Since an adsorption of particles onto the membrane surface, especially at higher cross flow rates, may not be excluded, the recovery rate represents another important parameter that has to be considered. Hereby, the UV-Vis detector signal was used, which is proportional to the particle amount. Initially, particles were injected at a constant detector flow rate without cross and focus flow. Under these conditions the sample elutes without being affected by any separation force and virtually any membrane interaction. For reproducibility reasons, five injections were performed successively. As described in Equation (4), the recovery rate can be easily calculated from the ratio of the peak areas determined either with or without cross/focus flow.
with
Re—recovery rate,
x1—peak area determined with cross/focus flow,
x2—peak area determined without cross/focus flow.
3.1. Separation of Polystyrene Nanoparticles
Our investigations started with the optimization of separation conditions for polystyrene (PS) nanoparticles in a conventional AF4 channel. A polystyrene nanoparticles standard mixture was used for the separation (50 nm, 0.80 mg·mL
−1; 100 nm, 0.16 mg·mL
−1 and 400 nm, 0.04 mg·mL
−1). Several parameters were considered: the cross flow, the elution exponent and the injection time. The cross flow is the part of the main or channel flow that passes the membrane perpendicular to the channel flow. It represents the main force that is responsible for the separation. The elution exponent characterizes the rate at which the cross flow decreases. It can be described by Equation (5):
with
Vx(
t)—cross flow at given time,
Vx,i—initial cross flow,
Vx,f—final cross flow,
ti—start time of cross flow,
tf—end time of crossflow,
n—elution exponent.
Since a constant (high) cross flow would result in long retention times mainly of larger particles, a decrease of the separation force during the elution has to be enabled. In our experiments, different cross flow rates and elution exponents were applied. Moreover, the injection time was varied. This parameter plays an important role. Particles need sufficient time to get equilibrated while focusing in the injection zone, whereas too long injection times are supposed to induce aggregation or at least agglomeration.
By means of these parameters, summarized in
Table 1,
Table 2 and
Table 3, a better description of the separation performance of the AF4 system can be given. The results in
Table 1 indicate that the highest channel resolution can be achieved at higher cross flows. However, at these conditions the lowest recovery rates were obtained, which might be attributed to an adsorption of nanoparticles on the membrane and/or by irreversible diffusion into membrane pores. Simultaneously, the time for an analysis increases up to 60 min. Lower cross flow rates (e.g., 1.0 mL·min
−1) significantly increase the recovery rates to above 90%, but on the other hand decrease peak resolution. As shown in
Table 2 and
Table 3, the influence of the elution exponent and the injection time on the separation parameters is comparatively small. To keep the resolution and recovery rates sufficiently high, the following standard separation method for the conventional channel was used: cross flow 2.5 mL·min
−1, injection time 4 min, elution exponent 0.2, total elution time 60 min.
