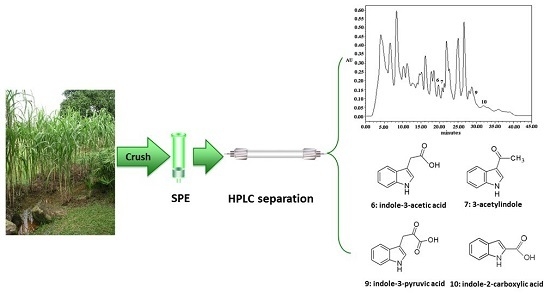
Analyses of Indole Compounds in Sugar Cane (Saccharum officinarum L.) Juice by High Performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry after Solid-Phase Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. HPLC Instrumental Set-Up and Procedure
2.3. LC-MS/MS Conditions
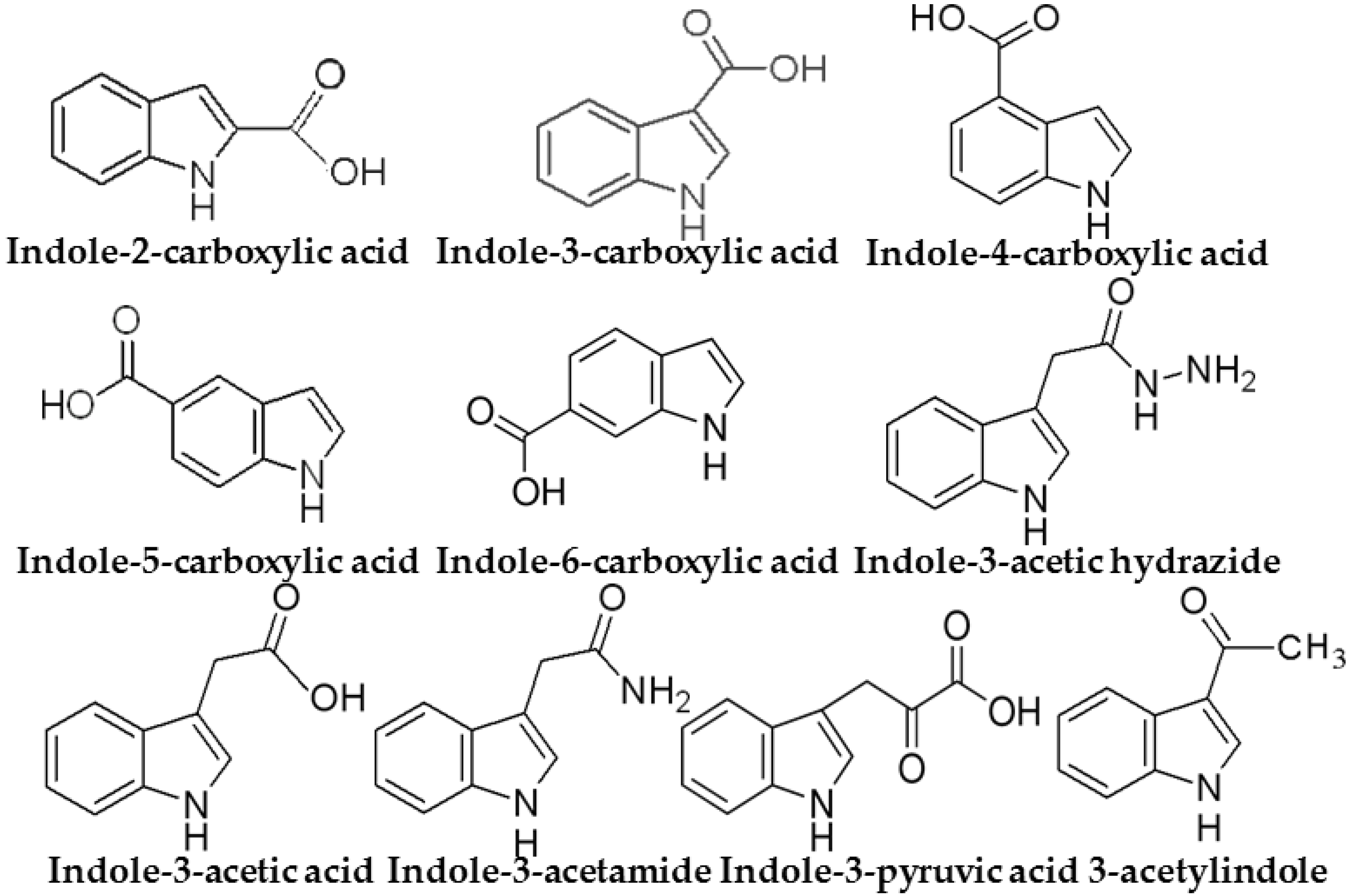
