Abstract
The extraction yield of a microextraction technique depends on thermodynamic and kinetics factors. Both of these factors have been the focus of intensive research in the last few years. The extraction yield can be increased by synthesizing and using novel materials with favorable distribution constants (one of the thermodynamic factors) for target analytes. The extraction yield can also be increased by improving kinetic factors, for example, by developing new extraction modes. Microextraction techniques are usually non-exhaustive processes that work under the kinetic range. In such conditions, the improvement of the extraction kinetics necessarily improves the performance. Since the extraction yield and efficiency is related to how fast the analytes diffuse in samples, it is crucial to stir the sample during extraction. The stirring can be done with an external element or can be integrated with the extraction element in the same device. This article reviews the main recent advances in the so-called extraction/stirring integrated techniques with emphasis on their potential and promising approaches rather than in their applications.
1. Introduction
Microextraction techniques are physicochemical processes based on the mass transference between, at least, two different phases. Mass transference is driven by thermodynamic and kinetics factors, both of which have a dramatic impact on the extraction yield [1]. Thermodynamics defines the maximum amount of analytes that can be extracted by a technique while kinetics defines the rate at which this transference occurs. The microextraction thermodynamics is mainly described by the distribution constant and many factors, such as the use of secondary reactions or the proper selection of the working pH, may affect this partitioning equilibrium. However, microextraction techniques usually work under diffusion controlled conditions. This situation, a direct consequence of the size difference between the sample and extractant phases (the diffusion paths of the analytes become larger), is of paramount practical importance. In short, a thermodynamically favored but slow technique is not suitable for analytical purposes, as it would provide a very low sample throughput. Therefore, kinetic factors must be deeply considered in any microextraction development. Among these factors, the extraction surface and the Nernst boundary layer can be highlighted. On one hand, the extraction kinetics are dependent on the area of the interface between the sample and the extractant. This phenomenon is behind the development of dispersive techniques [2] where the liquid or solid extractant is dispersed in the form of fine droplets or small-sized particles into the sample. On the other hand, the thickness of the boundary layer between the bulk sample and extractant also affects the extraction rate. In this case, the higher the thickness, the lower the rate. The boundary layer thickness can be effectively reduced if the sample is agitated, enhancing the mass transference during the extraction. In fact, agitation is common to all of the microextraction techniques.
Agitation can be done in two different ways. In most cases, the sample is agitated using an internal mechanical element (e.g., inert stir bar) or an external energy source (e.g., ultrasound) independent of the extraction element. However, some techniques are based on the integration of the agitation and extraction elements in the same device. This integration simplifies the extraction to a large extent, avoids analyte losses due to retention on external devices, and enhances the extraction yields. These techniques, that can be named as extraction/stirring integrated techniques, are the topic of this review article. This contribution reviews the recent developments of these techniques rather than focusing on the applications reported in the field.
2. Stir Bar Sorptive Extraction
Stir bar sorptive extraction (SBSE) was the first technique based on the integration of the extraction and stirring elements in the same device. It was proposed in 1999 as an alternative to solid phase microextraction (SPME) with which it shares some extraction principles [3]. The basic SBSE device consists of a stir bar coated with a polymeric phase that acts as the extractant. The stir bar is introduced and stirred into the sample and the analytes are extracted in an enhanced diffusion medium. The similarities between SPME and SBSE are based on the use of almost the same coatings which makes the application field similar. However, both techniques operate with different workflows and also differ based on the sorptive phase thickness. The last fact is of paramount importance and has thermodynamic and kinetics connotations. On the one hand, the extraction recoveries in SBSE are higher than in SPME, as the volume of the sorptive phase is 50–250 times higher in the first technique. However, the extraction kinetics are somewhat affected by the thickness since the diffusion of the analytes in the polymeric coating is hindered [4].
Polydimethylsiloxane (PDMS), a classic SPME coating, is extensively reported in SBSE applications, which are focused, as a direct consequence of the hydrophobic nature of the coating, on the extraction of non-polar compounds [5]. The in-lab synthesis of new coatings to extend the applicability of SBSE has been the main research topic during the last decade. The most salient coatings are presented in the following sections. However, it is necessary to highlight that some of the new coatings are not commercially available since they are not as robust as the classical PDMS one.
2.1. Selective Coatings in SBSE
Conventional PDMS lacks extraction selectivity as the analytes are isolated by hydrophobic interactions. However, selectivity is an important analytical feature in extraction methods that becomes critical in the microextraction context where the sorptive capacity is limited. This situation is even more complicated when complex samples comprising hundreds of interferents are processed [6]. Under these circumstances, a selective coating allows the use of the limited sorption capacity to the target analytes, avoiding sorbent saturation by the matrix components. The enhancement of sorption selectivity in SBSE has been accomplished following several strategies, the most important among them being the use of molecularly imprinted polymers (MIPs) or selective molecules.
Molecularly imprinted polymers can be defined as polymeric networks containing selective chemical cavities for the target analyte and structurally related compounds. These cavities are ad-hoc prepared by incubation, prior to the polymerization, of a special monomer with the target compound. In these conditions, the polymer grows around the template creating a cavity sterically and chemically compatible with the template. This cavity is released by the washing of the polymer, a critical step.
The use of MIPs as SBSE coatings was first proposed by Zhu et al. in 2006 [7]. This approach exploited the switchable solubility of nylon in order to create a multi-cavity network towards monocrotophos. A precursor solution containing the template and the polymer was prepared in formic acid where nylon was completely soluble. The stir bar was subsequently immersed into the solution and finally in pure water. The solvent change over induces the gelation of the polymer, which is not soluble in water, around the template molecules creating the selective cavities. The resulting MIP showed a high porosity which was beneficial for the extraction kinetics.
Although the previous approach is simple and useful, MIPs-bars have been mainly synthesized by co-polymerization of an appropriate monomer and a crosslinker in the presence of the template molecule. The bar must be previously treated in order to achieve a strong retention of the MIP over its surface which is crucial during the extraction (mechanical stability) and elution (chemical stability) steps. This strategy was followed by Xu et al. in order to fabricate MIP-bar towards ractopamine [8]. In the last few years, this workflow has been proposed for the extraction of triazines in rice [9], thiabendazole in citrus samples [10], and melamine in powdered milk [11]. The selectivity of MIPs restricts their applicability to compounds with similar chemical structures. In some cases, the analyst may be interested in the extraction of two or more different families of compounds while maintaining a high selectivity level. Dual template MIPs try to address this situation by creating a polymeric network with two types of cavities, each one selective for a family of compounds. The process is quite similar to that previously described although in this case two templates, instead of one, are added to the synthesis medium. This approach has been proposed to create stir bars that are selective towards different estrogenic compounds in water and plastic samples [12]. Seng et al. have also proposed an alternative synthetic method that consists of the preparation of silica particles coated with the MIP which are finally loaded into the stir bar. This approach was applied for the extraction of bisphenol A from water with excellent results [13].
Other chemical structures can be used to enhance the extraction selectivity. In this sense, a polymeric composite containing α-cyclodextrin as a selective element has been reported as an SBSE coating for the extraction of polychlorinated biphenyls (PCBs) from water samples [14]. α-Cyclodextrin is a cyclic (R-1,4)-linked oligosacharide that consist of six glucopyranose subunits that presents a cage-like structure. The hydrophobic cavity can host analytes with a mechanism where the molecular size plays a key-role.
Aptamers, which are artificial nucleic acid (DNA or RNA) ligands towards specific analytes, have been used as biorecognition elements in SBSE for the extraction of selected PCBs from fish samples [15].
2.2. The Potential of Nanoparticles in SBSE
Nanoparticles (NPs) can be defined as those particles that present one or more dimensions in the nanometric range (using 100 nm as an arbitrary reference), which provide them with special properties not observed in the bulk material [16]. The use of NPs in sample treatment has been the focus of extensive research during the last decade [17,18], since their properties are different than the classical materials. In SBSE, NPs may play two different and, in some cases, complementary roles. On the one hand, they can act as the active sorptive phase introducing new interaction chemistries that boost the extraction of the target analytes. On the other hand, the inclusion of NPs in the polymeric SBSE coating avoids the normal stacking of the polymer network, creating a more porous structure which makes the diffusion of the analytes easier with evident kinetics benefits.
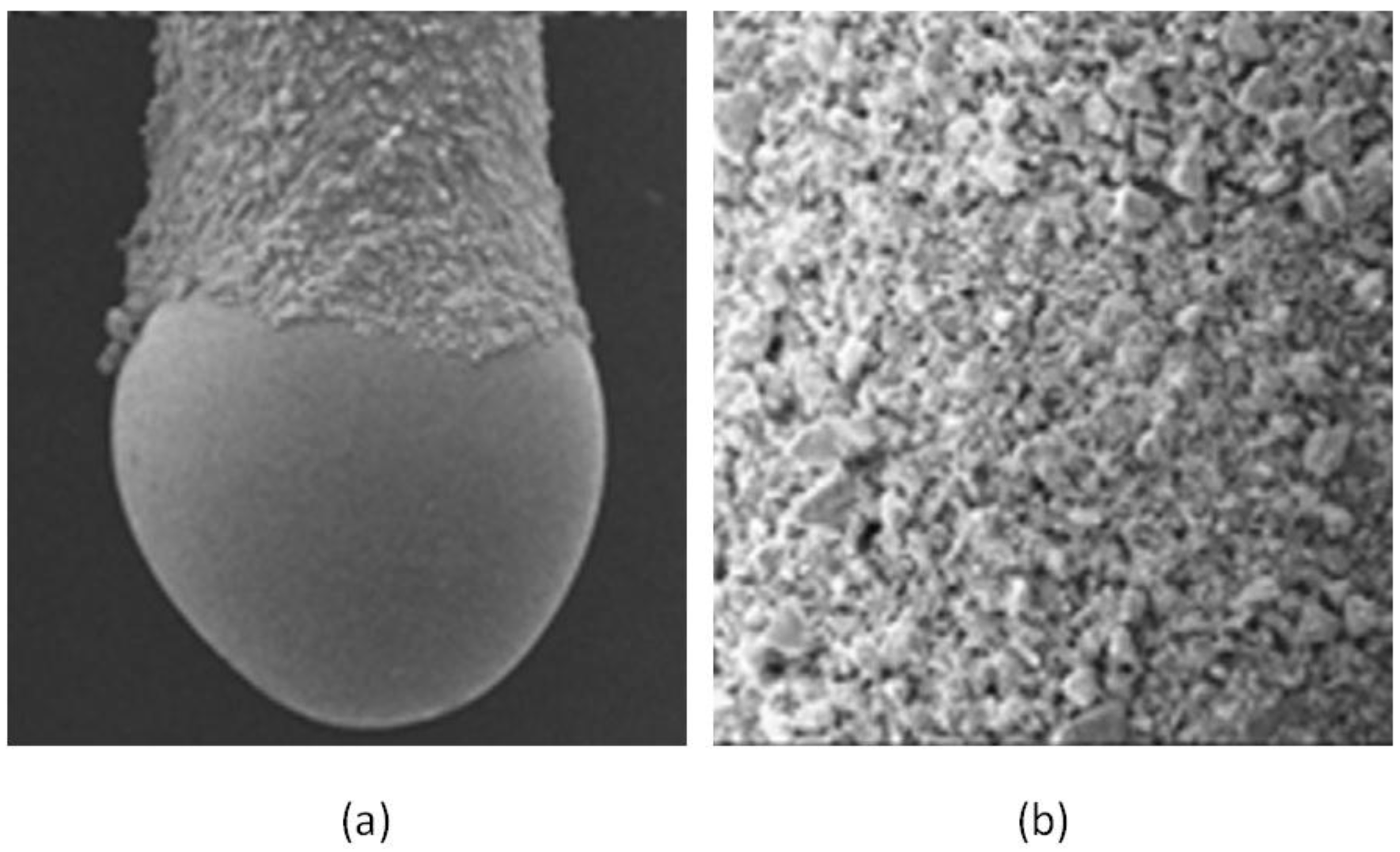
Nanoparticles can be classified according to different criteria. Considering their chemical composition, they can be divided into inorganic or carbon based NPs. In this sense, inorganic NPs have been proposed as components of SBSE coatings. Li et al. reported the use of zirconia NPs as the sorptive phase for the extraction of polar organophosphorous compounds in water samples [19]. The location of ZrO2 NPs, with sizes in the range of 10–20 nm, over a PDMS phase generates a rough coating with a superficial area of 103 m2/g and a pore size of 5 nm. Figure 1 shows an SEM picture of the dumbbell-shaped stir bar and a closer view of the coating. ZrO2 NPs prevail over PDMS in the interaction with the polar analytes, which can be extracted by three mechanisms (cation/anion/ligand exchange) depending on the working pH. The enrichment factors (742–1583) were excellent.