In order to evaluate the suitability of a miniaturized channel design to separate nanoparticle mixtures, an optimization on the basis of available literature was performed. Since the miniaturized channel was expected to be very sensitive to overloading, suitable particle concentrations had to be found first. Therefore, different mixtures of three polystyrene nanoparticles with a size of 50, 100 and 400 nm were applied for separation under the following conditions: cross flow 0.5 mL·min
−1, injection time 4 min, elution exponent 0.2. The results of the calculation of the separation parameters are listed in
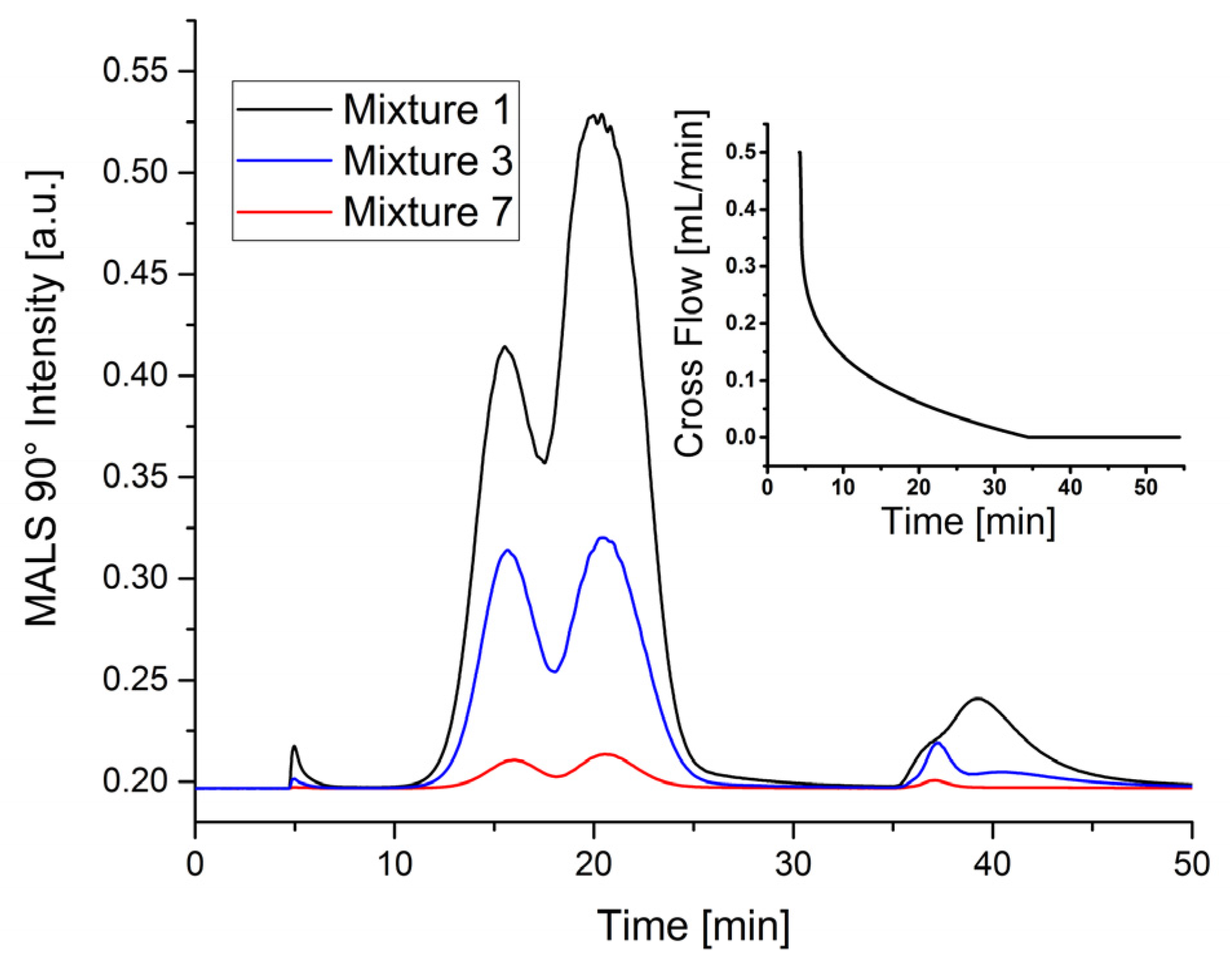
Table 4. Three representative fractograms are shown in
Figure 1. In particular, the measurement performed with latex mixture 1 reveals both a low resolution and a low plate number compared to other measurement data, and clearly shows that the amount of particles has a pronounced influence on the fractograms, when a critical limit is exceeded.
