical, environmental, and toxicological laboratories.
1. Introduction
In modern analytical methods, sensitive techniques as well as new analytical instruments are used for the determination of various analytes in a plethora of different and complex matrices. However, the direct determination of very low concentrations has been broadly recognized as the Achilles heel of the analysis and is mainly associated with matrix interferences or inadequate sensitivity. On the other hand, it is well known that sample preparation is a key step of chemical analysis and somehow is considered as the bottleneck of the whole analytical cycle being the most tedious and time consuming stage and affecting significantly the precision as well as the accuracy of the overall analysis [1,2]. Thus, a preliminary step of preconcentration and separation of analytes from the original sample matrix or matrix simplification is usually required.
Among sample pretreatment techniques which are used prior to determination, solid–phase extraction (SPE) is recognized as an advantageous alternative to classic liquid–liquid extraction (LLE), which is undesirable due to the recent green analytical chemistry (GAC) regulations, regarding the use of organic solvents [3,4]. SPE has been widely accepted thanks to several inherent advantages like simplicity, the ability to extract polar compounds, no requirement of phase separation, lower volumes of organic solvent which can be reached even at microliter level (50–100 μL) in automated procedures, lower sample pretreatment time, as well as lower cost of analysis. Moreover, SPE facilitates miniaturization and automation [5,6].
Following the trends of analytical chemistry on miniaturization, several approaches of sorbent microextraction have been developed such as solid-phase microextraction (SPME) [7,8,9], stir-bar sorptive extraction (SBSE) [10], thin film microextraction (TFME) [11] and related techniques as well as magnetic solid-phase extraction (MSPE) [12]. Despite the fact that SPME is a well-established microextraction technique, in some applications, it may not provide the desired sensitivity due to the small sorbent mass and sample capacity as well as mechanical distortion resulting in poor precision and sensitivity [13].
In order to increase the volume of the extraction phase as well as the active surface area, Pawliszyn et al. proposed a TFME approach based on a thin sheet of a polydimethylsiloxane (PDMS) organic polymer membrane as an extraction phase [11]. The membrane was attached to a stainless-steel rod which was immersed into the sample solution containing the target analytes. The extraction procedure took place in both direct and headspace extraction mode. After the extraction procedure, the membrane was rolled around the rod and placed into a gas chromatography (GC) injector for thermal desorption. In comparison with SPME, it presents higher extraction efficiency as well as shorter equilibrium time, thanks to the larger extraction phase (25–125 times more than a fiber). In 2012, Kermani and Pawliszyn reported a modification of TFME based on the distribution of a polymeric sorbent consisting of a mixed carboxen/polydimethylsiloxane (CAR/PDMS) and polydimethylsiloxane/divinylbenzene (PDMS/DVB), spread onto a glass wool fabric support as the substrate [14]. The extraction procedure was similar to TFME for GC-MS analysis. The resulted samplers presented better stability and robustness thanks to the incorporation of the fabric substrate in their thin film structure. Recently, a carbon mesh support was presented by Grandy et al., as an alternative substrate for the TFME technique [15] for GC-toroidial ion trap MS (GC-TMS) analysis. The proposed carbon substrate seems to be more durable than other TFME designs.
In 2014, Kabir and Furton [16] developed a novel highly promising and versatile sample preparation sorbent extraction technique named as fabric phase sorptive extraction (FPSE). FPSE successfully combines the advantages of sol–gel derived sorbents used in microextraction and the wide variety of fabric substrates, resulting in a highly efficient and green sample pretreatment technique [13,17]. Two main limitations of sorptive extraction techniques have been addressed by FPSE, the low sorbent capacity and long sample preparation time. The inherent porous surface of cellulose or polyester used as fabric substrate together with the strength of sol–gel derived hybrid sorbents uniformly dispersed as an ultra-thin film within the fabric substrate, results in a plethora of sorbent materials with significant analyte retention capacity and very fast extraction equilibrium. In comparison with a typical SPME fiber, the sorbent loading in FPSE media is about 400-times higher. Also unlike SPME, the extraction sorbent is dispersed homogeneously on the surface of nanometer size polyester/cellulose micro-fibrils of FPSE [13]. Regarding the elution of the retained analytes from the FPSE media in organic solvents, this is also fast without the potential carryover risk. Highly acidic or basic chemical environments as well as any organic solvent can be used as eluents. The advantages of the FPSE technique include: (a) simplicity, low cost, minimal consumption of solvents; (b) sample preparation can be completed by directly introducing the FPSE media into the vessel containing the sample matrix; (c) enhanced efficiency by sonication, magnetic stirring; (d) a plethora of organic solvents can be used as eluent; (e) minimization of sample preparation steps, reducing potential sources of errors; (f) a variety of effective sol–gel coatings can be employed as sorbent; (g) high analyte preconcentration factors; (h) high chemical resistance of the FPSE media thanks to a strong chemical bonding between the sorbent phase and the substrate.
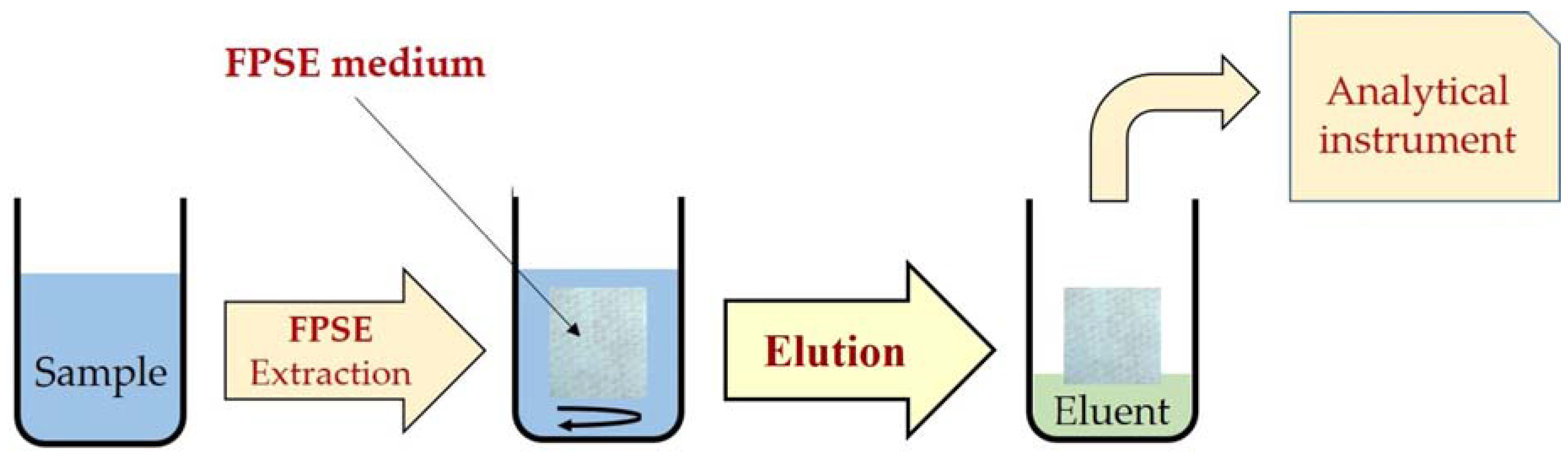
Typically, the FPSE procedure starts with the immersion of the FPSE medium in a solvent system to clean any unwanted residue, followed by subsequent rinsing with deionized water to remove residual organic solvents. An amount of sample containing the target analytes is taken into a screw-capped glass vial. The FPSE medium is inserted into the vial along with a clean Teflon-coated magnetic stir bar. The sample solution is stirred for a defined extraction time for the sorption of the analytes. The FPSE medium is then removed from the extraction vial and is inserted into another vial containing the eluting solvent, for 4–10 min. Finally, the eluent is centrifuged and filtered to remove any particulate matter prior to injection into HPLC or other systems [18]. The FPSE procedure is shown in Figure 1. The FPSE medium can be reused by washing with the solvent system or it can be left to dry on a watch glass and stored in an air-tight glass container for future use.
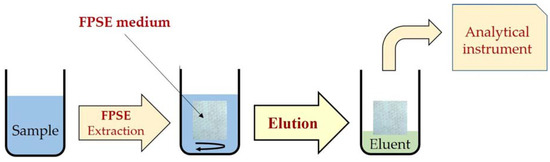
Figure 1.
Schematic presentation of the main steps involved in FPSE process.
The recent trend of solid-phase extraction and microextraction is related to the development and characterization of new sorbent materials. The main targets in the search of novel sorbents depend on the extraction mode as well as on the analytes and samples, including better selectivity (or even specificity towards definite target species), improved sorptive or adsorptive capacity, as well as enhanced thermal, chemical or mechanical stability of the extractive media [19].
Sol–gel technology has many features in producing new materials of high purity and homogeneity, in forms of bulk, fibers, sheets, coating films as well as particles [20]. In analytical chemistry, the sol–gel process is commonly used in the synthesis of materials as sorbents for sample preparation techniques like SPE, SPME, and SBSE. The first demonstration of sol–gel technology for preparation of SPME fibers was presented by Malik and co-workers [21]. Since then, a wide variety of sol–gel sorbents with high selectivity, extraction sensitivity and FPSE applications have been presented.