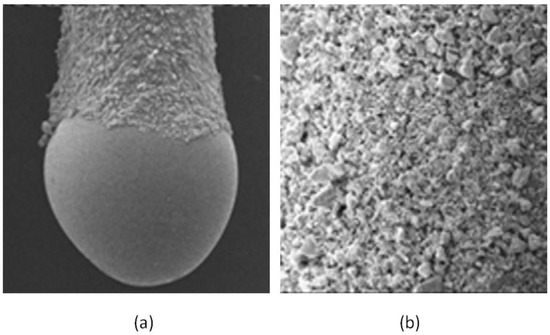
Figure 1.
Scanning electron microscopy (SEM) picture of the (a) dumbbell-shaped stir bar (magnification 40×) and (b) the surface of the coating (magnification 150×). Reproduced with permission from [19], copyright Elsevier, 2012.
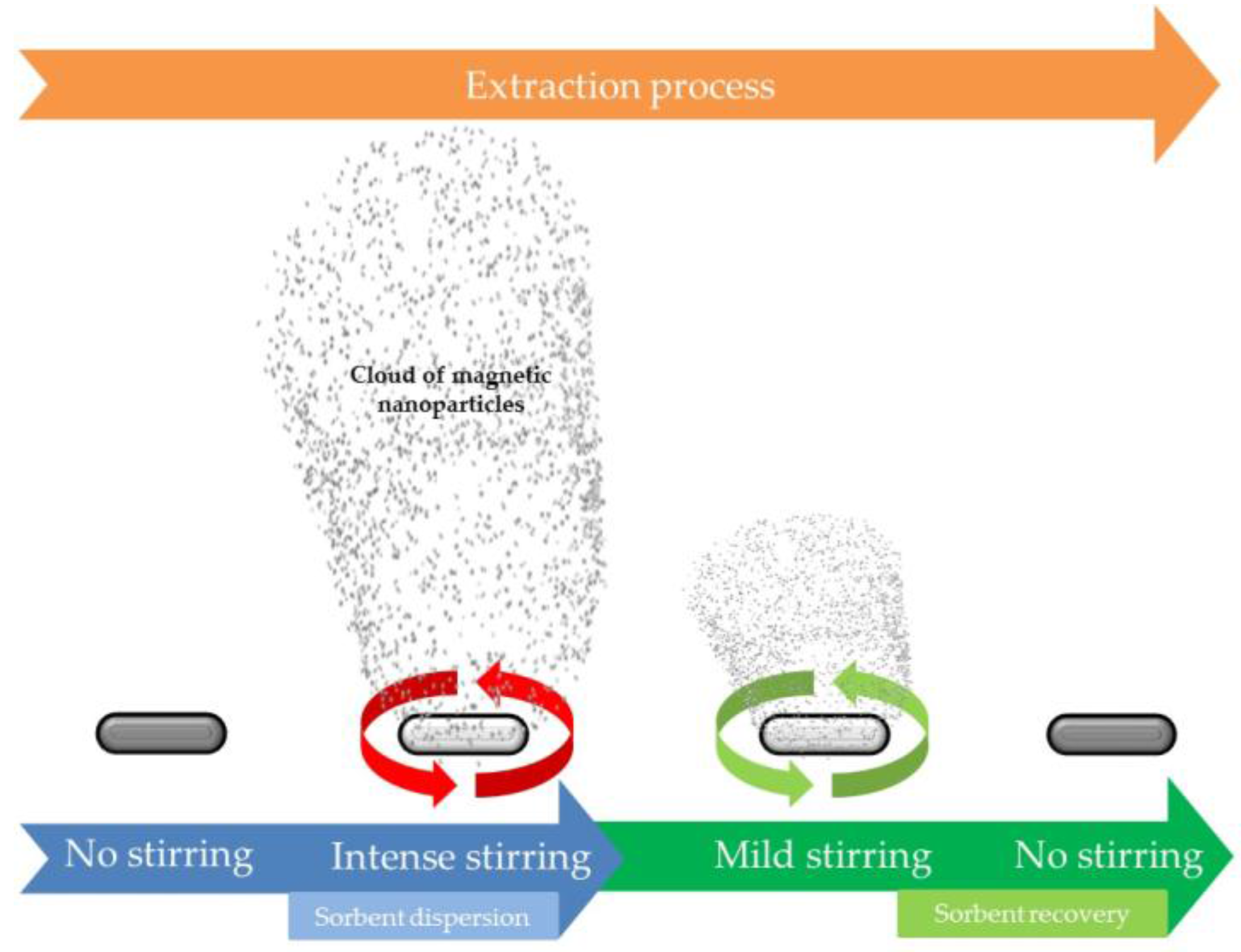
Magnetic nanoparticles have also found applications in SBSE in an elegant approach proposed by Benedé et al. [20], which combines the benefits of classical dispersive phase microextraction and SBSE. The extraction workflow, which is schematically shown in Figure 2, consists of several subsequent steps. Initially, the oleic acid coated CoFe2O4 NPs are attached to the stir bar by magnetic forces. Once the stir bar is introduced into the sample, the bar is stirred at high velocities inducing the detachment of the magnetic nanoparticles (MNPs) which are dispersed into the sample, extracting the target analytes. After that, the stirring rate is reduced inducing again the attachment of the MNPs on the bar for the final elution. This approach reduces the treatment time as the isolation takes place under a perfect dispersion of the sorbent. In addition, it is versatile since virtually any MNP can be applied. In fact, the same authors have evaluated the potential of magnetic nylon 6 composites under this format [21].
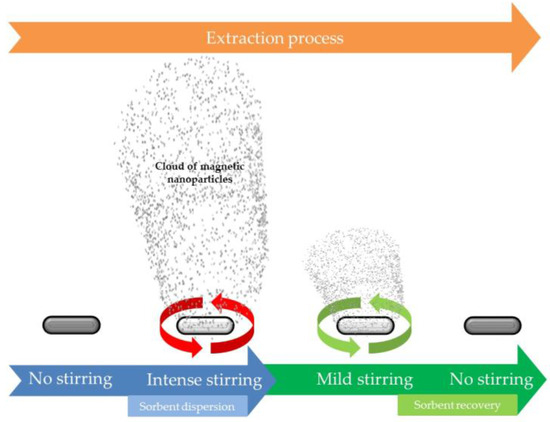
Figure 2.
Scheme of stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles. Reproduced with permission of the Microextraction Tech blog.
Carbon based nanomaterials can develop special interaction chemistries that can be used to enhance the extraction selectivity. The capacity of carbon nanotubes (CNTs) to interact by π–π bonds with analytes containing aromatic domains has been exploited in SBSE [22,23,24]. The introduction of CNTs in the sorptive phase can be done in several ways. Hu et al. [22] introduced amino-modified CNTs in a PDMS matrix, where they are entrapped, in order to make the extraction of polar phenols feasible. The presence of CNTs in the polymer creates a rougher surface with a higher extraction capacity. Farhadi and co-workers preferred a different approach based on the electro-polymerization of aniline over a steel pin in the presence of CNTs, which are also introduced in the polymeric network [23]. Although the inclusion of CNTs enhances the extraction capacity, the amount of CNTs that can be loaded is somewhat limited by mechanical stability issues. In fact, the resulting composite becomes unstable when the amount of CNTs is high. This stability can be improved if covalent bonding is selected to anchor the CNTs into the polymeric network [24]. This synthetic path requires a modification of the CNTs which are previously oxidized to include carboxylic groups on their surface. Carboxyl groups are finally transformed into the acyl chloride form, allowing their inclusion into a polyethylene glycol (PEG) phase.
Graphene oxide (GO) has been also proposed as a SBSE coating element. Fan et al. evaluated a polyethylene glycol (PEG)/GO composite for the extraction of fluoroquinolones from chicken muscle and liver [25]. The composite, which is synthesized by co-blending, is finally immobilized over the stir bar by the sol-gel reaction. It provides the best extraction of the target analytes compared to commercial and lab-made PDMS coatings thanks to its superior hydrophobic/hydrophilic balance. The presence of PEG also stabilizes GO avoiding losses of the nanomaterial during stirring. The introduction of GO into the coating can be also done with a different strategy. Zhang et al. proposed the use of polydopamine coated over a stainless steel bar as a support for the covalent GO immobilization [26], providing a better distribution of the nanomaterial in the polymeric network. The resulting composites provided better efficiencies (signal enhanced by factors higher than 10) than the naked polymeric coating. Although GO has an active role on the extraction in the above reported applications, it may also play other secondary but positive roles in the extraction. In this sense, it has been also proposed as a dopant for enhancing the extraction capabilities of an MIP stir bar coating [27] through improvement of the coating porosity.
2.3. ICE Concentration Linked with Extractive Stirrer
The improvement of the extraction yield can be also achieved by the proper modification of the experimental conditions. Recently, Logue et al. have proposed the so-called ice concentration linked with an extractive stirrer (ICECLES) which combines the advantages of freeze concentration and SBSE [28].
Freeze concentration can be considered as an innovation in analytical sample preparation although it is frequently used in the food industry to concentrate liquid products. It is based on a simple and well known principle, freezing-point depression. This principle states that solutions have lower freezing points than pure water. As a consequence, when a sample is cooled down, ice starts to form, excluding the solutes which are concentrated in the remaining liquid.