Overloading might also be one reason for the long tailing of 400 nm particles (
Figure 1 at 40 min). Since the radius of gyration
Rg of this peak determined by a MALS detector is around 155 nm corresponding to a geometric diameter of 400 nm, when assuming a spherical shape, this tailing cannot be attributed to agglomerates (see
Figure S1 in Supplementary Information). The measurements performed with latex mixture 3 (mixture used for the conventional channel) and 7 (blue and red lines in
Figure 1) were less influenced by overloading than it could be seen for mixture 1. As a result, the peak at 37 min is only slightly deformed. Similar effects are revealed considering the plate number. Overloading drastically reduces the number of theoretical plates. Thus, to achieve better separation efficiency using the miniaturized AF4 channel, measurements have to be performed with considerably lower particle concentrations. The difference of the concentration between mixture 3 and 7 also correlates with the significantly smaller separation area of the miniaturized channel.
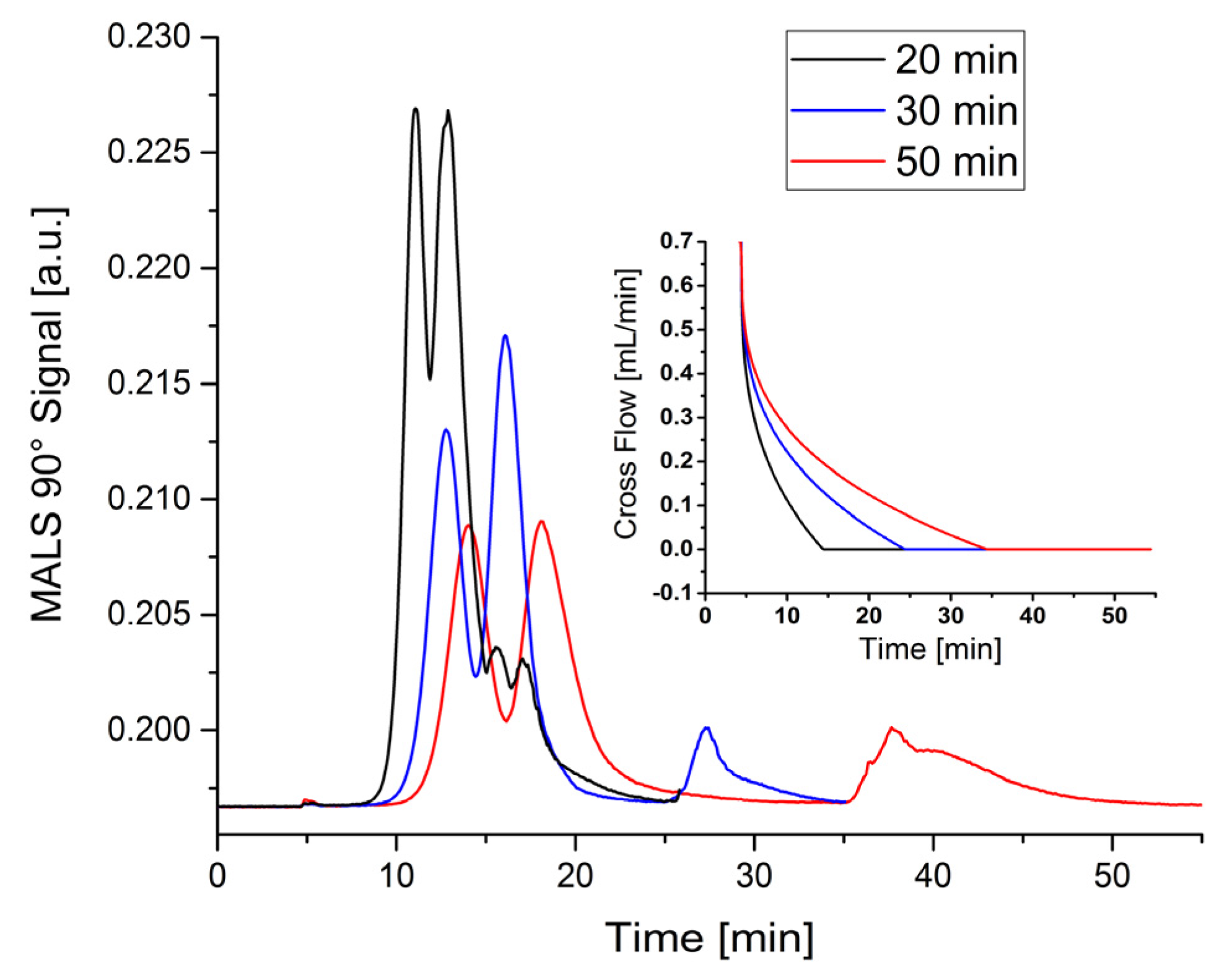
Therefore, mixture 7 was used for the separation in the miniaturized channel. One major advantage of this channel was supposed to be a significantly shorter analysis time accompanied by less solvent consumption. Thus, the whole elution process was improved based on the previously determined chromatographic parameters. The procedure consisted of three steps. In the first step, the cross flow remained constant for 0.2 min, while the focus flow was gradually reduced to zero mL·min
−1. This was followed by a decrease of the cross flow to zero mL·min