2.4. Retention of Indole Compounds on SPE Cartridges
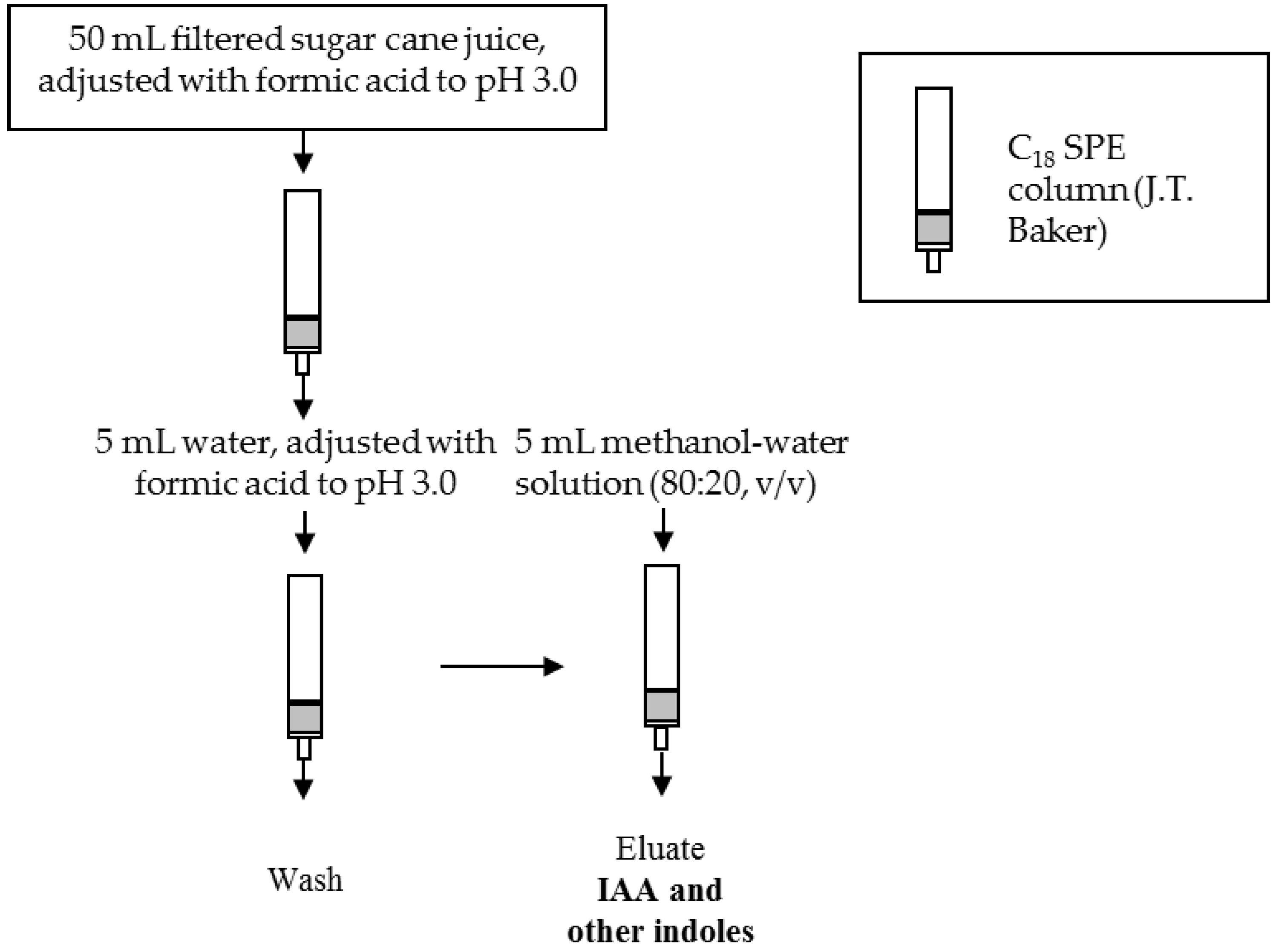
2.5. Sample Preparation
2.6. Recovery Study of SPE Procedure
3. Results and Discussion
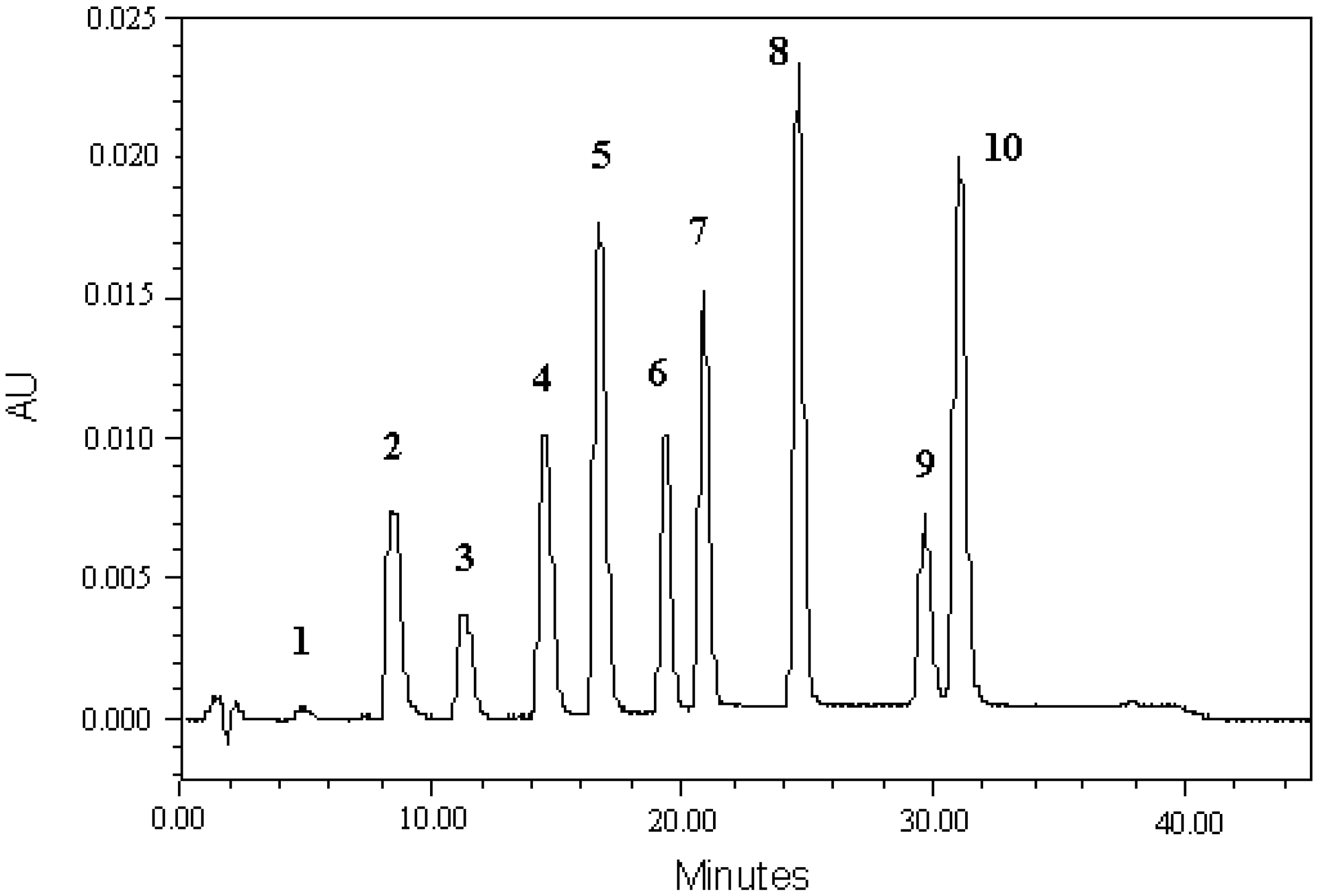
3.1. Development HPLC-PDA Method
3.2. Validation of the Method
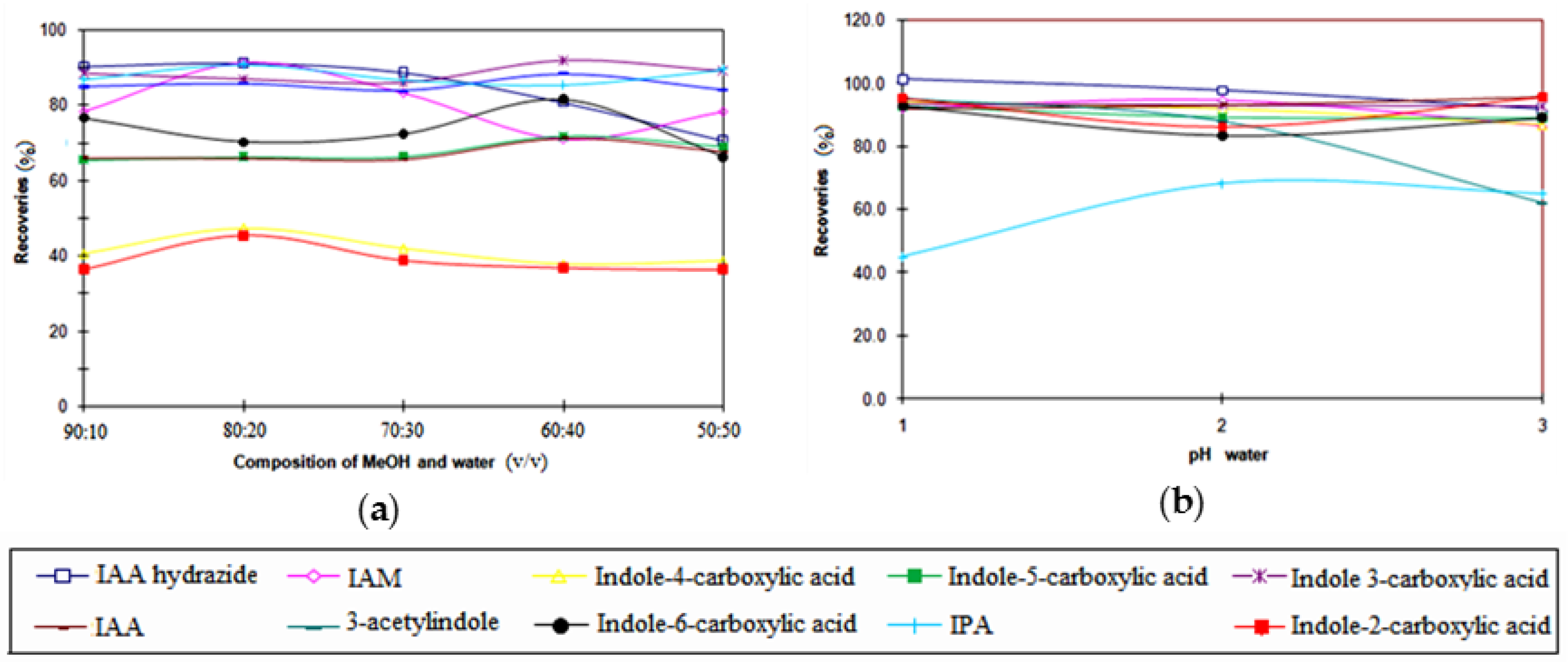
3.3. Optimization of the SPE Conditions
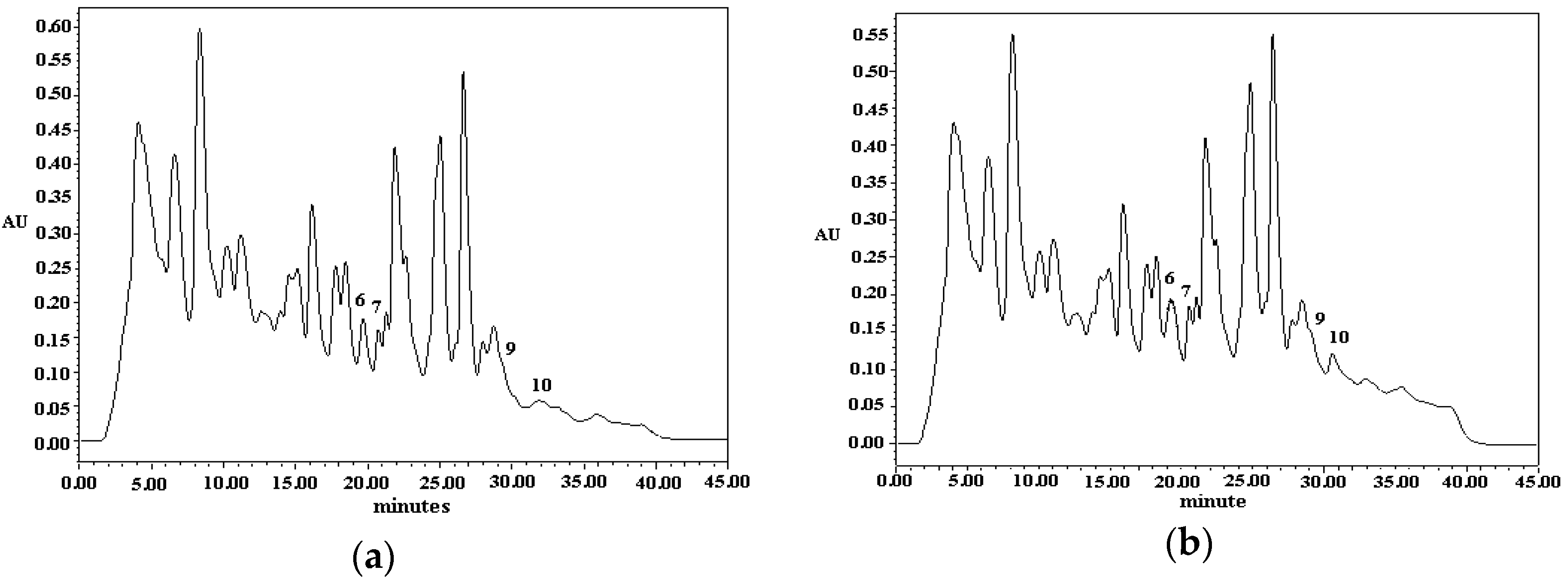