In FPSE, a large pool of sol–gel sorbent materials with unique properties is available including a variety of polymers coated on either hydrophilic or hydrophobic substrates. The characteristics of the FPSE media used in the developed FPSE methods are given in Table 1.

Table 1.
Characteristics of the FPSE media used in the developed methods.
The objective of the present review article is to address, from both a presentative and critical point of view, the FPSE technique as well as its applications in diverse fields of analytical chemistry, either in batch or automated mode. In addition, the most important information regarding the principles of the FPSE technique and the sol–gel sorbent materials used for this purpose along with the process of preparing each substrate and the procedure of sol–gel coating are also presented.
2. Sol–gel Technology in Developing Microextraction Sorbents
Sol–gel technology is an interesting approach for the synthesis of inorganic polymers and organic–inorganic hybrid porous products of various sizes, shapes and formats like films, fibers, particles and monoliths, by employing mild reaction conditions [22]. By carefully modifying the synthesis process, the resulted materials are thermally and chemically stable with better homogeneity and purity, tunable porosity, and selectivity. The most important advantages of sol–gel technology for sorbent micro-extraction are the strong retention of the coating onto the substrate due to chemical bonding as well as the reduction of the extraction equilibrium time and the fast mass transfer thanks to the inherent porous structure [17].
A sol–gel process involves the catalytic hydrolysis of sol–gel precursor(s) and subsequent polycondensation of the hydrolyzed precursor(s), resulting in the transition of a liquid colloidal (solid particles with diameter of 1–100 nm) suspension known as “sol” into a 3D network of solid matrix “gel” with pores of sub-micrometer dimensions and polymeric chains whose average length is greater than a micrometer [23].
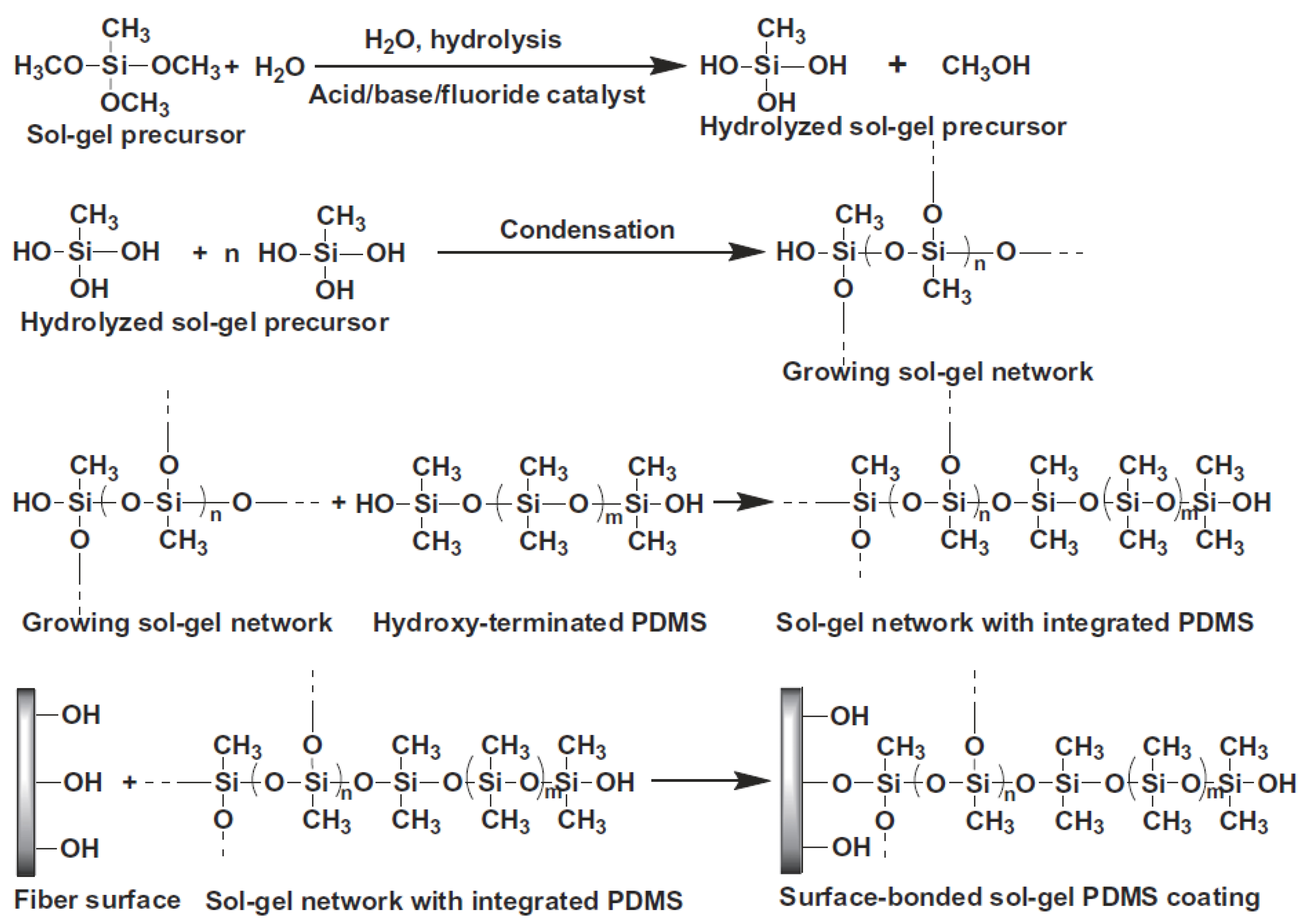
Initially, in the sol–gel process an inorganic or organically-modified inorganic precursor, for example methyltrimethoxysilane (MTMOS) is mixed with water and solvent in the presence of acid/base/fluoride catalyst. Afterwards, the hydrolyzed sol–gel precursor is condensed leading to the formation of a growing sol–gel network, into which a sol–gel active organic polymer, like hydroxy-terminated PDMS, can become chemically integrated. The reactions of the sol–gel process are schematically represented in Figure 2. As a result, a surface-bonded sol–gel hybrid organic-inorganic polymeric network is created and a new sol–gel hybrid material is synthesized.
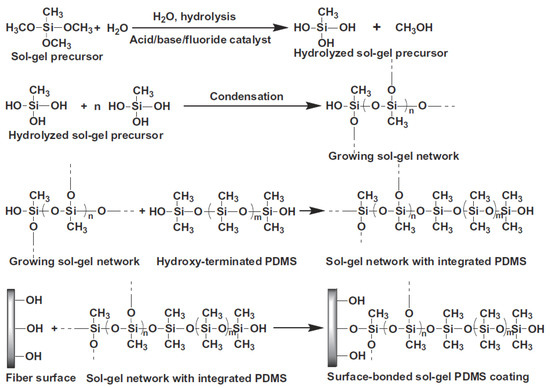
Figure 2.
Schematic representation of chemical reactions involved in the synthesis of sol–gel hybrid organic–inorganic sorbents. Reproduced from [17] with permission of Elsevier.
A wide variety of available organically modified sol–gel precursors with different polarities can be used in the sol solution design to complement the overall polarity of the extraction sorbent [23]. The characteristics and the chemical properties of sol–gel hybrid organic-inorganic sorbents are affected by several factors, including the nature and type of precursors, the precursor to water ratio, the type of catalyst and its concentration, the pH of sol-solution, the organic solvent, the temperature and humidity during reactions, as well as the post-gelation aging conditions. The chemical structure of the produced sol–gel matrix depends on the type of the catalyst, used in the sol solution. The most commonly used precursors as well as different types of catalysts employed in sol–gel technology are given in Table 2.

Table 2.
Types of precursors and catalysts used in a sol–gel process.
3. Preparation of FPSE Media
The preparation of FPSE media involves two main steps: (1) pretreatment of fabric substrates for sol–gel coating and (2) design and preparation of the sol solution for sol–gel coating process.
3.1. Pretreatment of Fabric Substrates
The segments of fabric substrates (e.g., cellulose, polyester) are first soaked with deionized water under sonication in order to become thoroughly wet. Fabric pieces are cleaned with a high amount of deionized water so that chemical residues are removed. Then, a process called mercerization follows, by treating the fabric with 1.0 mol·L−1 NaOH under sonication and the mercerized fabric is washed several times with plenty of deionized water. The next step is treating the fabric with 0.1 mol·L−1 HCl under sonication, washing again with deionized water, and finally drying overnight in an inert atmosphere. Dried fabric substrates are stored in clean glass airtight containers until they are coated with the appropriate sol–gel sorbent.
3.2. Preparation of the Sol Solution for the Sol–gel Coating Process
Designing the sol solution is the main step on the way to the development of a sol–gel sorbent due to the fact that its composition and the relative ratio of the constituents, define the porosity as well as the selectivity and specificity of the resulting sorbent [24]. For an effective sol–gel sorbent, the selection of the sol–gel active organic polymer, the inorganic or organically modified inorganic sol–gel precursor, the solvent/solvent system, the catalyst, the amount of water, as well as an appropriate relative molar ratio of the constituents must be considered. The composition of the sol solutions for the FPSE media preparation is given in details in Table 3.