−1 simultaneously with the elution progress. During this step, the 50 nm and 100 nm particles eluted. The ‘no cross flow’ conditions were kept constant for additional 10 min in order to ensure that the 400 nm particles can elute completely (see insert in
Figure 2). In other words, the time of the second elution step was varied, whereas both the elution exponent (0.3), and the cross flow (0.7 mL·min
−1) were kept constant for all measurements. The obtained fractograms are shown in
Figure 2 and the corresponding chromatographic parameters are listed in
Table 5. The results show that with shorter elution time the retention ratio increases, which indicates that particles elute faster. However, it can be simultaneously observed that the resolution and the plate number decrease with decreasing elution times. These assumptions are supported by
Figure 2, where the peaks of the 50 nm and 100 nm particles can hardly be separated using an elution time of 20 min. In order to perform the separation reasonably fast and to keep the resolution high, a total elution step of 30 minutes was supposed to be the best value for the separation of latex mixtures in a miniaturized channel.
From our previous data (
Table 3), it was concluded that the injection time does not have much influence on the separation. Thus, only the cross flow rates and the elution exponent were further optimized. Finally, a standard separation method was obtained using the miniaturized channel (cross flow 0.7 mL·min
−1, injection time 4 min, elution exponent 0.3, total elution time 30 min).
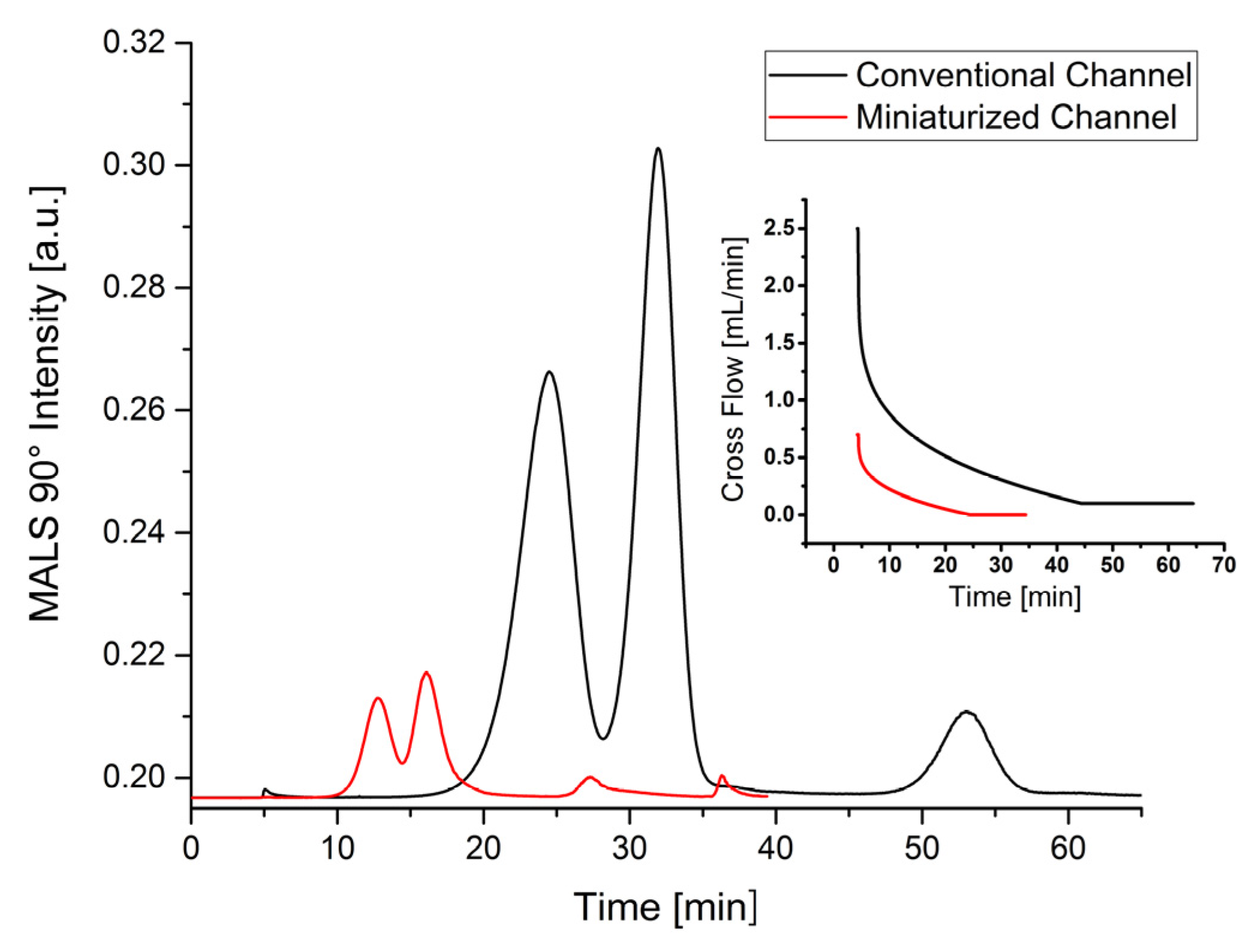
The chromatographic parameters and fractograms obtained with both standard separation methods are compared in
Table 6 and