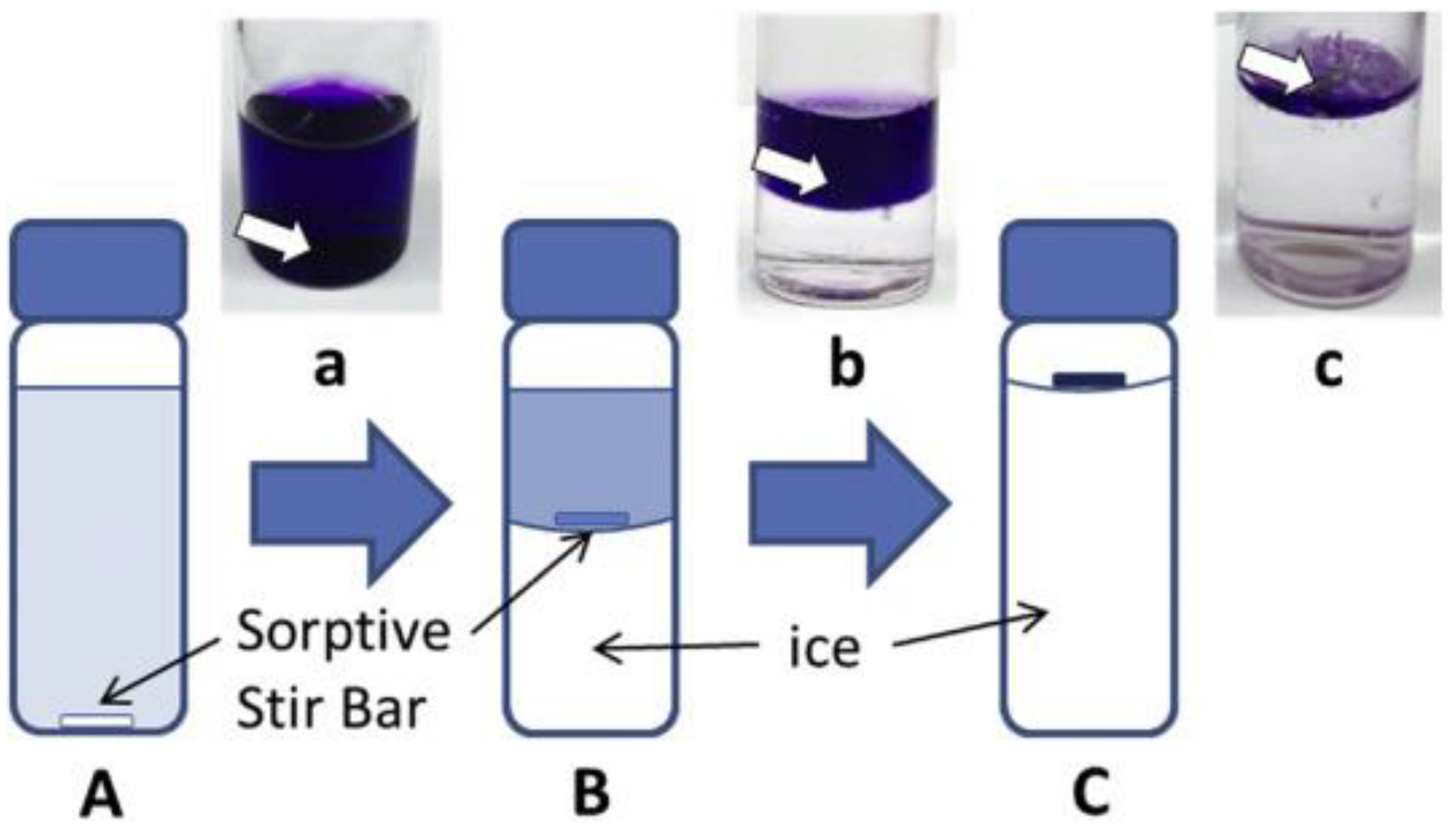
ICECLES operates in a simple and well established workflow (see Figure 3). A sorptive bar is introduced into a liquid sample and is stirred continuously. During the extraction, the sample is cooled down, inducing the freeze concentration. As a result, the solution is enriched with the analytes establishing a higher concentration gradient with the stir bar, thus increasing the extraction yield. In addition, the lower temperature increases the sorption of the analytes as this process is exothermic.
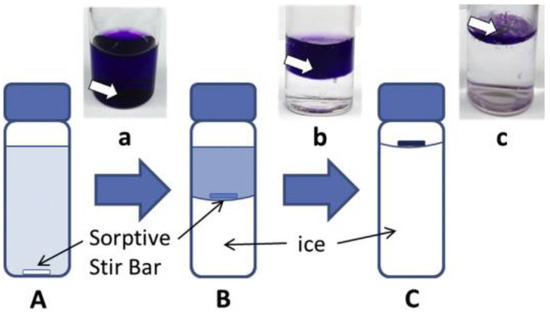
Figure 3.
Scheme of ice concentration linked with the extractive stirrer technique. The stirrer is located in the bottom of the vial (A) which is cooled down. As a consequence, ice is created (B and C) leaving a more concentrated solution than the original sample. The pictures show the extraction of methyl violet as the model analyte: (a) initial state, (b) extraction and (c) final state. Reproduced with permission from [28], copyright Elsevier, 2016.
ICECLES provides signal enhancement factors of 450 over conventional SBSE for the extraction of (semi)volatile compounds, which makes it a very promising technique in the coming years.
3. Stir Membrane Extraction
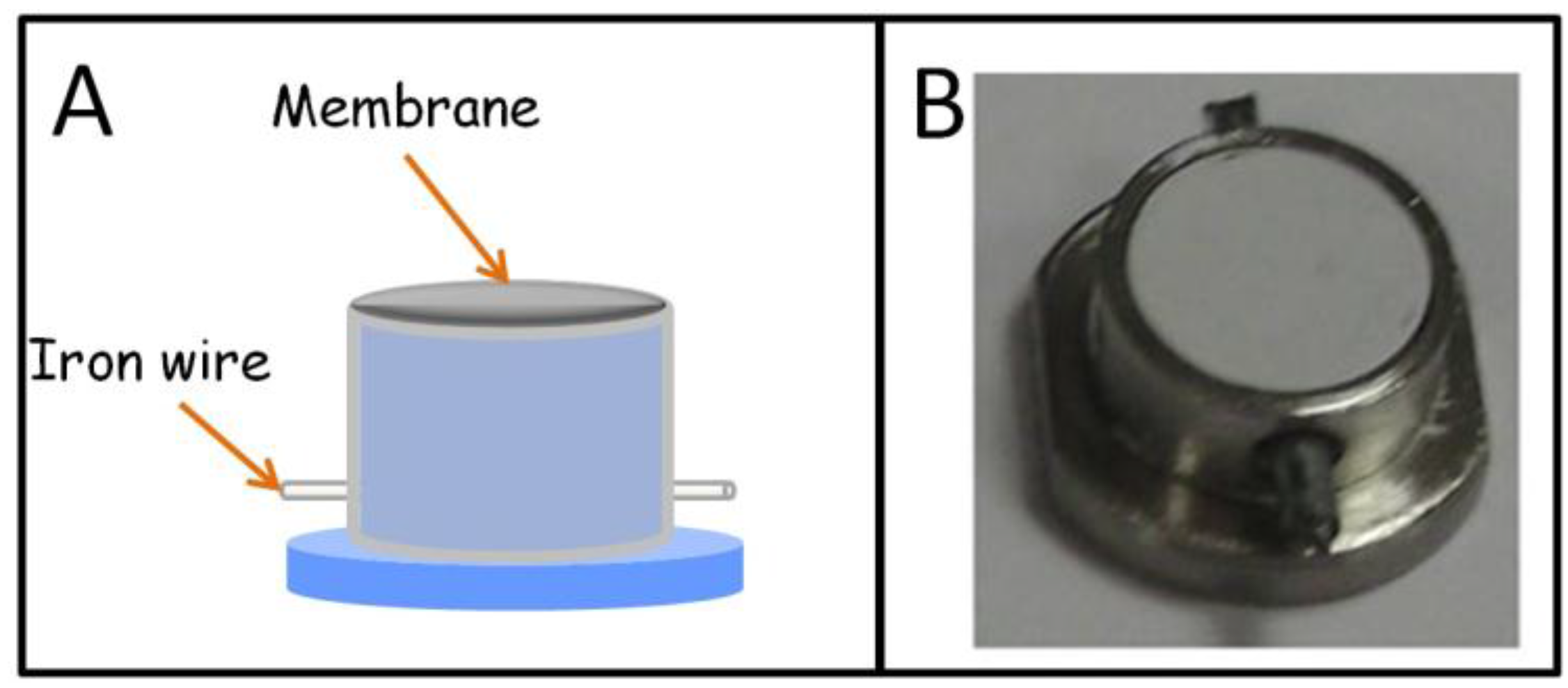
Stir membrane extraction (SME) was proposed by our research group in 2009 as a new stirring technique based on the use of polymeric membranes as sorptive phases [29]. Membranes present a high surface-to-volume ratio and permeability, especially if they are compared with classical SBSE coatings, which are advantageous characteristics from the kinetics point of view. To be applied, SME requires a special device that integrates stirring (a protected iron wire) and extraction (membrane) elements. The first unit, schematically shown in Figure 4A, was designed in polypropylene using commercial products as precursors. In its first application, aimed at determining selected polycyclic aromatic hydrocarbons (PAHs) in water samples, SME provided better results than stir bars fabricated by the same membrane polymer (PTFE). Although SME can be applied in the normal extraction/elution workflow, it can be directly combined with spectroscopic techniques that allow the in-membrane detection of the targets without a previous elution. This strategy was reported for the determination of the hydrocarbon index in water by infrared spectroscopy [30]. For this purpose, the SME unit was built in stainless steel (Figure 4B) to promote the retention of the analytes into the membrane which presents a low thickness (40 µm), to permit the transmission of the infrared beam. The versatility of SME is based on the great availability of commercial and lab-made membranes. In fact, the use of fabric phases, a porous hybrid inorganic-organic sorbent material chemically bonded to the flexible and permeable substrate matrix, [31] has been also proposed as a sorptive phase in SME [32]. This combination allowed the extraction of several triazine herbicides from water samples with enrichment factors in the range from 444 to 1410.
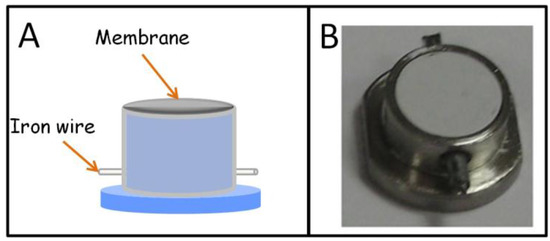
Figure 4.
Stir membrane extraction device. (A) Diagram of the unit built in plastic; (B) picture of the unit built in stainless steel (reproduced with permission from [30], copyright Springer, 2010).