3.4. Analysis of Indole Compounds in Sugar Cane Juice Using HPLC
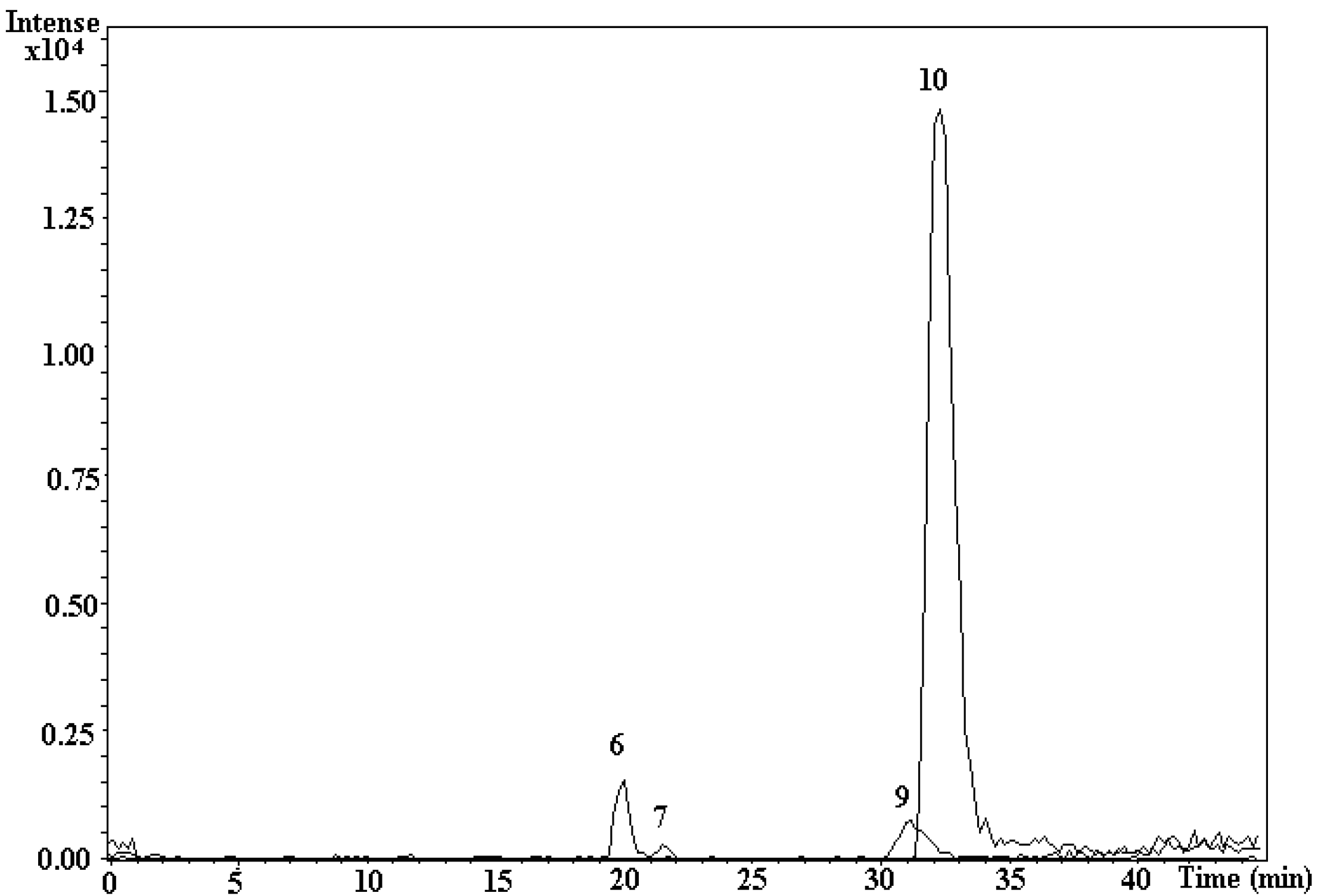
3.5. LC-ESI-MS/MS Measurement
3.6. Recovery Study of SPE Procedure
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Duarte-Almeida, J.M.; Novoa, A.V.; Linares, A.F.; Lajolo, F.M.; Genovese, M.I. Antioxidant activity of phenolics compounds from sugar cane (Saccharum officinarum L.) juice. Plant Food Hum. Nutr. 2006, 61, 187–192. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K. Analysis of sugar cane flavonoids by capillary zone electrophoresis. J. Chromatogr. 1993, 634, 107–112. [Google Scholar] [CrossRef]
- Naik, G.R. Sugarcane Biotechnology; Agritech Publications: Shrub Oak, NY, USA, 2001. [Google Scholar]
- Arditti, J.; Ernst, R. Micropropagation of Orchids; John Wiley & Sons, Inc.: New York, NY, USA, 1993; pp. 47–48. [Google Scholar]
- Ang, S.L.P.; Yong, J.W.H. A protocol for in vitro germination and sustainable growth of two tropical mistletoes. Plant Cell Tissue Organ Cult. 2005, 80, 221–228. [Google Scholar] [CrossRef]
- Available online: http://en.wikipedia.org/wiki/Indole (accessed on 1 December 2016).