Figure 3. It can be shown that both elution times (60 min to 30 min) and solvent consumption (from 71.5 mL to 21 mL) can be reduced to at least 50%. This is accompanied by a significantly reduced sample consumption of approximately 87.5%. The chromatographic data also reveal that after minor optimization polystyrene nanoparticles can be almost baseline separated using the conventional channel (
Rs = 1.2). Simultaneously, the recovery rate is between 70%–90%. Using a miniaturized channel, the peak resolution is significantly lower (−28%), which might result from the shorter elution process. The recovery rates obtained with a miniaturized channel were also lower as with a conventional channel.
The reproducibility of channel membranes was checked by comparative measurements of polystyrene nanoparticle mixture 7 performed by triplicate runs on one 10 kDa Polyether sulfone (PES) membrane and between three 10 kDa PES membranes (see
Supplementary Information, Figure S2). These results clearly show a high reproducibility of repetitive measurements both in the conventional and the miniaturized channel. In contrast to that, the comparison of measurements performed on different membranes shows a shift of retention times of smaller NPs, whereas their peak resolution remains almost constant (see
Supplementary Information, Table S1). Moreover, the separation of larger NPs also seems to be affected, since one experiment shows a strong tailing at 40 min retention time. In this particular case, there might obviously be a quality issue regarding the membrane material. In the production process, membranes might have ‘micro-crevices’ on their surface, which can trap the nanoparticles and prolong the retention time [
25]. Moreover, compression of the membrane and the spacer can result in different effective channel thicknesses, which can change retention times accordingly [
22].