3.1. Stir Membrane Extraction in the Liquid Phase Microextraction Context
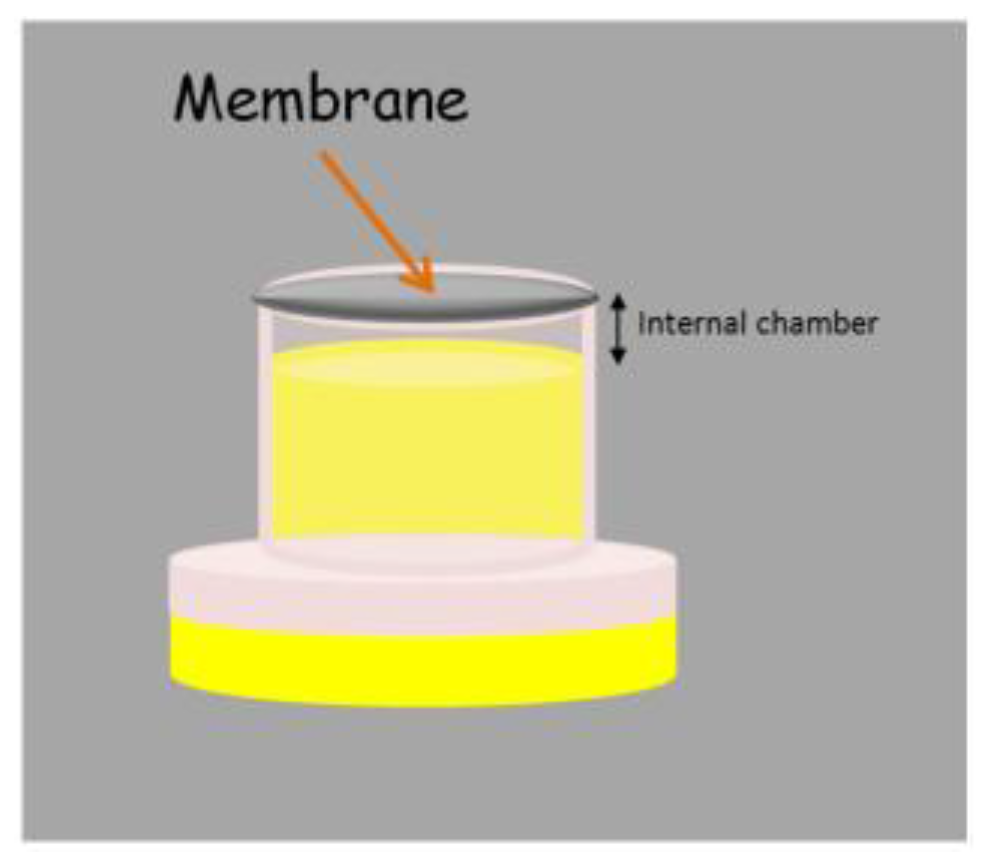
The original SME device can be adapted to the liquid phase microextraction (LPME) context with slight modifications. The simple introduction of a cap in the lower part of the unit creates a small chamber where an extractant phase can be located, as can be observed in Figure 5. The membrane is used for achieving the confinement of the liquid extractant phase in the chamber while the unit is stirred in the sample. The stir membrane unit in LPME can operate under the two [33] and three [34,35] phase mode. In the two phase mode, an organic solvent is located in the chamber, wetting the membrane, and the analytes are extracted by the different solubilities they present in the aqueous (sample) and organic (extractant) phases. This mode is specially indicated for the extraction of non-polar compounds and it is fully compatible with gas chromatography. In the three phase mode, the organic solvent only wets the membrane (forming the so-called supported liquid membrane, SLM) while an aqueous phase is located in the internal chamber. This mode, which is based on the transference of the analytes through the SLM thanks to an existing pH gradient between the sample and the extractant (both of aqueous nature), is useful for the extraction of ionisable non-polar compounds.
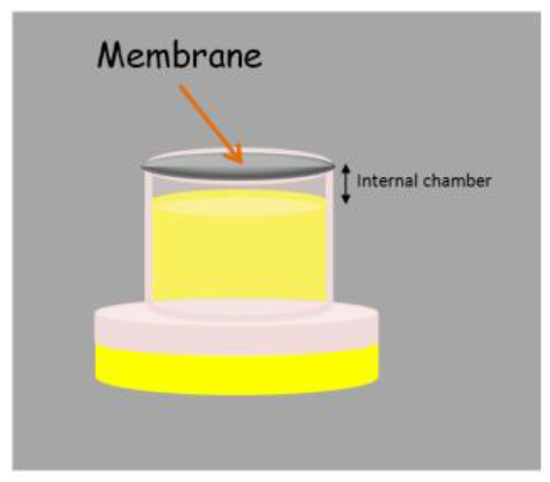
Figure 5.
Stir membrane extraction device working in the liquid phase microextraction context.
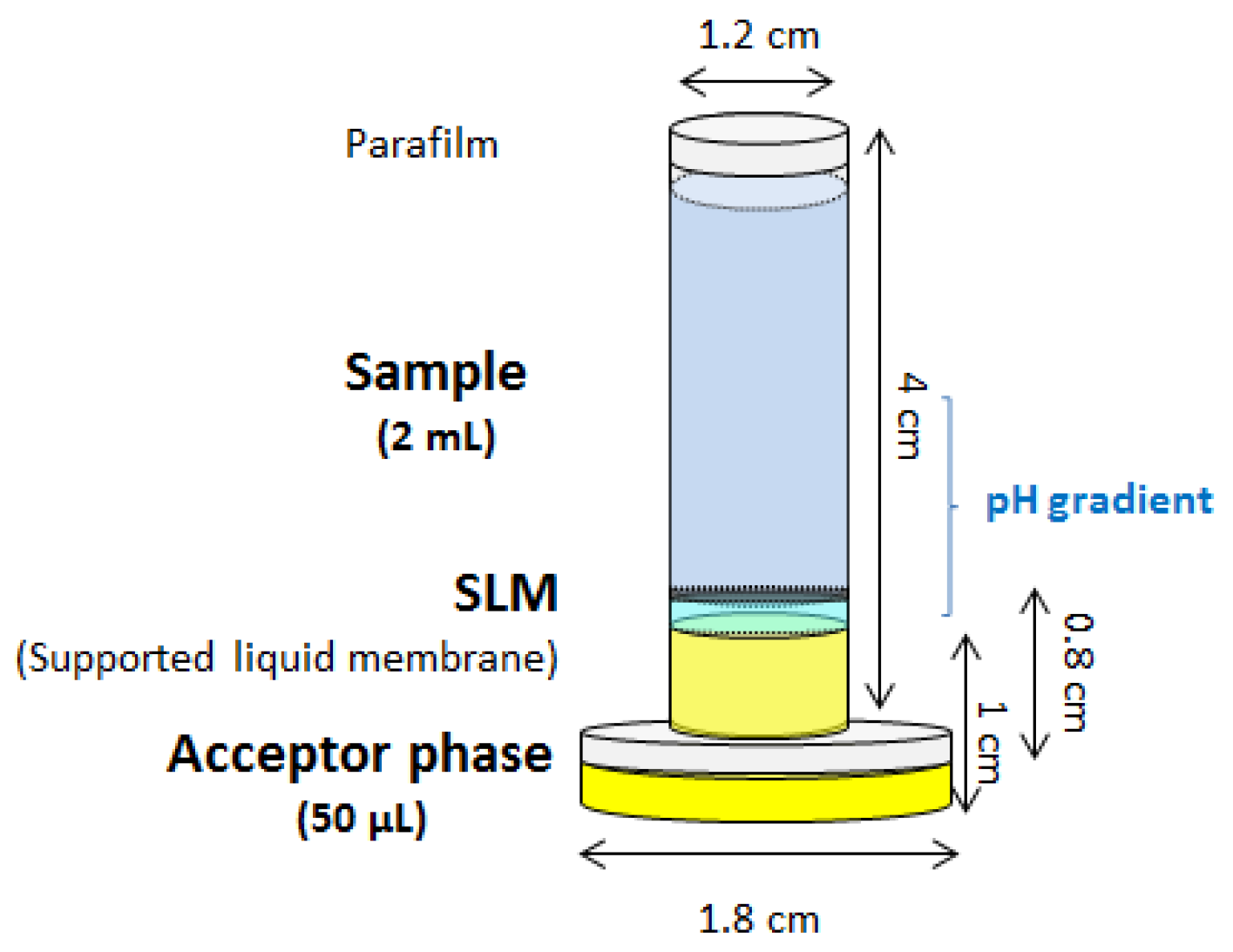
The conventional stir membrane unit, both in the solid and liquid phase microextraction context, is stirred in the sample. Sample volumes larger than 20 mL (the exact volume depends on the extraction vessel) are required which restricts the application to some biological samples with limited volume. For this reason, the stir membrane extraction has been also adapted to this scenario. This was accomplished by slightly modifying the device, as can be observed in Figure 6, by just increasing the height of the plastic adapter. This modification allows the creation of an upper chamber with a small volume where the sample is located [36]. Although this approach, which was evaluated for the extraction of paracetamol from saliva samples, cannot be strictly considered as a stirring device (the unit is agitated in a vortex), it clearly shows the versatility of the membrane-based units.
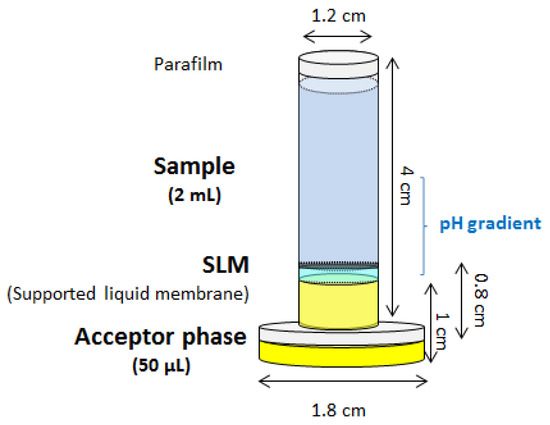
Figure 6.
Modification of the stir membrane liquid-liquid-liquid extraction device to process a low volume of sample. SLM, supported liquid membrane.