Table 3.
Composition of the sol solutions for FPSE media preparation.
The coating procedure is integrated by inserting gently the treated fabric pieces into the vial containing the sol solution. As a result, a three dimensional network is formed throughout the porous substrate matrix (cellulose, polyester etc.). After a predetermined coating time the fabric is taken away from the sol solution, dried, and placed in a desiccator overnight for solvent evaporation and for aging of the sol–gel coating. The objective of this step is to complete the condensation reaction and remove solvents and unreacted residuals from the sol–gel matrix, ensuring a clean, surface bonded sol–gel sorbent free of structural deformation and internal stress. The coated FPSE media is then rinsed with the appropriate solvent system under sonication for a few minutes in order to remove residual sol solution ingredients from the coated surface. The FPSE media is then cut into 2.5 cm × 2.0 cm pieces and stored in clean airtight containers, for future use.
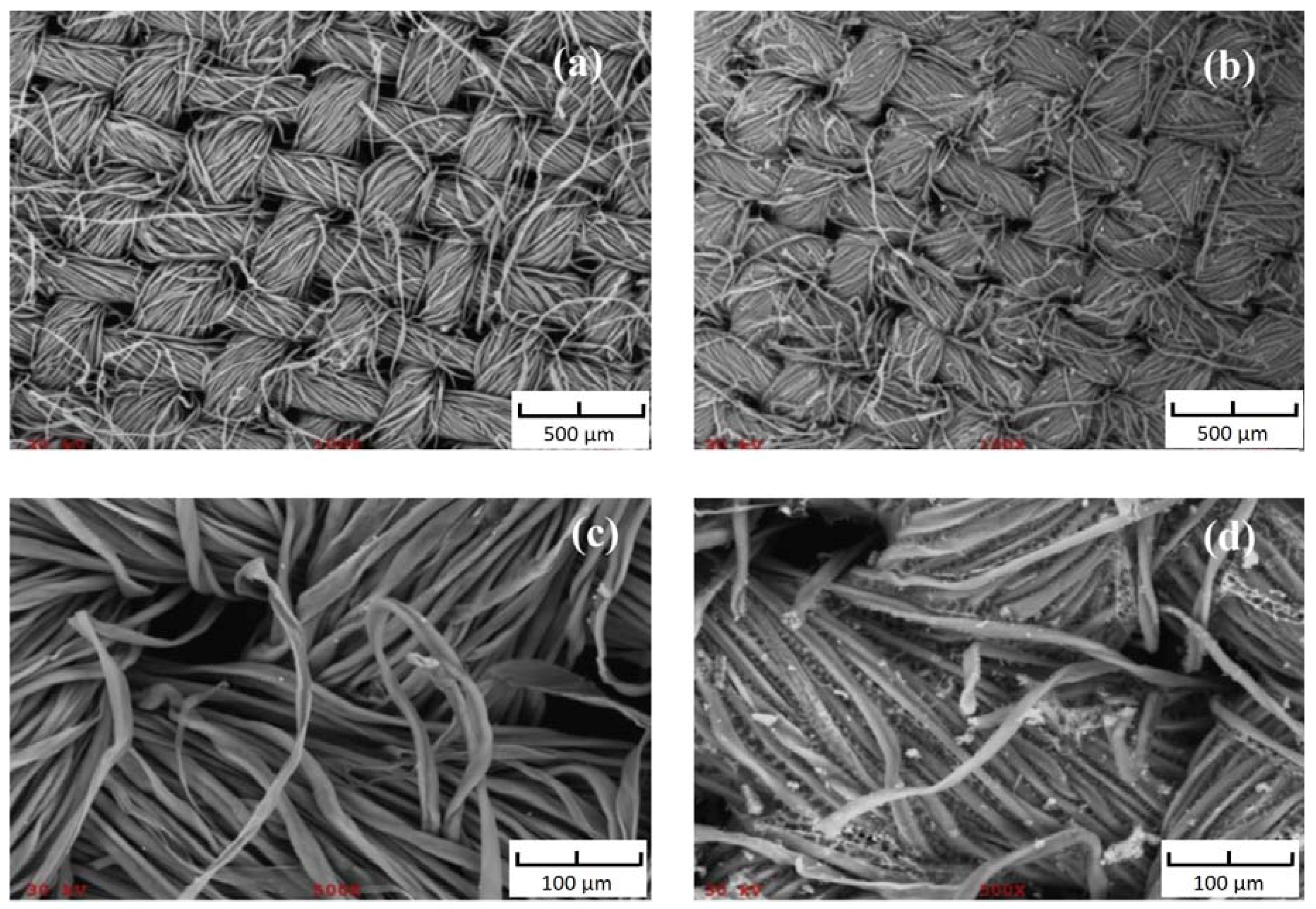
Images of the surface by scanning electron microscope (SEM) of (a) uncoated surface of cellulose fabric substrate at 100× magnification; (b) sol–gel poly-PEG coated surface of FPSE media at 100× magnification; (c) uncoated surface of cellulose fabric substrate surface at 500× magnification; and (d) sol–gel poly-PEG coated surface of FPSE media at 500× magnification are presented in Figure 3. Both low and high magnification SEM images of the sol–gel PEG coated FPSE media clearly demonstrate that the ultra-thin coating of the sol–gel sorbent is uniformly distributed throughout the substrate matrix without blocking the pores of the fabric. The easy access of the low viscosity sol solution into the fabric matrix helps in achieving this uniform ultrathin sol–gel PEG coating. The sol–gel PEG coating does not clog the through-pores of the fabric substrate, allowing an easy permeation of sample matrix through its body, which consequently helps to accomplish extraction equilibrium in a very short period of time [18].
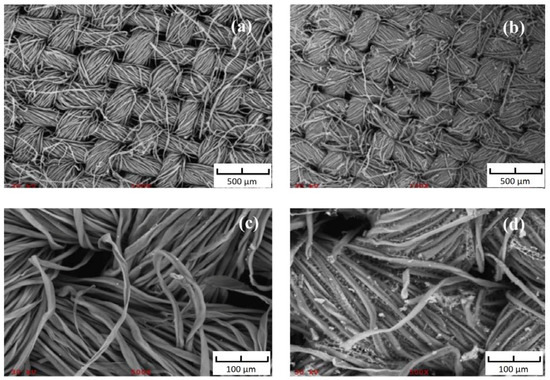
Figure 3.
SEM images of (a) uncoated cellulose at 100× magnification; (b) PEG coated FPSE media at 100× magnification; (c) uncoated cellulose at 500× magnification; (d) PEG coated FPSE media at 500× magnification. Reproduced from [18] with permission of Elsevier.
4. Applications of Fabric Phase Sorptive Extraction
Since the presentation of FPSE in 2014 as a novel sample pretreatment technique prior to high performance liquid chromatography for the determination of selected estrogens, various applications for organic and inorganic analytes have been reported in the literature. All reported FPSE methods are presented briefly in Table 4, containing information about all FPSE procedures for sample preparation, the main parameters with the analytical performance characteristics, as well as the analytes and the types of samples. Most of the reported methods were applied for drugs and pharmaceuticals using HPLC or UPLC coupled with DAD, MS or MS/MS.

Table 4.
Applications of the FPSE technique.
In the first FPSE application, Kumar et al. [13] developed a FPSE-HPLC-FLD method for selected estrogen (BPA, E2 and EE2) determination in urine and environmental (ground, river and drinking) water samples by HPLC coupled with an FLD detection system. The estrogens which are medium polar compounds, were extracted using a medium polar FPSE medium prepared by sol–gel PTHF coated on a cellulose substrate. The FPSE procedure took place in a glass vial containing the target analytes along with the coated FPSE medium and followed by magnetic stirring for an efficient adsorption. A volume of 500 μL of methanol was used to elute the analytes from the fabric. After 5 min centrifugation and filtration of the eluent, it was injected into the HPLC system. The proposed method presented good analytical characteristics as shown in Table 4.
Samanidou et al. [26] used the FPSE technique for the determination of selected benzodiazepines, APZ, BRZ, DZP, and LRZ in blood serum by HPLC-DAD. Benzodiazepines are commonly prescribed drugs with sedative, anti-depressive, tranquilizing, hypnotic, and anticonvulsant properties. Their determination in biological fluids is of great importance not only in clinical assays, but also in forensics and toxicological studies. Three different FPSE sorbents were examined; the sol–gel PEG and sol–gel PTHF coated on cellulose substrates and the sol–gel PDMDPS coated on a polyester substrate. The optimization experiments showed that sol–gel PEG was the most appropriate FPSE medium to extract benzodiazepines. After conditioning the FPSE medium, it was inserted into a glass vial containing the sample solution along with a Teflon-coated magnetic stirrer. The sample was stirred for about 20 min and then, elution followed by immersing the FPSE medium into another vial containing an elution solvent system of acetonitrile: methanol (50:50 v/v). The elution time was 10 min. The extracted benzodiazepines were analyzed directly in the HPLC instrument with photodiode array detector (DAD) operated at 240 nm. In this work, the FPSE sol–gel PEG medium was introduced directly into the matrix without prior deproteinization, resulting in the minimization of sample preparation steps as well as in the elimination of probable errors during the sample pretreatment procedure. However, it is not mentioned if the FPSE medium is replaced for each analytical cycle as it could become clogged from complex matrices like blood, milk, etc.