3.2. Separation of Gold Nanoparticles
The previously chosen conditions (cross flow 2.0 mL·min
−1, injection time 6 min, elution exponent 0.2) were checked for their suitability to separate a gold nanoparticle mixture (I) containing particles with three different sizes (10 nm, 17.2 µg·mL
−1; 30 nm, 16.08 µg·mL
−1 and 60 nm, 17.28 µg·mL
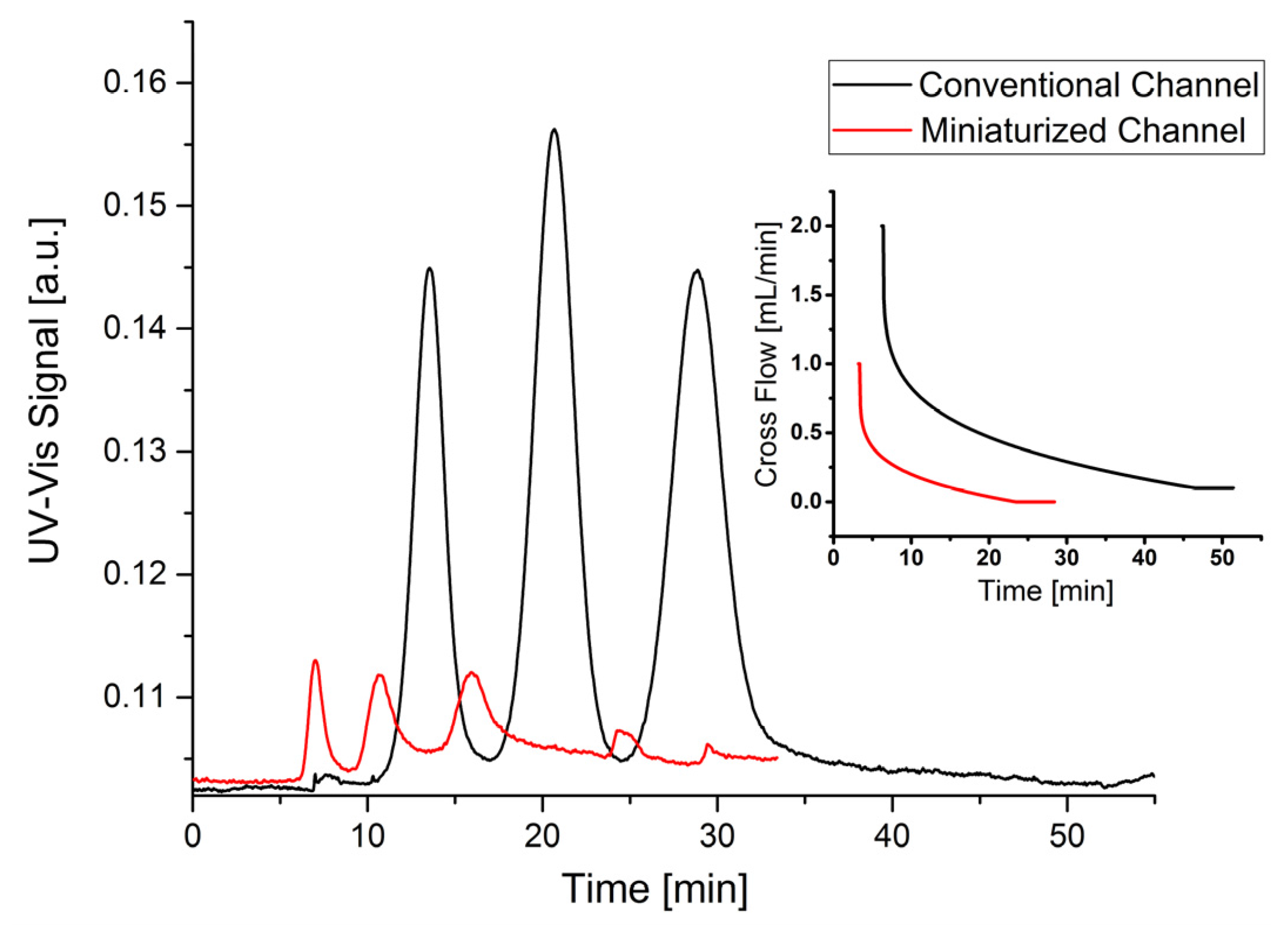
−1) in a conventional channel. The fractogram is shown in
Figure 4 (black line). The calculated separation parameters are listed in
Table 7. It can be seen that the gold nanoparticle mixture can be almost baseline separated. The recovery rates of the particles are between 70%–80%, whereby the value for the 60 nm particles is slightly lower than those of the smaller particles. A possible reason might be that the larger particles could be compressed closer to the membrane, because of the weaker diffusion force that retracts particles from the membrane throughout the elution. Therefore, the unwanted interaction between 60 nm particles and the membrane might also be slightly stronger. The retention times are comparable to those of the polystyrene latex particles (28.7 min for 60 nm Au and 24.6 min for 50 nm PS). These results also indicate that after some minor optimization both polystyrene and gold nanoparticle mixtures could be almost baseline separated using a conventional AF4 channel. The major drawback, however, is the long analysis time of 50–60 min accompanied by a comparatively large solvent consumption of more than 60 mL per analysis.
These optimized conditions (cross flow 1.0 mL·min
−1, injection time 3 min, elution exponent 0.2) were used for the separation of a gold nanoparticle mixture in a miniaturized channel. For this purpose the gold nanoparticle mixture I was diluted (1:8
v:
v), which resulted in the following nanoparticle concentrations: 10 nm, 2.15 µg·mL
−1; 30 nm, 2.01 µg·mL
−1 and 60 nm, 2.16 µg·mL
−1 (gold nanoparticle mixture (II)). The fractogram is shown in