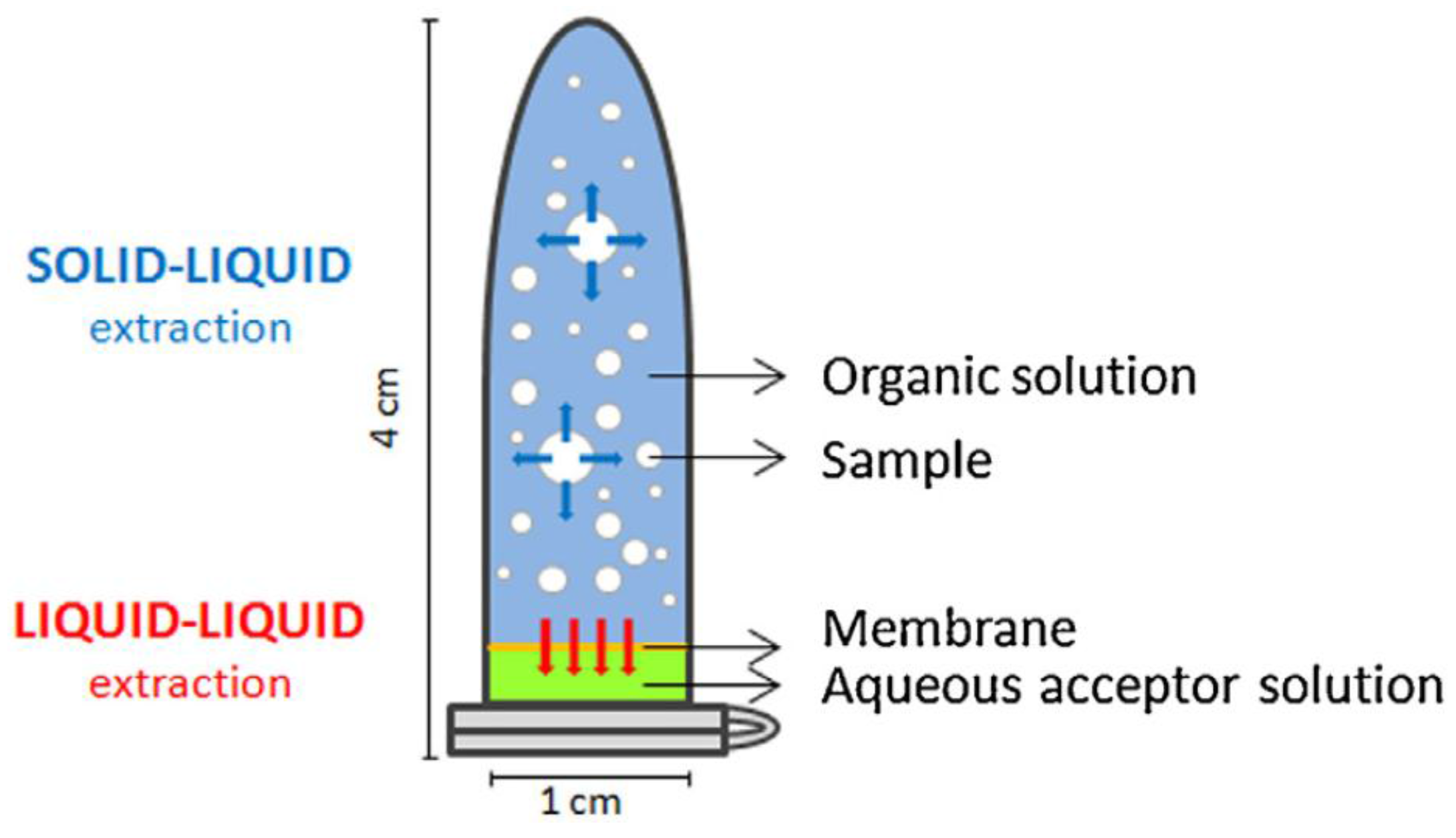
In the same way, the original device has been adapted for solid-liquid-liquid extraction [37]. Although the main idea remains the same, the unit was built using a different construction block. In this case, a conventional plastic eppendorf tube is used as extraction device (Figure 7) where the solid sample is dispersed in an organic solvents mixture. An aqueous extractant phase is situated in the eppendorf cap and protected by a polymeric membrane. Once deployed, the unit is rotated and agitated. During the extraction, the analytes are transferred from the solid sample to the organic solvent and then from the organic solvent to the aqueous phase, passing through the membrane. This approach was initially used for the determination of parabens in lyophilized human breast milk with very good sensitivity.
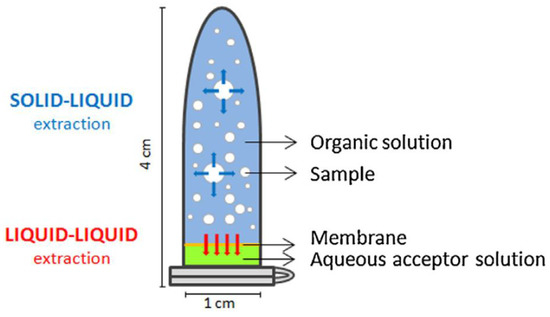
Figure 7.
Device for stir membrane solid-liquid-liquid extraction. Reproduced with permission from [37], copyright Elsevier, 2014.
3.2. Adaptations of Stir Membrane Units
The stir membrane unit has been adapted to other formats. For example, the polymeric membrane can be substituted by a borosilicate disk to develop liquid phase microextraction in a solvent film. In this case, the disk is derivatized with octadecyl groups that make the physical immobilization of an organic solvent, the active extractant, on the disk surface easier. This approach provides enrichment factors towards selective triazine herbicides in the range from 79 to 839 [38].
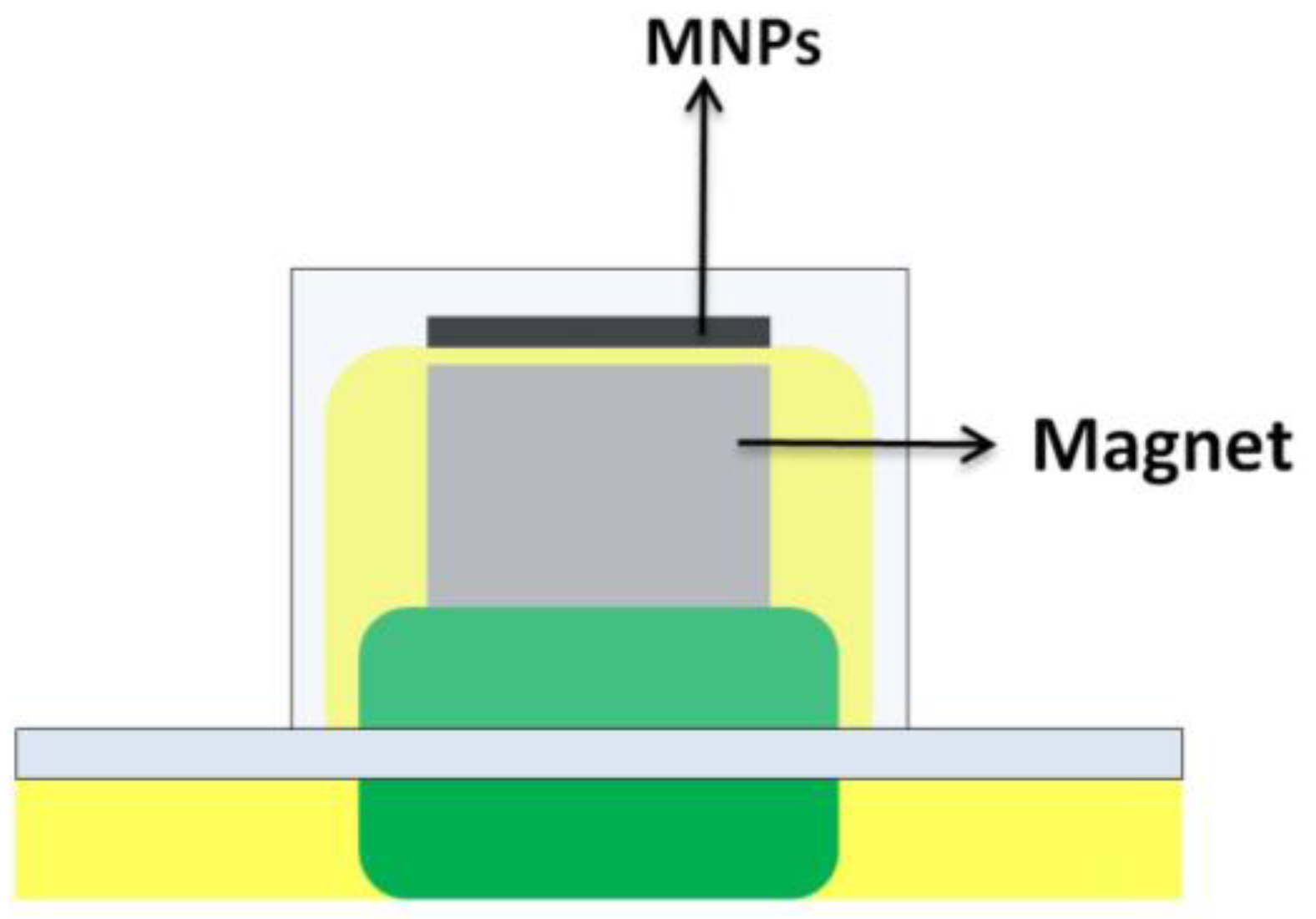
In addition, the use of small magnets within the unit (Figure 8) has allowed the development of the so called magnetically confined extraction [39,40]. The magnet allows the stirring of the device and the confinement of the hydrophobic magnetic nanoparticles (extractant) over the unit. This approach exploits the extraction capacity of these nanoparticles which are hard to disperse into an aqueous media due to hydrophobic attractions.
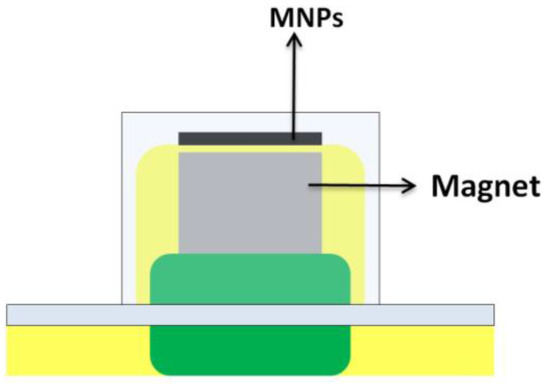
Figure 8.
Device for microextraction of magnetically confined hydrophobic nanoparticles. MNPs, magnetic nanoparticles.
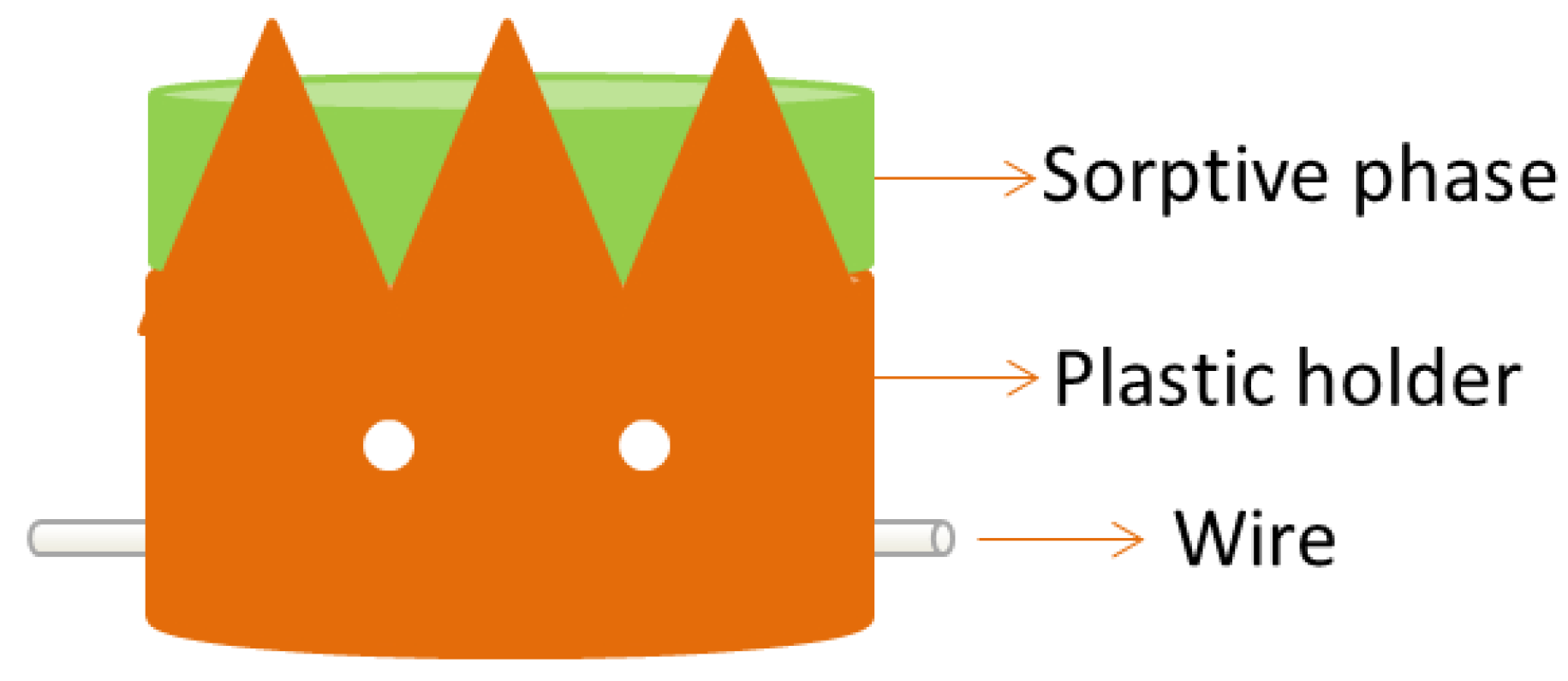
4. Stir Cake Sorptive Extraction
Stir cake sorptive extraction (SCSE) was first proposed in 2011 by Huang et al. [41]. It is very similar to stir bar sorptive extraction but, in this case, the sorbent is a monolithic cake placed in a homemade holder with a protected iron wire (Figure 9). This configuration avoids the direct contact of the sorptive phase with the sample vessel. As a result, higher stirring rates can be used during the extraction. In addition, the life span of the monolith is increased allowing its reuse up to 300 times, while the typical reusability of a stir bar is ca. 60.