Guedes-Alonso et al. [35] developed a simple, fast and sensitive method for the quantification of natural and synthetic steroid hormones, androgens and progestogens by coupling sol–gel PTHF coated FPSE medium with UHPLC-MS/MS. Steroid hormones have been widely used in both human and veterinary medicine and their effective determination in water samples is of crucial importance for the assessment of the concentration levels and their related ecological risk. A UHPLC system coupled to a triple quadrupole detector was used for the quantification of selected androgens and progestogens, NORET, NOR, MGA, PRO, BOL, NAN, ADTD, DHEA, TES, and AND, in tap water and wastewater treated with different techniques as well as in urine samples. The FPSE procedure took place in glass vials with a Teflon coated magnetic stirrer. After submerging the FPSE medium into the sample solution (water or urine samples), it was stirred at 1000 rpm, with an extraction efficiency maximum achieved in 20 min and elution in 3 min. Methanol in a small quantity, approximately 0.75 mL, was used as the elution solvent. The recovery of the developed method ranged from 65.9% to 121.2%. Both repeatability values (intra-and inter-day) were in all type of samples lower than 20%.
The FPSE technique was also applied for the determination of four non-steroidal anti-inflammatory drugs, namely: IBU, NAP, KET, and DIC, in environmental water samples in combination with GC-MS [25]. Up to now, this is the only method that associates FPSE with the GC-MS analytical technique. Three different sorbents, sol–gel PDMDPS on a polyester substrate, sol–gel PTHF and sol–gel PEG on cellulose substrates, were investigated. The sol–gel PEG coated FPSE medium was found to be the most efficient one for the NSAIDs analytes independently of the pH and the ionic strength. The FPSE extraction was performed in a glass screw-cap vial by immersing the FPSE medium into the sample solution with the help of a tweezers. The solution was magnetically stirred at 500 rpm for 2 h. Ethyl acetate was used to elute the analytes with an elution time of 15 min. For the elution of the compounds, the FPSE medium was introduced into a 1 mL sample of ethyl acetate for 15 min. Subsequently, the FPSE medium was removed from the vial and the extract was evaporated to dryness by nitrogen stream. The residue was reconstituted into a 100 μL insert with 40 μL of ethyl acetate and 10 μL of MTBSTFA. The derivatization reaction took place in an oven at 60 °C for 60 min. After cooling to room temperature, the extract was ready for GC-MS analysis. The total runtime was 38.2 min. The instrument was a gas chromatograph equipped with a capillary column, combined with a quadrupole mass spectrometer. Recoveries were in the range of 82%–116% and RSDs between 3.5% and 18%.
Samanidou et al. [18] reported a facile sol–gel synthesis to incorporate short-chain PEG into the sol–gel matrix and anchor the growing sol–gel PEG network to a flexible hydrophilic cellulose substrate, resulting in a homogenous and ultra-thin, highly polar FPSE medium. The sol–gel PEG FPSE medium was applied for the first time to assess the concentration of amphenicol drugs in raw milk samples. The extraction of amphenicols residues, TAP, FF, and CAP from raw milk followed by HPLC-DAD was investigated. The sol–gel PEG FPSE medium together with an aliquot of milk sample (0.5 g) with 500 μL DI water (or 0.5 g milk spiked with 500 μL of amphenicols standard solution) was magnetically stirred for 30 min. Methanol was used as the elution solvent and elution was performed for 10 min. The extract was centrifuged for 15 min and filtered prior to HPLC. The FPSE medium could be reused by washing with 2 mL acetonitrile: methanol (50:50 v/v) for 5 min. The proposed method showed good linearity and sensitivity. The precision was evaluated within a day and between days and ranged from 1.0%–10.7% and 7.6%–14.0% for TAP, FF, and CAP respectively. In this study, no protein precipitation or solvent evaporation and sample reconstitution were necessary, thanks to the FPSE technique which reduces the sample preparation steps, ending up in a short extraction equilibrium time pretreatment technique.
Karageorgou et al. [24] developed a simple FPSE-HPLC-UV method for the isolation of three sulfonamides, SMTH, SIX, and SDMX, from untreated milk samples. The highly polar sol–gel PEG coated on a cellulose substrate was used for the extraction of the selected sulfonamides. The FPSE procedure was the same as the previous method [18]. After the extraction, the FPSE medium was first inserted into a clean vial with 250 μL MeOH for 8 min and then into another vial with 250 μL ACN for a further 5 min for the elution of the analytes. The resulting solution was filtered prior to HPLC injection to remove any particulate matter. The same medium could be reused up to 30 times without any significant loss in extraction performance. The precision of the method was estimated for within-day repeatability and between-day repeatability with RSDs ranging from 5.6% to 6.7% respectively. The flexibility of the FPSE medium facilitates the easy insertion of the fabric into the sample solution, resulting in the fast extraction of the analytes. In addition, neither prior pretreatment of the sample was carried out including protein precipitation, nor solvent evaporation was performed followed by sample reconstitution. Another application from the same group, using the sol–gel PEG FPSE medium on cellulose substrate and offering the same advantages regarding the reduction of sample preparation steps, was reported for the extraction of four penicillin antibiotic residues (PENG, CLO, DICLO, and OXA) from cows’ milk [37]. Sol–gel PEG FPSE medium was adopted for method optimization and validation among sol–gel C18 and sol–gel PEG-PPG-PEG all coated on cellulose substrates, as the first one provided better absolute recovery values ranging between 22% and 58%, than 5% provided by the two other sorbent FPSE media.
FPSE has been also employed for the preconcentration of four endocrine disruptor alkylphenol molecules, namely, 4-TBP, 4-SBP, 4-TAP, and 4-CP in aqueous and soil samples followed by HPLC-UV detection [33]. The sol–gel PTHF on a cellulose substrate was selected for the extraction procedure. The sample was stirred at a speed of 1000 rpm for an extraction time of 25 min and then elution with methanol followed, for 6 min. The eluent was centrifuged for 5 min, filtered with a syringe filter and finally it was injected into the HPLC system. Relative recoveries were satisfactory and ranged from 91% to 97% in aqueous samples, while for soil and sludge samples they were lower between 89% and 91%, probably due to the complicated sample matrix.
Another interesting application of FPSE using UPLC-MS(QqQ) is for migration analysis of several non-volatile additives in food packaging materials [34]. Three different sol–gel coated FPSE media with diverse polarities were investigated: sol–gel PDMS (non-polar), sol–gel PTHF (medium polar) and sol–gel PEG (highly polar) all coated on cellulose substrates as well as several extraction parameters were optimized. The proposed method was applied for 18 plastic additives: eight plasticizers (DEP, TBC, DBM, TBoAC, TXIB, DBP, 2EHAdip, 2EHSeb), five antioxidants (IRGA38, TOPAC, IRGA1076, IRGA168 and IRGA1010), four UV absorbers (TINU326, CHIMA81, TINU327, CYA1084) and one antistatic agent (HAA C12). Three different food simulants with concentration of 300 μg·L−1 of the target analytes: A, ethanol 10%; B, acetic acid 3%, and D1, ethanol 50% were prepared to optimize the FPSE protocol. Better retention was achieved using sol–gel PTHF and sol–gel PEG coated FPSE media with analytes dissolved in ethanol 10% or acetic acid 3%. For these simulants, the retention efficiency was over 75%. On the other hand, they were slightly retained using ethanol 50% as simulant. The extraction of these compounds with the low polar sol–gel PDMS was, as expected, lower than the other FPSE media, especially for DEP. Generally, compounds with low logP values proved to have higher enrichment factors, especially with sol–gel PTHF and sol–gel PEG FPSE media. The use of sol–gel PDMS improved the enrichment capacity, in the case of compounds with high logP values. Sample extraction assisted by magnetic stirring at 700 rpm was optimized at 20 min and 10 min for solvent elution assisted by ultrasound. Acetonitrile was adopted as the elution solvent since recoveries were higher than 70% for 13 out of the 18 selected compounds in all FPSE media. The best extraction recovery values were achieved when analytes were dissolved in 3% aqueous acetic acid solution, where 17 out of 18 compounds showed improved sensitivity and 10 of them obtained enrichment factors higher than 3 for all tested FPSE media. When FPSE eluents were evaporated using nitrogen, 11 out of 18 compounds reached EFs higher than 100. This significant improvement of the sensitivity was based on the combination of FPSE technique with nitrogen evaporation allowing the determination of such analytes at very low concentrations in various types of samples.