Figure 4 (red line). As already shown for the polystyrene latex particles, the separation of gold nanoparticles in the miniaturized channel proceeded much faster. However, it can also be seen from the data in
Table 7 that the peak resolution and the recovery rates of the miniaturized channel are lower than those of the conventional channel. A possible reason could be the comparatively high cross flow, which on the one hand improves the resolution, but on the other hand may induce agglomeration of particles. This assumption is supported by additional peaks at 25 min (see
Figure 4). The calculated radius of gyration (
Rg) was in a range from 40.2 to 87.4 nm, which corresponds to diameters of agglomerates from 103.7 to 225.5 nm. In order to exclude any additional contribution from plasmon resonance, the AF4-UV-Vis-MALS system was calibrated and normalized with certificated 10, 30 and 60 nm NIST Au nanoparticles before (calibration curve, see
Figure S3 in Supplementary Information).
The limit of detection (LOD) and limit of quantification (LOQ) of gold nanoparticles in a conventional and a miniaturized channel were determined by measuring the signal to noise ratio (S/N) of the UV-Vis signals. For this purpose, the concentration of gold nanoparticles was diluted stepwise and applied for separation with the standard method. The concentrations at which the S/N ratios are equal to 3 and 10, respectively, were defined as LOD and LOQ. Since standard NIST gold nanoparticles were measured, the corresponding particle concentration can be calculated according to their certified particle size. The obtained LOD and LOQ for both channels are listed in
Table 8. An example fractogram of LOD and LOQ determination is shown in
Figure S4 in Supplementary Information.
Our results indicate that the LOD and LOQ obtained with a miniaturized channel are nearly 50% lower than those of a conventional channel. The peak width obtained using a miniaturized channel is narrower, which causes particles to pass the detector cell within a shorter time span. This results in a higher concentration in a certain time, which increases the S/N ratio.