- Guerrero, J.R.; Garcia-Ruiz, P.; Sanchez-Bravo, J.; Acosta, J.M.; Arnao, M.B. Quantification of indole-3-acetic acid by LC with electrochemical detection in etiolated hypocotyls of Lupinus Albus. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 3095–3104. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones: Biosynthesis, Signal Transduction, Action! Kluwer Academic Publishers: Norwell, MA, USA, 2004. [Google Scholar]
- Kende, H.; Zeevarrt, J.A.D. The five “classical” plant hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Manulis, S.; Shafrir, H.; Epstein, E.; Lichter, A.; Barash, I. Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology 1994, 140, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Matsumoto, T.; Chujo, T.; Nojiri, H.; Omori, T.; Minamisawa, K.; Nishiyama, M.; Yamane, H. Isolation and characterization of low-indole-3-acetic acid-producing mutants from Bradyrhizobium elkanii. Biosci. Biotechnol. Biochem. 2001, 65, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, S.; Neu, D.; Weiler, E. Molecular cloning and characterization of an amidase from Arabidopsis thaliana capable of converting indole-3-acetamide into the plant growth hormone, indole-3-acetic acid. Phytochemistry 2003, 62, 293–300. [Google Scholar] [CrossRef]
- Silvestri, R.; Mento, G.; Raffaele, M.; Luca, G.D.; Buttini, G.; Casella, C.; Tisano, A.; Domenico, P.D.; Rosa, A.D.; Perri, R.D. Indole-3-pyruvic acid as a possible hypnotic agent in insomniac subjects. J. Int. Med. Res. 1991, 19, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Lapin, I.P.; Politi, V. Anxiolytic effect of indole-3-pyruvic acid (IPA) in mice. Pharmacol. Res. 1993, 28, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Politi, V.; Deluca, G.; Gallai, V.; Puca, O.; Comin, M. Clinical experiences with the use of indole-3-pyruvic acid. Adv. Exp. Med. Biol. 1999, 467, 227–232. [Google Scholar] [PubMed]
- Bartolini, B.; Corniello, C.; Sella, A.; Somma, F.; Politi, V. The enol tautomer of indole-3-pyruvic acid as a biological switch in stress responses. Adv. Exp. Med. Biol. 2003, 527, 601–608. [Google Scholar] [PubMed]
- Kaminski, R.; Wolinska, E.; Gasior, M.; Kleinrok, Z.; Czuczwar, S.J. NMDA receptor glycine site antagonist (indole-2-carboxylic acid) enhances the anticonvulsive activity of antiepileptic drugs. Neurosci. Res. Commun. 1998, 22, 137–145. [Google Scholar] [CrossRef]
- Stetinova, V.; Smetanova, L.; Grossmann, V.; Anzenbacher, P. In vitro and in vivo assessment of the antioxidant activity of melatonin and related indole derivatives. Gen. Physiol. Biophys. 2002, 21, 153–162. [Google Scholar] [PubMed]
- Rajendra, N.G.; Aditi, S. Oxidation chemistry of indole-2-carboxylic acid: Mechanism and products formed in neutral aqueous solution. Electrochim. Acta 2004, 50, 2135–2143. [Google Scholar]
- Rani, P.; Srivastava, V.K.; Kumar, A. Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur. J. Med. Chem. 2004, 39, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Siatra-Papastaikoudi, T.; Tsotinis, A.; Raptopoulou, C.; Sambani, C.; Thomou, H. Synthesis of new alkylaminoalkyl thiosemicarbazones of 3-acetylindole and their effect on DNA synthesis and cell proliferation. Eur. J. Med. Chem. 1995, 30, 107–114. [Google Scholar] [CrossRef]
- Moussata, J.; Wang, Z.; Wang, J. Development and validation of an HPLC method for the simultaneous quantification of indole-3-carbinol acetate, indole-3-carbinol, and 3,3′-diindolylmethane in mouse plasma, liver, and kidney tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 958, 1–9. [Google Scholar]
- Gomez, F.J.V.; Hernández, I.G.; Cerutti, S.; Silva, M.F. Solid phase extraction/cyclodextrin-modified micellar electrokinetic chromatography for the analysis of melatonin and related indole compounds in plants. Microchem. J. 2015, 123, 22–27. [Google Scholar] [CrossRef]
- Grunwald, C.; Lockard, R.G. Analysis of indole acid derivatives by gas chromatography using liquid phase OV-101. J. Chromatogr. A 1970, 52, 491–493. [Google Scholar] [CrossRef]