Recently, Montesdeoca-Esponda et al., applied the FPSE methodology followed by UHPLC-MS/MS detection for the determination of benzotriazole UV stabilizers (BUVSs) in sewage samples [27]. BUVSs are classified as emerging pollutants used in different personal care products such as sunscreens, soaps, shampoos, lip gloss, hair dyes or makeup that can affect the aqueous environmental ecosystem in various ways. The target analytes were: UV P, UV 329, UV 326, UV 328, UV 327, UV 571, and UV 360. The non-polar sol–gel PDMDPS coated on a polyester substrate was used to extract the analytes. A sample of volume 10 mL together with the fabric were stirred at 1000 rpm for 60 min and the analytes were eluted with 1.0 mL methanol for 5 min. Under these conditions, the preconcentration factor was 10 times. A UPLC system coupled with a triple quadropole detector with an ESI interface was used to determine the target analytes. The proposed method was applied to sewage samples with recoveries ranging from 42% to 99%.
Garcia-Guerra et al. [28] reported a FPSE-UHPLC-MS/MS method for benzotriazole UV stabilizers’ (UV P, UV 329, UV 326, UV 328, UV 327, and UV 360) determination in seawater samples collected from beaches used by tourists where the direct input of these compounds may be significant. Three different coated FPSE media; sol–gel PDMDPS, sol–gel PTHF, and sol–gel PEG were evaluated using aqueous solutions at pH 6 as initial conditions. Among them, sol–gel PDMDPS coated on a polyester substrate, showed the greatest capability to extract the non-polar target analytes, so it was selected for further experiments. For 60 min extraction time and 10 min elution in methanol the developed method provided absolute recoveries in the range from 40.9% to 44.3%, except for UV P and UV 329 whose values were between 9.30% and 20.6%.
Lakade et al. [31] presented a comparative study of four FPSE media: non-polar sol–gel PDMDPS, medium-polar sol–gel PTHF, polar sol–gel PEG-PPG-PEG triblock, and polar sol–gel PEG to extract a group of PPCPs (logKow values range from −0.6 to 6.1) from environmental water samples. The FPSE sol–gel PEG media was selected as the most appropriate sorbent material for the target PPCPs (MPB, CBZ, PrPB, DHB, BzPB, DHMB, DICLO, BP-3, TCC, and TCS) in the developed method. The analytes were determined in river and wastewater samples by LC-MS/MS. A portion of 1.0 mL of methanol was used as the elution solvent with a time of 5 min and the total extraction time was extremely long, for about 4 h.
5. Stir Fabric Phase Sorptive Extraction
In order to increase extraction kinetics, various interesting approaches have been reported by increasing the contact surface area of micro-extraction devices. Pawliszyn et al. [11] proposed the thin-film microextraction technique where a thin membrane of PDMS was used as the extracting surface by enhancing the interaction between the sample and the active sorptive phase. In this context, as FPSE is a typical diffusion extraction process, it can be improved by the stirring of the whole extraction system simultaneously including the fabric sorbent medium as well as the sample.
The potential combination of FPSE media with the advantages of stir membrane extraction (SME) [38] was investigated by Roldan-Pijuan et al. [30]. The proposed stir fabric phase sorptive extraction (SFPSE), which integrates sol–gel hybrid organic–inorganic coated FPSE media with a magnetic stirring mechanism, is presented for the first time and demonstrated for determination of seven triazine herbicides. Two flexible fabric substrates, cellulose and polyester were used as the host matrix for three different sorbents, sol–gel PTHF, sol–gel PEG, and sol–gel PDMDPS. Results showed that the analytes were better extracted by sol–gel PEG, so it was selected for further studies.
The SFPSE unit was constructed using a section of a polypropylene SPE cartridge, an FPSE medium, an external element cut from a pipette tip, and an iron wire to allow magnetic stirring of the unit. The configuration of the extraction device as well as the extraction procedure, are depicted in Figure 4. The extraction time was fixed at 60 min, while elution time with 1.0 mL methanol at 5 min. The absolute recovery values were in the range 22.2%–70.5%. In this new approach, following the SME model, fast analyte diffusion as well as high contact surface area are provided thanks to the device design, leading to the enhancement of the extraction efficiency as well as to the reduction of total extraction time.

Figure 4.
Configuration of stir FPSE device (a) along with the extraction procedure (b). Reproduced from [30] with permission of Elsevier.
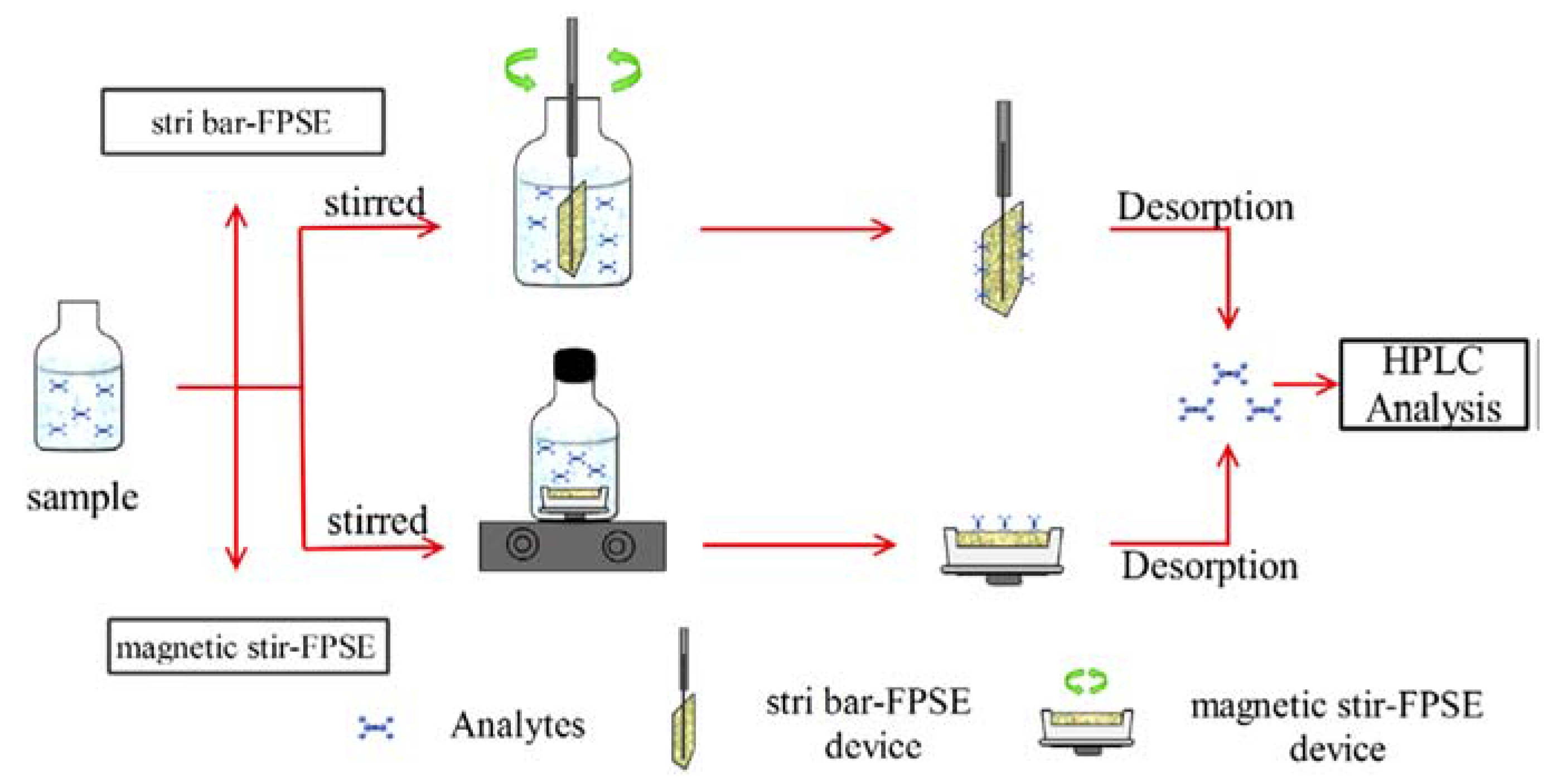
Huang et al. [32] presented a stir FPSE system similar to that presented previously [30] to extract brominated flame retardants: TBBPA, TBBPA-BAE, and TBBPA-BDBPE, from wastewater and reservoir water samples, followed by HPLC-DAD analysis. In the same work [32] and in the context of stirring of the fabric, Huang et al., presented an alternative extraction device called as stir-bar fabric phase sorptive extraction (stir bar-FPSE). Briefly, the FPSE media was cut into a house shape, clamped, and fixed by using a stir bar. Schematic diagram of the two extraction procedures stir-bar FPSE and magnetic stir-FPSE are shown in Figure 5.
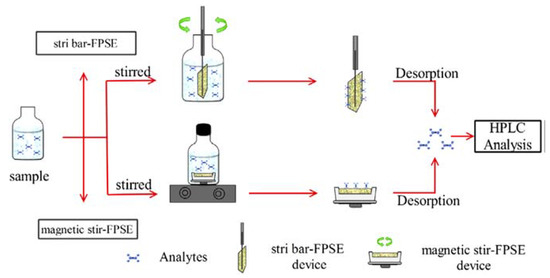
Figure 5.