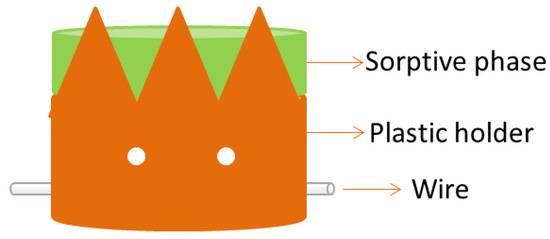
Figure 9.
Device for stir cake sorptive extraction. Reproduced with permission of the Microextraction Tech blog.
Monoliths are continuous porous structures which are easily obtained by the polymerization of a monomer mixture with a porogen solvent into a given holder (capillary, spin column, pipette tip, stir cake). Their chemistry can be tailored by the proper selection of the monomers and also the porosity can be controlled during the synthetic process. Monoliths can be classified in three groups: organic, silica, and hybrid monoliths, depending on the nature of their ingredients. Their potential in separation techniques has been widely studied [42].
The preparation of the monolith-based SCSE unit is very simple [41]. The monolithic cake is synthesized and located in a cake-shaped plastic holder (see Figure 9). The holder is finally pierced by an iron wire that allows for magnetic stirring of the system. In addition, small holes can be drilled through the holder to promote the flow of the sample through the monolithic cake. Before its first use, the monolith must be conditioned with the appropriate solvents. Once conditioned, the sorptive cake is introduced in the sample for the isolation of the analytes, which are finally eluted for instrumental analysis.
Different monoliths have been used in this SCSE format, with polymeric ionic liquids and organic-based polymers being the most reported in the literature.
Ionic liquids (ILs) can be considered as a group of non-molecular solvents which present a melting point below 100 °C [43]. Ionic liquids have been extensively used in microextraction techniques, both in the solid and liquid phase formats. In recent years polymeric ionic liquids (PILs), obtained by the polymerization of IL cations, have been also proposed in this context. In comparison with the ILs, PILs exhibit higher viscosity, thermal stability, and mechanical strength which are important characteristics for sorptive phases in miniaturized solid-phase extraction techniques [44]. PILs were first proposed as an active phase in solid phase microextraction (SPME) in 2010 [45]. In 2012, Wang et al. reported the first application of PILs-monolith (PILM) in SCSE for the extraction of inorganic anions from water [46]. The PILM was in situ obtained by the copolymerization of 1-allyl-3-methylimidazolium chloride (AMIC) and ethylene dimethacrylate (EDMA) providing a final structure that exhibited a strong anionic exchange capacity towards the analytes (F−, Cl−, Br− NO2−, NO3−, SO4−, PO43−). The presence of imidazolium cations in the structure was behind this capacity. Following a similar procedure, traces of antimony can be extracted from environmental water using a PILM containing 3-(1-ethyl imidazolium-3-y)propyl-methacryamido bromide (EPB) and EDMA as precursors [47]. In this specific example, the amino groups of the PILM coordinate to Sb, favoring its extraction. PILM-SCSE has also demonstrated its applicability for the pre-concentration of organic compounds. The selection of the monomers is crucial to obtain an adequate sensitivity and selectivity. The monolith described in [46] was also efficient for the extraction of preservatives (sorbic, benzoic, and cinnamic acids) in fruit juices and soft drinks [48]. In this case, the hydrophobic and anion exchange interactions are the driving forces of the extraction procedure. Similar analytes can also be extracted using 1-ally-3-vinylimidazolium chloride (AV) polymerized in-situ with divinylbenzene (DVB) to form the PILM-SCSE [49]. Other relevant contributions in the field are the determination of benzimidazole anthelmintics in water, honey, and milk samples [50], and estrogens in environmental water [51].
PILM-SCSE can be ad-hoc synthesized depending on the target analytes. A multi interaction cake can even be fabricated if analytes of different nature are intended to be extracted. [52]. In this sense, if 1-vinylbenzyl-3-methylimidazolium chloride (VBMI) and divinylbenzene (DB) are used as precursors, the resulting PILM may interact with the target compounds via π–π hydrophobic, hydrogen bonding, dipole-dipole, and anion exchange interactions.
Conventional monolithic phases have also been synthesized for SCSE. Polar phenols were extracted from environmental water by means of an allylthiourea and DB polymer using DMF as a porogen solvent and AIBN as an initiator [53]. Β-agonists have been extracted in milk and swine urine samples by SCSE using a poly(4-vinylbenzoic acid-divinylbenzene) monolith [54], while a novel boron-rich monolith has been synthesized and characterized for the determination of fluoroquinolones in environmental water and milk samples [55].
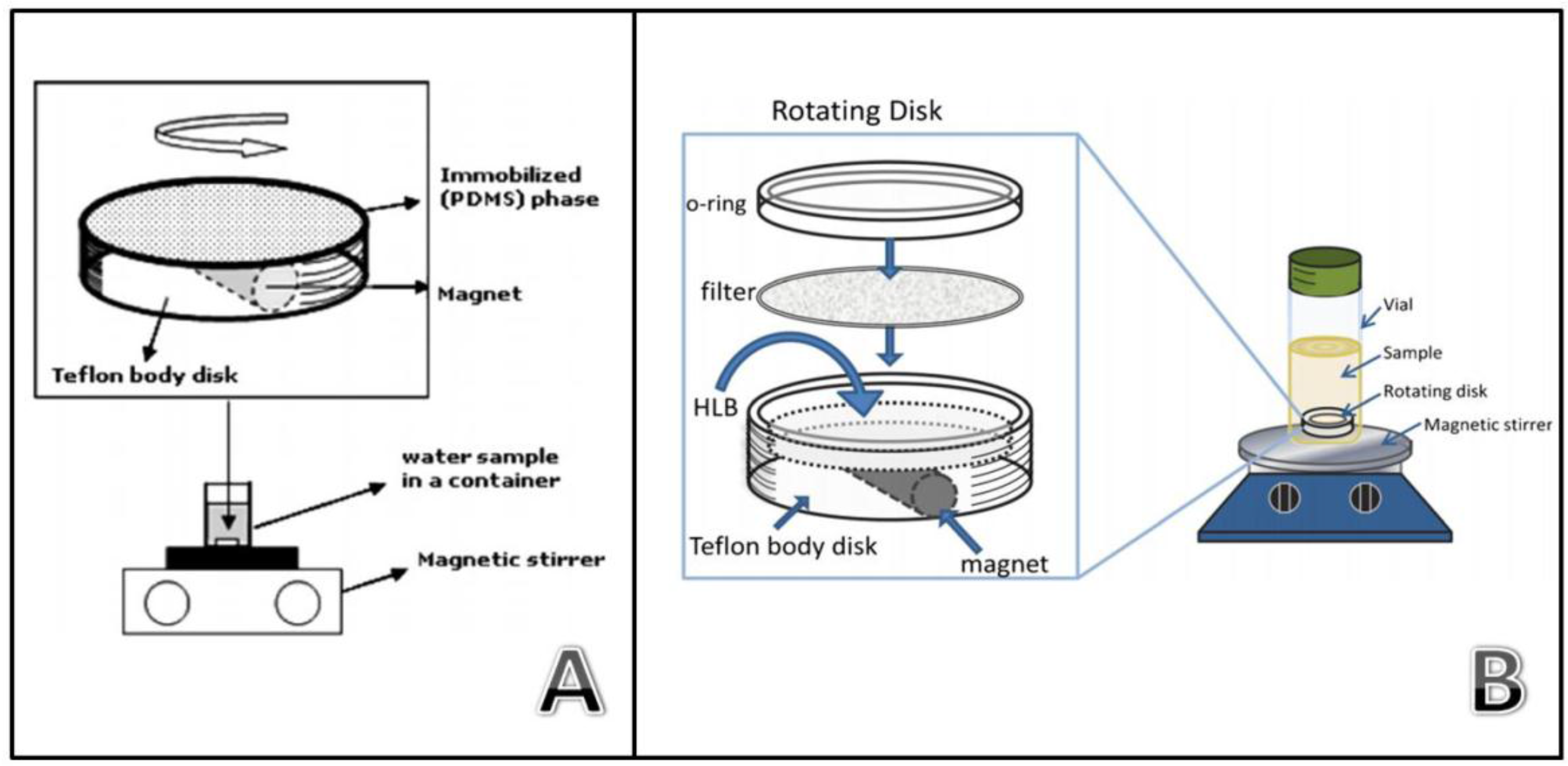
5. Stir Disk Extractions
Disks offer a higher superficial area than bars and, in some cases, they present a higher porosity that enhances the diffusion of the analytes through the sorptive phase. Rotating disk sorptive extraction (RDSE) was the first approach in this context [56]. The RDSE unit (Figure 10A) consists of a thin film of PDMS deposited over a PTFE disk containing an integrated magnetic bar. Similarly to SCSE, this configuration protects the sorptive phase from direct contact with the vial, allowing for the application of faster stirring rates than conventional SBSE. RDSE operates in a similar fashion than SBSE since the unit is stirred into the sample for a defined period of time for the isolation of the target compounds. After the extraction the unit is recovered for the final instrumental analysis. When chromatographic techniques are employed, the analytes must be eluted with a proper solvent [57,58]. However, the configuration of the unit allows for the detachment of the film after the extraction for its direct spectroscopic analysis, avoiding the dilution inherent to the elution step [59,60]. RDSE has evolved in the last few years following two different trends, namely: the development of new extraction phases and the potential automation of the technique [61].