- Gallagher, C.A.; Hough, L.B.; Keefner, S.M.; Seyed-Mozaffari, A.; Archer, S.; Glick, S.D. Identification and quantification of the indole alkaloid ibogaine in biological samples by gas chromatography-mass spectrometry. Biochem. Pharmacol. 1995, 49, 73–79. [Google Scholar] [CrossRef]
- Müller, A.; Düchting, P.; Weiler, E. A multiplex GC-MS/MS technique for the sensitive and quantitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana. Planta 2002, 216, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J.; Georgiev, M.; Bley, T. Metabolite and hormonal status of hairy root cultures of Devil’s claw (Harpagophytum procumbens) in flasks and in a bubble column bioreactor. Process Biochem. 2008, 43, 15–23. [Google Scholar] [CrossRef]
- Tan, S.N.; Yong, J.W.H.; Ge, L. Analyses of phytohormones in coconut (Cocos Nucifera L.) water using capillary electrophoresis-tandem mass spectrometry. Chromatography 2014, 1, 211–226. [Google Scholar] [CrossRef]
- Fei, X.Q.; Li, C.; Yu, X.D.; Chen, H.Y. Determination of heterocyclic amines by capillary electrophoresis with UV-DAD detection using on-line preconcentration. J. Chromatogr. B 2007, 854, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Graffeo, A.P.; Karger, B.L. Analysis for indole compounds in urine by high-performance liquid chromatography with fluorometric detection. Clin. Chem. 1976, 22, 184–187. [Google Scholar] [PubMed]
- Hanna, M.; Alicja, K.; Ksenia, P. Determination of indole-3-acetic acid in the Gulf of Gdańsk by high-performance liquid chromatography of its 4-methyl-7-methoxycoumarin derivative. J. Chromatogr. A 1997, 766, 261–266. [Google Scholar]
- Matsuda, F.; Miyazawa, H.; Wakasa, K.; Miyagawa, H. Quantification of indole-3-acetic acid and amino acid conjugates in rice by liquid chromatography-electrospray ionization-tandem mass spectrometry. Biosci. Biotechnol. Biochem. 2005, 69, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, K.; Noppe, H.; Aluwé, M.; Millet, S.; Bussche, J.V.; Brabander, H.F.D. Development and validation of a method for simultaneous analysis of the boar taint compounds indole, skatole and androstenone in pig fat using liquid chromatography-multiple mass spectrometry. J. Chromatogr. A 2007, 1174, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Versini, G. Determination of indole-3-acetic acid, tryptophan and other indoles in must and wine by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 1999, 855, 227–235. [Google Scholar] [CrossRef]
- Gerasimenko, I.; Sheludko, Y.; Unger, M.; Stöckigt, J. Development of an efficient system for the separation of indole alkaloids by high performance liquid chromatography and its applications. Phytochem. Anal. 2001, 12, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Yusof, S.; Shian, L.S.; Osman, A. Changes in quality of sugar-cane juice upon delayed extraction and storage. Food Chem. 2000, 68, 395–401. [Google Scholar] [CrossRef]
- Ma, Z.; Ge, L.; Lee, A.S.Y.; Yong, J.W.H.; Tan, S.N.; Ong, E.S. Simultaneous analysis of different classes of phytohormones in coconut (Cocos nucifera L.) water using high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry after solid-phase extraction. Anal. Chim. Acta 2008, 610, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, W.T.; Arshad, M. (Eds.) Phytohormones in Soils: Microbial Production and Function; Marcel Dekker, Inc.: New York, NY, USA, 1995; pp. 17–136.
- Sun, J.; Baker, A.; Chen, P. Profiling the indole alkaloids in yohimbe bark with ultra-performance liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Preti R. Core-shell columns in high-performance liquid chromatography: Food analysis applications. Int. J. Anal. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Indole Compounds | MRM Transition (m/z) |
|---|---|
| Indole-2-carboxylic acid | 160→115 |
| Indole-3-carboxylic acid | 160→115 |
| Indole-4-carboxylic acid | 160→115 |
| Indole-5-carboxylic acid | 160→115 |
| Indole-6-carboxylic acid | 160→115 |
| Indole-3-acetic acid (IAA) | 173→129 |
| Indole-3-acetic hydrazide | 188→115 |
| Indole-3-acetamide (IAM) | 173→129 |