Schematic presentation of stir-bar FPSE and magnetic stir-FPSE procedure. Reproduced from [32] with permission of Elsevier.
Three FPSE sorbents: sol–gel PTHF, sol–gel PEG, and sol–gel PDMDPS, were prepared on cellulose fabric substrates. Based on the medium polarity of three BFRs, the sol–gel PTHF fabric was selected for further studies. In both analytical procedures 300 μL acetonitrile was used to elute the BFRs with an elution time of 15 min. Due to the large sorbent loading capacity and unique stirring performance, both techniques possessed high extraction capability and fast extraction equilibrium. Due to the low solvent consumption, the proposed methods could meet the green analytical criteria.
6. Dynamic Fabric Phase Sorptive Extraction
As already mentioned, FPSE technique has been applied for the extraction of various analytes from different types of samples providing satisfactory results by achieving better extraction recoveries in several cases than in other sample preparation techniques. However, the main drawback of FPSE is the extraction time, which takes up to four hours (see Table 4) for extraction equilibrium.
In order to overcome this drawback of the long extraction time, Lakade et al. [29] proposed a new mode of FPSE, called dynamic phase sorbent extraction (DFPSE). DFPSE uses 47 mm circular disks of FPSE media in a filtration assembly instead of 25 mm × 20 mm fabric media introduced directly into the sample solution. The retained analytes on the FPSE disks are eluted by passing a volume of elution solvent through them. This configuration decreases the extraction time extremely as the interfacial area is highly increased. The performance efficiency of the DFPSE technique was evaluated for the extraction of pharmaceuticals and personal care products (PPCPs), MPB, CBZ, PrPB, DHB, BzPB, DHMB, DICLO, BP-3, TCC, and TCS, from river and wastewater samples, followed by LC-MS/MS detection. Taking into account that the majority of the target PPCPs are either highly polar (PARA, CAFF, APy, MPB, CBZ) or medium polar (PROP, PrPB, DHB, BzPB), a hydrophilic substrate such as cellulose, would be a suitable choice. In addition, a polar polymer PEG was selected as organic polymer from a large number of polymer candidates.
Initially, three FPSE disks were placed into the filtration assembly conditioned by passing MeOH followed by ultrapure water and then they were dried by applying vacuum. For the extraction procedure, 50 mL of sample solution was loaded into the filtration assembly and left for 10 min in contact with the FPSE disks for the adsorption of analytes. After that, the sample was passed entirely through the FPSE disks by vacuum. Subsequently, the FPSE disks were dried by an air flow generated by vacuum. The retained analytes were eluted by passing 10 mL of EtOAc, as it took less time to be evaporated in comparison with other solvents that were examined. The analysis was performed by HPLC-MS/MS in MRM mode in positive or negative ionization mode. Recovery values were better than those provided by static FPSE except for PARA and CAFF whose recoveries were lower than 38%. The extraction time was significantly reduced from 240 min to 10 min [31], proving that the proposed dynamic mode provides promising results and that it can be used for the determination of various target analytes in different kinds of samples, with shorter equilibrium time and higher retention than static FPSE.
7. Automated Fabric Phase Sorptive Extraction
Current trends in analytical chemistry, are mainly focused on three significant objectives namely miniaturization, simplification, and automation. During recent years, noteworthy progress has been made in order to enhance the quality of analytical results and follow the concept of green analytical chemistry. The implementation of flow-based sample pretreatment methodologies used for fluidic manipulation and on-line sample/reagent pretreatment holds many advantages in contrast with the batch mode of sample preparation. These include low consumption of solvents and reagents and thus low cost of total analysis as well as a significant improvement of the repeatability of the extraction procedure. The combination of flow injection analysis (FIA), sequential injection analysis (SIA), and related techniques with atomic spectrometry (AS) [6,39] provide unique capabilities and enhanced performance of the developed methods. Despite the numerous advantages of the FPSE technique, there is a need for automation in order to reduce sample preparation time extremely and considerably improve the analytical characteristics of each developed method.
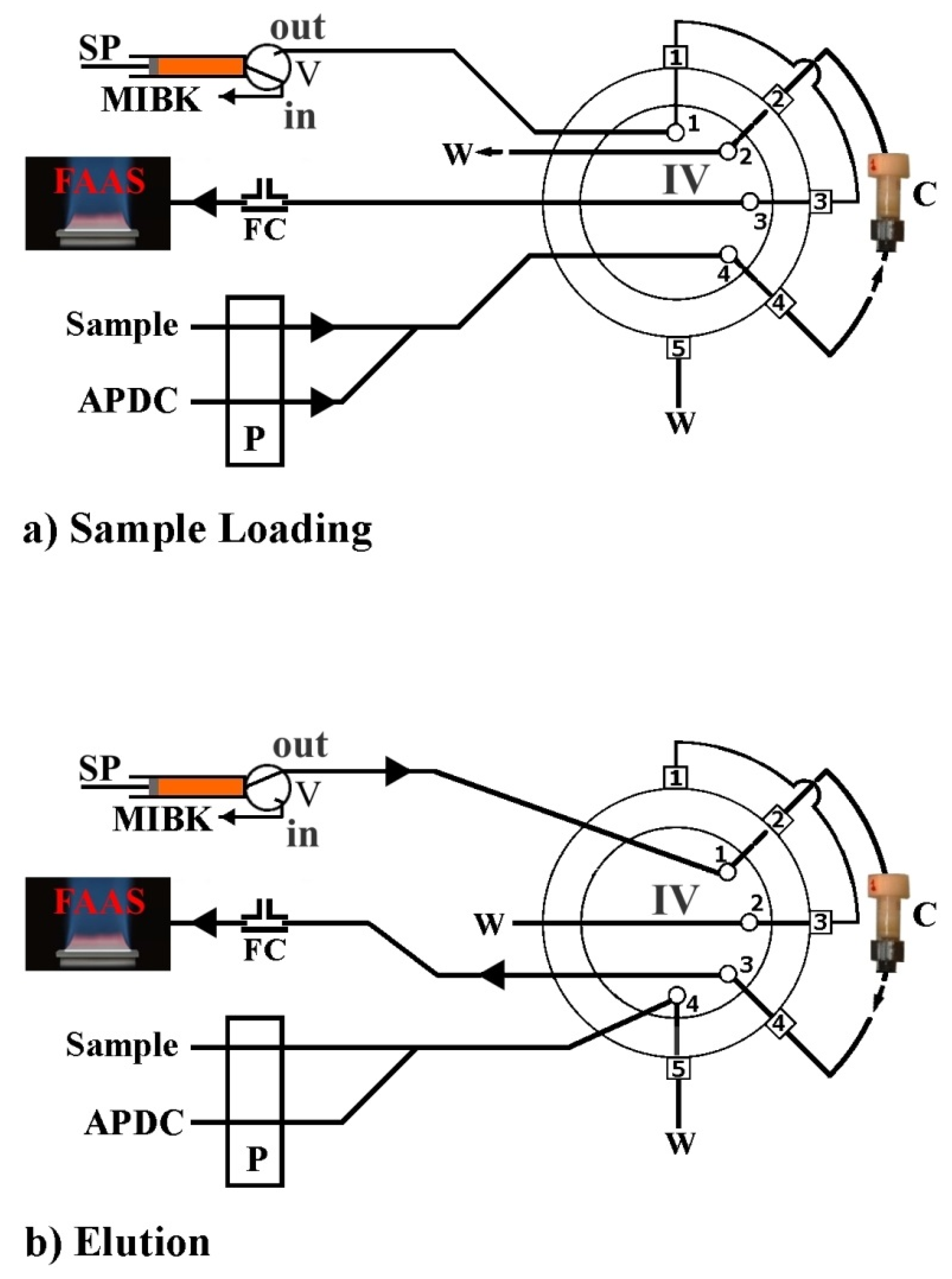
Very recently, Anthemidis et al. [36], demonstrated and successfully evaluated for the first time, an automated platform using the FPSE technique in an on-line column preconcentration system. The novel automated on-line flow injection fabric disk sorptive extraction system (FI-FDSE) coupled with flame atomic absorption spectrometry (FAAS), was applied for the preconcentration and determination of lead and cadmium in environmental water samples. Generally, the automation of the FPSE technique is based on the effective packing of a minicolumn with FPSE sorbent media in a shape of disks packed in a series and fixed appropriately. The minicolumn was incorporated onto the FIA system as shown in Figure 6. Four different FPSE sorbent media, sol–gel PDMDPS coated on a polyester substrate, sol–gel PTHF, sol–gel PEO-PPO-PEO triblock copolymer, and sol–gel graphene coated on cellulose substrates, were examined through the developed FDSE platform. Sol–gel PDMDPS presented the highest extraction sensitivity and very good reproducibility in comparison to the other FPSE media, so it was selected for further experiments. The way of FDSE media preparation as well as the way of the minicolumn packing are given elsewhere [36].