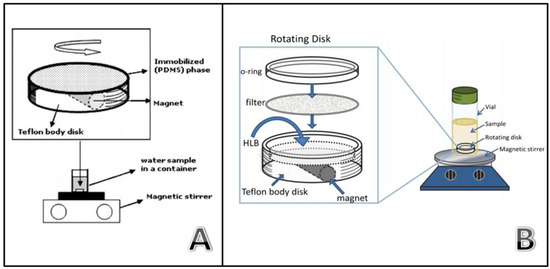
Figure 10.
Device for rotating disk sorptive extraction using thin films (Reproduced with permission from [56], copyright Elsevier, 2009) (A) and sorbent particles (Reproduced with permission from [63], copyright Springer, 2014) (B).
PDMS has a clear potential as a sorptive phase but presents some disadvantages that have been previously described. The development of new phases in RSDE will increase the versatility of the technique, opening the door to the extraction of analytes of intermediate or high polarity. The first approach is this context involved the use of conventional C18 solid phase extraction (SPE) disks for the extraction of hexachlorobenzene from water [62]. These SPE disks, which are membranes with embedded particles, are linked to the device using silicone as a binder. The results were promising but these disks have non-polar compounds as targets. Some polymeric SPE sorbents have been specially designed for the extraction of polar compounds. Richter et al. proposed an evolution of the classic RDSE in order to exploit this characteristic. In this case, a cavity is dug into the unit and finally loaded with polymeric sorbent (HLB) particles (Figure 10B) [63]. The cavity is covered with a filter to confine the particles, avoiding their losses during the extraction. The great variety of commercially available SPE sorbents and their easy loading on the unit makes this approach highly versatile. In addition, lab-synthesized sorbents can be also applied. In this sense, researchers have proposed the use of MIPs [64] and ionic liquids intercalated in montmorillonite [65] as a sorptive phase in this device.
Polyethylene disks, conventionally used as frits in solid phase extraction cartridges, can be also used as stirred units by simply piercing them with a metallic wire. This material is hydrophobic and it is indicated for the extraction of hydrophobic compounds [66]. The material exhibits sufficient thermal stability to allow for its mild thermal desorption in a conventional headspace vial.
Borosilicate disks can also be excellent supports for RDSE due to their mechanical strength. However, as polar materials, they are not able to extract organic compounds from aqueous samples. Borosilicate disks have been modified with carbon nanohorns on the surface for the extraction of benzophenone-3 from swimming pool water [67]. These modified disks can be easily adapted to a portable drill (Figure 11) that stirs them into the sample for the on-site extraction of the target compound. The enrichment factor and extraction recovery, which are 1379 and 68.9%, respectively, reveal the great potential of the technique.

Figure 11.
Borosilicate disk modified with carbon nanohorns extraction device. Reproduced with permission of from [67], copyright Elsevier, 2014.
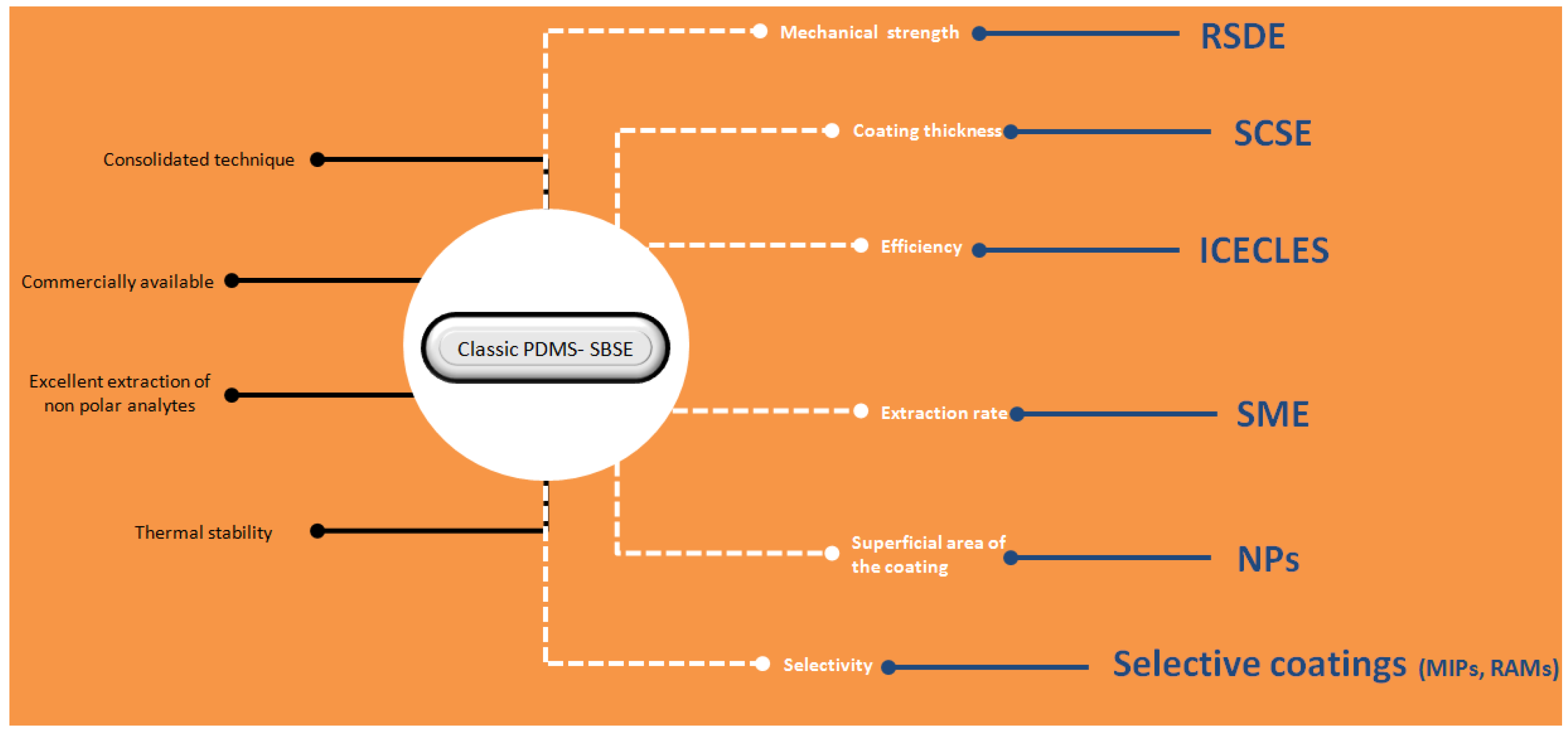
6. Conclusions
The approaches described in this review article clearly demonstrate the advantages of extraction/stirring integrated techniques. Since its proposal in 1999, new microextraction formats and new sorptive materials have been developed. No doubt, the combination of both aspects results in an analytical measurement processes with enhanced basic (sensitivity, selectivity) and productivity-related (rapidity, environmental friendship) analytical properties. Monolithic solids, polymers, nanoparticles, and ionic liquids can be cited among the most novel and efficient sorptive phases. SCSE and RDSE are, on the other hand, the most competitive approaches in solid phase approaches as they minimizes the friction of the coating with the extraction vessel, allowing for the use of higher agitation speeds. SME has proven to be a versatile configuration as it is compatible with solid and liquid phase microextraction approaches. Moreover, it permits the processing of low sample volumes by a simple re-design of the unit.
Future trends will be focused on the application of new materials and new extraction strategies. In this context, ICECLES is especially useful.
Figure 12 shows a brief summary of the techniques presented in this review article. The discussion has been focused on the main advantages provided by the novel techniques compared with classical SBSE. Despite these advantages, it is necessary to point out that SBSE is a consolidated technique due to its commercial availability and robustness.
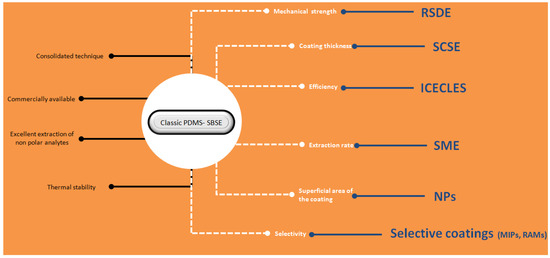
Figure 12.
Brief summary of the techniques described in the article. The advantages of classic polydimethylsiloxane (PDMS) based stir bar sorptive extraction (SBSE) are shown in black letters, the characteristics that may be compromised in several applications are indicated in white characters, and the techniques/material that can overcome these limitations are presented in blue. For further details, read the text.
Acknowledgments
Financial support from the Spanish Ministry of Economy and Competitiveness (CTQ2014-52939R) is gratefully acknowledged.