| Indole-3-pyruvic acid (IPA) | 202→157 |
| 3-acetylindole | 158→115 |
| Indole Compounds | Retention Times a | Peak Area a | Equation of Calibration Curve b | R2 | Linear Range (μM) | LOQ (μM) c | ||
|---|---|---|---|---|---|---|---|---|
| Mean (min) | RSD (%) | Mean (μAu·s) | RSD (%) | |||||
| Indole-3-acetic hydrazide | 4.827 | 0.10 | 11,362 | 6.0 | y = 31.8x + 9717.1 | 0.998 | 25–200 | 1.09 |
| Indole-3-acetamide | 8.432 | 0.08 | 290,551 | 0.4 | y = 6231.3x − 9126.4 | 0.999 | 12.5–200 | 0.08 |
| Indole-4-carboxylic acid | 11.331 | 0.15 | 135,491 | 1.5 | y = 2920.1x − 13671 | 0.989 | 12.5–200 | 1.64 |
| Indole-5-carboxylic acid | 14.574 | 0.17 | 333,576 | 0.7 | y = 7086.2x − 14788.3 | 0.999 | 12.5–200 | 1.50 |
| Indole-3-carboxylic acid | 16.723 | 0.18 | 545,307 | 0.6 | y = 11269.9x − 13352.6 | 0.999 | 12.5–200 | 1.34 |
| Indole-3-acetic acid | 19.358 | 0.19 | 284,600 | 0.3 | y = 5999.5x − 13144.3 | 0.999 | 12.5–200 | 1.15 |
| 3-acetylindole | 20.867 | 0.18 | 398,848 | 1.0 | y = 8465.8x − 13598.7 | 0.999 | 12.5–200 | 1.49 |
| Indole-6-carboxylic acid | 24.659 | 0.24 | 589,492 | 1.7 | y = 12959x − 17287.8 | 0.992 | 12.5–200 | 1.48 |
| Indole-3-pyruvic acid | 29.637 | 0.22 | 208,408 | 2.8 | y = 5459.3x − 61613.2 | 0.996 | 12.5–200 | 1.72 |
| Indole-2-carboxylic acid | 31.044 | 0.21 | 615,529 | 0.2 | y = 13249.1x − 35713.8 | 0.999 | 12.5–200 | 0.11 |
| Indole Compounds | Intra-Day Variability a | Inter-Day Variability d | ||
|---|---|---|---|---|
| RSD (tR b %) | RSD (pa c %) | RSD (tR %) | RSD (pa %) | |
| Indole-3-acetic hydrazide | 0.10 | 6.0 | 0.12 | 7.1 |
| Indole-3-acetamide | 0.08 | 0.4 | 0.09 | 1.3 |
| Indole-4-carboxylic acid | 0.15 | 1.5 | 0.16 | 1.5 |
| Indole-5-carboxylic acid | 0.17 | 0.7 | 0.19 | 2.1 |
| Indole-3-carboxylic acid | 0.18 | 0.6 | 0.19 | 1.6 |
| Indole-3-acetic acid | 0.19 | 0.3 | 0.20 | 0.9 |
| 3-acetylindole | 0.18 | 1.0 | 0.20 | 1.2 |
| Indole-6-carboxylic acid | 0.24 | 1.7 | 0.25 | 1.9 |
| Indole-3-pyruvic acid | 0.22 | 2.8 | 0.23 | 1.4 |
| Indole-2-carboxylic acid | 0.21 | 0.2 | 0.23 | 0.8 |
| Indole Compounds | Estimated Concentrations (µM) | Recovery (%) | Estimated Original Concentrations (µM) |
|---|---|---|---|
| Indole-3-acetic hydrazide | n.d. | 92% | Below 1.18 |
| Indole-3-acetamide | n.d. | 80% | Below 0.10 |
| Indole-4-carboxylic acid | n.d. | 79% | Below 2.08 |
| Indole-5-carboxylic acid | n.d. | 65% | Below 2.31 |
| Indole-3-carboxylic acid | n.d. | 78% | Below 1.72 |
| Indole-3-acetic acid | 1.05 ± 0.23 | 68% | 1.54 ± 0.34 |
| 3-acetylindole | 0.57 ± 0.11 | 62% | 0.92 ± 0.18 |
| Indole-6-carboxylic acid | n.d. | 87% | Below 1.70 |
| Indole-3-pyruvic acid | 0.21 ± 0.08 | 61% | 0.34 ± 0.13 |
| Indole-2-carboxylic acid | 0.34 ± 0.17 | 82% | 0.41 ± 0.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, J.W.H.; Ge, L.; Wong, W.S.; Ma, Z.; Tan, S.N. Analyses of Indole Compounds in Sugar Cane (Saccharum officinarum L.) Juice by High Performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry after Solid-Phase Extraction. Separations 2017, 4, 7. https://doi.org/10.3390/separations4010007
Yong JWH, Ge L, Wong WS, Ma Z, Tan SN. Analyses of Indole Compounds in Sugar Cane (Saccharum officinarum L.) Juice by High Performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry after Solid-Phase Extraction. Separations. 2017; 4(1):7. https://doi.org/10.3390/separations4010007
Chicago/Turabian StyleYong, Jean Wan Hong, Liya Ge, Wei San Wong, Zhen Ma, and Swee Ngin Tan. 2017. "Analyses of Indole Compounds in Sugar Cane (Saccharum officinarum L.) Juice by High Performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry after Solid-Phase Extraction" Separations 4, no. 1: 7. https://doi.org/10.3390/separations4010007
APA StyleYong, J. W. H., Ge, L., Wong, W. S., Ma, Z., & Tan, S. N. (2017). Analyses of Indole Compounds in Sugar Cane (Saccharum officinarum L.) Juice by High Performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry after Solid-Phase Extraction. Separations, 4(1), 7. https://doi.org/10.3390/separations4010007