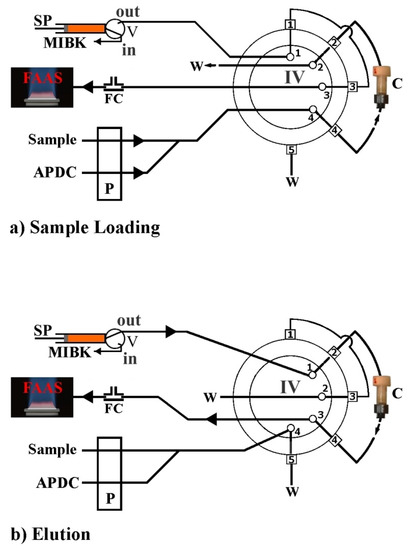
Figure 6.
Schematic diagram of the FI-FDSE-FAAS manifold for metal preconcentration and determination by FAAS. APDC, aqueous solution 0.2% (m/v) ammonium pyrrolidine dithiocarbamate; MIBK, methyl isobutyl ketone; W, waste; P, peristaltic pump; SP, syringe pump; IV, injection valve in the load or elution position; V, two-position valve; FC, flow compensation unit; C, FDSE minicolumn. (a) Sample loading step, (b) elution step. Reproduced from [36] with permission of Elsevier.
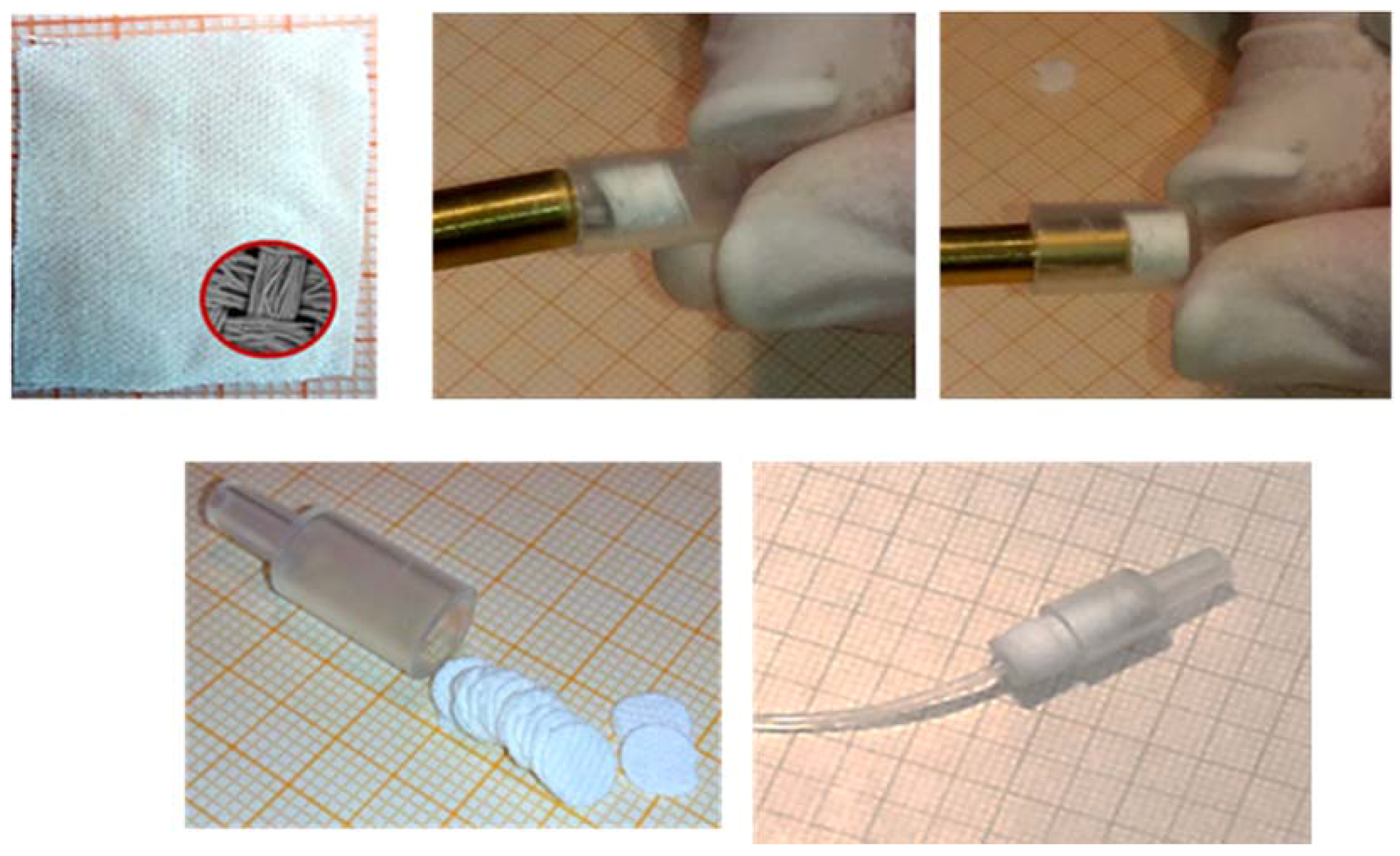
Briefly, the FDSE minicolumn was created using the body of a polyethylene syringe (4.0 mm i.d.) shortened to 1.5 cm and packed with 38–40 disks FPSE disks in a row as shown in Figure 7. No frits or glass wool were used at either end of the column to block fabric disks.
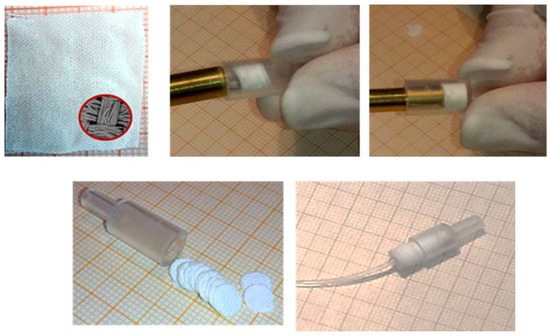
Figure 7.
FDSE technique: The FPSE fabric (up–left); the construction of the minicolumn packed with the FPSE media in the form of disks in a row; the minicolumn with FPSE disks ready for use (down–right). Reproduced from [36] with permission of Elsevier.
This configuration of the minicolumn provides limited backpressure due to the easy permeation of the incoming flow through the pores of the FPSE substrate. Thus, high loading flow rates can be applied, resulting in higher extraction efficiency and lower time of analysis. Enrichment factors of 140 and 38 and detection limits of 1.8 and 0.4 μg·L−1 were achieved for lead and cadmium determination, respectively, with a sampling frequency of 30 h−1 for 90 s preconcentration time. The precision as relative standard deviation (RSD) was 3.1% and 3.3% for lead and cadmium respectively. The FDSE minicolumn was efficient and stable for at least 500 sorption/elution cycles.
8. Conclusions and Future Outlook
Fabric phase sorptive extraction is a newly developed technique used for isolation and preconcentration of different analytes, from various matrices where even untreated samples demonstrate high extraction efficiency, operational flexibility, simplicity, and a shortened sample pretreatment scheme. FPSE has successfully eliminated inherent errors of conventional sample preparation as well as excessive time consumption. Submerging the FPSE media directly into the sample solution for analyte extraction offers great flexibility and simplicity, decreasing drastically potential analyte loss. The FPSE is easy to use with chromatographic techniques (e.g., liquid chromatography and gas chromatography) coupled with various detection systems such as spectrometry, mass spectrometry and atomic absorption spectrometry.
The inherent porosity of sol–gel sorbent and characteristic permeability of flexible cellulose or polyester fabric substrate result in rapid extraction of target analytes and complete the extraction equilibrium in a short time. Small volumes of organic solvent for elution purposes, elimination of solvent evaporation, and a sample reconstitution step, make the technique environmentally friendly and cost effective in accordance with Green Analytical Chemistry requirements. This review of the literature dealing with FPSE and its applications clearly shows that the technique has achieved great importance. Most of the applications have been developed for the selective extraction of drugs, pharmaceuticals, and various chemical compounds in water, and in biological and food samples.
In the future, research will have the challenge to develop new sol–gel coatings and materials to determine any type of analyte in complex sample matrices. The new automated approach opens up the possibility of sample preparation and total analysis without human intervention, increasing throughput, and improving several analytical performance characteristics.