Author Contributions
Both authors contributed equally to the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucena, R. Extraction and stirring integrated techniques: Examples and recent advances. Anal. Bioanal. Chem. 2012, 403, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Vera, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Sample treatments based on dispersive (micro)extraction. Anal. Methods 2011, 3, 1719–1728. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcol. Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Wells, M.J.M. Sample Preparation Techniques in Analytical Chemistry; Mitra, S., Ed.; John Wiley & Sons: Hobojen, NY, USA, 2003. [Google Scholar]
- Nogueira, J.M.F. Stir bar sorptive extraction and related techniques. In Analytical Microextraction Techniques; Valcárcel, M., Cárdenas, S., Lucena, R., Eds.; Bentham Science: Sharjah, UAE, 2017. [Google Scholar]
- Lucena, R. Making biosamples compatible with instrumental analysis. J. Appl. Bioanal. 2015, 1, 72–75. [Google Scholar] [CrossRef]
- Zhu, X.; Cai, J.; Yang, J.; Su, Q.; Gao, Y. Films coated with molecular imprinted polymers for the selective stir bar sorption extraction of monocrotophos. J. Chromatogr. A 2006, 1131, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, Y.; Hu, Y.; Li, G. Investigation of ractopamine molecularly imprinted stir bar sorptive extraction and its application for trace analysis of β2-agonists in complex samples. J. Chromatogr. A 2010, 1217, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Hu, Y.; Hu, Y.; Li, G. Online desorption of molecularly imprinted stir bar sorptive extraction coupled to high performance liquid chromatography for the trace analysis of triazines in rice. J. Sep. Sci. 2012, 35, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted stir bars for selective extraction of thiabendazole in citrus samples. J. Sep. Sci. 2012, 35, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, G.; Wei, F.; Yang, J.; Hu, Q. Determination of melamine in powdered milk by molecularly imprinted stir bar sorptive extraction coupled with HPLC. J. Colloid Interface Sci. 2015, 454, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, Z.; Liu, Z. Development of dual-templates molecularly imprinted stir bar sorptive extraction and its application for the analysis of environmental estrogens in water and plastic samples. J. Chromatogr. A 2014, 1358, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sheng, N.; Wei, F.; Zhan, W.; Cai, Z.; Du, S.; Zhou, X.; Li, F.; Hu, Q. Dummy molecularly imprinted polymers as the coating of stir bar for sorptive extraction of bisphenol A in tap water. J. Sep. Sci. 2012, 35, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; He, M.; Chen, B.; Hu, B. Polyaniline/cyclodextrin composite coated stir bar sorptive extraction combined with high performance liquid chromatography-ultraviolet detection for the analysis of trace polychlorinated biphenyls in environmental waters. Talanta 2016, 150, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gan, N.; Zhang, J.; Qiao, L.; Chen, Y.; Cao, Y. Aptamer-Functionalized stir bar sorptive extraction coupled with gas chromatography-mass spectrometry for selective enrichment and determination of polychlorinated biphenyls in fish samples. Talanta 2016, 149, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Bottero, J.; Lowry, G.V.; Jolivet, J.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Valverde, M.T.; Lucena, R.; Cardenas, S.; Valcarcel, M. Titanium-Dioxide nanotubes as sorbents in (micro)extraction techniques. Trends Anal. Chem. 2014, 62, 37–45. [Google Scholar] [CrossRef]
- Lasarte Aragonés, G.; Lucena, R.; Cardenas, S.; Valcarcel, M. Nanoparticle-based microextraction techniques in bioanalysis. Bioanalysis 2011, 3, 2533–2548. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, B.; Li, X. Zirconia coated stir bar sorptive extraction combined with large volume sample stacking capillary electrophoresis-indirect ultraviolet detection for the determination of chemical warfare agent degradation products in water samples. J. Chromatogr. A 2012, 1247, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Giokas, D.L.; Salvador, A. Development of stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles and its analytical application to the determination of hydrophobic organic compounds in aqueous media. J. Chromatogr. A 2014, 1362, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Chisvert, A.; Giokas, D.L.; Salvador, A. Stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles–nylon 6 composite for the extraction of hydrophilic organic compounds in aqueous media. Anal. Chim. Acta 2016, 926, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Chen, B.; He, M.; Hu, B. Amino modified multi-walled carbon nanotubes/polydimethylsiloxane coated stir bar sorptive extraction coupled to high performance liquid chromatography-ultraviolet detection for the determination of phenols in environmental samples. J. Chromatogr. A 2013, 1300, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, K.; Firuzi, M.; Hatami, M. Stir bar sorptive extraction of propranolol from plasma samples using a steel pin coated with a polyaniline and multiwall carbon nanotube composite. Microchim. Acta 2015, 182, 323–330. [Google Scholar] [CrossRef]
- Ekbatani Amlashi, N.; Hadjmohammadi, M.R. Sol-Gel coating of poly(ethylene glycol)-grafted multiwalled carbon nanotubes for stir bar sorptive extraction and its application to the analysis of polycyclic aromatic hydrocarbons in water. J. Sep. Sci. 2016, 39, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; He, M.; Wu, X.; Chen, B.; Hu, B. Graphene oxide/polyethyleneglycol composite coated stir bar for sorptive extraction of fluoroquinolones from chicken muscle and liver. J. Chromatogr. A 2015, 1418, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Zhang, J.; Meng, J.; Bao, T.; Chen, Z. Covalent immobilization of graphene onto stainless steel wire for jacket-free stir bar sorptive extraction. J. Chromatogr. A 2014, 1351, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; He, M.; You, L.; Zhu, X.; Chen, B.; Hu, B. Water-Compatible graphene oxide/molecularly imprinted polymer coated stir bar sorptive extraction of propranolol from urine samples followed by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2016, 1443, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maslamani, N.; Manandhar, E.; Geremia, D.K.; Logue, B.A. ICE concentration linked with extractive stirrer (ICECLES). Anal. Chim. Acta 2016, 941, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Stir membrane extraction: A useful approach for liquid sample pretreatment. Anal. Chem. 2009, 81, 8957–8961. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lendl, B.; Lucena, R.; Cárdenas, S. Valcárcel, Sensitive in-surface infrared monitoring coupled to stir membrane extraction for the selective determination of total hydrocarbon index in waters. Anal. Bioanal. Chem. 2010, 398, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractors (FPSE). US Patent Application No. 14.216,121, 2014. [Google Scholar]
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A 2015, 1376, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Stir membrane liquid-liquid microextraction. J. Chromatogr. A 2011, 1218, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Determination of phenols in waters by stir membrane liquid–liquid–liquid microextraction coupled to liquid chromatography with ultraviolet detection. J. Chromatogr. A 2011, 1218, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Riaño, S.; Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Determination of non-steroidal anti-inflammatory drugs in urine by the combination of stir membrane liquid–liquid–liquid microextraction and liquid chromatography. Anal. Bioanal. Chem. 2012, 403, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Alcudia-León, M.C.; Lucena, R.; Cárdenas, S. Valcárcel, Stir-membrane liquid microextraction for the determination of paracetamol in human saliva samples. Bioanalysis 2013, 5, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, R.; Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A.; Valcárcel, M. Stir-Membrane solid–liquid–liquid microextraction for the determination of parabens in human breast milk samples by ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1354, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Lucena, R.; Alcudia-León, M.C.; Cárdenas, S.; Valcárcel, M. Stir octadecyl-modified borosilicate disk for the liquid phase microextraction of triazine herbicides from environmental waters. J. Chromatogr. A 2013, 1307, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S. Valcárcel, M. Magnetically confined hydrophobic nanoparticles for the microextraction of endocrine-disrupting phenols from environmental waters. Anal. Bioanal. Chem. 2013, 405, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Determination of parabens in waters by magnetically confined hydrophobic nanoparticle microextraction coupled to gas chromatography/mass spectrometry. Microchem. J. 2013, 110, 643–648. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Lin, F.; Yuan, D. Novel extraction approach for liquid samples: Stir cake sorptive extraction using monolith. J. Sep. Sci. 2011, 34, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Dario Arruda, R.; Causon, T.J.; Hilder, E.F. Recent developments and future possibilities for polymer monoliths separation science. Analyst 2012, 137, 5179–5189. [Google Scholar]
- Welton, T. Room-Temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.J.; Pino, V.; Ayala, J.H.; Afonso, A.M. Ionic liquids in the microextraction context. In Analytical Microextraction Techniques; Valcárcel, M., Cárdenas, S., Lucena, R., Eds.; Bentham Science: Sharjah, UAE, 2017; pp. 70–134. [Google Scholar]
- López-Darias, J.; Pino, V.; Anderson, J.L.; Graham, C.M.; Afonso, A.M. Determination of water pollutants by direct-immersion solid-phase microextraction using polymeric ionic liquid coatings. J. Chromatogr. A 2010, 1217, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, L.; Yuan, X.; Si, S. Preparation of a new polymeric ionic liquid-based monolith for stir cake sorptive extraction and its application in the extraction of inorganic anions. J. Chromatogr. A 2012, 1248, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, M.; Ouyang, T.; Huang, X. Preparation of a new polymeric ionic-liquid-based sorbent for stir cake sorptive extraction of trace antimony in environmental water samples. Talanta 2016, 161, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Nong, S.; Huang, X.; Yuan, D. Sensitive determination of organic acid preservatives in juices and soft drinks treated by monolith-based stir cake sorptive extraction and liquid chromatography analysis. Anal. Bioanal. Chem. 2013, 405, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, X. Preparation of a polymeric ionic liquid-based adsorbent for stir cake sorptive extraction of preservatives in orange juices and tea drinks. Anal. Chim. Acta 2016, 916, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Huang, X.; Yuan, D. Preparation of stir cake sorptive extraction based on polymeric ionic liquid for the enrichment of benzimidazole anthelmintics in water, honey and milk samples. Anal. Chim. Acta 2014, 840, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mei, M.; Huang, X.; Yuan, D. Sensitive determination of estrogens in environmental waters treated with polymeric ionic liquid-based stir cake sorptive extraction and liquid chromatographic analysis. Talanta 2016, 152, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Hong, Q.; Liu, Y.; Yuan, D. Preparation a new sorbent based on polymeric ionic liquid for stir cake sorptive extraction of organic compounds and inorganic anions. J. Chromatogr. A 2013, 1314, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Yuan, D.; Li, X.; Nong, S. New monolithic stir-cake-sorptive extraction for the determination of polar phenols by HPLC. Anal. Bioanal. Chem. 2013, 405, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, L.; Yuan, D. Preparation of stir cake sorptive extraction based on poly(4-vinylbenzoic acid-divinylbenzene) monolith and its application in sensitive determination of β-agonists in milk and swine urine samples. J. Hazard. Mater. 2013, 262, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Huang, X. Determination of fluoroquinolones in environmental water and milk samples treated with stir cake sorptive extraction based on a boron-rich monolith. J. Sep. Sci. 2016, 39, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Leiva, C.; Choque, C.; Giordano, A.; Sepúlveda, B. Rotating-Disk sorptive extraction of nonylphenol from water samples. J. Chromatogr. A 2009, 1216, 8598–8602. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Richter, P.; Ahumada, I. Determination of pesticides in river water using rotating disk sorptive extraction and gas chromatography-mass spectrometry. Talanta 2011, 85, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Jachero, L.; Sepúlveda, B.; Ahumada, I.; Fuentes, E.; Richter, P. Rotating disk sorptive extraction of triclosan and methyl-triclosan from water samples. Anal. Bioanal. Chem. 2013, 405, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Manzo, V.; Navarro, O.; Honda, L.; Sánchez, K.; Toral, M.I.; Richter, P. Determination of crystal violet in water by direct solid phase spectrophotometry after rotating disk sorptive extraction. Talanta 2013, 106, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Toral, M.I.; Ahumada, I.; Richter, P. Rotating disk sorptive extraction of Cu-bisdiethyldithiocarbamate complex from water and its application to solid phase spectrophotometric quantification. Anal Sci. 2014, 30, 613–617. [Google Scholar] [PubMed]
- Manzo, V.; Miró, M.; Richter, P. Programmable flow-based dynamic sorptive microextraction exploiting an octadecyl chemically modified rotating disk extraction system for the determination of acidic drugs in urine. J. Chromatogr. A 2014, 1368, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Cañas, A.; Richter, P. Solid-Phase microextraction using octadecyl-bonded silica immobilized on the surface of a rotating disk: Determination of hexachlorobenzene in water. Anal. Chim. Acta 2012, 743, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Cañas, A.; Valdebenito, S.; Richter, P. A new rotating-disk sorptive extraction mode, with a copolymer of divinylbenzene and N-vinylpyrrolidone trapped in the cavity of the disk, used for determination of florfenicol residues in porcine plasma. Anal. Bioanal. Chem. 2014, 406, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Manzo, V.; Ulisse, K.; Rodríguez, I.; Pereira, E.; Richter, P. A molecularly imprinted polymer as the sorptive phase immobilized in a rotating disk extraction device for the determination of diclofenac and mefenamic acid in wastewater. Anal. Chim. Acta 2015, 889, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Fiscal-Ladino, J.A.; Obando-Ceballos, M.; Rosero-Moreano, M.; Montaño, D.G.; Cardona, W.; Giraldo, L.F.; Richter, P. Ionic liquids intercalated in montmorillonite as the sorptive phase for the extraction of low-polarity organic compounds from water by rotating-disk sorptive extraction. Anal. Chim. Acta 2017, 953, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Stir frit microextraction: An approach for the determination of volatile compounds in water by headspace-gas chromatography/mass spectrometry. J. Chromatogr. A 2012, 1251, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Micro-Solid phase extraction based on oxidized single-walled carbon nanohorns immobilized on a stir borosilicate disk: Application to the preconcentration of the endocrine disruptor benzophenone-3. Microchem. J. 2014, 115, 87–94. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).