Author Contributions
Viktoria Kazantzi wrote the review article and Aristidis Anthemidis reviewed and revised the article. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
2EHAdip, Bis(2-ethylhexyl) adipate; 2EHSeb, Bis(2-ethylhexyl) sebacate; 3-CPTEOS, 3-cyanopropyltriethoxysilane; 4-CP, 4-Cumylphenol; 4-TAP, 4-tert-amylphenol; 4-SBP, 4-sec-butylphenol; 4-TBP, 4-tert-butylphenol; ADTD, Androstenedione; AND, Androsterone; APDC, Ammonium pyrrolidinedithiocarbamate; APy, Antipyrine; APZ, Alprazolam; BOL, Boldenone; BPA, Bisphenol A; BP-3, Benzophenone; BRZ, Bromazepam; BzPB, Benzylparaben; C18-MTMS, Octadecyltrimethoxysilane; C8-TMS, Octyltrimethoxysilane; CAFF, Caffeine; CAP, Chloramphenicol; CBZ, Carbamazepine; CHIMA81, Chimassorb 81; CLO, Cloxacillin; CYA1084, Cyassorb 1084; DBM, Dibutyl maleate; DBP, Dibutyl phthalate; DEP, Diethyl phthalate; DFPSE, Dynamic fabric phase sorptive extraction; DHB, 2,4-dihydroxybenzophenone; DHEA, Dehydroepiaandrosterone; DHMB, 2,2-dihydroxy-4-4-methoxybenzophenone; DIC, Diclofenac; DICLO, Dicloxacillin; DZP, Diazepam; E2, 17β-Εstradiol; EE2, 17α-Εthynylestradiol; FF, Florfenicol; FDSE, Fabric disk sorptive extraction; FPSE, Fabric phase sorptive extraction; GAA, Glacial acetic acid; HAA C12, N-Lauryldiethanolamine; IBU, Ibuprofen; IRGA38, Irgafos38; IRGA168, Irgafos 168; IRGA1010, Irganox 1010; IRGA1076, Irganox 1076; KET, Ketoprofen; LRZ, Lorazepam; MGA, Megestrol acetate; MIBK, Methyl isobutyl ketone; MPB, Methylparaben; MTBSTFA, N-Methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide; MTMS, Methyltrimethoxysilane; NAN, Nandrolone; NAP, Naproxen; NOR, Norgestrel; NORET, Norethisterone; OXA, Oxacillin; PARA, Paracetamol; PDMDPS, Poly(dimethyldiphenylsiloxane); PDMS, Poly(dimethylsiloxane); PDPS, Poly(diphenylsiloxane); PEG, Poly(ethyleneglycol); PEG-PPG-PEG, Poly(ethyleneglycol)–block-poly(propyleneglycol)–block-poly(ethyleneglycol); PENG, Penicillin-G; PEO-PPO-PEO, Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer; PPCPs, Pharmaceuticals and personal care products; PRO, Progesterone; PROP, Propranololhydrochloride; PrPB, Propylparaben; PTHF, Poly(tetrahydrofuran); PTMOS, Phenyltrimethoxysilane; SDMX, Sulfadimethoxine; SFPSE, Stir fabric phase sorptive extraction; SIX, Sulfisoxazole; SMTH, Sulfamethazine; TAP, Thiamphenicol; TBBPA, Tetrabromobisphenol A; TBBPA-BAE, Tetrabromobisphenol A bisallylether; TBBPA-BDBPE, Tetrabromobisphenol A bis(2,3-dibromopropyl)ether; TBC, Tributyl citrate; TBoAC, Tributyl-o-acetyl citrate; TCC, Triclocarban; TCS, Triclosan; TEOS, Tetraethoxysilane; TES, Testosterone; TFA, Trifluoroacetic acid; TINU326, Tinuvin 326; TINU327, Tinuvin 327; TMSPA, (Trimethoxysilyl)propylamine; TOPAC, Topanol CA; TXIB, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate; UV 326, 2-tert-butyl-6-(5-chlorobenzotriazol-2-yl)-4-methylphenol; UV 327, 2,4-ditert-butyl-6-(5-chlorobenzotriazol-2-yl)phenol; UV 328, 2-(benzotriazol-2-yl)-4,6-bis(2-methybutan-2-yl) phenol; UV 329, 2-(benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl) phenol; UV 360, 2-(benzotriazol-2-yl)-6-[[3-(benzotriazol-2-yl)-2-hydroxy-5-(2,4,4-trimethylpentan-2-yl)phenyl]methyl]-4-(2,4,4-trimethylpentan-2-yl)phenol; UV 571 , 2-(benzotriazol-2-yl)-6-dodecyl-4-methyl phenol; UV P, 2-(benzotriazol-2-yl)-4-methylphenol; VTEOS, Vinyltriethoxysilane.
References
- Płotka-Wasylka, J.; Szczepańska, N.; Guardia de la, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC–Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; Guardia de la, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC–Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namiesnik, J. The 12 principles of green analytical chemistry and the significance mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Miró, M. Recent developments in flow injection/sequential injection liquid-liquid extraction for atomic spectrometric determination of metals and metalloids. Appl. Spectrosc. Rev. 2009, 44, 140–167. [Google Scholar] [CrossRef]
- Miró, M.; Hansen, E.H. On-line sample processing involving microextraction techniques as a front-end to atomic spectrometric detection for trace metal assays: a review. Anal. Chim. Acta 2013, 782, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.K.; Kaur, V.; Verma, N. A review on solid phase microextraction—High performance liquid chromatography as a novel tool for the analysis of toxic metal ions. Talanta 2006, 68, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Shen, Z.; Wu, D.; Guan, Y. Recent developments in solid-phase microextraction for on-site sampling and sample preparation. Trends Anal. Chem. 2011, 30, 1568–1574. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aquatus samples: Theory and principles. J. Microcolumn Sep. 1999, 10, 737–747. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-film microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Giakisikli, G.; Anthemidis, A.N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kermani, F.R.; Pawliszyn, J. Sorbent Coated Glass Wool Fabric as a Thin Film Microextraction Device. Anal. Chem. 2012, 84, 8990–8995. [Google Scholar] [CrossRef] [PubMed]
- Grandy, J.J.; Pawliszyn, J. Development of a Carbon Mesh Supported Thin Film Microextraction Membrane As a Means to Lower the Detection Limits of Benchtop and Portable GC/MS Instrumentation. Anal. Chem. 2016, 88, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractor (FPSE). U.S. Patent and Trademark Office 14,216,121, 17 March 2014. [Google Scholar]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol–gel microextraction phases for solvent-free sample preparation in analytical chemistry. Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Samanidou, V.; Galanopoulos, L.D.; Kabir, A.; Furton, K.G. Fast extraction of amphenicols residues from raw milk using novel fabric phase sorptive extraction followed by high-performance liquid chromatography-diode array detection. Anal. Chim. Acta 2015, 855, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Augusto, F.; Carasek, E.; Silva, R.G.C.; Rivellino, S.R.; Batista, A.D.; Martendal, E. New sorbents for extraction and microextraction techniques. J. Chromatogr. A 2010, 1217, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. TrAC–Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Chong, S.L.; Wang, D.; Hayes, J.D.; Wilhite, B.W.; Malik, A. Sol–gel coating technology for the preparation of solid-phase microextraction fibers of enhanced thermal stability. Anal. Chem. 1997, 69, 3889–3898. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gaurav; Malik, A.K.; Tewary, D.K.; Singh, B. A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal. Chim. Acta 2008, 610, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, M.; Cárdenas, S.; Lucena, R. Novel Sol–gel Sorbents in Sorptive Microextraction Analytical Microextraction Techniques. In Analytical Microextraction Techniques; Bentham Science: Sharjah, United Arab Emirates, 2016; pp. 28–69. [Google Scholar]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Racamonde, I.; Rodil, R.; Quintana, J.B.; Sieira, B.J.; Kabir, A.; Furton, K.G.; Cela, R. Fabric phase sorptive extraction: A new sorptive microextraction technique for the determination of non-steroidal anti-inflammatory drugs from environmental water samples. Anal. Chim. Acta 2015, 865, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Kaltzi, I.; Kabir, A.; Furton, K.G. Simplifying sample preparation using fabric phase sorptive extraction technique for the determination of benzodiazepines in blood serum by high-performance liquid chromatography. Biomed. Chromatogr. 2015, 30, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Guerra, R.B.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana- Rodríguez, J.J. Rapid monitoring of residual UV-stabilizers in seawater samples from beaches using fabric phase sorptive extraction and UHPLC-MS/MS. Chemosphere 2016, 164, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A 2015, 1376, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marce, R.M. Comparative study of different fabric phase sorptive extraction sorbents to determine emerging contaminants from environmental water using liquid chromatography-tandem mass spectrometry. Talanta 2015, 144, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Dong, S.; Zhang, M.; Zhang, H.; Huang, T. Fabric phase sorptive extraction: Two practical sample pretreatment techniques for brominated flame retardants in water. Water Res. 2016, 101, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Kabir, A.; Furton, K.G.; Malik, A.K. Development of a fabric phase sorptive extraction with high-performance liquid chromatography and ultraviolet detection method for the analysis of alkyl phenols in environmental samples. J. Sep. Sci. 2015, 38, 3228–3238. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Alonso, R.; Ciofi, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Del Bubba, M.; Kabir, A.; Furton, K.G. Determination of androgens and progestogens in environmental and biological samples using fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1437, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Michaelidou, K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction of selected penicillin antibiotic residues from intact milk followed by high performance liquid chromatography with diode array detection. Food Chem. 2017, 224, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Stir membrane extraction: A useful approach for liquid simple preparation. Anal. Chem. 2009, 81, 8957–8961. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Wang, J.H. Recent advances in flow-based sample pretreatment for the determination of metal species by atomic spectrometry. Chi. Sci. Bull. 2013, 58, 1992–2002. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).